Abstract
Lowering the CP level in piglet diets reduces the risk of postweaning diarrhea and N excretion to the environment. The question remains at what point CP becomes limiting. An experiment was designed with 2 standardized ileal digestible (SID) Lys levels (10 and 11 g) and 6 CP levels (140, 150, 160, 170, 180, 190 g/kg) in a 2 × 6 factorial design (with 6 pens of 6 animals each per treatment). Linear and quadratic (QP) mixed models of performance in function of CP were fitted to study the effect of SID Lys and CP and their interaction. To determine optima, QP models and broken line models with linear (BLL) or quadratic (BLQ) ascending portions were fitted through the data. It was hypothesized 1) that the response to a decreasing digestible CP level could be described with broken line models and 2) that the break point of these models is dependent on the dietary SID Lys level. Decreasing the CP level decreased ADG (P < 0.001). For G:F, the effect of decreasing CP level depended on the SID Lys level (P of the interaction = 0.028 in the linear model and P = 0.002 in the QP model). According to the BLL model, with 11 g SID Lys in the diet, G:F started to decline with CP levels < 176 g CP [SID Lys:CP = 0.062, SID Lys:apparent total tract digestible (ATTD) CP = 0.077], and with 10 g SID Lys, CP levels < 165 g/kg (SID Lys:CP = 0.061, SID Lys:ATTD CP = 0.075) depressed performance. Serum creatinine levels showed a linear decrease with increasing SID Lys:CP levels (P < 0.001). Across both SID Lys levels, when fitting a BLL model, minimal serum urea levels were reached at an SID Lys:CP ratio of 0.064. This seems to be the point where CP and not Lys limits muscle deposition. The small difference in break point between serum urea level and performance suggests that the composition of nonessential AA may also be at stake. The effect of decreasing CP level depends on SID Lys, and using a maximal SID Lys:CP ratio may be useful for optimizing the AA profile of dietary CP. When the SID Lys:CP ratio exceeds 0.064 (SID Lys:ATTD CP > 0.079), protein and not individual AA limits growth in most piglets between 4 and 9 wk of age.
Keywords: crude protein, performance, pig, serum urea, SID Lys
INTRODUCTION
Because piglet feed intake is low shortly after weaning, diets need to be dense so that the animals receive sufficient nutrients for growth. Crude protein is an important nutrient in pig nutrition. Still, high CP levels are associated with higher risk for disturbed intestinal health in newly weaned piglets (Nyachoti et al., 2006; Wellock et al., 2006; Heo et al., 2008, 2009). In older animals, excessive CP levels are linked with increased urea excretion and ammonia production (Millet et al., 2018). Therefore, it is of utmost importance to find the optimal CP level in the diet. It is generally accepted that requirements are based in the first place on the intake of a complete set of AA instead of CP. Decreasing the dietary CP content while maintaining optimal standardized ileal digestible (SID) AA concentrations has been proven successful for maintaining optimal performance. In a study of Gloaguen et al. (2014), a reduction in CP from 176 to 135 g/kg was possible without significantly affecting performance by piglets weighing 10 to 20 kg. In a similar experiment, Jansman et al. (2016) could not reduce CP beyond 160 g/kg before performance of 8 to 25 kg piglets started to decline significantly. In both studies, SID Lys level was well below the optimum for performance (9.2 and 10 g SID Lys/kg, respectively). Results of both studies suggest that meeting the essential AA demand alone does not suffice. Wu (2014) stated that minimal levels are also required for nonessential AA (NEAA). In most studies, only 1 Lys level is used to yield a minimum CP level (Gloaguen et al., 2014; Nemechek et al., 2014; Jansman et al., 2016). As the amount of N needed is probably dependent on the amount of muscle deposition and thus the SID Lys level, the minimum amount of CP needed might depend on the SID Lys level. Therefore, it was hypothesized that using a ratio of SID Lys to CP is more accurate than a fixed minimum CP level. An experiment was designed to get more insight into the relationship between dietary SID Lys and minimum CP level on performance and N metabolism. The design consisted of 2 SID Lys and 6 CP levels. It was hypothesized 1) that the response to a decreasing digestible CP level could be described with broken line models and 2) that the break point of these models is dependent on the dietary SID Lys level.
MATERIALS AND METHODS
This study was approved by the Ethics Commission of the Flanders Research Institute for Agriculture, Fisheries and Food (ILVO) (EC: 2016/282).
Experimental Design
Diets.
The experiment compared 12 treatment groups: 2 SID Lys levels (10 and 11 g SID Lys/kg) × 6 CP levels (140, 150, 160, 170, 180, 190 g/kg). The SID Lys levels were chosen below the optimum for maximal performance (Millet et al., 2017) to make sure that Lys was the first limiting AA. Diets were formulated to be isocaloric to ensure that Lys was the first limiting factor. To avoid essential AA deficiencies, their contents were formulated to slightly exceed the levels required by the ideal AA profile (Gloaguen et al., 2013). A safety margin (at least 0.02 above the ideal SID AA ratio to SID Lys) was included to anticipate small errors made during sampling, analysis or mixing the ingredients and the diets. Before formulation, AA composition of the major feed ingredients was analyzed, and SID AA levels per ingredient were calculated using tabular digestibility values (CVB, 2007). First, 4 diets were formulated; for both SID Lys levels, one with the highest (190 g/kg) and one with the lowest (140 g/kg) CP level (Tables 1 and 2). Then, the diets with 180, 170, 160, and 150 g/kg CP were produced by mixing the extreme diets in ratios of 80:20, 60:40, 40:60, and 20:80, respectively, per SID Lys level. This ensured a linear decrease in CP level. Moreover, by formulating the high and low CP diet, a similar ratio of essential SID AA to SID Lys was obtained in all diets.
Table 1.
Ingredient composition of the 4 extreme diets with highest and lowest CP level at 2 standardized ileal digestible (SID) levels1
| Low Lys, low CP | Low Lys, high CP | High Lys, low CP | High Lys, high CP | |
|---|---|---|---|---|
| SID Lys, g/kg | 10 | 10 | 11 | 11 |
| CP, g/kg | 140 | 190 | 140 | 190 |
| Wheat | 25.0 | 17.6 | 21.4 | 12.6 |
| Barley | 25.0 | 15.0 | 25.0 | 19.7 |
| Corn | 25.0 | 25.0 | 25.0 | 26.0 |
| Soybean meal | 5.4 | 18.2 | 4.6 | 20.0 |
| Wheat middlings | 4.9 | |||
| Rapeseed meal | 2.6 | 5.0 | 5.0 | |
| Wheat gluten feed | 1.8 | 0.5 | ||
| Palm kernel meal | 1.7 | |||
| Premix2 | 6.0 | 6.0 | 6.0 | 6.0 |
| Beet molasses | 3.0 | 3.0 | 4.0 | 3.0 |
| Soy oil | 1.9 | 3.1 | 2.0 | 3.0 |
| Silicon oxide | 1.0 | 1.0 | 1.0 | 1.0 |
| Salt | 0.28 | 0.28 | ||
| L-Lysine HCL, 78% | 0.83 | 0.40 | 1.01 | 0.49 |
| Limestone | 0.80 | 0.73 | 0.83 | 0.73 |
| Monocalcium phosphate | 0.63 | 0.50 | 0.63 | 0.49 |
| Bicarbonate | 0.56 | 0.58 | ||
| l-Threonine, 98% | 0.39 | 0.22 | 0.49 | 0.30 |
| l-Valine, 96.5% | 0.38 | 0.17 | 0.48 | 0.26 |
| l-Leucine,99% | 0.38 | 0.54 | 0.13 | |
| dl-Methionine, 99% | 0.30 | 0.12 | 0.39 | 0.21 |
| l-Isoleucine, 99% | 0.28 | 0.01 | 0.38 | 0.08 |
| l-Phenylalanine, 99% | 0.21 | 0.30 | 0.04 | |
| l-Tryptophan, 98% | 0.15 | 0.09 | 0.18 | 0.12 |
| l-Tyrosine, 99% | 0.13 | 0.19 | 0.03 | |
| l-Histidine, 99% | 0.11 | 0.16 | 0.03 | |
| Phytase3 | 0.01 | 0.01 | 0.01 | 0.01 |
1The other experimental diets were produced by mixing the high and low CP diets (per SID Lys level) in the required amounts to reach CP levels of 150, 160, 170, and 180 g/kg (150 = 0.8 × 140 + 0.2 × 190; 160 = 0.6 × 140 + 0.4 × 190; 170 = 0.4 × 140 + 0.6 × 190; 180 = 0.8 × 140 + 0.2 × 190).
2The premix contained 80% dairy products and 20% vitamin and mineral premix, providing the following quantities of vitamins and minerals per kilogram of diet: vitamin A, 15,000 IU; vitamin D3, 2,000 IU; vitamin E, 100 mg; vitamin K, 2 mg; vitamin B1, 2.5 mg; vitamin B2, 7.5 mg; vitamin B5, 20 mg; vitamin B6, 5 mg; vitamin B12, 0.04 mg; vitamin C, 100 mg; vitamin PP, 30 mg; choline, 324 mg; folic acid, 3 mg; biotin, 0.15 mg; Ca, 516 mg; P, 419 mg; Mg, 165 mg; Na, 353 mg; Cl, 1,375 mg; K, 1,227 mg; S, 234 mg; Fe, 100 mg; Cu, 160 mg; Mn, 60 mg; Zn, 100 mg; I, 2 mg; Se, 0.4 mg.
3Ronozyme Hiphos. The concentration added provided 1,000 FYT units/kg.
Table 2.
Nutrient composition1 of the 4 extreme diets with the highest and lowest CP level at 2 standardized ileal digestible (SID) Lys levels2 (g/kg, unless otherwise mentioned)
| Low Lys, low CP | Low Lys, high CP | High Lys, low CP | High Lys, high CP | |
|---|---|---|---|---|
| DM | 881 | 880 | 888 | 881 |
| Crude ash | 54 | 59 | 54 | 59 |
| Crude fiber | 34 | 40 | 33 | 39 |
| CP | 139 | 188 | 139 | 192 |
| Ether extract | 40 | 57 | 41 | 55 |
| ADF | 38 | 47 | 38 | 43 |
| NDF | 107 | 111 | 117 | 104 |
| ADL | 7.2 | 8.6 | 7.3 | 7.2 |
| Calcium | 5.5 | 5.5 | 5.5 | 5.5 |
| Phosphorus | 4.8 | 5.1 | 5 | 5.1 |
| Digestible phosphorus | 3.5 | 3.5 | 3.5 | 3.5 |
| LYS | 10.5 | 11.3 | 11.7 | 12.0 |
| M+C | 6.7 | 7.3 | 7.5 | 7.8 |
| MET | 4.6 | 3.9 | 5.3 | 4.6 |
| THR | 7.4 | 8.5 | 7.9 | 9.3 |
| TRP | 2.6 | 3.1 | 2.9 | 3.2 |
| LEU | 12.5 | 13.8 | 13.3 | 14.7 |
| ILE | 7.0 | 7.5 | 7.3 | 7.8 |
| VAL | 8.9 | 9.9 | 9.4 | 10.2 |
| ARG | 5.9 | 10.2 | 5.5 | 10.0 |
| HIS | 3.7 | 4.5 | 3.9 | 4.7 |
| PHE | 7.0 | 8.3 | 7.4 | 8.3 |
| TYR | 4.6 | 5.0 | 5.1 | 5.2 |
| GLU | 24.3 | 36.3 | 23.1 | 33.3 |
| SID3 LYS | 9.8 | 10.2 | 11.0 | 10.9 |
| SID M + C | 6.2 | 6.5 | 7.0 | 7.0 |
| SID MET | 4.4 | 3.6 | 5.1 | 4.2 |
| SID THR | 6.7 | 7.4 | 7.2 | 8.2 |
| SID TRP | 2.4 | 2.8 | 2.7 | 2.8 |
| SID LEU | 11.4 | 12.0 | 12.2 | 12.9 |
| SID ILE | 6.2 | 6.6 | 6.6 | 6.8 |
| SID VAL | 8.2 | 8.8 | 8.7 | 9.1 |
| SID ARG | 5.3 | 9.2 | 4.9 | 9.2 |
| SID HIS | 3.3 | 3.9 | 3.6 | 4.1 |
| SID PHE | 6.4 | 7.3 | 6.8 | 7.3 |
| SID TYR | 4.2 | 4.4 | 4.6 | 4.6 |
| SID GLU | 22.2 | 33.3 | 21.0 | 30.3 |
| NE, MJ/kg | 9.85 | 9.85 | 9.85 | 9.85 |
1DM, CP, and total AA (except Tyr) were analyzed on the final diets. Tyr was only analyzed in the basal ingredients. Other values were based on near-infrared spectroscopy analyses of the basal ingredients and published digestibility coefficients (CVB, 2007).
2The other experimental diets were produced by mixing the high and low CP diets (per Lys level) in the required amounts to reach CP levels of 150, 160,170 and 180 g/kg (150 = 0.8 × 140 + 0.2 × 190; 160 = 0.6 × 140 + 0.4 × 190; 170 = 0.4 × 140 + 0.6 × 190; 180 = 0.8 × 140 + 0.2 × 190).
3SID = standardized ileal digestible.
After mixing, the diets were pelleted using cold pelleting equipment (Promill Type A36; Promill-Stolz S.A., Serville, France; die characteristics: 6.0-mm hole size, 60.0-mm wall thickness, 10 rows, 124 holes per row, 490.0 mm flange diameter, 359.0 mm i.d., 155.0-mm width, and 78.0-mm track width), generating pellets with 6 mm diameter.
Housing and animals.
The experiment, conducted at ILVO (Melle, Belgium), was spread over 3 weaning rounds. A total of 408 weanling pigs (Piétrain boar × RA-SE genetics hybrid sow) were used. The average BW at weaning was 8.3 ± 0.2 kg (mean ± SE). In both of the first 2 rounds, 144 piglets were divided over 24 pens (3 barrows and 3 gilts per pen), blocked per weight class (12 heavyweight and 12 lightweight pens). In the third round, due to a shortage in piglets, the heavyweight pens contained only 5 piglets (3 barrows and 2 gilts). Per BW class and per round, each pen was randomly assigned to one of the 12 treatments. In total, 6 pen replicates per treatment were used. Pens measured 1 m × 1.8 m (1.8 m2). The slatted floor was covered with a synthetic coating. The temperature in the compartments ranged from 26 °C at the start to 22 °C at the end of the experiments; the light schedule was natural daylight, supplemented with artificial light between 7 h 30 and 15 h 30. Feed and water were provided ad libitum.
Measurements
Performance.
All pigs were weighed individually at weaning (4 wk of age), 5, 7, and 9 wk of age. At the end of each period, feed leftovers were recorded to calculate feed consumption per pen and per period. Average daily gain, ADFI, and G:F were calculated per pen for each period and for the total period.
Serum urea and creatinine level.
Blood samples were collected in the morning at 7 wk of age from 1 male and 1 female pig per pen by puncture of the jugular vein. Blood samples were collected in a 10-mL serum tube with a silicone-coated interior (Becton, Dickinson and Company, Franklin Lakes, NJ). Serum was obtained by centrifugation at 1,499 × g for 10 min at 4 °C. Serum was stored at −80 °C until analysis. Serum urea and creatinine were measured in a commercial laboratory using the commercial “Cobas Ureal” kit and “Cobas CREJ2” kit, respectively (Roche, Basel, Switzerland). The detection limit was 3.0 mg/dL for urea and 0.17 mg/dL for creatinine. Samples below the detection limit were set at 2.12 for urea and 0.12 for creatinine (detection limit divided by the square root of 2; Hornung and Reed, 1990).
Amino acid analysis.
Before formulating the diets, major ingredients were analyzed using a validated Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry (HILIC-LC-MS) method, whereas final diets were analyzed according to European Commission Directives 98/64 and 2000/45. The HILIC–LC–MS method proved cost-effective because no preoxidation or postcolumn derivatization step was needed. Within this method, AA were hydrolyzed under vacuum using a 3 M HCl-3.8% thioglycolic acid solution except for Trp analysis, where an alkaline vacuum hydrolysis (4 M LiOH) was applied. Thioglycolic acid prevents oxidation of Met during vacuum hydrolysis (Joergensen and Thestrup, 1995). All samples were hydrolyzed for 4 h at 150 °C, followed by cleanup on an OASIS HLB solid-phase cartridge (200 mg, 6 cc, Waters). The eluent was analyzed by LC–MS2 (Nexera LC-MS 8040). Separation was done on a HILIC column (2.6 µm, 150 × 2.1 mm, Kinetex, Phenomenex) with 50 mM ammonium formate as mobile phase A and acetonitrile as mobile phase B. Amino acids were detected by MS after electrospray ionization. Amino acid composition of the complete diets was analyzed in an external laboratory (AVEVE Merksem, Belgium). The AA (exclusive Tyr and Trp) were obtained with HPLC after acid hydrolysis (EC, 1998) and Trp after alkaline hydrolysis (EC, 2000).
Apparent total tract crude protein digestibility
Acid-insoluble ash (silicon oxide, Sipernat 2200, Evonik, Germany) was used as digestibility marker. During the fourth week of the experiment, fresh fecal grab samples were collected from each pen for 4 consecutive days. After these 4 d, samples from each pen were pooled and homogenized. This homogenized sample was freeze dried and ground to pass through a sieve with 1-mm mesh size, for further analysis of DM (EC, 1971), AIA (McCarthy et al., 1974), and CP (ISO 5983-2, 2009). Apparent total tract digestibility (ATTD) of DM and CP was then calculated as ATTDNU = 100 × [1 − (NUF × AIAD)/(NUD × AIAF)], where ATTDNU = apparent digestibility of the nutrient, NUF = fecal concentration of the nutrient, AIAD = dietary concentration of AIA, NUD = dietary concentration of the nutrient, AIAF = fecal concentration of AIA (Kong and Adeola, 2014).
Statistical Analysis
For statistical analysis, the pen was considered as experimental unit. The data distribution was assumed to be sufficiently normal and homoscedastic based on graphical examination of the residuals of the fitted models.
For performance results (ADG, ADFI, G:F, BW), linear and quadratic (QP) mixed models were fitted with BW at weaning, SID Lys level, CP level, and the interaction between SID Lys and CP levels as fixed factors and weaning round as random factor. The QP model also included (CP level)2 and the interaction between SID Lys and (CP level)2. If an interaction was not significant, the analysis was repeated without the interaction term. In addition, for determining the CP level corresponding with maximal efficiency, QP models and broken line models with linear (BLL) or quadratic (BLQ) ascending portions were fitted for each SID Lys level using a nonlinear least squares approach (NLS function) in R (Version 1.0.153; R Core Team, 2015). For digestibility, a mixed model was fitted with BW at weaning, SID Lys level, CP level, and the interaction between SID Lys and CP levels as fixed factors and weaning round as random factor.
Urea and creatinine levels were analyzed using a model that included sex, BW at weaning, SID Lys level, CP level, and the interaction between SID Lys and CP levels as fixed factors. Pen within weaning round was included as random factor to correct for repeated measurements (different animals) within the pen. Nonsignificant interactions were excluded from the final models. In addition, to obtain the optimal CP levels, QP, BLL, and BLQ models were fitted using the NLS function in R (R Core Team, 2015), with SID Lys:CP level as independent variable. For all parameters, differences were considered significant if P < 0.05.
RESULTS
Performance
Both dietary SID Lys and CP level affected performance (Tables 3 and 4). With decreasing CP levels, ADG decreased linearly (P < 0.001). It was not possible to show a significant effect of SID Lys level on ADG (P = 0.101 in the linear model and P = 0.084 in the quadratic model). SID Lys and CP level interacted on G:F: at low CP levels, differences between the 2 dietary SID Lys levels were small, but with increasing CP level, piglets receiving 11 g SID Lys showed higher G:F. The positive effect of CP on G:F diminished with increasing CP level (P-value of CP2 < 0.001). Average daily feed intake was not affected by diet. Body weight at start significantly affected both ADG and ADFI (P < 0.001 for both), but not G:F (P = 0.705; data not shown).
Table 3.
The effect of dietary CP or standardized ileal digestible (SID) Lys level on performance between 4 and 9 wk of age (n = 6 pens per treatment)
| 10 g SID Lys | 11 g SID Lys | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP, g/kg | 139 | 149 | 159 | 168 | 178 | 188 | 139 | 149 | 160 | 170 | 181 | 192 | SEM |
| ADFI, g | 570 | 568 | 564 | 619 | 568 | 595 | 576 | 572 | 601 | 572 | 583 | 607 | 9 |
| ADG, g | 344 | 352 | 367 | 416 | 376 | 381 | 354 | 359 | 404 | 394 | 418 | 424 | 6 |
| G:F, g/g | 0.606 | 0.622 | 0.653 | 0.675 | 0.663 | 0.645 | 0.616 | 0.632 | 0.674 | 0.689 | 0.716 | 0.699 | 0.006 |
Table 4.
P-values for the linear and quadratic models fitted through the performance data
| Linear model | Quadratic model | |||||||
|---|---|---|---|---|---|---|---|---|
| Lys | CP | Lys × CP | Lys | CP | CP2 | Lys × CP | Lys × CP2 | |
| ADFI, g | 0.724 | 0.206 | NS | 0.734 | 0.929 | 0.883 | NS | NS |
| ADG, g | 0.101 | <0.001 | NS | 0.084 | 0.173 | 0.241 | NS | NS |
| G:F, g/g | 0.061 | 0.057 | 0.028 | 0.006 | 0.475 | <0.001 | 0.002 | NS |
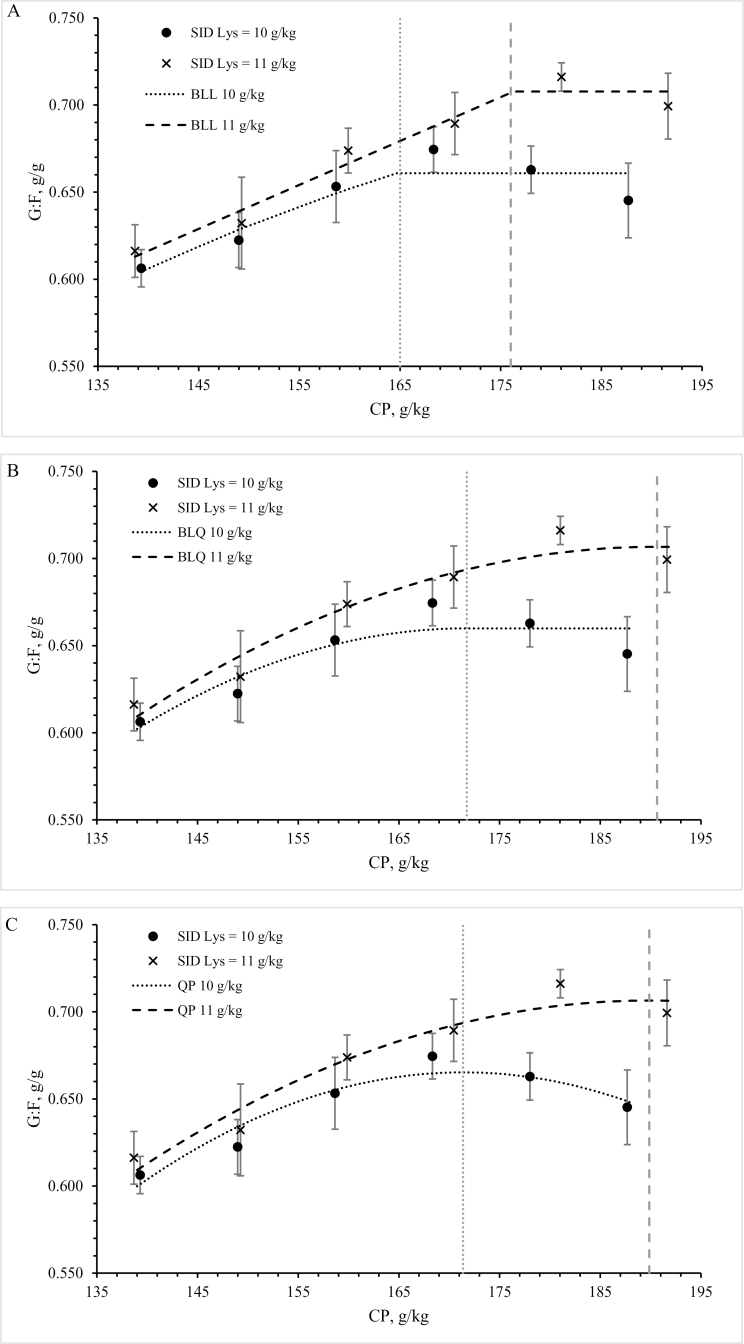
It was possible to fit QP, BLL, and BLQ models for SID Lys at 10 and 11 g/kg. With SID Lys = 10 g/kg, a solution for the BLL model was only found with G:F in function of SID Lys:CP. For visual presentation, this function was back-transformed in function of dietary CP level (Fig. 1).
Figure 1.
Effect of dietary CP level on G:F of piglets between 4 and 9 wk of age. Piglets receiving 10 or 11 g standardized ileal digestible (SID) Lys/kg diet are presented, together with their corresponding broken line models with linear (BLL; A) or quadratic (BLQ; B) ascending portions, or quadratic model (QP; C). Vertical gray lines represent break points for BLL and BLQ, or optima for QP.
With 10 g SID Lys, the break point was at 171, 165, and 174 g/kg CP for the QP, BLL, and BLQ models, respectively (corresponding with 0.059, 0.061, and 0.058 SID Lys:CP, Table 5). With 11 g SID Lys, the break point was at 190, 176, and 191 g/kg CP for the QP, BLL, and BLQ models, respectively (corresponding with 0.057, 0.062, and 0.058 g SID Lys:CP). Within 1 SID Lys level, the Akaike information criterion, the Bayesian information criterion, and the correlation coefficient were fairly similar for the 3 fitted models (Table 5).
Table 5.
Descriptors of different models studying the effect of standardized ileal digestible (SID) Lys:CP ratio on G:F and serum urea level
| 10 g SID Lys | 11 g SID Lys | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| QP1 | BLL | BLQ | QP | BLL | BLQ | QP | BLL | BLQ | |
| G:F | |||||||||
| r 2 | 0.26 | 0.25 | 0.24 | 0.43 | 0.44 | 0.43 | |||
| AIC2 | −127 | −126 | −126 | −121 | −122 | −122 | |||
| BIC3 | −120 | −120 | −119 | −116 | −116 | −116 | |||
| Breakpoint CP | 171 | 165 | 174 | 190 | 176 | 191 | |||
| Breakpoint SID Lys:CP | 0.059 | 0.061 | 0.058 | 0.057 | 0.062 | 0.058 | |||
| Serum urea level | |||||||||
| r 2 | 0.76 | 0.77 | 0.76 | 0.63 | 0.65 | 0.66 | 0.72 | 0.74 | 0.74 |
| AIC | 360 | 358 | 360 | 343 | 339 | 338 | 701 | 691 | 693 |
| BIC | 369 | 367 | 369 | 352 | 348 | 347 | 713 | 704 | 705 |
| Breakpoint CP | 142 | 155 | 142 | 150 | 173 | 162 | |||
| Breakpoint SID Lys:CP | 0.069 | 0.064 | 0.069 | 0.073 | 0.063 | 0.068 | 0.072 | 0.064 | 0.068 |
1QP = quadratic models; BLL = broken line models with linear ascending portion; BLQ = broken line models with quadratic ascending portion.
2AIC = Akaike information criterion.
3BIC = Bayesian information criterion.
Digestibility
The ATTD of DM decreased with increasing CP level, whereas ATTD of CP was not significantly affected by SID Lys or CP level (Table 6). Therefore, to calculate values on ATTD CP level, 1 average digestibility coefficient (0.807) was used.
Table 6.
Apparent total tract digestibility (ATTD; %) of DM and CP of the 12 experimental diets differing in standardized ileal digestible (SID) Lys levels or dietary CP fed to piglets between 4 and 9 wk of age (n = 6 pens per treatment)
| 10 g SID Lys | 11 g SID Lys | P-value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CP, g/kg | 139 | 149 | 159 | 168 | 178 | 188 | 139 | 149 | 160 | 170 | 181 | 192 | SEM | Lys | CP |
| ATTD DM | 87.18 | 87.35 | 86.09 | 87.21 | 84.12 | 85.61 | 86.19 | 86.37 | 86.34 | 85.33 | 86.38 | 85.57 | 0.13 | 0.387 | <0.001 |
| ATTD CP | 80.35 | 81.08 | 80.02 | 82.32 | 78.64 | 80.29 | 80.47 | 81.88 | 81.22 | 79.85 | 81.51 | 80.71 | 0.22 | 0.201 | 0.426 |
Serum Urea and Creatinine
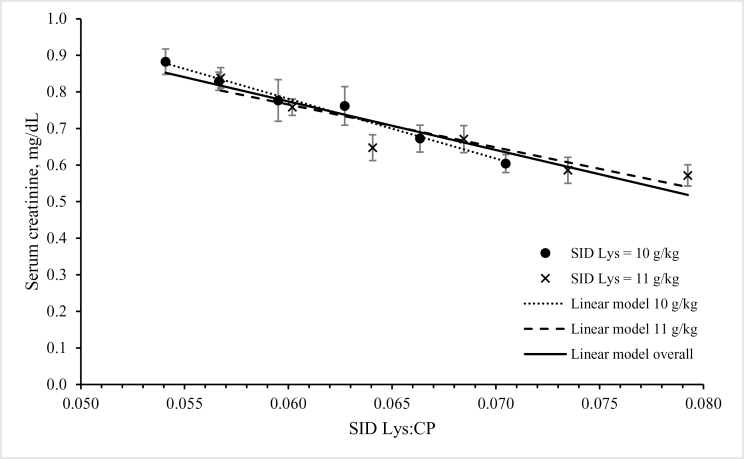
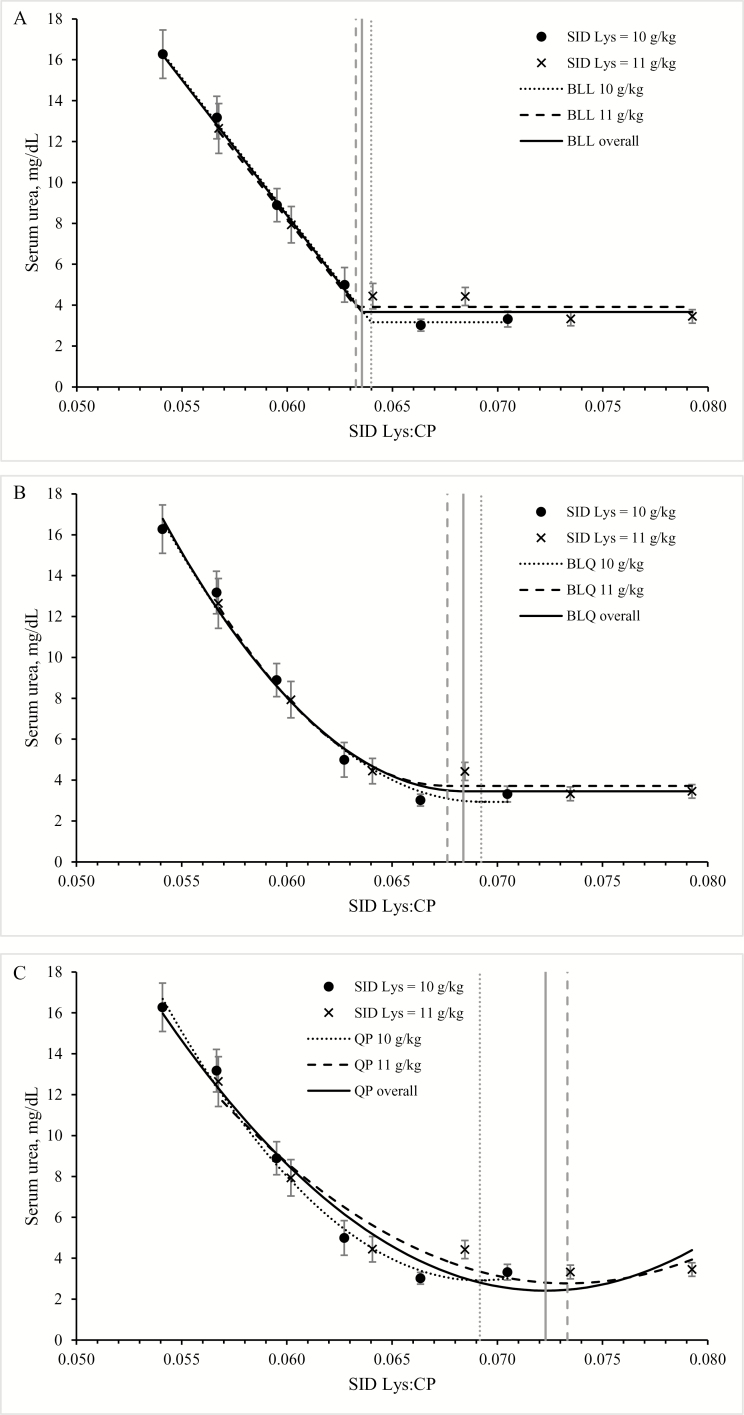
Both dietary SID Lys and CP level significantly affected serum urea and creatinine level (P < 0.001 for all). For serum creatinine levels, a linear regression in function of SID Lys:CP ratio best described the results (Fig. 2), when analyzed per SID Lys level and when analyzed across all 12 groups. Serum creatinine level increased with increasing CP level (P < 0.001) and was lower in pigs receiving the 11 g SID Lys compared with the 10 g SID Lys level (P < 0.001). For serum urea level, an interaction between CP and SID Lys levels was noted (P < 0.001): >155 g CP/kg serum urea levels were higher in pigs receiving the 10 g SID Lys level, whereas below that CP content, serum urea concentrations were similar for the 2 SID Lys groups. Quadratic, BLL, and BLQ models could be fitted for serum urea level as a function of SID Lys:CP ratio for SID Lys at 10 and 11 g/kg and across both SID Lys levels (Table 5, Fig. 3).
Figure 2.
Effect of dietary standardized ileal digestible (SID) Lys to CP ratio on serum creatinine levels in piglets at 7 wk of age. Piglets receiving 10 or 11 g SID Lys/kg diet are presented, together with their corresponding and overall linear-plateau model.
Figure 3.
Effect of dietary standardized ileal digestible (SID) Lys to CP ratio on serum urea levels in piglets at 7 wk of age. Piglets receiving 10 or 11 g SID Lys/kg diet are presented, together with their corresponding and overall broken line models with linear (BLL; A) or quadratic (BLQ; B) ascending portions, or quadratic model (QP; C). Vertical gray lines represent break points for BLL and BLQ, or optima for QP.
Hence, with 10 g SID Lys, the break point or maximum was at 0.069, 0.064, and 0.069 SID Lys:CP for the QP, BLL, and BLQ models, respectively (CP level of 142, 155, and 142 g/kg). With 11 g SID Lys, the break point was at 0.073, 0.063, and 0.068 SID Lys:CP for the QP, BLL, and BLQ models, respectively (CP level of 150, 173, and 162 g/kg). Overall, the break point was at 0.072, 0.064, and 0.068 for the QP, BLL, and BLQ models, respectively. Over both SID Lys levels, the Akaike and Bayesian information criteria were slightly higher and the correlation coefficient slightly lower for the quadratic model versus the broken line models (Table 5).
DISCUSSION
Reducing the CP level reduces the risk of postweaning diarrhea (Nyachoti et al., 2006; Heo et al., 2008, 2009) and reduces N excretion to the environment (Henry and Dourmad, 1993). Indeed, AA given in excess are deaminated, and the resulting urea is excreted in the urine (van Milgen and Dourmad, 2015). Therefore, the animals’ protein requirements are based on the intake of a complete set of AA instead of CP. Free Lys, Thr, Met, and Trp have long been available for commercial use, and their requirements have been studied extensively, enabling a considerable reduction in CP. In addition, Val has become commercially available since 2009. Uncertainty about the requirements for the next limiting AA necessitates provision of sufficient CP in the diet. Henry and Dourmad (1993) suggested a ratio of Lys:CP of 65 to 68 g/kg “to limit the risk for deficiencies in NEAA or in essential AA that have not been taken into account.” With increasing knowledge on these next limiting AA, this ratio might increase, resulting in lower CP levels in the pig diet. The latter is only valid to the extent that a feed can be formulated with a maximum level of CP and a minimum level for each essential AA, given the available ingredients. In the present experiment, we achieved this goal by using food grade AA, which are not yet available for commercial feed production.
Decreasing the CP level increased the DM digestibility. This is probably a result of the chosen ingredients, with slightly lower crude fiber levels in the low CP diets, which may affect DM digestibility (Zhang et al., 2013). In contrast, no effect on CP digestibility was observed. Crude protein level was decreased by lowering the inclusion of soybean meal (of which CP digestibility is generally higher than corn, wheat, or barley; CVB, 2016), but also increased the amount of free AA that are considered almost entirely digestible, eventually leading to no detectable difference in CP digestibility.
Diets were formulated for identical SID Lys content, but identical formulation might not be possible in reality. For example, diets may be differently affected by heat treatment, leading to lower availability than estimated from apparent ileal digestibility (D’Mello, 1993). As cold pelleting was applied, this seems unlikely to have occurred in the present study. With the changing ingredient and fiber composition, it can also not be fully excluded that the true digestible AA content was affected. Indeed, Blank et al. (2012) showed that endogenous Thr losses were affected by both fiber level and fiber source. Still, using SID AA levels might be the best approximation of available AA content at the moment (Stein et al., 2007). With decreasing CP and soybean meal level, and hence lower fiber levels, lower endogenous losses might have been anticipated, which would result in better rather than worse performance. Fiber level also affects the amount of N that is fermented versus the amount retained in the large intestine as bacterial protein (Jha and Berrocoso, 2016), and therefore, it may affect ATTD CP. These theoretical differences might have been too small to be detected in the CP digestibility.
Both SID Lys and CP level affected ADG and G:F. For the latter, an interaction between SID Lys and CP levels was found. This is in agreement with the hypothesis that at low CP levels, protein rather than Lys limits performance. It was possible to describe the G:F response with QP and broken line models. Break points differed between the models used, but in all cases, the break point for pigs receiving 10 g SID Lys was lower than the break point for pigs receiving 11 g SID Lys. This corresponds with our initial hypothesis. When expressed as a ratio, the calculated break points for both SID Lys levels were fairly close together. The question arises which model is best suited to deduct recommendations. Based on the correlation coefficient, Akaike and Bayesian information criteria, the 3 models described the response equally well. Still, the interpretation differs: the QP model assumes that performance improves to a maximum and then declines. Although, theoretically, the energy costs of removing excess N as urea in urine may explain a decrease in performance, one may wonder whether this is already at play in the measured CP range. Broken line models assume an increase in performance up to a maximum followed by a plateau, at least within the measured range. The BLL model gives nutritional requirements which are always lower than the BLQ model (Pesti et al., 2009). The latter probably describes the response of a population of animals, taking stochasticity into account, whereas the model with linear ascending function may depict the response of 1 “average” animal (Pomar et al., 2003). Thus, although the average piglet in the population showed maximal G:F at a SID Lys:CP ratio of 0.061 to 0.062, a ratio of 0.058 would be needed to cover the N requirement of all animals in the population. However, as both Lys and CP levels affect G:F in the same direction (higher G:F with higher Lys or CP levels), different requirements may be obtained for the optimal SID Lys:CP ratio if studied by varying either the Lys or the CP level. By varying the Lys level, a SID Lys:CP ratio for optimal performance can be expected >0.062, whereas by varying the CP level (as done in the present study), a SID Lys:CP ratio for maximal performance < 0.061 was seen. It can be anticipated that the ratios estimated with the 2 approaches, but with use of a model with a linear ascending portion, would be closer together. From a practical point of view, even if a ratio of 0.058 would be needed to observe maximal performance from all animals, this would also mean that most animals would have consumed excessive N, as reflected in the high serum urea concentration. Also, the marginal gain from moving from the optimum deducted via models with linear ascending portion to the optimum deducted via models with quadratic ascending portion is small (0.002 or 0.008 difference in G:F for 10 or 11 g SID Lys, respectively).
Serum urea level also decreased with decreasing CP levels until a plateau was reached. Urea is an end product of protein breakdown that has not been used for protein synthesis. Previously, urea has been suggested as a useful parameter to determine protein quality (Pedersen and Boisen, 2001). Lower urea levels indicate better N efficiency and lower excretion to the environment.
The quadratic model seems less suitable to depict the evolution in serum urea level, according to the modeling criteria as well as from a physiological point of view. To our knowledge, there is no physiological basis for expecting a rise in serum urea level after reaching a minimum. Therefore, models with a plateau are most appropriate to describe the response. For both SID Lys levels, the break point was close together, and therefore, the relationship could be described by 1 formula across all treatment groups. The high correlation coefficient for both the BLL and BLQ models indicates that SID Lys:CP level is a good predictor of serum urea level. Again, although the model with linear descending portion may represent the response of the population average, it can be expected that lowering the CP level further to the optimum of the BLQ model would only yield marginal extra gains. Again, although using the ratio deducted from the model with quadratic descending portion (SID Lys:CP ratio of 0.068) may ascertain that all animals in the population show minimal serum urea levels (and thus maximal N efficiency), at this point, most animals will experience protein deficiency, which corresponds with the lower G:F. Hence, although N efficiency may be maximal in this point, the efficiency of the total diet (and thus most nutrients) may be compromised. Therefore, we propose using 0.064, which is the ratio deducted with the BLL model as set point for maximal N efficiency.
For the “average piglet” receiving 11 g SID Lys, the break point for G:F (176 g CP) and serum urea level (173 g CP) was fairly close. For the piglets receiving 10 g SID Lys, performance started to decline at a point where maximal N efficiency was not yet reached (break point for G:F at 165 g and for serum urea level at 156 g CP). Thus, although serum urea levels suggested that some of the dietary protein was still in excess, performance was already declining. It was hypothesized that readily available NEAA are used more efficiently than transaminated AA to yield the required levels of nonessential AA. Indeed, when CP level limits growth, part of the essential AA will be “excessive” and may be transaminated to yield NEAA. The profile of NEAA may therefore affect the observed response to decreasing CP level, even at a point where CP itself is not yet limiting. This agrees with the statement of Wu (2014), namely, that NEAA should be taken into account when formulating for optimal performance.
The observed dietary CP level at which performance starts to decline was higher than the levels reported by Nemechek et al. (2014), Gloaguen et al. (2014), or Jansman et al. (2016). Different reasons may be found for this. First, the models used may play an important role. In some of the abovementioned studies, treatments were considered as categorical variables, and post hoc tests were used to discriminate between treatment groups. These tests are normally used to prove differences between groups, but the absence of a significant effect does not mean that there is no effect. In addition, by considering treatment as categorical variable, the linear nature of the independent variable is not explored. For example, in the first experiment of Gloaguen et al. (2014), a linear relationship might be deduced from setting out reported G:F in function of CP level, suggesting a linear decrease in performance between the highest and the lowest levels. Second, differing SID Lys levels have been used throughout studies. For this type of studies, it is critical that Lys is the first limiting AA. Failing to obtain this will overestimate the maximal SID Lys:ATTD CP level. Third, the NEAA profile may differ between studies and as such affect performance, as explained earlier. Last, between-laboratory differences in CP or AA level may explain some of the variation.
Apart from serum urea level, we also measured serum creatinine as a measure for muscle turnover. Creatinine is produced via the catabolism of creatine phosphate in the muscle. With higher SID Lys levels and with lower CP levels, creatinine level decreased. In contrast with serum urea levels, it followed a linear pattern with no clear plateau. The present results suggest that not only CP by itself but also the composition of CP may affect serum creatinine levels. This is in contrast with the study of Toledo et al. (2014), who did not observe any response of CP reduction in piglets on serum creatinine level. The reason for this is not entirely clear. Despite similarly formulated SID Lys levels, it cannot be excluded that absorbed Lys differed between diets, as discussed earlier. Serum creatinine is possibly influenced by the substrate for creatine synthesis. Creatine synthesis requires 3 AA: Gly, Met, and Arg (Brosnan et al., 2011). With increasing protein levels, an increasing amount of these AA might be used to produce creatine in the liver, eventually leading to a higher creatine and creatine phosphate turnover, yielding higher creatinine levels.
The findings of the present study have scientific and practical consequences. First, when performing Lys requirement studies—which are typically assumed to be performed with energy as first limiting factor (Roth et al., 1999; Oresanya et al., 2007)—in reduced CP diets, one may actually be determining the optimal SID Lys:CP instead of SID Lys:NE ratio. Next, the results suggest that in practical piglet feeding, not AA but protein may be limiting performance. Indeed, to limit the risks of postweaning diarrhea in the absence of antibiotics, reducing the CP level is widely used in European diets (in Belgium, CP level in commercial diets is estimated at 175 g/kg, Millet et al., 2018). When using the ratio of 0.064 SID Lys:CP, this means that SID Lys levels > 11.2 g SID Lys/kg might not lead to better performance, even when this level is below the AA requirement for maximal performance (NRC, 2012). Thus, while accepting lower performance to diminish the risk for diarrhea, using the ratio of 0.064 for SID Lys:CP may be used for optimizing the AA composition within this reduced protein level.
As there was no clear linear effect on protein digestibility, the use of SID Lys:CP instead of SID Lys:ATTD CP did not affect data analysis nor interpretation. The choice for expressing SID Lys on total CP level was made mainly because both nutrients are commonly used in feed formulation and hence allows for direct implementation in feeding practice. Besides, in most other studies, CP digestibility is not given. Therefore, a comparison between studies is only possible based on a total CP level. Apparent total tract digestible CP may be a better parameter than total CP, as Mansilla et al. (2015) showed that N absorbed from the large intestine could be used when animals are fed diets deficient in dispensable AA. Therefore, we also reported the results in function of ATTD CP, and we recommend measuring and reporting CP digestibility in similar experiments as well to allow for correct comparison and interpretation. In the present study, the ratio of 0.064 corresponded with 0.079 g SID Lys:ATTD CP. In studies where ingredient choices lead to differences in CP digestibility, models should be fitted in function of ATTD CP. Although it is assumed that this would be a better descriptor, more research is necessary to get further insights.
In conclusion, both Lys and CP levels affected performance of piglets between 4 and 9 weeks of age. The effect of decreasing CP level depends on SID Lys, and using a maximal SID Lys:CP ratio may be useful for optimizing the AA profile of dietary CP. When the SID Lys:CP ratio exceeds 0.064 (SID Lys:ATTD CP 0.079), protein rather than individual AA is limiting growth in piglets of 4 to 9 wk of age.
Footnotes
This research was funded by the Own Capital fund of Flanders Research Institute for Agriculture, Fisheries and Food (EV ILVO). The authors thank B.D.B., S.D.N., J.D., K.D., R.L., H.U., and G.V.d.W. for their excellent practical support. We thank M. Levenson for English-language editing.
LITERATURE CITED
- Blank B., Schlecht E., and Susenbeth A.. 2012. Effect of dietary fibre on nitrogen retention and fibre associated threonine losses in growing pigs. Arch. Anim. Nutr. 66:86–101. doi: 10.1080/1745039X.2012.663669 [DOI] [PubMed] [Google Scholar]
- Brosnan J. T., da Silva R. P., and Brosnan M. E.. 2011. The metabolic burden of creatine synthesis. Amino Acids 40:1325–1331. doi: 10.1007/s00726-011-0853-y [DOI] [PubMed] [Google Scholar]
- CVB 2007. Table on feed ingredients. Information about chemical composition, digestibility and feeding value. Centraal veevoederbureau, Lelystad, The Netherlands. [Google Scholar]
- CVB 2016. Table on feed ingredients. Information about chemical composition, digestibility and feeding value. Centraal veevoederbureau, Lelystad, The Netherlands. [Google Scholar]
- D’Mello J. P. F. 1993. Amino acid supplementation of cereal-based diets for non-ruminants. Anim. Feed Sci. Technol. 45:1–18. doi: 10.1016/0377-8401(93)90068-U [DOI] [Google Scholar]
- EC 1971. Determination of moisture. Commission Directive 71/393/EEC establishing Community methods of analysis for the official control of feedingstuffs. Off. J. Eur. Communities L279:3–5. [Google Scholar]
- EC 1998. Establishing Community methods of analysis for the determination of amino-acids, crude oils and fats, and olaquindox in feeding stuffs, and amending. Directive 71/393/EEC. Commission Directive 98/64/EC. Off. J. Eur. Communities L257:14–23. [Google Scholar]
- EC 2000. Establishing Community methods of analysis for determination of vitamin A, vitamin E and tryptophan in feeding stuffs. Commission Directive 2000/45/EC. Off. J. Eur. Communities L174:32–50. [Google Scholar]
- Gloaguen M., Le Floc’h N., Corrent E., Primot Y., and van Milgen J.. 2014. The use of free amino acids allows formulating very low crude protein diets for piglets. J. Anim. Sci. 92:637–644. doi: 10.2527/jas.2013-6514 [DOI] [PubMed] [Google Scholar]
- Gloaguen M., Le Floc’h N., and van Milgen J.. 2013. Update on amino acid requirements for piglets fed with low crude protein diets. INRA Prod. Anim. 26:277–288. https://www6.inra.fr/productions-animales_eng/2013-Volume-26/Issue-3-pp.-237-300/Update-on-amino-acid-requirements-for-piglets-fed-with-low-crude-protein-diets [Google Scholar]
- Henry Y., and Dourmad J. Y.. 1993. Feeding strategy for minimizing nitrogen output in pigs. In: Verstegen M. W. A., den Hartog L. A., van Kempen G. J. M., and Metz J. H. M., editors, Nitrogen flow in pig production and environmental consequences. EAAP Publ. No. 69. Pudoc Sci. Publ, Wageningen, The Netherlands: p. 137–150. [Google Scholar]
- Heo J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. 2008. Effects of feeding low protein diets to piglets on plasma urea nitrogen, faecal ammonia nitrogen, the incidence of diarrhoea and performance after weaning. Arch. Anim. Nutr. 62:343–358. doi: 10.1080/17450390802327811 [DOI] [PubMed] [Google Scholar]
- Heo J. M., Kim J. C., Hansen C. F., Mullan B. P., Hampson D. J., and Pluske J. R.. 2009. Feeding a diet with decreased protein content reduces indices of protein fermentation and the incidence of postweaning diarrhea in weaned pigs challenged with an enterotoxigenic strain of Escherichia coli. J. Anim. Sci. 87:2833–2843. doi: 10.2527/jas.2008-1274 [DOI] [PubMed] [Google Scholar]
- Hornung R. W., and Reed L. D.. 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg. 5:46–51. doi:10.1080/1047322X.1990.10389587 [Google Scholar]
- ISO 5983–2 2009. Animal feeding stuffs – Determination of nitrogen content and calculation of crude protein content – Part 2: Block digestion and steam distillation method. International Organization for Standardization, Geneva, Switzerland. [Google Scholar]
- Jansman A., van Diepen J., Rovers M., and Corrent E.. 2016. Lowering the dietary protein content in piglets: How far can we go? In: Skomiał J. and Lapierre H., editors. Energy and protein metabolism and nutrition. Wageningen: Wageningen Academic Publishers; p. 161–162. [Google Scholar]
- Jha R., and Berrocoso J. F. D.. 2016. Dietary fiber and protein fermentation in the intestine of swine and their interactive effects on gut health and on the environment: A review. Anim. Feed Sci. Technol. 212:18–26. doi: 10.1016/j.anifeedsci.2015.12.002 [DOI] [Google Scholar]
- Joergensen L., and Thestrup H. N.. 1995. Determination of amino acids in biomass and protein samples by microwave hydrolysis and ion-exchange chromatography. J. Chromatogr. A 706:421–428. doi: 10.1016/0021-9673(94)01107-P [DOI] [Google Scholar]
- Kong C., and Adeola O.. 2014. Evaluation of amino acid and energy utilization in feedstuff for swine and poultry diets. Asian-Australas. J. Anim. Sci. 27:917–925. doi: 10.5713/ajas.2014.r.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla W. D., Columbus D. A., Htoo J. K., and de Lange C. F.. 2015. Nitrogen absorbed from the large intestine increases whole-body nitrogen retention in pigs fed a diet deficient in dispensable amino acid nitrogen. J. Nutr. 145:1163–1169. doi: 10.3945/jn.115.212316 [DOI] [PubMed] [Google Scholar]
- McCarthy J. F., Aherne F. X., and Okai D. B.. 1974. Use of Hcl insoluble ash as an index material for determining apparent digestibility with pigs. Can J Anim Sci. 54:107–109. doi:10.4141/cjas74-016 [Google Scholar]
- Millet S., Aluwé M., Le Gall E., Corrent E., Lambert W., De Sutter J., Ampe B., and De Campeneere S.. 2017. Le besoin en lysine digestible iléale standardisée des porcelets de 8 à 24 kg. Journées Recherche Porcine, Paris, France: p. 121–122. [Google Scholar]
- Millet S., Aluwé M., Van den Broeke A., Leen F., De Boever J., and De Campeneere S.. 2018. Review: Pork production with maximal nitrogen efficiency. Animal 12:1060–1067. doi: 10.1017/S1751731117002610 [DOI] [PubMed] [Google Scholar]
- Nemechek J. E., Tokach M. D., Dritz S. S., Goodband R. D., and DeRouchey J. M.. 2014. Evaluation of standardized ileal digestible valine:lysine, total lysine:crude protein, and replacing fish meal, meat and bone meal, and poultry byproduct meal with crystalline amino acids on growth performance of nursery pigs from seven to twelve kilograms. J. Anim. Sci. 92:1548–1561. doi: 10.2527/jas.2013-6322 [DOI] [PubMed] [Google Scholar]
- NRC (National Research Council) 2012. Nutrient requirements of swine. 11th rev. ed Natl. Acad. Press, Washington, DC. [Google Scholar]
- Nyachoti C. M., Omogbenigun F. O., Rademacher M., and Blank G.. 2006. Performance responses and indicators of gastrointestinal health in early-weaned pigs fed low-protein amino acid-supplemented diets. J. Anim. Sci. 84:125–134. doi:10.2527/2006.841125x [DOI] [PubMed] [Google Scholar]
- Oresanya T. F., Beaulieu A. D., Beltranena E., and Patience J. F.. 2007. The effect of dietary energy concentration and total lysine/digestible energy ratio on the growth performance of weaned pigs. Can. J. Anim. Sci. 87:45–55. doi: 10.4141/A05-064 [DOI] [Google Scholar]
- Pedersen C., and Boisen S.. 2001. Studies on the response time for plasma urea nitrogen as a rapid measure for dietary protein quality in pigs. Acta Agric. Scand. A Anim. Sci. 51:209–216. doi:10.1080/09064700152717182 [Google Scholar]
- Pesti G. M., Vedenov D., Cason J. A., and Billard L.. 2009. A comparison of methods to estimate nutritional requirements from experimental data. Br. Poult. Sci. 50:16–32. doi: 10.1080/00071660802530639 [DOI] [PubMed] [Google Scholar]
- Pomar C., Kyriazakis I., Emmans G. C., and Knap P. W.. 2003. Modeling stochasticity: Dealing with populations rather than individual pigs. J. Anim. Sci. 81(14 Suppl 2):E178–E186. doi: 10.2527/2003.8114_suppl_2E178x [DOI] [Google Scholar]
- R Core Team 2015. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Roth F. X., Eder K., and Kirchgessner M.. 1999. The effect of energy density and the lysine to energy ratio of diets on the performance of piglets. J. Anim. Physiol. Anim. Nutri. 82:1–7. doi: 10.1046/j.1439-0396.1999.00211.x [DOI] [Google Scholar]
- Stein H. H., Fuller M. F., Moughan P. J., Sève B., Mosenthin R., Jansman A. J. M., Fernández J. A., and de Lange C. F. M.. 2007. Definition of apparent, true, and standardized ileal digestibility of amino acids in pigs. Livest. Sci. 109:282–285. doi: 10.1016/j.livsci.2007.01.019 [DOI] [Google Scholar]
- Toledo J. B., Furlan A. C., Pozza P. C., Carraro J., Moresco G., Ferreira S. L., and Gallego A. G.. 2014. Reduction of the crude protein content of diets supplemented with essential amino acids for piglets weighing 15 to 30 kilograms. R. Bras. Zootec. 43:301–309. doi:10.1590/S1516- 35982014000600004 [Google Scholar]
- van Milgen J., and Dourmad J. Y.. 2015. Concept and application of ideal protein for pigs. J. Anim. Sci. Biotechnol. 6:15. doi: 10.1186/s40104-015-0016-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellock I., Fortomaris P., Houdijk J., and Kyriazakis I.. 2006. The effect of dietary protein supply on the performance and risk of post-weaning enteric disorders in newly weaned pigs. Anim. Sci. 82:327–335. doi:10.1079/ASC200643 [Google Scholar]
- Wu G. 2014. Dietary requirements of synthesizable amino acids by animals: A paradigm shift in protein nutrition. J. Anim. Sci. Biotechnol. 5:34. doi: 10.1186/2049-1891-5-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Li D., Liu L., Zang J., Duan Q., Yang W., and Zhang L.. 2013. The effects of dietary fiber level on nutrient digestibility in growing pigs. J. Anim. Sci. Biotechnol. 4:17. doi: 10.1186/2049-1891-4-17 [DOI] [PMC free article] [PubMed] [Google Scholar]