Abstract
Altitude-induced pulmonary hypertension is a disease once thought to only occur at extremely high elevations (>1,600 m), but recently, it has been observed at moderate elevations of 1,200 to 1,600 m. Pulmonary arterial pressure (PAP) has been used as an indicator of tolerance to high altitude in mountainous beef production systems for over 30 yr. The trait is typically measured on yearling bulls and heifers with values ≤ 41 mmHg being favorable. These observations were historically only considered valid when they were recorded at elevations ≥ 1,600 m; however, if observations from lower (i.e., moderate) elevations were reliable indicators, a greater number of cattle records could be used in genetic improvement programs for high-altitude beef systems. The objectives of this study were to evaluate the relationship between PAP and elevation, as well as to determine whether PAP measures obtained at moderate elevations (ME) less than 1,600 m have a genetic relationship with PAP observations obtained at high elevations (HE) 1,600 m or greater. Elevation and PAP data from purebred Angus cattle (n = 14,665) from 349 contemporary groups were used in the analyses. Elevation and PAP averaged 1,887 ± 1.8 m and 43.0 ± 0.1 mm Hg, respectively. A univariate model containing the effects of sex, age, elevation category (HE vs. ME), elevation (continuous), and elevation category by elevation interaction along with a random direct genetic effect was utilized to determine the relationship between PAP and elevation. In this model, all main effects were found to be significant contributors of variation in PAP (P < 0.001). The interaction between elevation category and elevation was not a significant contributor to variability of PAP (P > 0.05). A bivariate animal model was then used to evaluate the relationship between PAP observations obtained between HE and ME groups. Heritability estimates for these 2 groups were 0.34 ± 0.03 and 0.29 ± 0.09, respectively, and their genetic correlation was 0.83 ± 0.15. Even though this is a strong genetic relationship, results of this study support the hypothesis that PAP observations collected at HE and ME are not perfectly, genetically related. Results suggest that PAP measures collected from 1,219 to 1,600 m may be useful as a correlated trait in a multitrait genetic evaluation to produce EPD useful for selection of animals with reduced susceptibility to pulmonary hypertension.
Keywords: Angus, cattle, elevation, genetic correlation, heritability, pulmonary arterial pressure
INTRODUCTION
Pulmonary hypertension is a physiological state that affects cattle in high (>1,600 m) elevation production systems. This condition manifests from reduced atmospheric oxygen as elevation increases, which causes the cardiopulmonary system to compensate in order to supply sufficient oxygen to the body. The healthy bovine cardiopulmonary system does not utilize oxygen efficiently; subsequently, alveolar-hypoxia induces pulmonary arterial vasoconstriction and, ultimately, vascular wall remodeling, which could also yield right-side heart failure and death (Viet and Farrell, 1978; Holt and Callan, 2007; Stenmark et al., 2013).
Pulmonary arterial pressure (PAP) is often measured in cattle located at high elevation as a measure of pulmonary hypertension. Most beef producers at low elevations do not PAP test their cattle, as these animals do not commonly experience pulmonary hypertension, and historically, PAP observations collected at these elevations have not been considered a reliable predictor of an individual’s susceptibility to pulmonary hypertension (Holt and Callan, 2007).
However, beef production systems in the Western United States typically move cattle from moderate to high elevations and vice versa for many reasons, such as summer vs. winter grazing locations, yearling heifer, and bull replacement procedures, etc. The latter often means that herd replacements are PAP tested at moderate elevations (approximately 1,200 to 1,600 m) before being moved to higher elevations. The relative value of PAP observations collected from cattle located at moderate elevations to improve tolerance to high elevation is unknown. Therefore, the first objective of this study was to evaluate the relationship between PAP values and elevation in Angus cattle. The second objective was to quantify the genetic relationship between high elevation PAP (HE-PAP) observations and moderate elevation PAP (ME-PAP) observations. An outcome of these assessments determined the level of sire re-ranking across elevations.
MATERIALS AND METHODS
Animal care and use committee approval was not obtained because the data used in this study were obtained from existing historical databases.
Data
Pulmonary arterial pressure observations, elevation, and management data were obtained from the American Angus Association (AAA; St. Joseph, MO), the Colorado State University Beef Improvement Center (CSU-BIC; Saratoga, WY), and Dr. Timothy Holt, DVM (TH; College of Veterinary Medicine and Biomedical Sciences, Colorado State University, Fort Collins, CO) historical databases.
Angus cattle were born during the years 1985 and 2015. The initial data contained individuals that were PAP tested at an average elevation of 1,994 m with minimum and maximum elevations of 1,219 to 2,896 m, respectively. Pulmonary arterial pressure observations were recorded on animals whose age ranged from 180 to 3,600 d of age. Holt and Callan (2007) suggested that PAP measurements are most accurate at indicating an animal’s susceptibility to high-altitude disease when they are measured on individuals over 16 mo of age. Typical ranch management practices, however, dictate the measurement of PAP on potential replacement animals at or around a year of age. As such and given the age range of these data, only observations recorded between 270 and 720 d of age were used in this study. This age requirement was implemented in an effort to be consistent with ages typical of replacement bulls and heifers for herds in the Western United States. In addition, use of PAP measurements from this age range helped to ensure observations was used on cattle that had fully developed cardiopulmonary systems thereby avoiding those cattle that could potentially be experiencing age-induced pulmonary arterial remodeling or loss of pulmonary arterial elasticity that results in increased PAP measurements (Neary et al., 2015a,b). Following the imposed age restrictions, the average age at PAP collection was 402 ± 102 d.
The distribution of phenotypic PAP observations has been shown to be a skewed right-tailed distribution (Zeng, 2016). In that body of work, the most effective transformation procedure to correct the non-normality and heterogeneous variance structure associated with nontransformed PAP observations was the power transformation. To determine the most appropriate power, a Box–Cox analysis was used (Box and Cox, 1964). In this study, violations of assumptions of normality were investigated using quantile–quantile plots, and following the work of Zeng (2016), a Box–Cox analysis was used to determine the most appropriate power transformation.
Given the differing entities from which the data in this study originated, a contemporary group (CG) definition using information common to each of the 3 data sources was used. In these data, CG was defined as the combination of herd, PAP date, and yearling date. In an effort to ensure the accuracy of the information in these groups, observations on individual animals were converted to unknown whether the particular group only contained the offspring of a single sire. Observations in CG with no variance for PAP observations were removed from the analysis. Formation of CG in this manner resulted in 349 unique groups with an average of 42 individuals per group. A 3-generation ancestral pedigree (n = 25,226) was constructed from individual animals in the final data file (n = 14,665). In this pedigree, there were 2,299 unique sires and 9,743 unique dams with an average inbreeding coefficient of 0.016.
Statistical Analysis
The statistical software package ASREML 3.0 (Gilmour et al., 2009) was used to implement models used in this study. Two separate evaluations were performed. First, because PAP observations were recorded in a number of different elevations, to differentiate between HE-PAP and ME-PAP, a univariate model was used to quantify the phenotypic relationship among PAP observations and various elevations. In this first objective, PAP was classified as having been observed in either HE (≥1,600 m) or ME (<1,600 m) based on observations of Holt and Callan (2007), to test whether PAP was statistically different between the 2 elevation categories (Objective 1). This first objective was subsequently followed with a bivariate analysis to determine the genetic relationship between PAP observations obtained from moderate and high elevations (Objective 2).
Univariate Analysis
A univariate mixed model was utilized to describe the relationship between elevation and PAP. This model is presented in matrix form below.
In the above equation, y was a vector of power transformed PAP observations; X and Z were known incidence matrices relating observations in y to fixed and random effects in b and u, respectively; and e was a vector of random residual errors unique to each observation. Fixed effects included in the aforementioned model consisted of sex (bull or heifer), a linear age of PAP measurement, an elevation class variable (HE or ME), a linear elevation covariate, and the interaction between elevation class and elevation covariate. The sole random effect included in this model consisted of a direct genetic effect for PAP. Wald F statistics were used to test the significance of fixed effects in the analysis. There were 147 distinct elevation levels, with a mean of 1,887 ± 1.8 m and a minimum and maximum of 1,219 and 2,896 m, respectively.
Bivariate Analysis
Subsequently, a bivariate animal model was used to estimate the genetic relationship between HE- and ME-classified phenotypes. This model is presented below in matrix form.
In the above equation, y1 and y2 were vectors of HE-PAP and ME-PAP observations, respectively; X1 and X2 were known incidence matrices relating the observations in y1 and y2 to unknown fixed effects in b1 and b2; Z1 and Z2 were known incidence matrices relating observations in y1 and y2 to random additive genetic effects in u1 and u2; and e1 and e2 were vectors of random residual errors unique to observations in y1 and y2. In the above model, random effects were assumed to have a mean of 0 and variances as presented below:
In the above equation, A was Wright’s numerator relationship matrix, was the direct additive genetic variance for HE-PAP, was the direct genetic variance for ME-PAP, was the additive genetic covariance between HE-PAP and ME-PAP, I1 was an identity matrix with an order equal to the number of HE-PAP observations, I2 was an identity matrix with an order equal to the number of ME-PAP observations, was the residual variance for HE-PAP, and was the corresponding residual variance for ME-PAP. In these data, individual animals only had 1 PAP observation; therefore, once they were classified into either HE-PAP or ME-PAP, no animal had a PAP observation in both elevation categories. Given the structure of the data, the residual covariance was set to 0.
Fixed effects included in the bivariate analysis were sex (bull or heifer), a linear age of PAP measurement, and CG. Additive direct genetic effects were included as a random effect. Estimated breeding values for sires in the pedigree were obtained from the results of the bivariate analysis of HE-PAP and ME-PAP groups. Spearman rank correlations were used to rank sires across each elevation classification.
RESULTS AND DISCUSSION
Table 1 summarizes the performance records for PAP from each of the 3 data sources. There were 14,665 PAP observations in the data, with each source approximately contributing a third of the total number of observations. The overall average PAP observation was 43 mmHg with minimum and maximum observations of 22 and 180 mmHg, respectively. The average observations (43 ± 10.72; 42 ± 9.79; 44 ± 11.94 mmHg) from each of the 3 independent data sources were similar.
Table 1.
Descriptive statistics of mean pulmonary arterial pressure (mmHg) observations in Angus cattle
| Source | N | Minimum | Mean | Maximum | SD |
|---|---|---|---|---|---|
| AAA1 | 4,511 | 30 | 43 | 180 | 10.72 |
| CSU-BIC2 | 5,344 | 22 | 42 | 139 | 9.79 |
| TH3 | 4,810 | 31 | 44 | 164 | 11.94 |
| Combined4 | 14,665 | 22 | 43 | 180 | 10.83 |
1Data obtained from the American Angus Association.
2Data obtained from Colorado State University Beef Improvement Center.
3Data obtained from Dr. Tim Holt, DVM.
4All 3 data sources combined.
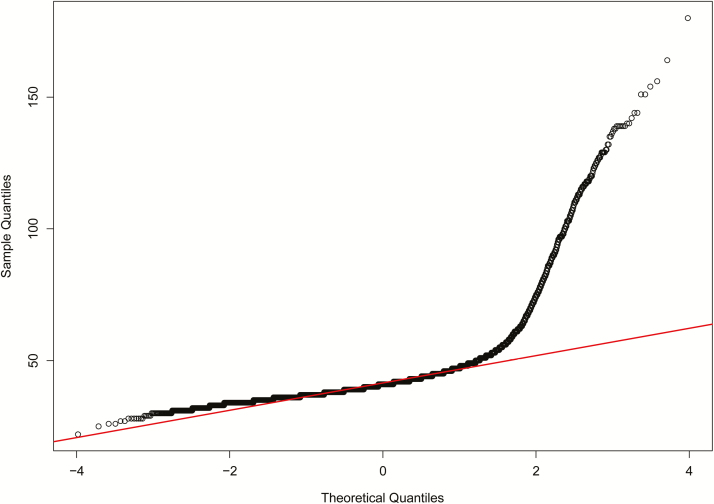
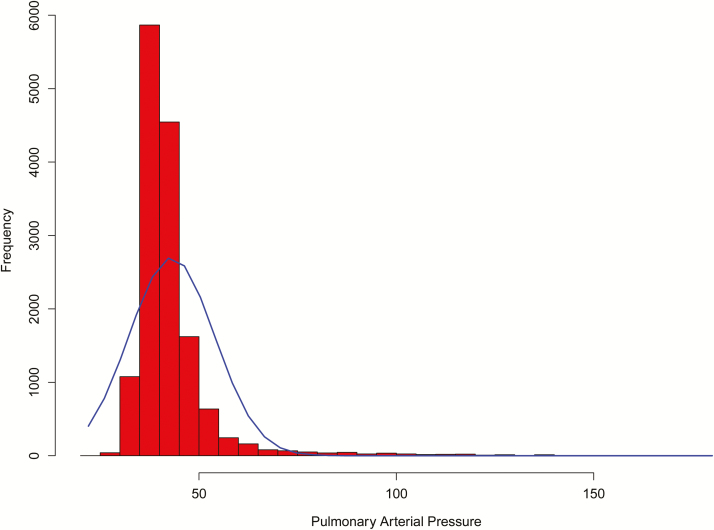
Figures 1 and 2 present the quantile–quantile plot and histogram of the raw pap observations, respectively. Similar to the results presented by Zeng (2016), PAP observations violated the assumption of normality due to the long, thin tail of extreme PAP measurements present on the right side of the distribution. We made the decision, following the recommendations of Zeng (2016), to not remove these extreme observations because these phenotypes are valuable to the understanding of the relationship between PAP and risk of high-altitude disease. Therefore, in an effort to accommodate this non-normality, data were transformed using a power transformation calculation. The most appropriate power transformation was determined using a Box–Cox analysis and suggested that raw PAP observations be raised to the power of −2.6.
Figure 1.
Pulmonary arterial pressure quantile–quantile plot with the theoretical normal distribution represented by the red line.
Figure 2.
Histogram of pulmonary arterial pressure measurements, with the blue line representing the corresponding probability density plot.
Univariate Analysis
In the univariate analysis, transformed PAP observations were evaluated using a model that contained sex, age, elevation class, elevation (continuous), and the interaction between elevation and elevation class. In this model, all main effects were found to be significant (P < 0.001). Specifically, elevation class solutions and elevation regression coefficients were found to be different from zero, meaning the intercept of the regression of PAP on elevation differed by elevation classification (HE or ME). In other words, based on these data, the average PAP phenotype observed at ME was different (P < 0.001) than the average PAP phenotype observed at HE. The interaction between elevation class and elevation was not significant (P > 0.05), which suggested that the slope of the regression line of PAP on elevation was similar between elevation classes.
In summary, these results indicate that PAP observations tended to be greater at higher elevations and a higher proportion of cattle located at elevations above 1,600 m most likely experienced pulmonary hypertension as was observed in prior research (Holt and Callan, 2007; Neary et al., 2013a,b). However, right-sided heart failure may occur at altitude levels less than 1,600 m (Neary et al., 2016) where PAP is only an indicator of high-altitude disease. As such, additional analyses were conducted to determine the genetic relationship between PAP measurements collected at high vs. moderate elevations.
Bivariate Analysis
Summary statistics of PAP observations made above and below 1,600 m of elevation are presented in Table 2. Raw unadjusted phenotypic mean PAP observations from both categories were the same (43 mmHg), whereas HE-PAP had a slightly larger range in observations than ME-PAP. As expected given historical emphasis and measurement of PAP at higher elevations, the majority (89%) of the recorded observations were classified into HE-PAP. As previously stated, PAP is an indicator of an individual animal’s susceptibility to pulmonary hypertension and is used as a tool to indicate whether or not an individual animal can adapt to lower oxygen environments associated with higher altitudes (Holt and Callan, 2007).
Table 2.
Descriptive statistics of pulmonary arterial pressure (PAP; mmHg) at high elevation (HE) and moderate elevation (ME) in Angus cattle
| Item | N | Minimum | Mean | Maximum | SD |
|---|---|---|---|---|---|
| HE-PAP1 | 13,088 | 22 | 43 | 180 | 10.87 |
| ME-PAP2 | 1,577 | 32 | 43 | 144 | 10.52 |
1PAP observations from elevations ≥ 1,600 m.
2PAP observations from elevations < 1,600 m.
Table 3 presents the number of sires by elevation, as well as average number of progeny per sire. Data classified into HE-PAP contained the largest number of sires, which is not surprising given the category also had the largest number of observations. There were a 112 sires represented in the ME-PAP category, and 74 sires had progeny in both categories.
Table 3.
Number of sires with progeny represented in high and moderate elevations, along with the average number of progeny per sire in each elevation category
| Elevation | N | Average number of progeny |
|---|---|---|
| High1 | 635 | 21 |
| Moderate2 | 112 | 14 |
| Both3 | 74 | 62 |
1Elevation ≥ 1,600 m.
2Elevation < 1,600 m.
3Sires with offspring in both elevation categories.
Heritabilities and genetic correlations from the bivariate analysis of HE-PAP and ME-PAP are presented in Table 4. The heritability estimate for HE-PAP observations was 0.34 ± 0.03, whereas the heritability estimate for ME-PAP observations was 0.29 ± 0.09. Both estimates were within the range of previously reported estimates in Angus cattle (0.20 to 0.46; Enns et al., 1992; Shirley et al., 2008; Crawford et al., 2016). Similar research by Williams et al. (2012) evaluated the genetic relationship between growth traits (weaning weight and postweaning gain) at differing altitudes in Angus cattle. In that study, the authors utilized the recommendation of Robertson (1959) that a genetic correlation coefficient less than 0.80 would be considered a biologically significant genotype by environment interaction. The estimate of the genetic correlation between HE-PAP and ME-PAP in the current study was 0.83 ± 0.15. When compared with the recommendation of Robertson (1959), the estimate obtained in this study was very close to the suggested threshold. An argument could be made either way as to whether or not HE-PAP and ME-PAP are distinct traits. An issue with these data is the minimum elevation of PAP observations used in this study at 1,219 m, which is still a greater elevation than most beef cattle production environments in the United States. Because of this, the relatively small number of ME-PAP observations (n = 1,577) may not be a thorough representation of PAP measured below the demarcation line of 1,600 m.
Table 4.
Heritability (diagonal; SE) and genetic correlation (above diagonal; SE) for HE-PAP and ME-PAP
| Item | HE-PAP1 | ME-PAP2 |
|---|---|---|
| HE-PAP1 | 0.34 (0.03) | 0.83 (0.15) |
| ME-PAP2 | 0.29 (0.09) |
1Pulmonary arterial pressure observations from elevations ≥ 1,600 m.
2Pulmonary arterial pressure observations from elevations < 1,600 m.
Holt and Callan (2007) suggested that PAP observations are only a reliable predictor of an animal’s susceptibility to pulmonary hypertension when obtained at elevations > 1,600 m. It has been reported in additional studies that PAP observations on calves, at or around a year of age, increase as altitude increases (Neary et al., 2015a,b). Because of this altitude-associated increase, if the recorded PAP observations are true indicators of an animal’s high-altitude adaptability, observations should be recorded at high elevation. These results do suggest, however, that PAP observations obtained below 1,600 m are useful at indicating an animal’s genetic merit for risk of developing high-altitude disease, but additional data and research are needed to better understand the genetic influences on PAP measured at various altitudes.
Pearson and Spearman’s rank correlation coefficients between sires HE-PAP EBV and ME-PAP EBV were 0.95 and 0.89, respectively. These values are high, yet suggest there was re-ranking among sires between environmental categories. In these data, there were only 27 sires with at least 10 progeny PAP tested in high and moderate elevations. Those sires with the greatest EBV re-ranking between elevations would be those individuals with progeny observations in the ME-PAP category and not represented in the HE-PAP category.
CONCLUSIONS
Results from this study indicated that both HE-PAP and ME-PAP are moderately heritable. These results also indicate a moderate to strong genetic relationship exists between the 2 traits. This relationship does result in some re-ranking of sires among groupings separated at 1,600 m and reinforces the heretofore perception that PAP observations recorded in lower elevations are not as useful as an indicator of pulmonary hypertension as are PAP observations recorded at higher elevations (Holt and Callan, 2007). Even though the genetic correlation between HE-PAP and ME-PAP was high, the use of a bivariate model for the prediction of a sire’s genetic merit for HE-PAP supported the use of observations recorded at lower elevations to predict an individual’s potential for use in higher elevations where pulmonary hypertension is a larger issue.
Footnotes
The authors thank the American Angus Association (i.e., Angus Genetics Inc.) for supplying data and financial support. We also thank Dr. Timothy Holt for supplying data, as well as Colorado State University’s John E. Rouse Endowments and its Beef Improvement Center for supplying both data and financial support of graduate students and faculty. This project was partially supported by Hatch Project Accession Number 1010007 from the USDA National Institute of Food and Agriculture.
LITERATURE CITED
- Box G. E., and Cox D. R.. 1964. An analysis of transformations. J. R. Stat. Soc. B (Methodol.) 26:211–252. [Google Scholar]
- Crawford N. F., Thomas M. G., Holt T. N., Speidel S. E., and Enns R. M.. 2016. Heritabilities and genetic correlations of pulmonary arterial pressure and performance traits in Angus cattle at high altitude. J. Anim. Sci. 94:4483–4490. doi: 10.2527/jas.2016-0703 [DOI] [PubMed] [Google Scholar]
- Enns R. M., Brinks J., Bourdon R., and Field T.. 1992. Heritability of pulmonary arterial pressure in Angus cattle. Proc. West. Sect. Am. Soc. Anim. Sci. 43:111–112. [Google Scholar]
- Gilmour A. R., Gogel B. J., Cullis B. R., and Thompson R.. 2009. ASReml user guide release 3.0. VSN Int. Ltd, Hemel Hempstead, UK: www.vsni.co.uk (accessed 17 May 2017). [Google Scholar]
- Holt T. N., and Callan R. J.. 2007. Pulmonary arterial pressure testing for high mountain disease in cattle. Vet. Clin. North Am. Food Anim. Pract. 23:575–596, vii. doi: 10.1016/j.cvfa.2007.08.001 [DOI] [PubMed] [Google Scholar]
- Neary J. M., Booker C. W., Wildman B. K., and Morley P. S.. 2016. Right-sided congestive heart failure in North American feedlot cattle. J. Vet. Intern. Med. 30:326–334. doi: 10.1111/jvim.13789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary J. M., Garry F. B., Holt T. N., Brown R. D., Stenmark K. R., Enns R. M., and Thomas M. G.. 2015a. The altitude at which a calf is born and raised influences the rate at which mean pulmonary arterial pressure increases with age. J. Anim. Sci. 93:4714–4720. doi: 10.2527/jas.2015-9217 [DOI] [PubMed] [Google Scholar]
- Neary J. M., Garry F. B., Holt T. N., Knight A. P., Gould D. H., and Dargatz D. A.. 2013a. Pulmonary arterial pressures, arterial blood-gas tensions, and serum biochemistry of beef calves born and raised at high altitude. Open Acc. Anim. Physiol. 5:1–8. doi: 10.2147/OAAP.S45513 [DOI] [Google Scholar]
- Neary J. M., Garry F. B., Holt T. N., Thomas M. G., and Enns R. M.. 2015b. Mean pulmonary arterial pressures in Angus steers increase from cow-calf to feedlot-finishing phases. J. Anim. Sci. 93:3854–3861. doi: 10.2527/jas.2015-9048 [DOI] [PubMed] [Google Scholar]
- Neary J. M., Gould D. H., Garry F. B., Knight A. P., Dargatz D. A., and Holt T. N.. 2013b. An investigation into beef calf mortality on five high-altitude ranches that selected sires with low pulmonary arterial pressures for over 20 years. J. Vet. Diagn. Invest. 25:210–218. doi: 10.1177/1040638713478608 [DOI] [PubMed] [Google Scholar]
- Robertson A. 1959. The sampling variance of the genetic correlation coefficient. Biometrics 15:469–485. [Google Scholar]
- Shirley K. L., Beckman D. W., and Garrick D. J.. 2008. Inheritance of pulmonary arterial pressure in Angus cattle and its correlation with growth. J. Anim. Sci. 86:815–819. doi: 10.2527/jas.2007-0270 [DOI] [PubMed] [Google Scholar]
- Stenmark K. R., Yeager M. E., El Kasmi K. C., Nozik-Grayck E., Gerasimovskaya E. V., Li M., Riddle S. R., and Frid M. G.. 2013. The adventitia: Essential regulator of vascular wall structure and function. Annu. Rev. Physiol. 75:23–47. doi: 10.1146/annurev-physiol-030212-183802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viet H. P., and Farrell R. L.. 1978. The anatomy and physiology of the bovine respiratory system relating to pulmonary disease. Cornell Vet. 68:555–581. [PubMed] [Google Scholar]
- Williams J. L., Bertrand J. K., Misztal I., and Lukaszewicz M.. 2012. Genotype by environment interaction for growth due to altitude in United States Angus cattle. J. Anim. Sci. 90:2152–2158. doi: 10.2527/jas.2011-4365 [DOI] [PubMed] [Google Scholar]
- Zeng X. 2016. Angus cattle at high altitude: Pulmonary arterial pressure, estimated breeding value and genome-wide association study. PhD Diss. Colorado State Univ, Fort Collins, CO. [Google Scholar]