Abstract
Dietary fat is known to modulate the hindgut microbiota in rodents; however, there is no clear evidence on the impact of high-fat diets on canine gut microbiota. The purpose of this study was to investigate the effect of feeding of diets differing in the amount of ME provided by fat and starch on the composition and activity of canine fecal microbiota. Twelve adult (3 to 7 yr of age) spayed Beagle dogs received a low-fat–high-starch diet (LF–HS; approximately 23%, 42%, and 25% ME provided by fat, starch, and CP, respectively) and a high-fat–low-starch diet (HF–LS; approximately 43%, 22%, and 25% ME provided by fat, starch, and CP, respectively) following a 2-period crossover arrangement. The higher amount of fat in the HF–LS diet was provided by lard, whereas the higher amount of starch in the LF–HS diet was provided primarily by maize and broken rice. Each period lasted 7 wk and included 4 wk for diet adaptation. Dogs were fed to meet their daily energy requirements (set at 480 kJ ME/kg BW0.75). Fecal samples were collected on weeks 5 and 6 of each period for the analysis of bacterial richness, diversity, and composition [by Ion-Torrent next-generation sequencing], bile acids, ammonia, and VFA. Additional fecal samples were collected from four dogs per diet and period to use as inocula for in vitro fermentation using xylan and pectin as substrates. Gas production was measured at 2, 4, 6, 9, 12, and 24 h of incubation. On week 7, blood samples were collected at 0- and 180-min postfeeding for the analysis of bacterial lipopolysaccharide (LPS). Feeding the HF–LS diet led to a greater (P < 0.05) fecal bile acid concentration compared with the LF–HS diet. Bacterial richness and diversity did not differ between diets (P > 0.10). However, dogs showed a lower relative abundance of Prevotella (P < 0.01), Solobacterium (P < 0.05), and Coprobacillus (P ˂ 0.05) when fed of the HF–LS diet. Fecal ammonia and VFA contents were not affected by diet (P > 0.10). Relative to the LF–HS diet, in vitro fermentation of xylan using feces of dogs fed the HF–LS diet produced less gas at 6 h (P < 0.01) and 9 h (P < 0.05). Blood LPS did not increase at 180-min postfeeding with either diet (P < 0.10). These findings indicate that feeding a HF–LS diet to dogs does not affect bacterial diversity or fermentative end products in feces, but may have a negative impact on Prevotella and xylan fermentation.
Keywords: bile acids, dog, fat, Prevotella, starch, xylan fermentation
INTRODUCTION
Major progress has been made in delineating the effects of diet on gut microbiota and host metabolism (Wu et al., 2011; Flint et al., 2012). Fat intake has been associated with the absorption of the inflammatory Gram-negative lipopolysaccharide (LPS) (Ghoshal et al., 2009), which contributes to the development of obesity (de la Serre et al., 2010). Feeding high-fat diets to rodents has been related to a decreased bacterial abundance and diversity, alongside an increase in Firmicutes and a decrease in Bacteroidetes (Turnbaugh et al., 2008). Although unsaturated fatty acids can have a negative effect on microbial growth (Enjalbert et al., 2017), fatty acids are majorly absorbed in the small intestine, and changes in the gut microbiota in response to fat have been linked to an increased flow of bile acids into the hindgut (Yokota et al., 2012; David et al., 2014). Thus, rats given oral doses of cholic acid (CA) showed changes in cecal microbiota resembling those found with high-fat diets along with a decreased cecal VFA content (Islam et al., 2011). However, high-fat diets are generally low in complex carbohydrates, including starch, contributing to the effects caused by these diets (Graf et al., 2015). It is acknowledged that part of starch may escape digestion and induce changes in the gut microbiota (Licht et al., 2006). To our knowledge, the effect of high-fat diets on canine gut microbiota remains uncertain. This study aimed to investigate the effect of feeding diets with a high or a low fat to starch content on the composition and activity of fecal microbiota and on blood LPS in dogs. We hypothesized that increases in dietary fat would affect the microbiota ecosystem, leading toward a decreased fermentative activity, primarily as a result of an increased bile acid secretion. Results concerning diet digestibility and dogs’ BW, BCS, and blood concentration of satiety-related hormones obtained from this study have been previously published (Schauf et al., 2018).
MATERIALS AND METHODS
Animals and Diets
Animal housing and experimental procedures were approved by the in-house Ethic Committee for Animal experimentation from the University of Zaragoza (CEAEA) and conformed to the Spanish Policy for Animal Protection (RD 1201/05), which meets the EU Directive 86/609 on the protection of animals used for experimental and scientific purposes. A total of 12 healthy adult (3 to 7 yr of age) spayed Beagle dogs with 13.70 ± 0.487 kg BW and 5.3 ± 0.22 BCS [using a 1 to 9 point scale, with 1 for cachectic to 9 for obese condition (Laflamme, 1997)] took part in this study. Dogs were individually housed in indoor concrete floor kennels (2.0 × 2.5 m) with outdoor access (2.0 × 5.0 m). Each pen was provided with a feeder, an automatic water dispenser, and an elevated plastic grid that allowed dogs to rest and stay dry. Indoor temperature was kept at 17 to 24 °C. Dogs were regularly vaccinated and dewormed and had received no medications expected to alter the gut microbiota (e.g., antibiotics) over the previous 2 mo. Two diets were formulated to contain differing levels of fat, in terms of ether extract (EE), and of starch, but to provide a similar amount of protein on a ME basis: low-fat–high-starch diet [LF–HS, 6.1 g fat, in terms of EE, 27.0 g starch, and 15.8 g CP/MJ estimated ME] and high-fat–low-starch diet (HF–LS, 11.7 g fat, 14.8 g starch, and 16.4 g CP/MJ estimated ME). Table 1 shows the ingredient and analyzed chemical composition and the estimated ME content of the diets. Differences in fat and starch contents were obtained by increasing the amount of lard in the HF–LS diet and the amount of maize and broken rice in the LF–HS diet. The dietary energy content was estimated based on NRC (2006) as the product of the determined GE and its digestibility coefficient (GED = [91.2 – (1.43 × % crude fiber, in DM)/100]), and assuming urinary energy losses of 4.35 kJ/g CP. The amount of food offered to dogs was calculated according to the estimated ME content of the diets. The contribution of CP, EE, and starch to total energy content (as % ME) was calculated by applying the Atwater factors 14.64, 35.56, and 14.64 kJ/g of CP, EE, and nitrogen-free extractives (NFE), respectively (NRC, 1985). As percentage of ME, the LF–HS diet provided approximately 23, 42, and 25% ME as EE, starch, and CP, respectively), whereas the HF–LS diet provided approximately 43, 22, and 25% ME as EE, starch, and CP, respectively).
Table 1.
Ingredient and analyzed chemical composition of the diets
| Low fat–high starch | High fat–low starch | |
|---|---|---|
| Ingredient composition, g/kg as fed | ||
| Poultry by-product meal (64% CP, 18% ash) | 200.0 | 200.0 |
| Maize | 340.0 | 180.0 |
| Lard | 40.0 | 150.0 |
| Broken rice | 170.0 | 107.0 |
| Whole barley | 97.5 | 120.0 |
| Soybean meal (48% CP) | 40.0 | 100.0 |
| Potato protein | 25.0 | 55.0 |
| Sugar-beet pulp | 30.0 | 30.0 |
| Hydrolized animal protein1 | 40.0 | 40.0 |
| Vitamin–mineral mixture2 | 16.5 | 16.5 |
| dl-Methionine | 1.0 | 1.5 |
| Analyzed chemical composition, g/kg DM | ||
| DM | 909.0 | 923.0 |
| Ash | 69.0 | 69.9 |
| CP | 259.0 | 300.0 |
| Ether extract | 99.3 | 214.0 |
| Crude fiber | 23.6 | 22.4 |
| Starch | 442.0 | 271.0 |
| Insoluble dietary fiber | 113.0 | 107.0 |
| Soluble dietary fiber | 10.4 | 20.8 |
| Energy content, MJ/kg DM | ||
| GE3 | 20.0 | 22.4 |
| ME4 | 16.4 | 18.3 |
1An Affinity Petcare product (Dog Pal SP 350500 Liquid).
2Supplying per kilogram: 5 g NaCl, 4 g KCl, 2.5 g choline chloride (60%); 0.360 g Mg, 173 mg Fe, 9.60 mg Cu, 190 mg Zn, 0.158 mg Co, 57.5 mg Mn, 0.75 mg Se, 8.10 mg vitamin A, 0.045 mg vitamin D3, 548 mg vitamin E, 0.450 mg vitamin K3, 46.0 mg vitamin B1, 22.8 mg vitamin B2, 170 mg vitamin B3, 44.5 mg vitamin B5, 13.6 mg vitamin B6, 0.137 mg vitamin B7, 5.76 mg vitamin B9, 0.127 mg vitamin B12, and 386 mg vitamin C.
3Determined by calorimetric bomb.
4ME = metabolizable energy. Estimated as GE × GE digestibility coefficient (91.2 − [1.43 × % crude fiber]/100) and assuming urinary losses of 4.35 kJ/g CP (NRC, 2006).
Diets were offered at 0900 h at a level of intake of 480 kJ ME/kg ideal BW0.75, which approximates the daily energy requirement reported by Jeusette et al. (2004) for spayed Beagle dogs. Ideal BW of dogs was estimated at the beginning of the study by increasing or decreasing their actual BW by 10% per unit BCS below or above an ideal BCS set at 5 (German et al., 2009).
Experimental Design and Procedure
Dogs received the LF–HS and the HF–LS diets in 2 consecutive experimental periods following a crossover arrangement (6 dogs per diet and period). Each experimental period lasted 7 wk and was preceded by a 2-wk washout period, during which dogs received a dry extruded maintenance diet [Brekkies Excel, Affinity Petcare; 140 g EE, 450 g NFE, 230 g CP, and 27.0 g crude fiber (CF)] per kg food, according to the manufacturer, providing 33, 45, and 22% ME as EE, NFE, and CP, respectively.
Each experimental period included 4 wk of diet adaption, during which dogs received half of the maintenance and of the experimental diets on the first week and then exclusively the experimental diets. Throughout weeks 5 and 6 of each period, fresh fecal samples (~60 g voided within 15 min) were collected from each dog in 2 sampling days (3 dogs assigned to each of the diets were sampled within the same week) for the characterization of fecal microbiota, by using next-generation sequencing (NGS), and for the determination of fermentative end products (VFA and ammonia), bile acids, and fecal pH. Additional samples were collected and stored at −80 °C to use as fecal inocula for in vitro fermentation. For the analysis of fecal microbiota, 5 g of feces from the first sampling day were snap frozen in liquid N, freeze-dried (LyoBeta 25, Telstar, Sant Cugat, Spain), according to the preservation procedures stated by Prates et al. (2010) for in vitro fermentation, and stored at −80 °C until DNA extraction. For VFA analysis, feces from each sampling day (~2 g) were added to a 10-mL screw-cap polypropylene tube (Starstedt, Numbrecht, Germany) containing 4 mL of a deproteinizing solution constituted by 20 mL/L of H3PO4 (85% purity) and 1 mL/L of 4-methylvaleric acid (ref. M-7396 SIGMA) as internal standard. For ammonia analysis, feces from both sampling days (~2 g) were homogenized in 4 mL of HCl 0.2 N. The resultant solutions were stored at −20 °C until analysis. For bile acid determination, feces from each sampling day (~4 g) were freeze-dried and stored at −80 °C until bile acid extraction. In each sampling occasion, 2 subsamples (~5 g) were collected for the determination of fecal DM content by freeze-drying. On week 7 of each period, blood samples were taken in the fasted state and at 180-min postfeeding for the determination of LPS. Blood (2 mL) was collected in serum separator tubes (BD Vacutainer, Plymouth, UK), centrifuged at 2,000 × g for 10 min at 4 °C, and stored at −80 °C until analysis.
Samples of both diets were collected weekly during each period and pooled per diet and period for analysis. Dogs were weighed weekly throughout the study, and their BCS was evaluated at the start and at the end of each period to assess their energy requirements.
Chemical Analysis of Food
Prior to analysis, food subsamples were ground to 1 mm. The DM content of food was determined by oven-drying at 105 °C for 24 h (UFE 500, Memmert GmbH, Schwabach, Germany). Analyses of ash, CP, EE, and CF were carried out according to the procedures outlined in the Association of Official Analytical Chemists (AOAC, 2005) (no. 942.05, 976.05, 954.02, and 978.10, respectively). Ash content was analyzed using a muffle furnace (10-PR/400serie 8B, Forns Hobersal, S.L., Caldes de Montbui, Barcelona, Spain). Analyses of CP and EE were determined using a 2300 Kjeltec Analyzer Unit (Foss TecatorAB, Höganäs, Sweden) and Majonnier flasks (V20129, Agora, S.A., Barcelona, Spain), respectively. Analysis of CF was carried out using Fibertec (1020, Foss Analytical AB, Höganäs, Sweden). Total dietary fiber (TDF) content was calculated as the sum of soluble and insoluble dietary fiber according to AOAC (1995) procedures no. 993.19 and 991.42, respectively, using the Megazyme Total Dietary Fiber kit (K-TDFR 100A/K-TDFR-200A, Megazyme International, Wicklow, Ireland). The starch content was analyzed enzymatically (K-TSTA kit, Megazyme International, Wicklow, Ireland) using the method 996.11 adopted by AOAC (2005). The GE content was determined in an adiabatic bomb calorimeter (IKA C-4000, Janke-Kunkel, Staufen, Germany).
Fecal DNA Extraction and Ion-Torrent NGS
Freeze-dried feces were disrupted by bead beating using a Mini-Beadbeater-16 (Biospec Products Inc., Bartlesville, OK) and subjected to DNA extraction using a QIAamp DNA Stool Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer’s instructions. The concentration and purity of the extracted DNA were verified using a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE). Sequencing of 16S rRNA gene was conducted following the procedure carried out by de la Fuente et al. (2014). Briefly, the bacterial V1–V2 region was amplified by PCR using bar-coded fusion primer pairs 27F and 338R (Wang et al., 2014). Sequencing of the PCR products was carried out on the Ion-Torrent Personal Genome Machine (PGM) system using the Ion PGM Sequencing 200 kit v2 (Life Technologies, Carlsbad, CA). Following sequencing, NGS amplicon reads were subjected to trimming, denoising, and chimera removal and clustered into Operational Taxonomic Units (OTU) at 97% identity using the UPARSE pipeline (Edgar, 2013). Reads were subjected to quality filtering (quality score of 20 in a 1 to 40 scale) and trimmed at a maximum length of 250 bp. Taxonomic assignation of 16S rRNA sequences was established by comparison against the Ribosomal Data Project II database (Cole et al., 2003), considering a bootstrap value over 0.65 for annotation, leaving successive taxon levels as unclassified. Amplicon sequences were deposited in the European Nucleotide Archive database and are available under the study accession number PRJEB19863.
Measurement of Fecal Ammonia, VFA, and Bile Acids
Fecal ammonia was measured by spectrophotometry following the method described by Chaney and Marbach (1962). Analysis of VFA was performed by GC using an Agilent 6890 gas liquid chromatograph (Agilent Technologies España, S.L., Madrid, Spain) fitted with a capillary column (HP-FFAP polyethylene glycol TPA-treated, 30 m × 530 µm i.d. × 1 µm film thickness) and a flame ionization detector. For bile acid analysis, feces from the 2-d collection were pooled per dog and subjected to bile acid extraction as described by Hagio et al. (2009). Total bile acids were quantified in an Olympus AU400 auto-analyzer (Olympus, Hamburg, Germany) using the Diazyme Laboratory Total Bile Acids Assay Kit (Diazyme Lab, Poway, CA). The relative concentration of bile acids was determined following the procedures outlined by Hagio et al. (2011) using a Ultra-Performance Liquid Chromatograph (Acquity UPLC, Waters, Milford, MA) fitted with a UPLC BEH C18 column (100 mm × 1.0 mm i.d., 1.7 µm BEH-bridge ethyl silicone hybrid structure-particle size) connected to an electrospray ionization device and a mass analyzer (Quattro Premier XE quadrupole tandem MS, Waters). Analyzed bile acids comprised primary bile acids in free form [CA and chenodeoxycholic acid (CDCA)] and conjugated form [taurocholic acid (TCA), glycocholic acid (GCA), taurochenodeoxycholic acid (TCDCA), and glycochenodeoxycholic acid (GCDCA)], secondary bile acids [deoxycholic acid (DCA) and lithocholic acid (LCA), derived from bacterial 7-α-dehydroxylation of CA and CDCA, respectively], and tertiary bile acids [taurodeoxycholic acid (TDCA), glycodeoxycholic acid (GDCA), taurolithocholic acid (TLCA), and glycolithocholic acid (GLCA)], derived from hepatic reconjugation of secondary bile acids. Glycocholic-2, 2, 4, 4-d4 acid was added to each sample as an internal standard, and quality control samples were evenly distributed over the batches to estimate any intrabatch drift. Internal standard corrected response in each batch was divided by its corresponding intrabatch drift trend, such that normalized bile acid concentration of the study samples were expressed with respect to the batch averaged quality control calibration (arbitrarily set to 1).
In Vitro Fermentation
Fermentative activity of feces was assessed by in vitro gas production at the end of each period (week 8) as described by Theodorou et al. (1994). The incubation solution was adapted from previously defined incubation media (Mould et al., 2005) following the modifications described by Suarez-Belloch et al. (2013). Frozen feces from 4 dogs per diet and period were chosen randomly to use as fecal inocula. Feces from the 2-d samplings were pooled and thawed in a 38 °C water bath and afterwards diluted in the incubation solution (1:10; wt:vol). A high-fermentable substrate (pectin from citrus fruits, 84% galacturonic content as is, P9135, Sigma-Chemical Co., St Louis, MO) and a low-fermentable substrate (xylan, from Birchwood, X0502, Sigma–Aldrich Chemie, Steinheim, Germany) were incubated. Before the assay, 13 mL of the incubation solution was dispensed under a stream of CO2 into gas-tight Pyrex glass culture bottles (32 mL total volume) containing 200 mg of substrate; bottles were sealed and maintained in a water bath for 1 h at 38 °C allowing complete hydration of the substrate. Bottles were then opened and filled with 7 mL of fecal inocula under a flow of CO2, sealed again, and incubated for 24 h at 38 °C. Gas production was measured at 2, 4, 6, 9, 12, and 24 h from the same tube using an HD8804 manometer with a TP804 pressure gauge (DELTA, OHM, Caselle di Selvazzano, Italy). Pressure readings (mbar) were converted to volume units (mL) using a pre-established linear regression equation [pressure = 1.301 + (75.10 × volume); R2 = 0.997; n = 35]. Two incubation runs were carried out for each period; in each run, fecal inocula of 2 dogs per diet and substrate were incubated in triplicate alongside 3 blank bottles with no substrate to correct for gas not arising from substrates.
Measurement of Blood LPS
Serum LPS concentration was determined in 6 dogs within each diet (n = 3 dogs per diet and period) using a kinetic chromogenic assay (Endosafe Detection System, Charles River, Ecully, France) composed of the Limulus Amebocyte extract and with a limit of sensitivity of 0.001 endotoxin units (EU)/mL.
Calculations and Statistical Analysis
Clustering analysis of the identified OTU was displayed using the Bray–Curtis similarity index. The relatedness between diets was evaluated on the dataset of OTU by permutational multivariate analysis of variance (Permanova) using Permanova + package (version 1.0.2; Primer-E, Ivybridge, UK). Richness (R), Shannon diversity, and Shannon equitability indices were calculated using normalized NGS data. The Shannon diversity index (H) was calculated as follows: , where pi is the proportion of 1 specific OTU (i) relative to the total number of OTU (R) in the sample. The Shannon equitability index (EH), which reflects the diversity of the bacterial community relative to a maximum level of diversity, was calculated as: EH = H/Hmax, where Hmax = ln (R). The coverage of the amplicon library (C) was estimated according to Good (1953) using the following equation: C = [1 − (n/N)] × 100, where n is the number of unique amplicon sequences (singletons) and N is the total number of sequences examined. Read sequences were assigned at phyla, class, family, and genera levels and presented as percentage of total sequences. Statistical analysis for fecal bacteria at each taxonomic level, fecal parameters (DM, bile acids, ammonia, VFA, and pH), gas production (for each type of fecal inocula and substrate at each time), and blood LPS (at 0 and 180 min) was performed using the mixed procedure (PROC MIXED) of the Statistical Analysis Systems software package version 9.2 (SAS Institute, Cary, NC). The model included diet, period, and dietary order as fixed effects and animal within dietary order as random effect. Differences between diets were established based on the LSD test. Level of significance was set at P < 0.05, and 0.05 ≤ P < 0.10 was considered a trend. Pearson’s correlations between bacterial sequences and bile acids were analyzed by the PROC CORR procedure, and only considered when │r│ ˃ 0.5. In tables, the SE of the difference for comparisons between diets is provided. Data along the text are presented as means ± SEM.
RESULTS
BW and BCS
The BW of dogs varied by less than 4% across each period. Mean BW and BCS of dogs throughout periods 1 and 2 were not affected by diet (P > 0.10); these results are shown in Schauf et al. (2018).
Characterization of Fecal Microbiota
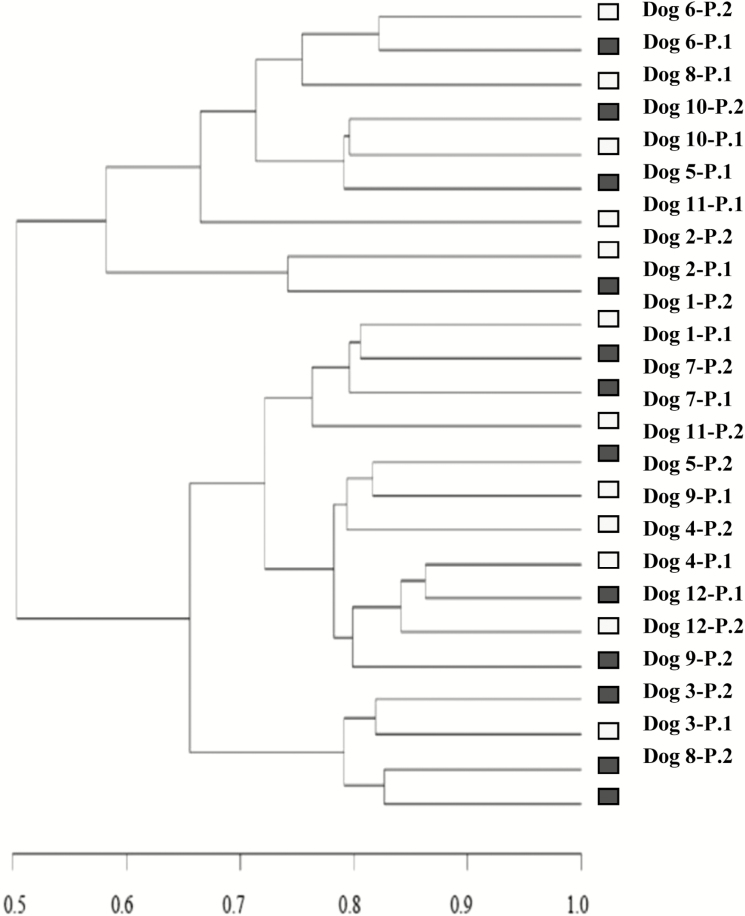
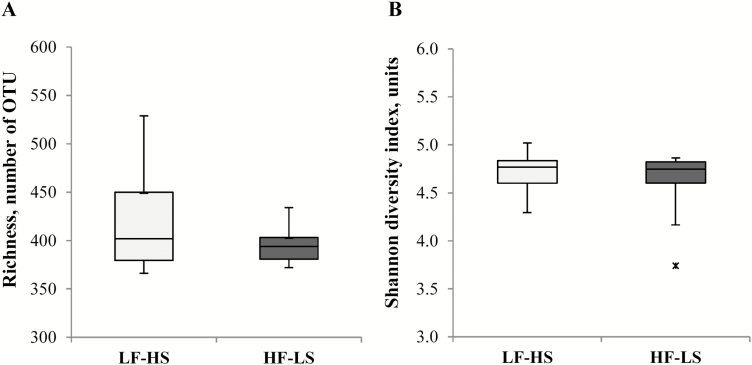
NGS dataset comprised on average 35,940 ± 6.8 read sequences per sample. At the 97% similarity level, V1–V2 samplings had a 99.85 ± 0.009% Good’s coverage (rarefaction curves showing amplicon library coverage are available in Supplementary Appendix 1). A total of 692 OTU were identified, with individual samples containing on average 407 ± 8.1 OTU. Clustering analysis of the OTU matrix grouped mostly by dog rather than by diet (Fig. 1). The richness and Shannon diversity index box plots of fecal microbiota of dogs with both diets are shown in Fig. 2. Diet did not affect richness (418 ± 15.0 vs. 396 ± 5.3, with LF–HS and HF–LS diet, respectively; P = 0.17) or Shannon diversity (4.72 ± 0.058 vs. 4.62 ± 0.097, with LF–HS and HF–LS diet, respectively; P = 0.11). Shannon equitability index remained similar in both diets (0.783 ± 0.009 with LF–HS and 0.772 ± 0.015 with HF–LS, P = 0.23).
Figure 1.
Dendrogram generated from the data set of OTU obtained by NGS representing paired compared similarities based on Bray–Curtis similarity index in dogs when fed a low-fat–high-starch diet (LF–HS; □) diet or a high-fat–low-starch diet (HF–LS; ■). Each diet was distributed to half of the dogs in period 1 (P1) and to the other half in period 2 (P2).
Figure 2.
Box plots for richness (A) and Shannon diversity index (B) estimated by NGS in 12 dogs fed a low-fat–high-fat diet (LF–HS; □) and a high-fat–low-starch diet (HF–LS; ■). Boxes represent 50% of all values between the 75th and 25th quartiles (median value showed as a horizontal line). The end point of the whiskers marks maximum and minimum values. The outlier (x) represents a data-point lower than 3 times the interquartile range (IQR = 75th to 25th values) subtracted from the 25th quartile.
Taxonomic Composition of Fecal Microbiota
Taxonomy-based analysis at phyla and class levels with both diets is shown in Table 2. Diet did not affect fecal bacterial composition at phyla or class levels (P ≥ 0.10). Firmicutes and Bacteroidetes were the predominant phyla, followed by Fusobacteria. Bacteroidia and Fusobacteria were the major bacterial classes, between the two contributing to more than 50% of total sequences. Among the phylum Firmicutes, the classes Erysipelotrichi and Clostridia were equally represented and were followed in number by Negativicutes and Bacilli. At a genera level, Bacteroides and Prevotella (class Bacteroidia) were the major bacteria in both diets. Along with Clostridium cluster XIX and Fusobacterium (class Fusobacteria), these 4 genera accounted for up to 50% of total sequences (Table 3). A lower relative abundance of Prevotella was evidenced in dogs fed the HF–LS diet (P = 0.008). However, this diet effect was influenced by the order in which dogs received the diets (dietary order, P = 0.049). Thus, in dogs that switched from the LF–HS to the HF–LS diet the relative abundance of Prevotella was reduced from 12.6 ± 1.46% to 4.10 ± 1.14% (P < 0.001), whereas in dogs that switched from the HF–LS to the LF–HS diet the relative abundance of Prevotella remained below 6% (from 5.03 ± 1.26% to 4.72 ± 1.67%, P = 0.86). Dogs fed the HF–LS diet showed a lower relative abundance of Solobacterium (P = 0.023) and Coprobacillus (P = 0.016) but a trend toward a higher relative abundance of Megamonas (P = 0.087) in relation to the LF–HS diet.
Table 2.
Effect of diet on prominent (˃0.01%) bacterial phyla and class in feces of dogs as determined by 16S rRNA gene Ion-Torrent next-generation sequencing
| Item, % of total sequences | Diet1,2 | SED3 | P-value | ||
|---|---|---|---|---|---|
| LF–HS | HF–LS | ||||
| Phyla | Class | ||||
| Firmicutes | 34.8 | 37.2 | 2.54 | 0.38 | |
| Erysipelotrichi | 10.6 | 10.3 | 0.776 | 0.70 | |
| Clostridia | 10.6 | 9.91 | 1.22 | 0.59 | |
| Negativicutes | 8.04 | 7.87 | 1.51 | 0.90 | |
| Bacilli | 4.73 | 5.75 | 2.15 | 0.65 | |
| Bacteroidetes | Bacteroidia | 34.3 | 31.5 | 3.16 | 0.42 |
| 33.7 | 31.1 | 3.14 | 0.42 | ||
| Fusobacteria | Fusobacteria | 24.0 | 24.9 | 1.34 | 0.50 |
| 24.0 | 24.9 | 1.31 | 0.50 | ||
| Proteobacteria | β-Proteobacteria | 6.76 | 6.23 | 1.07 | 0.63 |
| 3.10 | 3.37 | 0.449 | 0.57 | ||
| γ-Proteobacteria | 3.61 | 2.82 | 0.939 | 0.42 | |
| Actinobacteria | Actinobacteria | 0.014 | 0.027 | 0.008 | 0.10 |
| 0.014 | 0.027 | 0.008 | 0.10 | ||
1HF–LS = high fat–low starch; LF–HS = low fat–high starch.
2Least square means for N = 12 dogs per diet.
3SE of the difference for comparison between diets.
Table 3.
Effect of diet on prominent (˃0.01%) bacterial genera in feces of dogs as determined by 16S rRNA gene Ion-Torrent next-generation sequencing
| Item, % of total sequences | Diet1,2 | SED3 | P-value | ||
|---|---|---|---|---|---|
| LF–HS | HF–LS | ||||
| Family | Genera | ||||
| Bacteroidaceae | Bacteroides | 18.1 | 21.5 | 3.80 | 0.45 |
| Prevotellaceae | Prevotella | 8.63 | 4.57 | 1.22 | 0.008 |
| Paraprevotella | 3.25 | 2.27 | 0.657 | 0.15 | |
| Fusobacteriaceae | Clostridium cluster XIX | 17.5 | 17.6 | 1.88 | 0.95 |
| Fusobacterium | 6.18 | 6.97 | 0.864 | 0.39 | |
| Erysipelotrichaceae | Catenibacterium | 2.89 | 3.14 | 0.897 | 0.79 |
| Erysipelotrichaceae 4 | 2.29 | 2.35 | 1.38 | 0.94 | |
| Solobacterium | 1.30 | 0.583 | 0.286 | 0.023 | |
| Turicibacter | 1.38 | 0.492 | 0.661 | 0.19 | |
| Allobaculum | 1.19 | 1.28 | 0.343 | 0.81 | |
| Clostridium cluster XVIII | 0.136 | 0.107 | 0.029 | 0.34 | |
| Coprobacillus | 0.117 | 0.058 | 0.020 | 0.016 | |
| Peptostreptococcaceae | Clostridium cluster XI | 5.02 | 4.17 | 0.636 | 0.20 |
| Lachnospiraceae | Blautia | 1.73 | 1.93 | 0.366 | 0.49 |
| Clostridium cluster XlVa | 0.630 | 0.600 | 0.082 | 0.72 | |
| Roseburia | 0.342 | 0.833 | 0.327 | 0.16 | |
| Coprococcus | 0.208 | 0.158 | 0.036 | 0.20 | |
| Lachnospiracea 4 | 0.133 | 0.167 | 0.025 | 0.21 | |
| Hespellia | 0.125 | 0.150 | 0.028 | 0.40 | |
| Ruminococcaceae | Faecalibacterium | 0.375 | 0.317 | 0.082 | 0.42 |
| Acidaminococcaceae | Succinispira | 4.44 | 3.40 | 0.809 | 0.23 |
| Veillonaceae | Megamonas | 2.28 | 3.89 | 0.857 | 0.087 |
| Lactobacillaceae | Lactobacillus | 4.44 | 5.39 | 2.04 | 0.65 |
| Sutterellaceae | Parasutterella | 1.28 | 1.69 | 0.458 | 0.39 |
| Sutterella | 1.23 | 1.31 | 0.288 | 0.78 | |
| Enterobacteriaceae | Enterobacter | 1.13 | 1.24 | 0.653 | 0.87 |
| Escherichia/Shigella | 0.458 | 0.300 | 0.163 | 0.32 | |
| Succinivibrionaceae | Anaerobiospirillum | 0.975 | 0.933 | 0.277 | 0.88 |
1HF–LS = high fat–low starch; LF–HS = low fat–high starch.
2Least square means for N = 12 dogs per diet.
3SE of the difference for comparison between diets.
4Uncertain genera (incertae sedis).
Fecal DM and Consistency Score, pH, and Fecal Concentration of Ammonia and VFA
Fecal DM was similar in both diets (~28%) (Table 4). The fecal pH and fecal concentration of ammonia and VFA were not influenced by diet (P > 0.10), although a trend (P = 0.075) toward a higher proportion of butyrate was found with the LF–HS diet.
Table 4.
Effect of diet on fecal DM, pH, and fecal concentration of fermentative end products
| Item | Diet1,2 | SED3 | P-value | |
|---|---|---|---|---|
| LF–HS | HF–LS | |||
| DM, % | 28.0 | 27.8 | 0.530 | 0.69 |
| pH | 5.84 | 5.88 | 0.082 | 0.63 |
| NH3, µmol/g DM | 39.3 | 36.8 | 3.00 | 0.42 |
| VFA, µmol/g DM | ||||
| Total | 836 | 854 | 26.7 | 0.52 |
| Acetate | 471 | 478 | 17.6 | 0.71 |
| Propionate | 258 | 276 | 11.5 | 0.14 |
| Isobutyrate | 8.92 | 9.17 | 0.607 | 0.69 |
| Butyrate | 83.7 | 74.7 | 6.31 | 0.19 |
| Isovalerate | 11.8 | 12.0 | 0.876 | 0.78 |
| Valerate | 2.75 | 3.42 | 0.914 | 0.48 |
| VFA, mmol/mol | ||||
| Acetate | 562 | 561 | 13.0 | 0.93 |
| Propionate | 310 | 323 | 9.80 | 0.24 |
| Butyrate | 99.0 | 87.0 | 6.20 | 0.075 |
| BCFA4 | 28.0 | 29.0 | 2.00 | 0.47 |
1HF–LS = high fat–low starch; LF–HS = low fat–high starch.
2Least square means for n = 12 dogs per diet.
3SE of the difference for comparison between diets.
4Branched-chain fatty acids: calculated as the sum of isobutyrate, isovalerate, and valerate.
Fecal Concentration of Bile Acids
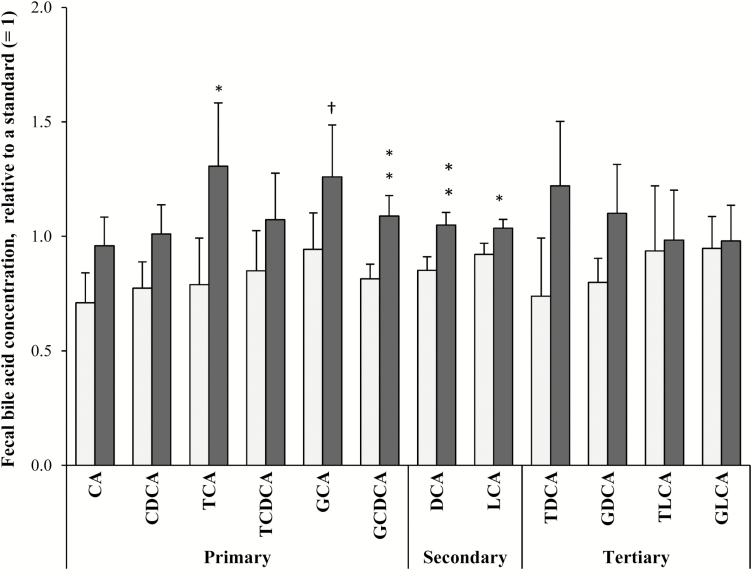
Total concentration of bile acids was higher in the HF–LS diet (25.9 ± 3.29 µmol/g fecal DM) than in the LF–HS diet (18.2 ± 2.37 µmol/g fecal DM) (P = 0.016). Dogs fed the HF–LS diet had a higher relative concentration of the primary bile acids GCDCA (P = 0.003) and TCA (P = 0.015) and of the secondary bile acids DCA (P = 0.003) and LCA (P = 0.033) (Fig. 3). Tertiary bile acids did not differ between diets (P > 0.10).
Figure 3.
Fecal concentration of primary, secondary, and tertiary bile acids (relative to a standard sample with a concentration set at 1) in 12 dogs fed a low-fat–high-starch diet (LF–HS; □) or a high-fat–low-starch diet (HF–LS; ■). †P < 0.10, *P < 0.05; **P < 0.01. CA = cholic acid; CDCA = chenodeoxycholic acid; TCA = taurocholic acid; TCDCA = taurochenodeoxycholic acid; GCA = glycocholic acid; GCDCA = glycochenodeoxycholic acid; DCA = deoxycholic acid; LCA = lythocholic acid; TDCA = taurodeoxycholic acid; GDCA = glycodeoxycholic acid; TLCA = taurolithocholic acid; GLCA = glycolythocholic acid.
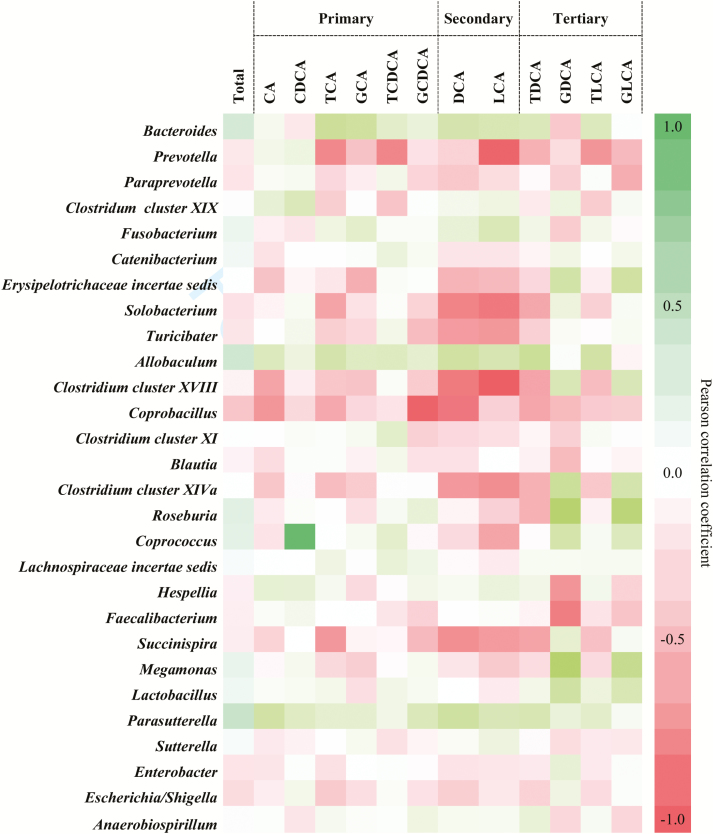
Correlations between Bacterial Composition and Bile Acid Concentration in Feces
The relationship between fecal bacterial groups and the relative fecal concentration of bile acids is shown in Fig. 4. Correlations affecting major bacterial groups (relative abundance >1%) are given below. Within the class Bacteroidia, Prevotella was negatively correlated with TCA (r = −0.513, P = 0.010) and TLCA (r = −0.526, P < 0.01), whereas Bacteroides correlated positively with TCA (r = 0.533, P ˂ 0.01). Within the class Erysipelotrichi, Clostridium cluster XVIII showed a negative relationship with DCA (r = −0.548, P < 0.01) and LCA (r = −0.634, P ˂ 0.001), whereas Allobaculum followed a positive relationship with TDCA (r = 0.527, P = 0.008). A positive correlation between Megamonas (class Negativicutes) and both GDCA (r = 0.752, P < 0.001) and GLCA (r = 0.585, P < 0.01) was found.
Figure 4.
Correlations between fecal microbiota and the relative fecal concentration of primary, secondary, and tertiary bile acids.
In Vitro Gas Production
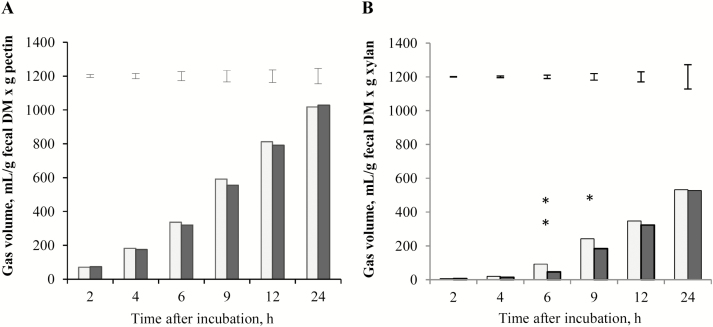
Fermentation of pectin resulted in greater gas production than that of xylan. Cumulative gas production using pectin as substrate did not differ between diets at any measured time (P > 0.10; Fig. 5A). However, when xylan was used as substrate, feces from dogs fed the LF–HS diet yielded higher gas volume at 6 h (P < 0.01) and 9 h (P = 0.012) compared with feces from dogs fed the HF–LS diet (Fig. 5B).
Figure 5.
In vitro fermentation of pectin (A) and xylan (B) using fecal inocula of 8 dogs fed a low-fat–high-starch diet (LF–HS; □) and a high-fat–low-starch diet (HF–LS; ■). Error bars represent the SE of the difference for comparisons between both types of fecal inocula at each measured time. Difference between diets *P < 0.05 and **P < 0.01.
Basal Concentration of LPS
Blood concentration of LPS was not affected by diet, neither in the fasted state (0.642 ± 0.159 and 0.479 ± 0.084 EU/mL, with LF–HS and HF–LS, respectively, P = 0.38) nor at 180-min postfeeding (0.514 ± 0.098 and 0.489 ± 0.125 EU/mL, with LF–HS and HF–LS, respectively, P = 0.84). At 180-min postfeeding, blood LPS did not differ from basal values with either diet (P = 0.43).
DISCUSSION
Most of the research carried out in dogs has focused on the effects of protein and fiber on fecal microbiota, which are the primary exogenous substrates the hindgut microbes survive on. In contrast, there is less information concerning fat and nonfibrous carbohydrates (Deng and Swanson, 2015), which are the main energy-delivery nutrients in dog food (Hervera et al., 2012). In the current study, we explored the effect of feeding of a low-fat–high-starch diet or a high-fat–low-starch diet on fecal microbiota composition and activity in dogs. Diets differed 2-fold and 1.6-fold in their fat and starch content, respectively, with differences being attributed to lard (3.8-fold higher in the HF–LS diet) and to maize and broken rice (1.9-fold and 1.6-fold higher in the LF–HS diet). The inclusion levels of fat and starch were within the range commonly used in dog food. However, the variation in dietary fat was comparatively lower than other studies carried out in mice, in which a 5-fold variation in the level of fat was applied (de La Serre et al., 2010; Murphy et al., 2010). Both diets were formulated with a similar level of TDF to circumvent a confounding effect of fiber fermentation on the studied parameters. Due to the higher energy density of the HF–LS diet in comparison with the LF–HS diet (Table 1), the amount of HF–LS diet offered to dogs was 12% higher (on DM basis) than that of LF–HS diet to ensure the same level of energy intake per kilogram metabolic BW with both diets. Throughout the study all dogs consumed the amount of food offered. Therefore, considering the referred food adjustments, daily fat intake was 1.9-fold higher with the HF–LS diet (5.63 vs. 2.92 g/kg BW0.75), whereas daily starch intake was 1.8-fold higher with the LF–HS diet (7.13 vs. 13.0 g/kg BW0.75). Daily intake of CP (7.61 and 7.89 g/kg BW0.75 with LF–HS and HF–LS, respectively) and TDF (3.63 and 3.36 g/kg BW0.75 with LF–HS and HF–LS, respectively) remained similar with both diets.
The proposed daily energy requirement, set at 480 kJ ME/kg BW0.75, was met with both diets and was adequate for the maintenance of BW.
Diets were distributed following a crossover arrangement, which permitted to increase the number of dogs assigned to each diet and to account for between animal variation, which constitutes a major source of variation in this kind of studies (Kerr et al., 2013). Microbial diversity and community profiles were assessed by NGS using fecal samples, following the same approach as previous studies in dogs (Middelbos et al., 2010; Handl et al., 2013; Panasevich et al., 2015). Both rarefaction curves and Good’s diversity test showed that the depth of sequencing was adequate to reach the diversity plateau.
Richness and Shannon diversity indices were greater than previous values in dogs (Middelbos et al., 2010; Handl et al., 2013) and were not affected by diet. Consistently, the dendrogram generated from NGS data (which considers the number and the relative abundance of coincident OTU) did not depict a cluster by diet. Shannon equitability index was 2-fold greater than the result obtained by Handl et al. (2013), reflecting an even distribution of the bacterial community with either diet.
In keeping with Middelbos et al. (2010), there was a strong dominance of the phyla Firmicutes, Bacteroidetes, and Fusobacteria, which alongside Proteobacteria and Actinobacteria constitute the predominant bacterial phyla in the canine gut (Suchodolski et al., 2008).
In contrast to the increase in Firmicutes and the decrease in Bacteroidetes reported in rodents in response to high-fat diets providing from 41 to 45% ME as fat mostly derived from lard, rich in both saturated (mainly palmitic and stearic) and unsaturated (mainly oleic) fatty acids (Turnbaugh et al., 2008; Hildebrandt et al., 2009) or oral doses of CA (Islam et al., 2011), in the current study feeding of the HF–LS diet (42% ME as fat primarily derived from lard) did not have an effect on fecal bacterial composition at phyla level. In humans, De Filippo et al. (2010) showed that Firmicutes and Proteobacteria are predominant on Western-type diets rich in fat and animal protein, whereas Bacteroidetes and Actinobacteria are the foremost phyla on diets rich in cereals. However, Firmicutes and Bacteroidetes can be affected by factors other than diet, such as age (Mariat et al., 2009) or obesity (Ley et al., 2005). At a lower taxonomic level, and in line with some other studies (Mozes et al., 2008; Hildebrandt et al., 2009), we evidenced a remarkable decrease in the genus Prevotella. In consistence with this finding, long-term consumption of an animal-based diet (rich in protein and fat) by humans has been associated with a lower relative abundance of Prevotella in comparison with plant-based diets (Wu et al., 2011; David et al., 2014). In the study of David et al. (2014), changes in gut microbiota were associated with increases in fecal DCA, corroborating a link between dietary fat, bile acid metabolism, and changes in intestinal microbiota. The negative effect of the HF–LS diet on Prevotella reported here was concurrent with a negative correlation between Prevotella and the relative fecal concentrations of TCA and TLCA, which reached higher levels with the HF–LS diet. These findings may suggest a negative impact of dietary fat on Prevotella related to an increased arrival of bile acids into the hindgut. Prior evidence has shown complete growth inhibition of Prevotella by 20% inclusion of bile salts in the culture medium (Shah and Collins, 1990). An effect of bile acids on microbial membrane lysis and cellular damage has been attributed to their amphipathic nature and detergent effect (Begley et al., 2005). Although fat per se can exert an inhibitory action on microbial communities (Enjalbert et al., 2017), fecal excretion of fat was similar with both diets (2.5 and 2.3 g fat/d with HF–LS and LF–HS diets, respectively). In addition, considering that dietary starch may reach the hindgut and be fermented by gut microbiota (Fuentes-Zaragoza et al., 2011), it is possible that the lower starch content provided by the HF–LS diet could have contributed to the decrease in Prevotella with this diet. Resistant starch content in maize (the main starch source differing between diets) has been related to its higher amylose: amylopectin ratio, which results in a structure that difficult its digestion by intestinal enzymes (Sajilata et al., 2006). In the study of De Filippo et al. (2010), Prevotella made up 53% of total sequences in children whose diet consisted mainly of cereals, but was not detected in children consuming a diet low in cereals. On the other hand, the fact that the relative abundance of Prevotella remained below 6% in dogs that switched from the HF–LS to the LF–HS diet may reflect a low or a slow capability of Prevotella to recover from an insult leading toward a decline in its population. In line with the results reported by Murphy et al. (2010) in mice exposed to a high-fat diet (45% ME fat), the genus Bacteroides was unaltered in dogs fed the HF–LS diet. However, diet-induced variations on Bacteroides are not clearly consistent among studies. Thus, a decrease in the genus Bacteroides has been reported in rodents fed high-fat diets (Hildebrandt et al., 2009), whereas an increase in Bacteroides has been shown in humans consuming a diet rich in protein and fat in comparison to a diet rich in carbohydrates (Wu et al., 2011; David et al., 2014). Although there is no clear evidence on the influence of fat quality on bile acid composition, and thereby on microbiota composition (Graf et al., 2015), discrepancies among studies could be partly related to differences in fat sources. Hence, Wu et al. (2011) reported that Bacteroides enterotype is positively correlated with the intake of saturated fats in humans. In addition, whereas certain species of Bacteroides exhibit successful growth in culture media containing TCA (Narushima et al., 2006), bile-tolerance within Bacteroides may be species specific (Begley et al., 2005), which may result in changes at a species, but not at a genera level.
A negative impact of HF–LS diet on fecal VFA content was not evidenced, and molar proportions of fecal acetate, propionate, and butyrate were similar to those found by Bosch et al. (2009) in dogs fed low- and high-fermentable fiber diets. However, it is important to note that fecal VFA levels do not reflect luminal VFA content, as these compounds are rapidly absorbed by the colonic mucosa (Scheppach, 1994). The trend toward a higher proportion of butyrate in dogs fed the LF–HS diet could have been partly related to a higher arrival of potentially fermentable carbohydrates to the hindgut with this diet. Thus, in the study conducted by Weaver et al. (1997), administration of α-amylase inhibitor to humans resulted in an increased fecal concentration of butyrate. Nevertheless, a potential acclimation of the gut microbiota to dietary variations should not be discarded, as shown by Jakobsdottir et al. (2013), who reported a lower cecal content of butyrate in rats consuming a high-fat diet after 2 wk, but not after 4 wk.
Regarding the fermentative activity of the fecal microbiota, the similar gas rendered by both types of fecal inocula when pectin was used as substrate reflects a similar capacity of most bacterial groups in utilizing high-fermentable fiber. In contrast, the higher gas rendered by the fecal inocula of dogs fed the LF–HS diet during xylan fermentation could have been partly related to the higher relative abundance of Prevotella, considering the well-evidenced xylanase activity of this bacterial group (Flint et al., 2008).
None of the dogs presented any adverse event such as vomiting or diarrhea throughout the study. In consistency with this, no marked changes were found in fecal microbiota abundance and diversity, whose decrease has been related to intestinal bowel disease (IBD) in dogs (Suchodolski et al., 2012), or in the relative abundance of Bacteroidetes, whose decrease has been associated with IBD in humans (Frank et al., 2007) and with nonhemorragic diarrhea in dogs (Suchodolski, 2011). The similar fecal VFA content found with both diets, whose effects on gut morphology and function are well recognized (Scheppach, 1994), substantiates the lack of gastrointestinal adverse effects reported in this study. Furthermore, the fact that blood LPS remained similar at the fasted and postfeeding (180 min) states with both diets indicate that inclusion of dietary fat at the levels used in this study does not result in a low-grade metabolic endotoxemia. This finding differs from the results reported by Ghoshal et al. (2009) in which administration of an intragastric bolus of long-chain fatty acids to mice resulted in an enhanced absorption of LPS via chylomicron formation. In this respect, intestinal inflammation seems to be a precondition for the elevation of blood LPS in response to high-fat diets via an increase in intestinal permeability (de La Serre et al., 2010).
CONCLUSION
Canine fecal microbiota scarcely changed as a result of feeding of an HF–LS diet or an LF–HS diet at inclusion levels of fat and starch within the range conventionally found in commercial extruded diets. However, feces of dogs fed the HF–LS diet had a lower relative abundance of Prevotella and a lower potential to ferment xylan, suggesting a decreased capacity to ferment low-fermentable carbohydrates. Fecal concentration of VFA was not affected by diet, although a higher proportion of butyrate was found with the LF–HS diet.
SUPPLEMENTARY DATA
Supplementary data are available at Journal of Animal Science online.
Footnotes
The authors thank the Servicio de Apoyo a la Investigación (SAI), University of Zaragoza, for providing assistance during the study, and José Joaquin Cerón, Faculty of Veterinary of Murcia, for conducting the analysis of bile acids. This research was financially supported by Affinity Petcare (Project ID 2012/0579) and the Department of Industry and Innovation of the Government of Aragón (Project A19/Animal Nutrition) and the European Social Fund. A.S.-M. and C.T. are employees of Affinity Petcare.
LITERATURE CITED
- AOAC 1995. Official methods of analysis. 16th ed Assoc. Off. Anal. Chem, Washington, DC. [Google Scholar]
- AOAC 2005. Official methods of analysis. 18th ed Assoc. Off. Anal. Chem, Gaithersburg, MD. [Google Scholar]
- Begley M., Gahan C. G., and Hill C.. 2005. The interaction between bacteria and bile. FEMS Microbiol. Rev. 29:625–651. doi: 10.1016/j.femsre.2004.09.003 [DOI] [PubMed] [Google Scholar]
- Bosch G., Verbrugghe A., Hesta M., Holst J. J., van der Poel A. F., Janssens G. P., and Hendriks W. H.. 2009. The effects of dietary fibre type on satiety-related hormones and voluntary food intake in dogs. Br. J. Nutr. 102:318–325. doi: 10.1017/S0007114508149194 [DOI] [PubMed] [Google Scholar]
- Chaney A. L., and Marbach E. P.. 1962. Modified reagents for determination of urea and ammonia. Clin. Chem. 8:130–132. [PubMed] [Google Scholar]
- Cole J. R., Chai B., Marsh T. L., Farris R. J., Wang Q., Kulam S. A., Chandra S., McGarrell D. M., Schmidt T. M., Garrity G. M., et al. ; Ribosomal Database Project. 2003. The ribosomal database project (RDP-II): Previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442–443. doi:10.1093/nar/gkg039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L. A., Maurice C. F., Carmody R. N., Gootenberg D. B., Button J. E., Wolfe B. E., Ling A. V., Devlin A. S., Varma Y., Fischbach M. A.,. et al. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. doi: 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C., Cavalieri D., Di Paola M., Ramazzotti M., Poullet J. B., Massart S., Collini S., Pieraccini G., and Lionetti P.. 2010. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U.S.A. 107:14691–14696. doi: 10.1073/pnas.1005963107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente G., Belanche A., Girwood S. E., Pinloche E., Wilkinson T., and Newbold C. J.. 2014. Pros and cons of Ion-Torrent next generation sequencing versus terminal restriction fragment length polymorphism T-RFLP for studying the rumen bacterial community. PLoS One 9:e101435. doi: 10.1371/journal.pone.0101435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de La Serre C. B., Ellis C. L., Lee J., Hartman A. L., Rutledge J. C., and Raybould H. E.. 2010. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 299:G440–G448. doi: 10.1152/ajpgi.00098.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P., and Swanson K. S.. 2015. Gut microbiota of humans, dogs and cats: Current knowledge and future opportunities and challenges. Br. J. Nutr. 113 (Suppl):S6–S17. doi: 10.1017/s0007114514002943 [DOI] [PubMed] [Google Scholar]
- Edgar R. C. 2013. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10:996–998. doi: 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- Enjalbert F., Combes S., Zened A., and Meynadier A.. 2017. Rumen microbiota and dietary fat: a mutual shaping. J. Appl. Microbiol. 123:782–797. doi: 10.1111/jam.13501 [DOI] [PubMed] [Google Scholar]
- Flint H. J., Bayer E. A., Rincon M. T., Lamed R., and White B. A.. 2008. Polysaccharide utilization by gut bacteria: Potential for new insights from genomic analysis. Nat. Rev. Microbiol. 6:121–131. doi: 10.1038/nrmicro1817 [DOI] [PubMed] [Google Scholar]
- Flint H. J., Scott K. P., Louis P., and Duncan S. H.. 2012. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 9:577–589. doi: 10.1038/nrgastro.2012.156 [DOI] [PubMed] [Google Scholar]
- Frank D. N., St Amand A. L., Feldman R. A., Boedeker E. C., Harpaz N., and Pace N. R.. 2007. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. U.S.A. 104:13780–13785. doi: 10.1073/pnas.0706625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Zaragoza E., Sánchez-Zapata E., Sendra E., Sayas E., Navarro C., Fernández-López J., and Pérez-Alvarez J. A.. 2011. Resistant starch as prebiotic: A review. Starch/Stärke 63:406–415. doi:10.1002/star.201000099 [Google Scholar]
- German A. J., Holden S. L., Bissot T., Morris P. J., and Biourge V.. 2009. Use of starting condition score to estimate changes in body weight and composition during weight loss in obese dogs. Res. Vet. Sci. 87:249–254. doi: 10.1016/j.rvsc.2009.02.007 [DOI] [PubMed] [Google Scholar]
- Ghoshal S., Witta J., Zhong J., de Villiers W., and Eckhardt E.. 2009. Chylomicrons promote intestinal absorption of lipopolysaccharides. J. Lipid Res. 50:90–97. doi: 10.1194/jlr.M800156-JLR200 [DOI] [PubMed] [Google Scholar]
- Good I. J. 1953. The population frequencies of species and the estimation of population parameters. Biometrika 40:237–264. [Google Scholar]
- Graf D., Di Cagno R., Fåk F., Flint H. J., Nyman M., Saarela M., and Watzl B.. 2015. Contribution of diet to the composition of the human gut microbiota. Microb. Ecol. Health Dis. 26:26164. doi: 10.3402/mehd.v26.26164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagio M., Matsumoto M., Fukushima M., Hara H., and Ishizuka S.. 2009. Improved analysis of bile acids in tissues and intestinal contents of rats using LC/ESI-MS. J. Lipid Res. 50:173–180. doi: 10.1194/jlr.D800041-JLR200 [DOI] [PubMed] [Google Scholar]
- Hagio M., Matsumoto M., and Ishizuka S.. 2011. Bile acid analysis in various biological samples using ultra performance liquid chromatography/electrospray ionization-mass spectrometry (UPLC/ESI-MS). Methods Mol. Biol. 708:119–129. doi: 10.1007/978-1-61737-985-7_6 [DOI] [PubMed] [Google Scholar]
- Handl S., German A. J., Holden S. L., Dowd S. E., Steiner J. M., Heilmann R. M., Grant R. W., Swanson K. S., and Suchodolski J. S.. 2013. Faecal microbiota in lean and obese dogs. FEMS Microbiol. Ecol. 84:332–343. doi: 10.1111/1574-6941.12067 [DOI] [PubMed] [Google Scholar]
- Hervera M., Castrillo C., Albanell E., and Baucells M. D.. 2012. Use of near-infrared spectroscopy to predict energy content of commercial dog food. J. Anim. Sci. 90:4401–4407. doi: 10.2527/jas.2012-5106 [DOI] [PubMed] [Google Scholar]
- Hildebrandt M. A., Hoffmann C., Sherrill-Mix S. A., Keilbaugh S. A., Hamady M., Chen Y. Y., Knight R., Ahima R. S., Bushman F., and Wu G. D.. 2009. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137:1716–1724.e1. doi: 10.1053/j.gastro.2009.08.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam K. B., Fukiya S., Hagio M., Fujii N., Ishizuka S., Ooka T., Ogura Y., Hayashi T., and Yokota A.. 2011. Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141:1773–1781. doi: 10.1053/j.gastro.2011.07.046 [DOI] [PubMed] [Google Scholar]
- Jakobsdottir G., Xu J., Molin G., Ahrné S., and Nyman M.. 2013. High-fat diet reduces the formation of butyrate, but increases succinate, inflammation, liver fat and cholesterol in rats, while dietary fibre counteracts these effects. PLoS One 8:e80476. doi: 10.1371/journal.pone.0080476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeusette I., Detilleux J., Cuvelier C., Istasse L., and Diez M.. 2004. Ad libitum feeding following ovariectomy in female beagle dogs: Effect on maintenance energy requirement and on blood metabolites. J. Anim. Physiol. Anim. Nutr. (Berl) 88:117–121. doi: 10.1111/j.1439-0396.2003.00467.x [DOI] [PubMed] [Google Scholar]
- Kerr K. R., Forster G., Dowd S. E., Ryan E. P., and Swanson K. S.. 2013. Effects of dietary cooked navy bean on the fecal microbiome of healthy companion dogs. PLoS One 8:e74998. doi: 10.1371/journal.pone.0074998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme D. 1997. Development and validation of a body condition score system for dogs. Canine Pract. 22:10–15. [Google Scholar]
- Ley R. E., Bäckhed F., Turnbaugh P., Lozupone C. A., Knight R. D., and Gordon J. I.. 2005. Obesity alters gut microbial ecology. Proc. Natl. Acad. Sci. U.S.A. 102:11070–11075. doi: 10.1073/pnas.0504978102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht T. R., Hansen M., Poulsen M., and Dragsted L. O.. 2006. Dietary carbohydrate source influences molecular fingerprints of the rat faecal microbiota. BMC Microbiol. 6:98. doi: 10.1186/1471-2180-6-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariat D., Firmesse O., Levenez F., Guimarăes V., Sokol H., Doré J., Corthier G., and Furet J. P.. 2009. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 9:123. doi: 10.1186/1471-2180-9-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middelbos I. S., Vester Boler B. M., Qu A., White B. A., Swanson K. S., and Fahey G. C. Jr. 2010. Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PLoS One 5:e9768. doi: 10.1371/journal.pone.0009768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould F. L., Morgan R., Kliem K. E., and Krystallidou E.. 2005. A review and simplification of the in vitro incubation medium. Anim. Feed. Sci. Tech. 123:155–172. doi: 10.1016/j.anifeedsci.2005.05.002 [DOI] [Google Scholar]
- Mozes S., Bujnáková D., Sefcíková Z., and Kmet V.. 2008. Developmental changes of gut microflora and enzyme activity in rat pups exposed to fat-rich diet. Obesity (Silver Spring) 16:2610–2615. doi: 10.1038/oby.2008.435 [DOI] [PubMed] [Google Scholar]
- Murphy E. F., Cotter P. D., Healy S., Marques T. M., O’Sullivan O., Fouhy F., Clarke S. F., O’Toole P. W., Quigley E. M., Stanton C.,. et al. 2010. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut 59:1635–1642. doi: 10.1136/gut.2010.215665 [DOI] [PubMed] [Google Scholar]
- Narushima S., Itoha K., Miyamoto Y., Park S. H., Nagata K., Kuruma K., and Uchida K.. 2006. Deoxycholic acid formation in gnotobiotic mice associated with human intestinal bacteria. Lipids 41:835–843. doi: 10.1007/s11745-006-5038-1 [DOI] [PubMed] [Google Scholar]
- NRC.. 1985. Nutrient requirements of dogs and cats. Natl. Acad. Press, Washington, DC. [Google Scholar]
- NRC 2006. Nutrient requirements of dogs and cats. Natl. Acad. Press, Washington, DC. [Google Scholar]
- Panasevich M. R., Kerr K. R., Dilger R. N., Fahey G. C. Jr, Guérin-Deremaux L., Lynch G. L., Wils D., Suchodolski J. S., Steer J. M., Dowd S. E.,. et al. 2015. Modulation of the faecal microbiome of healthy adult dogs by inclusion of potato fibre in the diet. Br. J. Nutr. 113:125–133. doi: 10.1017/S0007114514003274 [DOI] [PubMed] [Google Scholar]
- Prates A., de Oliveira J. A., Abecia L., and Fondevila M.. 2010. Effects of preservation procedures of rumen inoculum on in vitro microbial diversity and fermentation. Anim. Feed. Sci. Tech. 155:186–193. doi: 10.1016/j.anifeedsci.2009.12.005 [DOI] [Google Scholar]
- Sajilata M. G., Singhal R. S., and Kulkarni P. R.. 2006. Resistant starch –A review. Compr. Rev. Food Sci Food Saf. 5:1–17. doi:10.1111/j.1541-4337.2006.tb00076.x [DOI] [PubMed] [Google Scholar]
- Schauf S., Salas-Mani A., Torre C., Jimenez E., Latorre M. A., and Castrillo C.. 2018. Effect of feeding a high-carbohydrate or a high-fat diet on subsequent food intake and blood concentration of satiety-related hormones in dogs. J. Anim. Physiol. Anim. Nutr. (Berl) 102:e21–e29. doi: 10.1111/jpn.12696 [DOI] [PubMed] [Google Scholar]
- Scheppach W. 1994. Effects of short chain fatty acids on gut morphology and function. Gut 35(1 Suppl):S35–S38. doi:10.1136/gut.35.1_Suppl.S35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah H. N., and Collins D. M.. 1990. Prevotella, a new genus to include Bacteroides melaninogenicus and related species formerly classified in the genus Bacteroides. Int. J. Syst. Bacteriol. 40:205–208. doi: 10.1099/00207713-40-2-205 [DOI] [PubMed] [Google Scholar]
- Suarez-Belloch J., Doti S., Rodriguez-Romero N., Guada J. A., Fondevila M., and Latorre M. A.. 2013. Hindgut fermentation in pigs induced by diets with different sources or starch. Minufiya J. Agric. Res. 11:780–789. doi: 10.5424/sjar/2013113-3958 [DOI] [Google Scholar]
- Suchodolski J. S. 2011. Companion animals symposium: Microbes and gastrointestinal health of dogs and cats. J. Anim. Sci. 89:1520–1530. doi: 10.2527/jas.2010-3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchodolski J. S., Camacho J., and Steiner J. M.. 2008. Analysis of bacterial diversity in the canine duodenum, jejunum, ileum, and colon by comparative 16S rRNA gene analysis. FEMS Microbiol. Ecol. 66:567–578. doi: 10.1111/j.1574-6941.2008.00521.x [DOI] [PubMed] [Google Scholar]
- Suchodolski J. S., Markel M. E., Garcia-Mazcorro J. F., Untereer S., Heilmann R. M., Dowd S. E., Kachroo P., Ivanov I., Minamoto Y., Dillman E. M.,. et al. 2012. The fecal microbiome in dogs with acute diarrhea and idiopathic inflammatory bowel disease. PLoS One 7:e51907. doi: 10.1371/journal.pone.0051907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorou M. K., Williams B. A., Dhanoa M. S., Mcallan A. B., and France J.. 1994. A simple gas-production method using a pressure transducer to determine the fermentation kinetics of ruminant feeds. Anim. Feed. Sci. Tech. 48:185–197. doi: 10.1016/0377-8401(94)90171-6 [DOI] [Google Scholar]
- Turnbaugh P. J., Bäckhed F., Fulton L., and Gordon J. I.. 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3:213–223. doi: 10.1016/j.chom.2008.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Linnenbrink M., Künzel S., Fernandes R., Nadeau M. J., Rosenstiel P., and Baines J. F.. 2014. Dietary history contributes to enterotype-like clustering and functional metagenomic content in the intestinal microbiome of wild mice. Proc. Natl. Acad. Sci. U.S.A. 111:E2703–E2710. doi: 10.1073/pnas.1402342111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver G. A., Tangel C. T., Krause J. A., Parfitt M. M., Jenkins P. L., Rader J. M., Lewis B. A., Miller T. L., and Wolin M. J.. 1997. Acarbose enhances human colonic butyrate production. J. Nutr. 127:717–723. doi: 10.1093/jn/127.5.717 [DOI] [PubMed] [Google Scholar]
- Wu G. D., Chen J., Hoffmann C., Bittinger K., Chen Y. Y., Keilbaugh S. A., Bewtra M., Knights D., Walters W. A., Knight R.,. et al. 2011. Linking long-term dietary patterns with gut microbial enterotypes. Science 334:105–108. doi: 10.1126/science.1208344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota A., Fukiya S., Islam K. B., Ooka T., Ogura Y., Hayashi T., Hagio M., and Ishizuka S.. 2012. Is bile acid a determinant of the gut microbiota on a high-fat diet?Gut Microbes 3:455–459. doi: 10.4161/gmic.21216 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.