Abstract
To evaluate the effects of a Saccharomyces cerevisiae fermentation product (SCFP; Original XPC, Diamond V, Cedar Rapids, IA) on growth performance and antioxidant defense of newly weaned beef cattle, 180 single-source steers (278 ± 21 kg; SD) were used in a 56-d receiving study. Seven days after arrival, steers were blocked by body weight (BW) to pens of 6 and randomly assigned to treatments: SCFP at 0 (CON), 14 (SCFP14), or 28 (SCFP28) g·steer−1·d−1. Pen was the experimental unit (n = 10 per treatment). On day 0, steers were boostered against Bovine Viral Diarrhea Virus (BVDV) Type 1 and 2 (Vista Once, Merck, Madison, NJ). Weights were collected on days 1, 0, 14, 27, 42, 55, and 56. One steer per pen was bled on days 0, 14, 27, 42, and 56 for analysis of BVDV antibody titers; blood from days 0, 27, and 56 was analyzed for red blood cell lysate superoxide dismutase (SOD) activity and glutathione (total = tGSH, oxidized = GSSG, and reduced = GSH) concentrations, plasma malondialdehyde (MDA) concentrations, and serum lysozyme activity. Performance and blood data were analyzed as a randomized complete block design using Proc Mixed of SAS with fixed effects of treatment and block and random effect of pen. Linear and quadratic contrast statements were used. Antibody titers were log transformed and analyzed as repeated measures. There were no treatment by day interactions (P ≥ 0.16), and no linear or quadratic effects of SCFP on feedlot performance, antibody titers, or lysozyme activity (P > 0.10). Day 27 MDA concentrations tended to linearly increase (P = 0.09). A quadratic effect of SCFP on day 56 SOD activity (P = 0.004) was driven by lesser activity for SCFP14-fed steers. On day 27, a tendency for a quadratic effect of SCFP (P = 0.09) on GSH was driven by greater concentrations for SCFP14-fed steers resulting in a lesser GSSG:GSH ratio (P = 0.05). Greater GSH for SCFP14-fed steers caused a tendency for a quadratic effect on day 56 (P = 0.07); however, this did not result in an effect of SCFP on the GSSG:GSH ratio (P ≥ 0.25). A tendency for a linear effect of SCFP on tGSH was noted on day 56 (P = 0.09). Morbidity data were analyzed using Proc Glimmix of SAS. There was a quadratic effect of SCFP on percentage of respiratory treatments prior to day 14 (P = 0.04). These results could indicate lesser levels of oxidative stress for steers receiving SCFP at 14 vs. 0 or 28 g/d. Under the conditions of this study, no performance benefit of SCFP was noted.
Keywords: antioxidants, newly weaned beef cattle, Saccharomyces cerevisiae fermentation product
INTRODUCTION
Beef cattle are exposed to a variety of stressors during the feedlot receiving period. Physiological and psychological stresses include recent weaning and introduction to novel feedstuffs, food and water deprivation during transportation, exposure to pathogens, and common processing procedures including vaccination, dehorning, and castration. Stress results in decreased dry matter intake (DMI) by newly received calves which negatively affects performance and immune function (Hutcheson and Cole, 1986; Duff and Galyean, 2007). Additionally, transit stress has been shown to increase markers of oxidative stress that are related to incidence of bovine respiratory disease (BRD) and mortality (Chirase et al., 2004).
The Saccharomyces cerevisiae fermentation product (SCFP) utilized in the current study is produced from anaerobic fermentation of yeast and contains beneficial metabolites such as vitamins, amino acids, nucleotides, lipids, organic acids, and oligosaccharides as well as yeast cell walls (Jensen et al., 2008). Supplementation of SCFP during the receiving phase improved average daily gain (ADG) and feed efficiency by 5.8% and 2.0%, respectively, in a meta-analysis of 9 experiments (Wagner et al., 2016). Although the antioxidant capacity of SCFP has not been observed in beef cattle, SCFP metabolites provided protection of red blood cells from oxidative damage in vitro (Jensen et al., 2008). Therefore, it was hypothesized that increasing inclusions of SCFP in diets of newly weaned beef steers would mitigate the negative impacts of receiving period stress. A 56-d study was designed to determine the effects of SCFP supplementation on feedlot performance, immune function, and antioxidant defense of steers.
MATERIALS AND METHODS
Animals and Experimental Design
All procedures and protocols for this experiment were approved by the Iowa State University Animal Care and Use Committee (7-15-8060-B). One hundred eighty newly weaned Angus-crossbred beef steers (278 ± 21 kg; SD) were utilized in a 56-d receiving study. Cattle were purchased from a single ranch in western Nebraska and transported approximately 860 km (9.5 h) to the Iowa State University Beef Nutrition Research Center (Ames, IA). Upon arrival (day 7), steers received long-stem grass hay followed by hay top-dressed with a corn-silage based total mixed ration (TMR; Table 1) on the following day. No additional hay was delivered 2 d after arrival to ensure relatively consistent intakes of the TMR by the start of the trial. Steers were weighed on days 1 and 0, blocked by initial body weight (BW) into partially covered concrete pens (3.7 × 12.2 m; 6 steers/pens; n = 10 pens/treatment), and pens randomly assigned to 1 of 3 daily doses of SCFP (Original XPC, Diamond V, Cedar Rapids, IA): 0 (CON), 14 (SCFP14), or 28 g/steer (SCFP28). The current recommended dose for receiving cattle is 14 g·steer−1·d−1. At trial initiation (day 0), steers were implanted with Component E-S (Elanco Animal Health, Greenfield, IN) containing 200-mg progesterone USP, 20-mg estradiol benzoate, and 29-mg tylosin tartrate. Additionally, steers were boostered against clostridial infections (Vision 7, Merck, Madison, NJ) and Bovine Viral Diarrhea Virus (BVDV) Type 1 and 2 (Vista Once, Merck); previous vaccinations had been given at branding and preconditioning. Steers were fed a common diet (Table 1) that was delivered once daily at approximately 0800 h via a mixer wagon with scale breaks of 0.05 kg and had ad libitum access to water. Treatments were delivered as part of the TMR using dried distiller’s grains as a carrier. Percent inclusions of treatment premixes were adjusted weekly based on the projected average treatment group DMI for the following week to ensure target intake of SCFP was maintained. Back calculated SCFP intake was 14.4 and 28.7 g·steer−1·d−1, for SCFP14 and SCFP28, respectively. Due to an outbreak of coccidiosis, Corid (Merial Inc., Duluth, GA) was included in the diet of all steers from days 12 through 16. During this time, lasalocid (Bovatec91, Zoetis, Parsipanny, NJ) was removed from the diet.
Table 1.
Ingredient composition of receiving diet
| DM, % | 58.2 |
|---|---|
| Ingredient, % DM basis | |
| Corn silage | 27 |
| WCGF1 | 20 |
| Dry-rolled corn | 20 |
| DDGS2 | 23.022 |
| Chopped grass hay | 8 |
| Limestone | 1.52 |
| Salt | 0.31 |
| Bovatec913 | 0.023 |
| Vitamin A premix4 | 0.11 |
| Trace mineral premix5 | 0.024 |
| Analyzed composition6, % | |
| Crude protein | 15.9 |
| NDF | 31.8 |
| Ether extract | 5.4 |
1Wet corn gluten feed.
2Dried distiller grains with solubles; carrier for microingredients and Saccharomyces cerevisiae fermentation product (Original XPC, Diamond V, Cedar Rapids, IA).
3Provided 300 mg lasalocid·steer−1·d−1 (Zoetis, Parsippany, NJ).
4Contained 4,400,000 IU/kg Vitamin A premix.
5Provided per kilogram of diet DM: 10 mg of Cu (copper sulfate), 30 mg of Zn (zinc sulfate), 20 mg of Mn (manganese sulfate), 0.5 mg of I (calcium iodate), 0.1 mg of Se (sodium selenite), and 0.1 mg of Co (cobalt carbonate).
6Based on TMR analysis from Dairyland, Inc., Arcadia, WI.
Steers were weighed individually in a hydraulic chute with scale breaks of 0.45 kg prior to feeding on 2 consecutive days at the beginning and end of the trial as well as on days 14, 27, and 42; pencil shrink was not applied to BW collected on any day. The average BW collected on days 1 and 0 served as the initial BW and the average BW collected on days 55 and 56 served as the final BW. ADG was calculated from days 0 (initial BW) to 27, 27 to 56 (final BW), and 0 to 56. Total feed offered and bunk scores were recorded daily, and TMR samples were collected weekly for dry matter (DM) determination by drying in a forced air oven at 70 °C for 48 h. Composites of weekly TMR samples were analyzed by a commercial laboratory (Dairyland, Inc., Arcadia, WI) via wet chemistry procedures for CP (method 990.03; AOAC, 1996), NDF (method 2002.04; AOAC, 2005), and ether extract (method 920.39; AOAC, 1996). Refusals were weighed and sampled in conjunction with weigh dates to determine pen DMI and feed efficiency (gain:feed; G:F). Animals were visually assessed for signs of respiratory illness including nasal discharge, cough, and lethargy. Steers were treated with tulathromycin (Draxxin, Zoetis) by trained personnel if visual symptoms were detected and rectal temperature was ≥39 °C.
Sample Collection and Analytical Procedures
One representative steer per pen was selected as a sampling animal for blood collection on days 0, 14, 27, 42, and 56; the same steer was sampled each time. Blood was collected via jugular venipuncture prior to feeding into vacuum tubes (serum, No. 366430; sodium heparin, No. 36784, Becton Dickinson, Franklin Lakes, NJ). Samples were transported to the laboratory on ice; blood collected for plasma analysis was immediately centrifuged at 1,200 × g for 20 min at 4 °C while blood collected for serum analysis was allowed to clot at room temperature for 90 min prior to centrifugation at 1,000 × g for 10 min at 4 °C. Plasma and serum from samples collected on days 0, 27, and 56 were removed, aliquoted, and stored at −80 °C until analysis of plasma malondialdehyde (MDA) concentrations (item number 700870, Cayman Chemical, Ann Arbor, MI) and serum lysozyme activity (item number E-22013, Molecular Probes, Eugene, OR) using commercially available kits. Intra- and inter-assay CV, respectively, were 4.5% and 7.8% for MDA concentrations, and 5.6% and 9.7% for lysozyme activity. Packed red blood cells from heparin tubes were lysed with 4 volumes of ice-cold molecular-grade water (Cayman Chemical), vortexed, and centrifuged at 10,000 × g for 15 min at 4 °C. The supernatant (red blood cell lysate [RBCL]) was removed, aliquoted, and stored at −80 °C until analysis of superoxide dismutase (SOD) activity (item number 706002, Cayman Chemical); intra- and inter-assay CV were 6.3% and 3.6%, respectively. A unit of SOD activity was defined as the amount of the enzyme required to dismutate 50% of the superoxide radical and the activity is expressed per gram of hemoglobin (1,000 units of SOD activity/g hemoglobin). Hemoglobin was determined using methods described by Hansen et al. (2010). Additionally, RBCL was deproteinated using meta-phosphoric acid solution prior to analysis of total glutathione (tGSH) and oxidized glutathione (GSSG) concentrations using a commercially available kit (item number 703002, Cayman Chemical); intra- and inter-assay CV, respectively, were 2.3% and 3.7% for tGSH, and 4.9% and 4.11% for GSSG. Reduced glutathione (GSH) concentrations were determined by subtracting GSSG from tGSH. The ratio of oxidized to reduced glutathione was calculated by dividing GSSG by GSH. Fresh serum samples collected on days 0, 14, 27, 42, and 56 were sent to the Iowa State University Veterinary Diagnostic Laboratory (Ames, IA) for analysis of BVDV Type 1 and 2 antibody titers via virus neutralization (method 9.104; Kalkwarf, 2014). All sampling steers tested negative for persistent infection with BVDV.
Statistical Analysis
Feedlot performance and blood measures were analyzed as a randomized complete block design using the MIXED procedure of SAS 9.4 (SAS Inst. Inc., Cary, NC) with pen as experimental unit. Performance data from one pen (SCFP28) were removed due to chronically poor performance, unrelated to treatment, by one steer. The model included the fixed effects of treatment and block and the random effect of pen. Orthogonal linear and quadratic contrast statements were constructed to compare treatment means. Least-squared means and SEM are reported. Blood samples from day 0 were used as covariates in analysis of all blood measures. All data were checked for normality using the Shapiro–Wilks test; antibody titers were log transformed to fit the assumption of normality and the log-transformed means are presented. Titers were analyzed as repeated measures using the autoregressive (AR1) covariance structure based on the lowest Akaike’s information criterion. Outliers were evaluated on a pen basis using Cook’s D statistic and removed if Cook’s D > 0.5. Morbidity data were analyzed using the GLIMMIX procedure of SAS 9.4 with pen as the experimental unit, the fixed effect of treatment, a logit link function, and binomial distribution. Steers that were treated more than once for respiratory illness were excluded from morbidity analyses. Significance was declared when P ≤ 0.05 and tendencies were declared when 0.05 < P ≤ 0.10.
RESULTS
Feedlot Performance and Health
Final BW did not differ by treatment (P ≥ 0.89; Table 2). No linear or quadratic effects of SCFP were noted for DMI, ADG, or G:F from days 0 to 27, 27 to 56, or 0 to 56 (P ≥ 0.12). There was a quadratic effect of SCFP on percentage of respiratory treatments prior to day 14 (P = 0.04; Table 2) with greater treatments for SCFP14. There was no effect of SCFP on percentage of respiratory treatments after day 14 (P ≥ 0.73). Two steers from CON and 1 steer from SCFP14 died during the course of the study from illness unrelated to treatment and were excluded from data analysis.
Table 2.
Effect of increased inclusions of a Saccharomyces cerevisiae fermentation product (SCFP) on feedlot performance and respiratory treatments of newly weaned beef steers during a 56-d receiving period
| Treatment1 | P-value | |||||
|---|---|---|---|---|---|---|
| CON n = 10 pens |
SCFP14 n = 10 pens |
SCFP28 n = 9 pens2 |
SEM3 | Linear | Quadratic | |
| Initial body weight, kg | 278 | 278 | 280 | 7.3 | 0.84 | 0.91 |
| Final body weight, kg | 381 | 379 | 380 | 7.8 | 0.91 | 0.89 |
| Dry matter intake, kg/d | ||||||
| Days 0 to 27 | 7.8 | 7.6 | 7.5 | 0.15 | 0.26 | 0.76 |
| Days 27 to 56 | 9.2 | 9.3 | 9.2 | 0.16 | 0.92 | 0.71 |
| Days 0 to 56 | 8.5 | 8.4 | 8.4 | 0.11 | 0.37 | 0.95 |
| Average daily gain, kg/d | ||||||
| Days 0 to 27 | 1.78 | 1.75 | 1.76 | 0.053 | 0.84 | 0.72 |
| Days 27 to 56 | 1.89 | 1.83 | 1.77 | 0.055 | 0.13 | 0.95 |
| Days 0 to 56 | 1.84 | 1.80 | 1.77 | 0.034 | 0.18 | 0.94 |
| Gain:Feed | ||||||
| Days 0 to 27 | 0.231 | 0.232 | 0.237 | 0.006 | 0.49 | 0.78 |
| Days 27 to 56 | 0.205 | 0.199 | 0.195 | 0.005 | 0.12 | 0.86 |
| Days 0 to 56 | 0.217 | 0.214 | 0.213 | 0.003 | 0.38 | 0.82 |
| Treated prior to day 14, % | 6.7 | 13.3 | 1.7 | 4.39 | 0.22 | 0.04 |
| Treated post day 14, % | 6.7 | 8.3 | 8.3 | 3.57 | 0.73 | 0.84 |
1CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
2One pen removed from analysis due to chronically poor performance by one steer, unrelated to treatment.
3Highest SEM of any treatment is reported.
Blood Measures
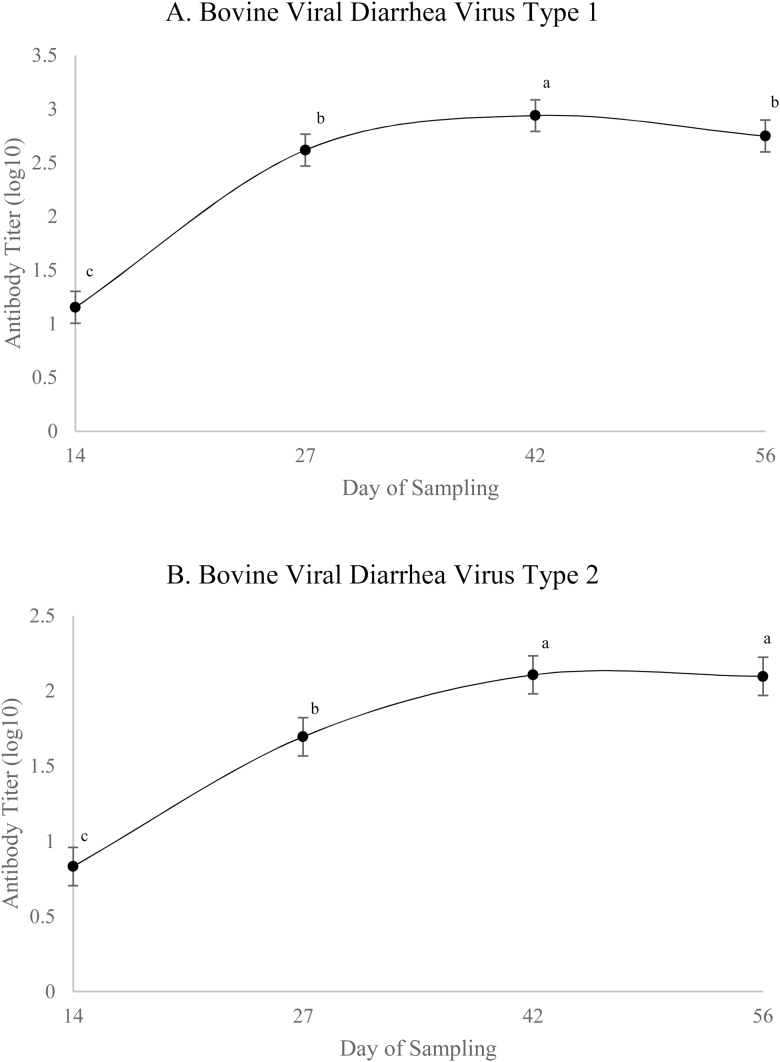
Day 0 values were used as covariates in the analysis of RBCL SOD (Table 3), GSH (Table 4), plasma MDA (Table 3), and serum lysozyme (Table 5); therefore, only means are presented for this sampling day. There was a quadratic effect of SCFP on RBCL SOD activity on day 56 (P = 0.004), driven by SCFP14-fed steers having lesser SOD activity vs. CON or SCFP28-fed steers. A tendency for a linear increase in MDA concentrations was observed due to treatment on day 27 (P = 0.09). On day 27, there was a tendency for a quadratic effect of SCFP on GSH concentrations (P = 0.09), driven by greater concentrations for SCFP14-fed steers, resulting in a quadratic effect of treatment on GSSG:GSH (P = 0.05). On day 56, a tendency for a linear effect of SCFP on tGSH was observed (P = 0.09) as well as a tendency for a quadratic effect on GSH (P = 0.07). Serum lysozyme activity did not differ due to treatment on day 27 or 56 (P ≥ 0.11). Serum BVDV Type 1 and 2 antibody titers were analyzed as repeated measures and no treatment × day effects were noted (P ≥ 0.16). The inclusion of SCFP did not affect antibody titers (P ≥ 0.23; Table 5); however, day effects were observed (P < 0.01; Figure 1) where titers peaked 42 d after a booster vaccine.
Table 3.
Effect of increased inclusions of a Saccharomyces cerevisiae fermentation product (SCFP) on superoxide dismutase activity and plasma malondialdehyde concentrations of newly weaned beef steers during a 56-d receiving period
| Treatment1 | P-value | |||||
|---|---|---|---|---|---|---|
| CON | SCFP14 | SCFP28 | SEM2 | Linear | Quadratic | |
| Red blood cell lysate | ||||||
| Superoxide dismutase3,4 | ||||||
| Day 0 | 19.5 | 22.2 | 19.7 | – | – | – |
| Day 27 | 9.8 | 9.4 | 9.8 | 0.66 | 0.95 | 0.60 |
| Day 56 | 12.0 | 9.8 | 11.0 | 0.45 | 0.11 | 0.004 |
| Plasma | ||||||
| Malondialdehyde, µM4 | ||||||
| Day 0 | 3.7 | 4.0 | 4.1 | – | – | – |
| Day 27 | 3.5 | 3.9 | 4.1 | 0.24 | 0.09 | 0.80 |
| Day 56 | 4.5 | 4.0 | 4.3 | 0.22 | 0.55 | 0.13 |
1CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
2Highest SEM of any treatment is reported.
3Superoxide dismutase activity; one unit of SOD activity (U) is defined as the enzyme required to dismutate 50% of the superoxide radical; reported as 1,000 U·g hemoglobin−1.
4Day 0 values used as a covariate in analysis.
Table 4.
Effect of increased inclusions of a Saccharomyces cerevisiae fermentation product (SCFP) on total (tGSH), oxidized (GSSG), and reduced (GSH) glutathione concentrations of newly weaned beef steers during a 56-d receiving period
| Treatment1 | P-value | |||||
|---|---|---|---|---|---|---|
| CON | SCFP14 | SCFP28 | SEM2 | Linear | Quadratic | |
| Red blood cell lysate | ||||||
| Glutathione3, µM | ||||||
| Day 0 | ||||||
| tGSH | 229.0 | 282.2 | 245.5 | – | – | – |
| GSSG | 67.6 | 72.5 | 69.3 | – | – | – |
| GSH | 161.4 | 209.7 | 176.2 | – | – | – |
| Ratio4 | 0.44 | 0.37 | 0.43 | – | – | – |
| Day 27 | ||||||
| tGSH | 173.6 | 203.8 | 187.0 | 11.04 | 0.38 | 0.11 |
| GSSG | 51.4 | 51.6 | 52.3 | 4.26 | 0.88 | 0.97 |
| GSH | 118.9 | 152.4 | 134.6 | 11.48 | 0.32 | 0.09 |
| Ratio | 0.50 | 0.34 | 0.47 | 0.055 | 0.65 | 0.05 |
| Day 56 | ||||||
| tGSH | 289.4 | 334.2 | 343.7 | 23.12 | 0.09 | 0.53 |
| GSSG | 88.7 | 93.4 | 98.2 | 4.86 | 0.19 | 0.99 |
| GSH | 198.9 | 244.2 | 227.1 | 12.91 | 0.13 | 0.07 |
| Ratio | 0.43 | 0.38 | 0.39 | 0.024 | 0.25 | 0.39 |
1CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
2Highest SEM of any treatment is reported.
3Day 0 values used as a covariate in analysis.
4Ratio of oxidized:reduced glutathione.
Table 5.
Effect of increased inclusions of a Saccharomyces cerevisiae fermentation product (SCFP) on serum lysozyme and Bovine Viral Diarrhea Virus Type 1 (BVDABT1) and 2 (BVDABT2) antibody titers of newly weaned beef steers during a 56-d receiving period
| Treatment1 | P-value | |||||
|---|---|---|---|---|---|---|
| CON | SCFP14 | SCFP28 | SEM2 | Linear | Quadratic | |
| Serum | ||||||
| Lysozyme, U/mL3 | ||||||
| Day 0 | 47.0 | 26.1 | 42.5 | – | – | – |
| Day 27 | 43.0 | 39.2 | 39.2 | 4.68 | 0.57 | 0.75 |
| Day 56 | 32.2 | 36.1 | 38.9 | 2.87 | 0.11 | 0.88 |
| BVDABT13,4 | 2.51 | 2.45 | 2.14 | 0.219 | 0.23 | 0.65 |
| BVDABT23,4 | 1.65 | 1.72 | 1.69 | 0.187 | 0.88 | 0.82 |
1CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
2Highest SEM of any treatment is reported.
3Day 0 values used as a covariate in analysis.
4Log transformed (log10); based on repeated measures analysis, treatment × day P ≥ 0.16 for all variables.
Figure 1.
Effect of day of sampling relative to booster with Vista Once (Merck, Madison, NJ) on steer Bovine Viral Diarrhea Virus Type 1 (A) and 2 (B) antibody titers. Day P < 0.0001. Values with unlike superscripts differ (P ˂ 0.05) across sampling days. Data from blood samples taken prior to vaccination used as a covariate in analysis (day 0 antibody titers for type 1 = 0.58 and type 2 = 0.45).
DISCUSSION
Oxidative stress, an imbalance between oxidants and antioxidants in favor of the oxidants (Sies, 2007), leads to damage of cellular components including lipids, proteins, and DNA. The cellular antioxidant defense system works to prevent an excess of reactive oxygen species through endogenous and dietary antioxidants, metal binding proteins, and free radical scavenging enzymes (Jacob, 1995). Because of the integrated nature of this system, the current study evaluated glutathione, an endogenous antioxidant; SOD, an antioxidant enzyme; and MDA, a product of lipid peroxidation, to gain a better understanding of the relationship between SCFP supplementation and oxidative stress. SCFPs have demonstrated antioxidant capacity both in vitro and in vivo; Original XP provided intracellular protection of red blood cells from oxidative damage (Jensen et al., 2008) and EpiCor, a SCFP for humans, increased serum antioxidant protection capacity 2 h after consumption (Jensen et al., 2011). Transit has been shown to increase markers of oxidative stress in cattle, horses, and dogs (Chirase et al., 2004; Onmaz et al., 2011; Fazio et al., 2015). Although steers were not exposed to transit during the current study, they had recently been transported approximately 860 km (9.5 h) to Iowa State University for this trial. Therefore, it is important to consider the effects of transit as well as treatment on markers of oxidative stress.
In the current study, plasma MDA concentrations remained relatively constant across sampling days regardless of treatment. Chirase et al. (2004) observed a 3-fold increase in MDA concentrations of steers immediately post-transit; however, MDA concentrations were only analyzed at one time point post-transit, so it is unclear how long MDA concentrations remained increased. Because the first blood sample was taken 7 d after arrival in the current study, it is possible that increased MDA concentrations caused by transit had already returned to pretransit levels. Glutathione must be in the reduced form to exert its antioxidant effect. Greater concentrations of reduced glutathione for SCFP14-fed steers on days 27 and 56 suggest greater antioxidant defense. A greater oxidized to reduced glutathione ratio is an indicator of redox status and a ratio greater than 0.1 is indicative of oxidative stress (Ithayaraja, 2011). Based on this value, steers in the current study were experiencing some degree of oxidative stress regardless of treatment or sampling day. The lesser oxidized to reduced glutathione ratio for SCFP14-fed steers on day 27 suggests that these steers were experiencing less oxidative stress than CON or SCFP28-fed steers. Russell et al. (2016) observed oxidized to reduced glutathione ratios that averaged 0.20 during the growing phase and 0.15 during the finishing phase. The greater ratios observed in the current study (0.42; average across sampling days and treatments) could be a function of a variety of factors including environmental stress and calf age.
SOD catalyzes the conversion of the superoxide anion into molecular oxygen and less reactive hydrogen peroxide (Harris, 1992). Lesser SOD activity on day 56 for SCFP14-fed steers could indicate less oxidative stress and thus a lesser need for antioxidant enzymes to combat reactive oxygen species. Regardless of treatment, SOD activity was numerically greater on days 0 vs. 27 and 56. This greater SOD activity could be a result of recent transportation to the feedlot. Chirase et al. (2004) evaluated the effects of transit on oxidative stress biomarkers by assembling 105 crossbred steer calves (207 ± 21.2 kg) at an order buyer barn and collecting blood samples prior to a 20-h shipping event as well as upon arrival at the feedlot. Serum total antioxidant capacity was decreased post-transit and continued to decrease up to day 28 of the trial. Russell et al. (2016) observed greater SOD activity observed at the beginning (3-d post-transit) vs. the conclusion of a 97-d finishing phase (30.98 vs. 23.3 U SOD activity/g hemoglobin), providing further evidence that transit may affect cellular redox state and the antioxidant defense system. Additional research is necessary to better understand how transit affects oxidative stress and the subsequent physiological impacts on newly received beef cattle.
Although SCFP14-fed steers seemed to exhibit greater antioxidant defense, these steers experienced a greater percentage of treatments for respiratory illness vs. CON or SCFP28-fed steers (21.6% vs. 13.4% and 10.0%, respectively). Chirase et al. (2004) observed calves who experienced ≥3 episodes of BRD had 2-fold higher MDA concentrations upon arrival than healthy calves, demonstrating that transportation resulted in an increase in lipid peroxidation that was negatively associated with animal health. In addition to BRD, oxidative stress has been associated with pneumonia and diarrhea in calves (Ledwozyw and Stolarczyk, 1992; Ranjan et al., 2006). As measures of antioxidant defense were not measured at time of treatment in the current study, it is unclear whether there was a relationship between oxidative stress and morbidity. Despite the greater treatment rate, SCFP14-steers maintained similar performance as CON and SCFP28-fed steers. Stressors encountered during the receiving period can enhance predisposing risks for BRD, and result in peak incidence within 14 d upon arrival (Snowder et al., 2006). It is possible that steers randomly assigned to SCFP14 were already host to pathogenic bacteria and viruses prior to the start of the study, resulting in an increased respiratory treatment rate prior to day 14. Variable effects of SCFP supplementation on receiving calf health have been observed by others. Zinn et al. (1999) observed a 48% decrease in morbidity and a 44% decrease in total sick days when providing Original XP at 28.4 g·steer−1·d−1 for 56 d. It is important to note that while Original XP is produced in a similar manner as the SCFP used in the current study, it is approximately 4 times less concentrated. Morbidity was not decreased in a study by Cole et al. (1992); however, morbid calves fed Original XP at 0.75% or 1.125% of DM required fewer days of antibiotic treatment (4.5 vs. 6.1 d) than control calves. Additionally, calves receiving Original XP maintained greater DMI and lost less weight after an intranasal challenge with infectious bovine rhinotracheitis virus (Cole et al., 1992). Supplementation of SCFP may affect animal health through a variety of mechanisms such as natural killer cell and B-cell activation (Jensen et al., 2008). B-cells are a critical component of the humoral immune response as they function to produce antibodies, though in the current study SCFP did not affect antibody titers to BVDV Type 1 or 2. Similarly, dairy calves fed Original XP at 2% of DM had a similar antibody response as control calves in response to an ovalbumin vaccination (Magalhães et al., 2008).
Under the conditions of this study, there were no effects of SCFP on growth performance. Although a meta-analysis examining the effects of SCFP supplementation during the receiving phase concluded that SCFP increased ADG and G:F (Wagner et al., 2016), previous research has shown variable growth performance responses. Similar to the current study, providing Original XP at 28.4 g·steer−1·d−1 had no effect on growth performance of shipping-stressed calves (Zinn et al., 1999). Alternatively, low-stress, ranch weaned calves provided SCFP at 14 g·steer−1·d−1 for 28 d had greater final BW and ADG (Belknap et al., 2007). The variable responses to SCFP supplementation are likely due to a variety of factors such as the amount of stress calves are experiencing, the product and rate of supplementation, diet composition, and calf genetics and nutritional status prior to entering the feedlot. The steers utilized in the current study were experiencing the psychological stress of recent weaning and transportation; however, the amount of physiological stress that calves were experiencing is unclear as cortisol concentrations were not measured. Steers were from a single-source, which may have minimized the amount of stress they were experiencing relative to typical marketing channels. To ensure adequate intake of the product, SCFP was not included in the diet until 7 d after arrival when feed intake had stabilized. A different response may have been observed if SCFP been included in the diet immediately upon arrival when stress was presumably the highest. Regardless of treatment, steers performed very well throughout the course of the study, indicating that there may not have been much opportunity for improved performance with SCFP supplementation.
There was no effect of SCFP on growth performance in the current study; however, markers of antioxidant capacity indicate lesser oxidative stress and greater antioxidant defense for steers fed SCFP at 14 g·steer−1·d−1. Further research is necessary to better understand how oxidative stress affects animal performance and health, the conditions under which supplementation of SCFP may be beneficial for newly received feedlot cattle, and the optimal supplementation dose.
Footnotes
This study was partially supported by Diamond V (Cedar Rapids, IA).
LITERATURE CITED
- Association of Official Analytical Chemists (AOAC).. 1996. Official methods of analysis, 16th ed AOAC Int, Rockville, MD, USA. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 2005. Official methods of analysis, 18th ed AOAC Int, Rockville, MD, USA. [Google Scholar]
- Belknap C. R., Scott R. R., and Forcherio J. C.. 2007. Effect of yeast culture on 28-day performance of newly weaned, low-stress beef calves. J. Anim. Sci. 85(Suppl. 1):551. [Google Scholar]
- Chirase N. K., Greene L. W., Purdy C. W., Loan R. W., Auvermann B. W., Parker D. B., Walborg E. F. Jr, Stevenson D. E., Xu Y., and Klaunig J. E.. 2004. Effect of transport stress on respiratory disease, serum antioxidant status, and serum concentrations of lipid peroxidation biomarkers in beef cattle. Am. J. Vet. Res. 65:860–864. doi: 10.2460/ajvr.2004.65.860 [DOI] [PubMed] [Google Scholar]
- Cole N. A., Purdy C. W., and Hutcheson D. P.. 1992. Influence of yeast culture on feeder calves and lambs. J. Anim. Sci. 70:1682–1690. doi: 10.2527/1992.7061682x [DOI] [PubMed] [Google Scholar]
- Duff G. C., and Galyean M. L.. 2007. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazio F. S. Casella, Giannetto C., Giudice E., and Piccione G.. 2015. Characterization of actue phase proteins and oxidative stress response to road transportation in the dog. Exp. Anim. 64:19–24. doi: 10.1538/expanim.14-0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S. L., Ashwell M. S., Moeser A. J., Fry R. S., Knutson M. D., and Spears J. W.. 2010. High dietary iron reduces transporters involved in iron and manganese metabolism and increases intestinal permeability in calves. J. Dairy Sci. 93:656–665. doi: 10.3168/jds.2009-2341 [DOI] [PubMed] [Google Scholar]
- Harris E. D. 1992. Regulation of antioxidant enzymes. Faseb J. 6:2675–2683. doi: 10.1096/fasebj.6.9.1612291 [DOI] [PubMed] [Google Scholar]
- Hutcheson D. P., and Cole N. A.. 1986. Management of transit-stress syndrom in cattle: nutritional and environmental effects. J. Anim. Sci. 62:555–560. doi: 10.2527/jas1986.622555x [DOI] [Google Scholar]
- Ithayaraja C. M. 2011. Mini-review: metabolic functions and molecular structure of gluathione reductase. Int. J. Pharm. Sci. Rev. Res. 9:104–115. [Google Scholar]
- Jacob R. A. 1995. The integrated antioxidant system. Nutr. Res. 15:755–766. doi: 10.1016/0271-5317(95)00041-G [DOI] [Google Scholar]
- Jensen G. S., Patterson K. M., and Yoon I.. 2008. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp. Immunol. Microbiol. Infect. Dis. 31:487–500. doi: 10.1016/j.cimid.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Jensen G. S., Redman K. A., Benson K. F., Carter S. G., Mitzner M. A., Reeves S., and Robinson L.. 2011. Antioxidant bioavailability and rapid immune-modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: results of a placebo-controlled double-blinded crossover pilot study. J. Med. Food 14:1002–1010. doi: 10.1089/jmf.2010.0174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalkwarf E. 2014. Procedure for virus neutralization (VN) test. Iowa State Univ. Diagn. Lab, Ames, IA, USA. [Google Scholar]
- Ledwozyw A., and Stolarczyk H.. 1992. The involvement of polymorphonuclear leukocytes in the pathogenesis of bronchopneumonia in calves. VI. Superoxide dismutase and lipoprotein lipase activities. Acta Vet. Hung. 40:267–277. [PubMed] [Google Scholar]
- Magalhães V. J. A., Susca F., Lima F. S., Branco A. F., Yoon I., and Santos J. E. P.. 2008. Effect of feeding yeast culture on performance, health, and immunocompetence of dairy calves. J. Dairy Sci. 91:1497–1509. doi: 10.3168/jds.2007-0582 [DOI] [PubMed] [Google Scholar]
- Onmaz A. C., Van Den Hoven R., Gunes V., Cinar M., and Kucuk O.. 2011. Oxidative stress in horses after a 12-hour transport period. Rev. Med. Vet. 162:213–217. [Google Scholar]
- Ranjan R., Naresh R., Patra R. C., and Swarup D.. 2006. Erythrocyte lipid peroxides and blood zinc and copper concentrations in acute undifferentiated diarrhoea in calves. Vet. Res. Commun. 30:249–254. doi: 10.1007/s11259-006-3185-8 [DOI] [PubMed] [Google Scholar]
- Russell J. R., Sexten W. J., Kerley M. S., and Hansen S. L.. 2016. Relationship between antioxidant capacity, oxidative stress, and feed efficiency in beef steers. J. Anim. Sci. 94:2942–2953. doi: 10.2527/jas.2016-0271 [DOI] [PubMed] [Google Scholar]
- Sies H, Jones DP. 2007. Oxidative stress. In: Fink G, editor, Encyclopedia of stress. 2nd ed Vol. 3 Amsterdam: Elsevier; p. 45–48. [Google Scholar]
- Snowder G. D., Van Vleck L. D., Cundiff L. V., and Bennett G. L.. 2006. Bovine respiratory disease in feedlot cattle: environmental, genetic, and economic factors. J. Anim. Sci. 84:1999–2008. doi: 10.2527/jas.2006-046 [DOI] [PubMed] [Google Scholar]
- Wagner J. J., Engle T. E., Belknap C. R., and Dorton K. L.. 2016. Meta-analysis examining the effects of Saccharomyces cerevisiae fermentation products on feedlot performance and carcass traits. Prof. Anim. Sci. 32:172–182. doi: 10.15232/pas.2015-01438 [DOI] [Google Scholar]
- Zinn R. A., Alvarez E. G., Rodriguez S., and Salinas J.. 1999. Influence of yeast culture on health, performance and digestive function of feedlot steers. Proc. West. Sec. Amer. Soc. Anim. Sci. 50:335–338. [Google Scholar]