Abstract
The aim of this study was to assess knife castration and knife castration + branding in 2-mo-old calves, and the effect of a single dose of s.c. meloxicam at mitigating pain indicators. Seventy-one Angus crossbred bull calves (128 ± 18.5 kg of BW) were used in a 3 × 2 factorial design where main factors included procedure: sham (control calves, CT; n = 23), knife (KN; n = 24) or knife + branding (BK; n = 24), and medication: single s.c. administration of lactated ringer solution (NM; n = 35) or a single dose of 0.5 mg/kg of s.c. meloxicam (M; n = 36). Physiological samples were collected at T0, 60, 90, 120, and 180 min and on days 1, 2, 3, and 7 after procedure, whereas behavioral observations were evaluated at 2 to 4 h and 1, 2, 3, and 7 days after procedure. A procedure × time effect (P < 0.01) was observed for cortisol, where KN and BK calves had greater (P ≤ 0.01) cortisol concentrations than CT calves 60 min after the procedure, whereas BK calves had the greatest (P < 0.05) cortisol concentrations, followed by KN calves and by CT calves 90, 120, and 180 min after the procedure. A procedure × time effect (P = 0.01) was observed for tail flicks, where KN and BK calves had a greater (P < 0.05) number of tail flicks than CT calves on days 1 and 3, whereas BK calves had the greatest number of tail flicks, followed by KN calves, and then by CT calves on day 2. Haptoglobin had a procedure × medication × time interaction (P = 0.05), where BK-NM calves had greater haptoglobin concentrations than BK-M, KN-M, and CT calves on days 1 and 3, whereas BK-NM and KN-NM calves had greater haptoglobin concentrations than BK-M, KN-M, and CT calves on day 2 after the procedure. Lying duration and tail flicks had a medication effect (P = 0.04; P < 0.01) where M calves had greater (P < 0.05) lying duration and lower (P < 0.05) number of tail flicks than NM calves 2 to 4 h after procedure. No medication effects (P > 0.10) were observed for salivary cortisol, substance P, and scrotal temperature minutes after the procedure or for cortisol, substance P, serum amyloid-A, stride length, or behavioral observations days after the procedure. Overall, BK calves presented greater physiological and behavioral indicators of acute pain than KN calves, suggesting that the combination of knife castration + branding was more painful. Meloxicam administered s.c. was effective at reducing physiological and behavioral indicators of acute pain associated with knife castration and knife castration + branding.
Keywords: acute pain, beef, behavior, branding, castration, pain mitigation
INTRODUCTION
Castration is a common husbandry procedure performed to reduce aggressive behavior, improve meat quality, and increase on farm safety (Jacobs et al., 1977; Stafford and Mellor, 2005). Common castration methods include band, knife, and bu r dizzo castration (Weaver et al., 2008) with knife castration being reported as the most common method conducted by veterinarians in the USA (Coetzee et al., 2010). In addition, multiple procedures such as ear tagging, vaccination, dehorning, and branding are typically carried out in combination with castration to reduce the number of times calves must be handled.
Hot-iron branding is a common method of permanent identification in beef cattle. In North America, branding is performed to establish ownership, and in Canada, it is also performed to meet the requirements for exporting cattle into the USA (Schwartzkopf-Genswein et al., 2012). A Western Canadian survey reported that over half of the calves (54%) were branded and only 4% of the respondents used pain mitigation (Moggy et al., 2017).
Both castration and branding are painful procedures (Schwartzkopf-Genswein et al., 1997a,b; Stafford and Mellor, 2005; Pang et al., 2006) usually carried out without the use of analgesia or anesthesia in North America. Meloxicam is a nonsteroidal anti-inflammatory drug (NSAID) and a practical option for producers due to its ease of administration (s.c.) and long-lasting half-life (22 ± 3 h) (Coetzee et al., 2012).
Therefore, the aim of this study was to assess acute pain indicators associated with castration alone and the combination of castration + branding and to assess the effect of meloxicam at mitigating these indicators in 2-mo-old beef calves. Our hypothesis was that the combination of multiple stressors would elicit a greater stress/pain response than castration alone and that a single s.c. dose of meloxicam would reduce pain indicators due to meloxicam’s analgesic and anti-inflammatory properties.
MATERIALS AND METHODS
This protocol was approved by the Animal Care Committees of the Lethbridge Research and Development Centre (ACC number 1410) and the University of Calgary (AC14-0159), and animals were cared for in accordance with the Canadian Council of Animal Care (CCAC, 2009).
Animal Housing and Management
Seventy-one Angus crossbred beef calves (128 ± 18.5 kg of BW, 67- to 87-d-old calves) and their dams were brought to the Lethbridge Research and Development Centre (LeRDC) from a neighboring ranch located 30 km from the LeRDC. Calves were separated into 2 groups of 36 and 35 calves as animals were castrated on different days 1 wk apart. Cow–calf pairs were housed in 6 experimental pens (treatments mixed within pen) containing a calf shelter (2.4 m × 3.6 m × 1.4 m), straw bedding, and a centrally located water system. Three of the pens measured 36.7 m × 22.2 m, and 3 pens measured 40 m × 27 m. Free choice alfalfa grass was available for the cows, whereas the calves diet consisted of free choice alfalfa grass, milk from suckling, and free choice salt blocks and loose minerals containing a coccidiostat (Diluted Rumensin Drug Premix 1100 (Medicated), HI-PRO FEEDS, Okotoks, AB, Canada) to prevent diarrhea caused by coccidiosis. The experiment took place from June 23 to July 7, 2015.
Calves were weighed in a portable chute (Pearsons Livestock Equipment, Thedford, NE) and sampled (saliva, blood, scrotal, and rectal temperature) while standing in a tip table (Calf Roper, Ram-Bull Ltd, Barons, AL, Canada) with a head lock. All calves were castrated and branded on a tipping table (Hi-Qual Manufacturing Canada Ltd, MB, Canada) while lying on their left side. Castration was performed first and consisted of making an incision in the scrotum with a Newberry knife (Syrvet Inc., Waukee, IA) and crushing and cutting of the cords with an emasculator. All castrations were performed by the same experienced veterinarian. Branding was performed with the use of an electric hot-iron based on 3 combined marks: a number, a symbol and a letter (3 = M) placed on the right rib cage when calves were tipped. Sham calves were handled in the same way as castrated and branded calves. The testicles were manipulated for a similar amount of time and the same iron used to make the brand, but unheated was placed on the calves simulating the pressure exerted with the hot-iron. Branding was performed by the same experienced person. Calves were castrated for an average time of 1.1 ± 0.19 min, branded for 0.5 ± 0.18 min, and sampled for 2.7 ± 2.64 min, for an average restraining time of 3.1 ± 2.75 min.
Calves were equally distributed by weight into treatments and pens and randomly assigned to treatments using a deck of cards. The experiment consisted of a 3 × 2 factorial design where main factors included procedure: sham (control calves, CT; n = 23), knife castration (KN; n = 24), or branding and knife castration (BK; n = 24) and medication: single dose of 0.5 mg/kg of s.c. meloxicam (Metacam 20 mg/mL, Boehringer Ingelheim, Burlington, ON, Canada; M; n = 36) or the corresponding volume of a single s.c. administration of lactated ringer solution (Lactated Ringer’s Irrigation, Baxter Canada, Mississauga, ON, Canada; NM; n = 35), to yield: CT-NM (n = 11), CT-M (n = 12), KN-NM (n = 12), KN-M (n = 12), BK-NM (n = 12), and BK-M (n = 12). Meloxicam and lactated ringer solution was administered immediately prior to the procedure.
Measurements of Acute Pain and Sample Collection
Cortisol.
Salivary samples were collected 24 h before castration (day −1), immediately before castration (T0), 60, 90, 120, 180 min and on days 1, 2, 3, and 7 after castration. Samples collected on days 1, 2, 3, and 7 were collected at the same time of day. Saliva was collected, stored, and analyzed as described by Meléndez et al. (2017b). The inter-assay CV was 13.2%, whereas the intra-assay CV was 9.9%.
Substance P, serum amyloid-A, haptoglobin, and complete blood count.
Blood samples were collected from all calves through jugular venipuncture on day −1, immediately before castration (T0), 60, 90, 120, 180 min and on days 1, 2, 3, and 7 after procedure. Samples for substance P were collected, centrifuged for 15 min at 1.5 × g at 0 °C, stored, and analyzed as previously described by Meléndez et al. (2017b). Briefly, samples were collected into a 6-mL tube containing EDTA (BD Vacutainer; Becton Dickinson Co., Franklin Lakes, NJ), where benzamidine hydrochloride was added to reduce substance P degradation. Samples were analyzed at Iowa State University, College of Veterinary Medicine (Ames, IA) with some modifications from the previously described procedure by Van Engen et al. (2014). The intra-assay CV was 11.9%, and the inter-assay CV was calculated at 24.2%.
Blood samples for serum amyloid-A (SAA) and haptoglobin were collected on days 1, 2, 3, and 7, stored, and analyzed as previously described by Meléndez et al. (2017b). Briefly, samples were collected into a 10-mL nonadditive tube (BD Vacutainer; Becton Dickinson Co., Franklin Lakes, NJ) and centrifuged for 15 min at 1.5 × g at 4 °C, and the serum was decanted and frozen at −80 °C for further analysis. The inter-assay CV for haptoglobin was 7.6%, whereas SAA intra-assay and inter-assay CV were 5.7% and 13.5%, respectively.
Blood samples for complete blood count (CBC) were collected into a 6-mL EDTA tube (BD Vacutainer; Becton Dickinson Co., Franklin Lakes, NJ) on days 1, 2, 3, and 7, and red blood cells (RBC), white blood cells (WBC), platelets (PLT), and neutrophil:lymphocyte (N:L) ratio were measured using a HemaTrueHematology Analyzer (Heska Co., Loveland, CO).
Scrotal area temperature.
Images of the area of the scrotum were collected on day −1, immediately before castration (T0), 60, 90, 120, 180 min, and on days 1, 2, 3, and 7 after castration. Images were collected and analyzed as previously described by Meléndez et al. (2017b). Briefly, a FLIR i60 infrared camera (FLIR Systems Ltd., Burlington, ON, Canada) was used to take infrared images of the scrotal area, and FLIR Tools version 5.1 (FLIR Systems Ltd.) was used to delineate the scrotal area and to record the maximum temperature.
Rectal temperature.
A digital thermometer (M750 Livestock Thermometer, GLA Agricultural Electronics, San Luis Obispo, CA) was used to collect rectal temperature on day −1, immediately before castration (T0), and on days 1, 2, 3, and 7 after the procedure.
Performance.
A portable scale (Pearsons Livestock Equipment) was used to obtain the initial (average of days −1 and 0) and final (day 7) BW. The ADG (kg/d) was calculated by subtracting the weights on day 7 from the average of days −1 and 0 and dividing the result by the number of days in the experiment (7 d).
Behavioral frequencies and visual analog scale.
Behavioral scoring during castration was collected as previously described by Meléndez et al. (2017b). Briefly, 2 experienced observers marked a line along a 10-cm continuum of their perception of the amount of pain calves were experiencing during castration and recorded the frequency of urination, defecation, leg movement, and vocalizations. Due to the experimental setting, observers could not be blind to the treatments.
Electronic reactivity measurements.
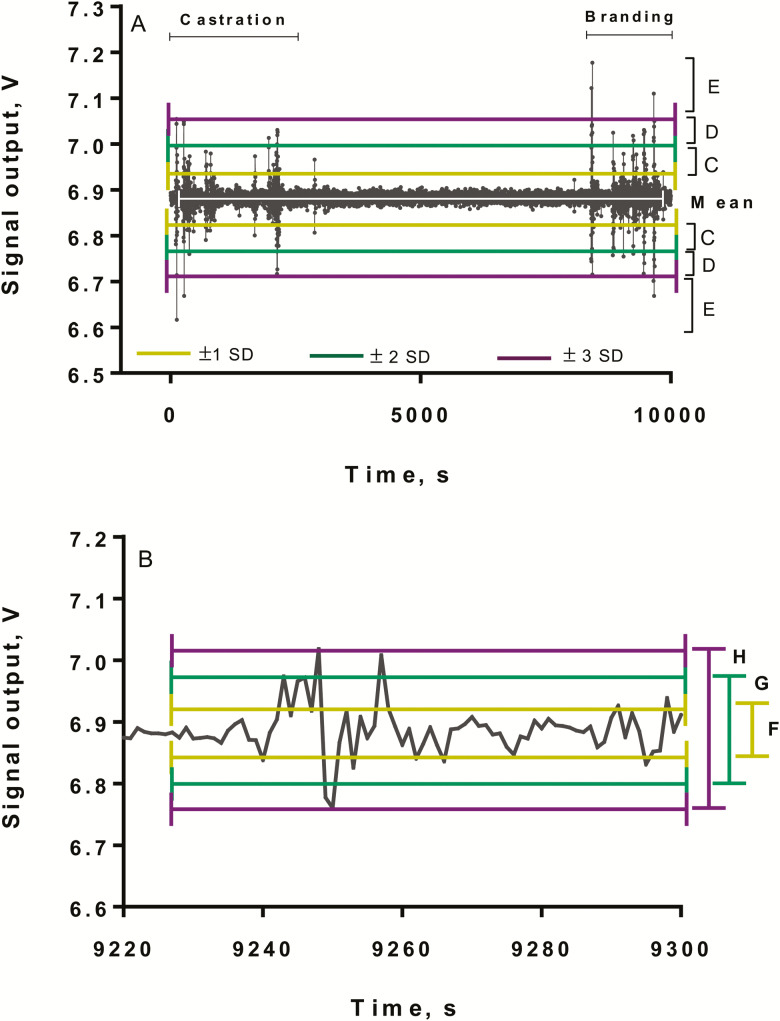
The tipping table was equipped with one 3D accelerometer, and the 3 forces were added to obtain an overall force during castration and branding procedures. Analog signals (V) from the accelerometer were sent to a computer at a rate of 100 samples/s. Data from control calves collected during sham castration and sham branding were used as the baseline for calves that were castrated and branded. Variables included number of peaks between 1 and 2 SD, 2 and 3 SD, and above or below 3 SD above and below the mean (Fig. 1A) and total area between the mean ± 1 SD, mean ± 2 SD, and mean ± 3 SD (Fig. 1B).
Figure 1.
Signal output in volts of the addition of 3 forces of a 3D accelerometer indicating movement of the tipping table by a calf (#74) during knife castration and branding. (A) C = number of peaks between 1 and 2 SD above and below the mean, D = number of peaks between 2 and 3 SD above and below the mean, and E = number of peaks above or below 3 SD above or below the mean. (B) F = total area between ± 1 SD, G = total area between ± 2 SD and H = total area between ± 3 SD.
Stride length.
Stride length was collected as previously described by Meléndez et al. (2017b). Briefly, calves were recorded when walking through an alley on day −1, immediately after castration, 180 min, and on days 1, 2, 3, and 7 after the procedure. Pictures of the back legs were taken with GOM player (GOM Lab, Gretech Corporation, Seoul, South Korea), whereas stride length was measured using Image J (National Institutes of Health Image, Bethesda, MD). Observers were blind to the treatments.
Behavioral observations.
Half of the animals of each treatment were recorded for behavioral observations, and focal animal sampling from continuous recordings (Martin and Bateson, 2007) were performed for frequencies of tail flicks, foot stamping, head turning and lesion licking, and duration of eating, lying, standing, and walking as described by Meléndez et al. (2017b). Briefly, the behaviors scored, and their definitions, were as follows: 1) eating: suckling from the udder or ingesting hay or straw from the ground or the feeder; 2) lying: either lateral (laying with hip and shoulder on the ground with at least 3 limbs extended) or ventral (laying in sternal recumbency with legs folded under the body or 1 hind or front leg extended) lying; 3) walking: walking forward more than 2 steps; 4) standing: standing on all 4 legs; 5) foot stamping: hind legs are lifted and forcefully placed on the ground or kicked outwards while standing; 6) head turning: head is turned and touches the side of the calf’s body when standing, including head turning to groom; 7) tail flicking: forceful tail movement beyond the widest part of the rump when standing, movement to one side is counted as one action; and 8) lesion licking: head turning to lick the lesion caused by castration while standing.
Two experienced observers scored behavior for a 2-h period on day 0 between 3 and 5 h relative to treatment application and for 4 min every 10 min for a 4-h period on days 1, 2, 3, and 7 for a subset of 6 animals per treatment. Observers were blind to the treatments. Inter-rater and intra-rater reliability were 0.95 and 0.91, respectively.
Standing and lying behavior.
Animals were equipped with accelerometers (Hobo pendant G, Onset Computer Corporation, Bourne, MA) to measure standing and lying bouts (number/d), total standing and lying duration (min/d) that was converted to a percentage (%), and mean standing and lying bout duration (min/d; UBC AWP, 2013) as previously described by Meléndez et al. (2017b). Briefly, accelerometers were placed on day −1 with Vet Wrap (Professional Preference, Calgary, AL, Canada) and removed on day 7. Only days with 24 h of information were included in the analysis (days 0 to 6).
Statistical Analysis
A power analysis was conducted for the outcomes of salivary cortisol and tail flicking. An α of 0.05, a power of 0.08, and the mean values and SD from a previous study of 2-mo-old beef calves under similar experimental conditions (Meléndez et al., 2017b) were used in the power calculation. Mean cortisol values were 3.3, 3.9, and 4.9 nmol/L and an SD of 0.62, whereas mean tail flicking values were 46.6, 62.6, and 116.6 tail flick counts and a SD of 5.6. The power analysis indicated that at least 6 to 12 calves per treatment were necessary to detect expected differences among treatments. Salivary cortisol, substance P, SAA, haptoglobin, CBC, stride length, and behavior the days after castration were analyzed using the MIXED procedure in SAS (SAS, version 9.4, SAS Institute Inc.) to evaluate the effect of procedure, medication, and time on all variables. Fixed effect included procedure, medication, time, and their interactions, whereas random effects included pen and calf within pen. Calves were divided into 2 groups and castrated 1 wk apart. All calves in 1 pen were castrated on the same day, and “group” was used as a covariate. Animals were the experimental unit as treatments were mixed within pen. All data were analyzed using the mixed repeated measures model (Proc Mixed of SAS) as samples were collected at different time points, with the exception of behavior during castration. Behavior during castration [visual analog scale (VAS), frequency of leg movement, urination, defecation, vocalizations, and electronic reactivity measurements (ERM)] and performance was analyzed as described above without time effect (as there were no repeated measures). Data were tested for normal distribution with PROC UNIVARIATE (SAS, version 9.4; SAS Institute Inc., Cary, NC), and physiological data that did not follow a normal distribution were log transformed, whereas behavioral data were square root + 1 transformed. The data collected on day −1 were used as a covariate for all physiological parameters and stride length. Electronic reactivity measurements collected for sham calves at the time of castration and branding were used as the mean for ERM for KN and BK calves. Urination and defecation were not analyzed as these behaviors were not present during castration or branding. The analysis with the covariance structure (unstructured, compound symmetry, and autoregressive order one) with the lowest Schwarz’s Bayesian criterion was selected as the analysis of choice. Data from the day of castration were analyzed separately from the data the days after castration as the time intervals between samples were different. A post-hoc test was run to separate the least square means using the PDIFF option in SAS. Effect of procedure, medication, and time was statistically significant when P ≤ 0.05 and considered a tendency when 0.05 < P ≤ 0.10. An intraclass correlation coefficient with a 95% confidence interval was used to calculate intra- and inter-observer reliability of 2 experienced observers using IBM SPSS statistics for Windows, version 22.0 (IBM Corp., Armonk, NY).
RESULTS AND DISCUSSION
Physiology
Salivary cortisol.
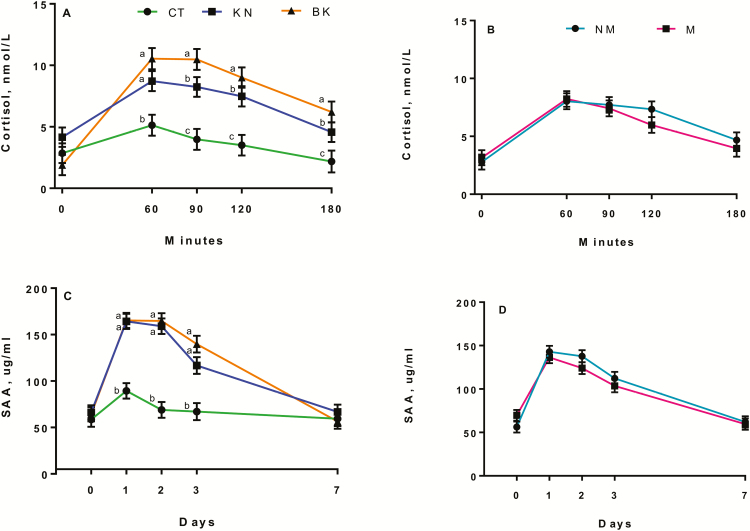
A procedure × time effect (P < 0.01) was observed for cortisol (Fig. 2A), where KN and BK calves had greater (P ≤ 0.01) cortisol concentrations than CT calves 60 min after the procedure. The BK calves had the greatest (P < 0.05) cortisol concentrations, KN calves had intermediate, and CT calves had the lowest concentrations 90, 120, and 180 min after the procedure. No medication effect (P > 0.10) was observed for cortisol 60, 90, 120, and 180 min (Fig. 2B) or on days 0, 1, 2, 3, and 7 after the procedure, and no procedure effect (P > 0.10) was observed for days after castration.
Figure 2.
Least square means and SEM for salivary cortisol (nmol/L) of (A) procedure and (B) medication immediately before treatment (T0), 60, 90, 120, and 180 min after treatment and serum amyloid-A (µg/mL) for (C) procedure and (D) medication on days 0, 1, 2, 3, and 7 after castration of noncastrated (CT, n = 23), knife (KN, n = 24), and branded and knife (BK, n = 24) castrated 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration. a–cLeast square means with differing superscripts differ (P ≤ 0.05).
Contrary to our findings, previous studies have reported a reduction in plasma cortisol concentrations in calves receiving NSAIDs prior to a painful procedure, such as surgically castrated calves receiving oral meloxicam compared with un-medicated surgically castrated 227-kg calves (Roberts et al., 2015), carprofen, in band castrated compared with un-medicated band-castrated 5.5-mo-old calves (Pang et al., 2006), burdizzo-castrated calves receiving ketoprofen compared with un-medicated bu r dizzo-castrated 11-mo-old calves (Ting et al., 2003), and dehorned calves receiving i.m. injection of meloxicam compared with un-medicated dehorned 6- to 12-wk-old dairy calves (Heinrich et al., 2009). However, in the previous studies, carprofen and ketoprofen were administered intravenously 20 min before castration, i.m. meloxicam was administered 10 min prior to castration, whereas oral meloxicam was given concurrently to castrated animals as a bolus administered directly into the rumen. Differences in results between our study and the results of Roberts et al. (2015), where meloxicam was administered at the time of castration could be due to differences between salivary and serum/plasma concentrations. Although a correlation has been observed between plasma and salivary cortisol concentrations in cattle, caution should be taken when comparing these results as there is a 10-min time lag between peak plasma and salivary cortisol concentrations (Hernandez et al., 2014), and plasma cortisol has been reported to be more sensitive than salivary cortisol to adrenal activity in pigs (Parrott et al., 1989).
Differences between studies could also be due to calves being older than the calves in the present study as a greater stress response has been reported in calves castrated after 6 mo of age compared with calves castrated at a younger age (Bretschneider, 2005). Differences could also be due to timing of meloxicam administration as the compendium for injectable meloxicam recommends the administration of meloxicam 10 to 20 min prior to the procedure for the reduction of pain caused by abdominal surgery. Based on these results, administering meloxicam s.c. immediately prior to castration may limit the analgesic effect of the drug. However, the results from the present study are consistent with the results from a previous study (Meléndez et al., 2017a) where no differences in salivary cortisol were found between animals receiving pre-emptive analgesia with s.c. meloxicam at 6, 3, or 0 h prior to knife castration up to 4 h following the procedure. Caution should be taken when interpreting these results as there was a lack of a control group that did not receive medication.
Similar to our results, Sutherland et al. (2013) did not see differences in cortisol concentrations between surgically castrated, dehorned, or surgically castrated + dehorned 3-mo-old calves 0, 24, and 72 h after treatment. Sutherland et al. (2013) suggested that lack of differences in cortisol concentrations could be due to a potential ceiling effect of the cortisol response to either castration or dehorning; however, cortisol area under the curve in castrated + dehorned calves was greater than only castrated or only dehorned calves up to 6 h after the procedure, providing some evidence that the combination of procedures is more painful. Similar results were reported by Mosher et al. (2013) who found a tendency for cortisol to be greater 60 min after castration in surgically castrated + dehorned 3- to 4-mo-old calves than those that were only castrated. Although different castration methods and painful procedures such as dehorning and branding can cause different physiological responses, both procedures are painful and stressful and therefore likely to increase cortisol concentrations.
Substance P.
No procedure or medication effects (P > 0.10) were observed for substance P minutes or days after the procedure (Table 1). These findings are similar to results reporting no differences in substance P levels 60 and 120 min and on day 7 after different castration methods (control, band, and knife) in 2-mo-old calves (Meléndez et al., 2017b), and on days 0, 1, and 7 after band castrated in medicated or un-medicated (oral meloxicam) weaned calves (Repenning et al., 2013). However, caution should be taken when comparing results as the age of the calves differ between experiments. In addition, lack of differences could be a result of other factors (alone or in combination) including high inter-assay CV; high individual animal variation in the measurements taken, which could mask treatment effects; sampling times being inadequate to detect differences among treatments; variables collected were not sensitive enough to detect differences among treatments or that no differences in substance P may suggest no pain markers the days following castration and branding.
Table 1.
Least square means (± SEM) of physiological samples taken after the procedure of noncastrated (CT, n = 23), knife (KN, n = 24), and branded and knife (BK, n = 24) castrated 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration1
| Treatment (T)2 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | KN | BK | P-value | |||||||
| Item | NM | M | NM | M | SEM3 | PRD | MED | PRD × T | MED × T | |
| Minutes after castration | ||||||||||
| Substance P, pg/mL | 81.8 | 80.1 | 79.4 | 82.6 | 78.0 | 0.06 | 0.63 | 0.35 | 0.54 | 0.45 |
| SCT, °C | 36.6 | 36.5 | 36.5 | 36.7 | 36.3 | 0.24 | 0.74 | 0.60 | 0.42 | 0.32 |
| Days after castration | ||||||||||
| Cortisol, nmol/L | 5.1 | 2.5 | 3.7 | 2.9 | 2.3 | 0.13 | 0.17 | 0.47 | 0.38 | 0.87 |
| Substance P, pg/mL | 82.2 | 78.7 | 75.8 | 84.5 | 81.4 | 0.07 | 0.25 | 0.64 | 0.29 | 0.15 |
| SCT, °C | 36.5 | 37.2 | 36.7 | 36.9 | 36.8 | 0.48 | 0.01 | 0.04 | 0.31 | 0.66 |
1Values in the table represent the mean of T0, 60, 90, and 120 min after procedure for substance P and scrotal temperature (SCT); and the means of days 1, 2, 3, and 7 after procedure for cortisol, substance P, and scrotal temperature (SCT).
2CT = sham noncastrated calves; KN = knife-castrated calves; BK = branded and knife-castrated calves; NM = single s.c. injection of lactated ringer’s immediately before procedure; M = single injection of s.c. meloxicam (0.5 mg/kg) immediately before procedure; PRD = procedure effect; MED = medication effect.
3The values correspond to nontransformed means; however, the SEM and the P-values correspond to ANOVA analysis using log-transformed data.
Serum amyloid-A and haptoglobin.
A procedure × time interaction (P < 0.01) was observed for SAA (Fig. 2C), where KN and BK calves had greater (P < 0.01) SAA concentrations than CT calves on days 1, 2, and 3, whereas no differences (P > 0.10) were observed between procedures on days 0 and 7. No medication effects (P > 0.10) were observed for SAA the days after procedure (Fig. 2D).
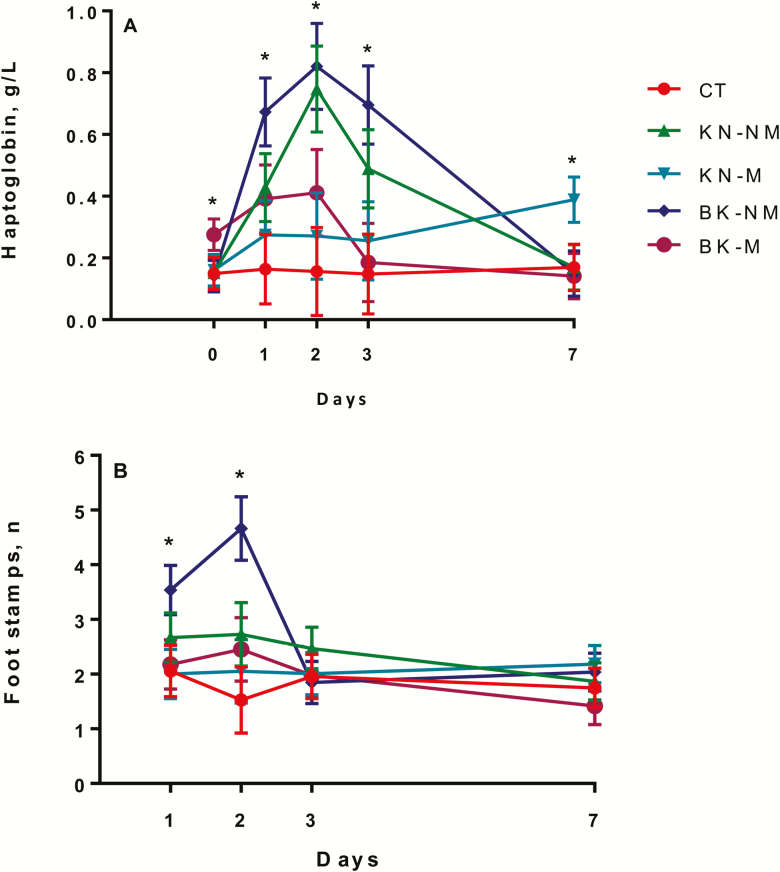
A procedure × medication × time effect (P = 0.05) was observed for haptoglobin (Fig. 3A), where BK-M calves had greater (P = 0.04) haptoglobin concentrations than BK-NM calves on day 0 (prior to castration). The BK-NM and the KN-NM calves had greater (P < 0.05) haptoglobin concentrations than BK-M, KN-M, and CT calves on days 1 and 2. The BK-NM calves had greater (P < 0.05) haptoglobin concentrations than BK-M, KN-M and CT calves on day 3, whereas KN-M calves had greater (P < 0.05) haptoglobin concentrations than BK-M calves on day 7.
Figure 3.
Least square means and SEM for (A) haptoglobin on days 0, 1, 2, 3, and 7 and (B) foot stamps on days 1, 2, 3, and 7 of noncastrated (CT, n = 23), knife (KN, n = 24), and branded and knife (BK, n = 24) castrated 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration. *P ≤ 0.05.
Both haptoglobin and SAA concentrations were above the normal range for healthy bovines (haptoglobin: <0.1 g/L and SAA: 1.3 ± 0.4 µg/mL) (Ceciliani et al., 2012) and followed the normal acute phase protein response, which increases 24 to 48 h after a challenge and returns to baseline levels approximately 4 to 7 d after (Petersen et al., 2004). Medication effects have been previously described for haptoglobin concentrations, where ketoprofen administration reduced haptoglobin concentrations 1 d after burdizzo castration in 13-mo-old calves (Ting et al., 2003) and up to 3 d after surgical castration in 5.5-mo-old calves (Earley and Crowe, 2002). Oral meloxicam has also been reported to decrease haptoglobin concentrations after surgical castration in calves at weaning weighing between 216 and 228 kg (Brown et al., 2015) and in 227-kg calves (Roberts et al., 2015). In contrast, there is a lack of literature evaluating the response of SAA after castration and pain mitigation. A study in 7- to 8-mo-old beef calves reported greater SAA concentrations than baseline levels after surgical castration, but no effect of time of s.c. meloxicam administration (6, 3, and 0 h before castration) on SAA concentrations (Meléndez et al., 2017a). Lack of differences in the previous study could be due to the fact that all treatments received meloxicam; however, no medication effect was observed for SAA in the present study, which assessed both medicated and un-medicated calves. A possible explanation could be that NSAIDs do not have the same effect in reducing the production of different APPs, which could explain the medication effect observed for haptoglobin but not for SAA.
Complete blood count.
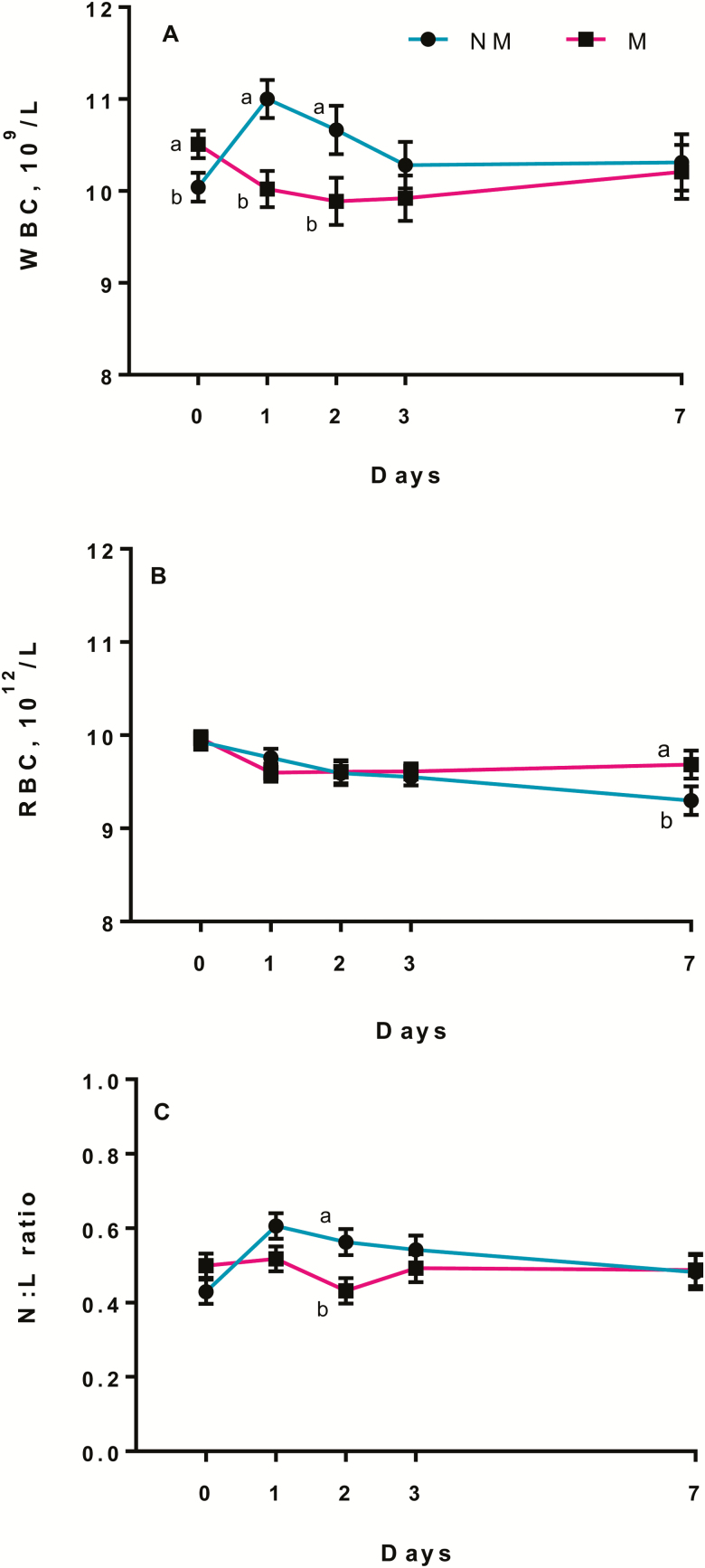
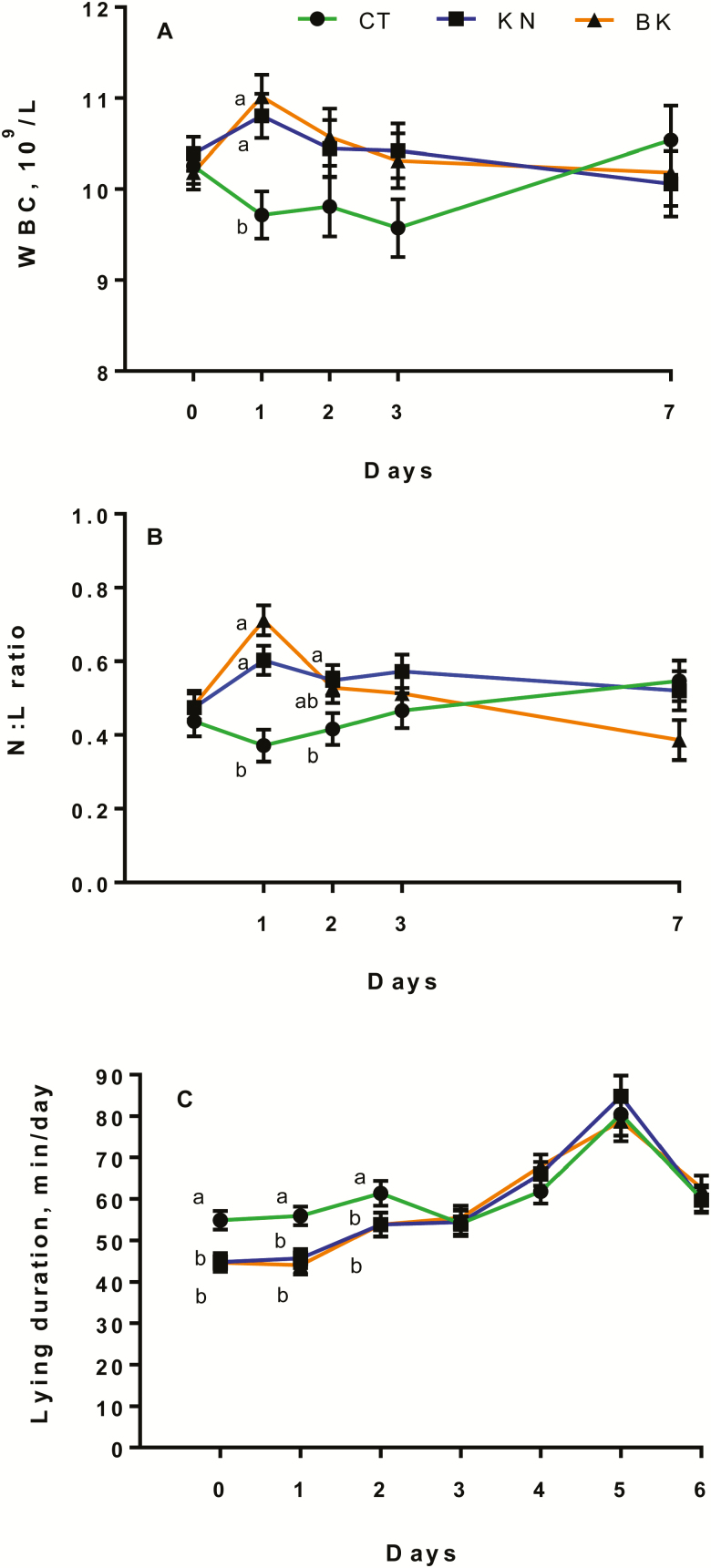
A medication × time effect (P < 0.01; P = 0.02; P = 0.02) was observed for WBC, RBC counts, and N:L ratio. The NM calves had greater (P < 0.05) WBC counts on days 1 and 2 (Fig. 4A), M calves had greater RBC counts than NM calves on day 7 (Fig. 4B), whereas NM calves had greater (P < 0.05) N:L ratio than M calves on day 2 after procedure (Fig. 4C). A procedure × time effect (P = 0.04; P < 0.01) was observed for WBC counts and N:L ratio, where KN and BK calves had a greater (P < 0.05) WBC and N:L ratio on day 1 compared with CT calves, whereas KN calves had a greater N:L ratio than CT calves on day 2 (Fig. 5A and B). No medication or procedure (P > 0.10) effects were observed for PLT.
Figure 4.
Least square means and SEM for (A) WBC, (B) RBC, and (C) N:L ratio on days 1, 2, 3, and 7 of noncastrated (CT, n = 23), knife (KN, n = 24), and branded and knife (BK, n = 24) castrated 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration. a,bLeast square means with differing superscripts differ (P ≤ 0.05).
Figure 5.
Least square means and SEM for (A) WBC and (B) N:L ratio on d 1, 2, 3, and 7, and (C) lying duration on d 0, 1, 2, 3, 4, 5, and 6 of noncastrated (CT, n = 23), knife (KN, n = 24), and branded and knife (BK, n = 24) castrated 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration. bLeast square means with differing superscripts differ (P ≤ 0.05).
Similar to our findings, Ballou et al. (2013) reported an increase in N:L ratio and total leukocytes in surgically castrated calves compared with noncastrated calves 6 h after castration and a reduction in leucocytes and N:L ratio following the administration of lidocaine and flunixine meglumin. Total WBC concentrations were lower in calves given lidocaine + flunixine meglumin before dehorning compared with calves dehorned without pain relief, but no differences were observed for calves castrated or castrated + dehorned with or without pain relief (Sutherland et al., 2013). In contrast, previous studies have reported no effect of NSAIDs on blood parameters after castration (Pang et al., 2006; Moya et al., 2014). Although levels of WBC, RBC, and N:L differed between treatments, levels were within the normal range (Smith, 2008), meaning that calves were not immunocompromised by castration or branding.
Scrotal temperature and rectal temperature.
No procedure or medication effects (P > 0.10) were observed for scrotal temperature (SCT) minutes after the procedure (Table 1). A medication effect (P = 0.04) was observed for SCT, where M (36.6 ± 0.46 °C) calves had lower (P < 0.05) SCT than NM (36.9 ± 0.46 °C) calves on days 1, 2, 3, and 7. A procedure effect (P = 0.01) was also observed where BK (36.9 ± 0.46 °C) and KN (36.9 ± 0.46 °C) calves had greater SCT than CT (36.5 ± 0.46 °C) calves on days 1, 2, 3 and 7. A medication × time interaction (P = 0.01) was observed for rectal temperature, where NM (39.4 ± 0.05 °C) calves had greater (P < 0.05) rectal temperature than M (39.2 ± 0.05 °C) calves on day 1 after treatment. A procedure × time interaction (P = 0.03) was observed for rectal temperature, where KN (39.4 ± 0.06 °C) and BK (39.3 ± 0.06 °C) calves had greater (P < 0.05) rectal temperature than CT (39.1 ± 0.06 °C) calves on day 1. No differences (P > 0.10) were observed for rectal temperature on days 0, 2, and 3 after treatment.
Some animals in the present study presented a fever (≥ 39.4 °C) (Smith, 2008) during the days after castration. NSAIDs are used in veterinary medicine to reduce body temperature in animals with fever (Lees et al., 2004); however, differences in rectal temperature and SCT between M and NM calves and CT, KN, and BK calves were so small that differences likely lack biological significance.
Weight and ADG.
A procedure × medication interaction (P = 0.01) was observed for ADG, where CT-M (1.3 ± 0.07), KN-NM (1.1 ± 0.08), and BK-M (1.3 ± 0.07), calves had greater (P < 0.05) ADG than KN-M (0.9 ± 0.07), and BK-NM (0.9 ± 0.08) calves, whereas CT-NM (1.2 ± 0.08) calves had greater (P < 0.05) ADG than BK-NM calves, but no differences (P > 0.10) were observed between CT-NM, CT-M, KN-NM, and BK-M calves, nor between CT-NM and KN-M calves. No medication or procedure effects (P > 0.10) were observed for initial and final BW.
The ADG was greater in CT-NM and CT-M calves as expected as the animals did not experience the trauma associated with surgery or burn. However, the BK-M calves had greater ADG than BK-NM calves, which may be due to the reduced pain that would motivate the calves to get up, walk, and suckle; however, we would also expect to see a greater ADG in KN-M calves compared with KN-NM calves. A possible explanation for the greater ADG observed in KN-NM calves compared with KN-M calves could be due to an increase in suckling in KN-NM calves as a way to cope with pain as suckling has been reported to increase oxytocin release (Lupoli et al., 2001), which can increase the nociceptive threshold (Uvnäs-Moberg et al., 1998). However, caution should be taken when interpreting these results as a difference of 0.2 kg/d may lack biological significance. A possible reason for the expected medication effect observed for the BK group but not in the KN group could be due to meloxicam being more effective at alleviating pain caused by branding (somatic pain) than pain caused by knife castration (somatic and visceral pain). However, the application of an NSAID, such as flunixine meglumin, did not have any effect on wound healing or pain response associated with branding (Tucker et al., 2014), and studies in cancer patients show that NSAIDs are effective at mitigating both somatic and visceral pain (Mercadante et al., 1999). Contrary to our findings, a study reported no differences in ADG in calves undergoing multiple painful procedures such as castration, dehorning and castration + dehorning in 3-to 4-mo-old dairy calves (Mosher et al., 2013).
Behavior
Behavioral frequencies and VAS.
A procedure × medication interaction (P = 0.04) was observed for leg movements, where the BK-M calves had a greater (P < 0.05) number of leg movements than CT, KN-NM, and KN-M calves during the procedures, but no differences (P > 0.10) were observed between BK-M and BK-NM calves (Table 2). The KN-M calves had greater (P < 0.05) number of leg movements than CT and KN-NM calves; however, no differences (P > 0.10) were observed between KN-M and BK-NM calves. A procedure effect (P < 0.01) was observed for VAS where BK (5.5 ± 0.07 cm) calves had greater (P < 0.05) VAS scores, followed by KN (2.6 ± 0.07 cm) calves, and then by CT (0.4 ± 0.07 cm) calves.
Table 2.
Least square means (± SEM) of VAS, leg movement and vocalizations during castration and behavioral observations assessed 2 to 4 h after procedure for a 2-h period of noncastrated (CT, n = 23), knife (KN, n = 24), and branded and knife (BK, n = 24) castrated 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration1
| Treatment2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CT | KN | BK | P-value | ||||||
| Item | NM | M | NM | M | SEM3 | PRD | MED | PRD × MED | |
| VAS, cm | 0.4 | 2.2 | 2.9 | 5.1 | 5.8 | 0.08 | <0.01 | 0.08 | 0.37 |
| Leg movement, n | 2.3d | 5.2c | 7.5b | 9.1ab | 10.8a | 0.13 | <0.01 | 0.03 | 0.04 |
| Vocalization, n | 2.3 | 2.2 | 1.5 | 6.8 | 9.9 | 0.17 | <0.01 | 0.29 | 0.10 |
| Behavioral observation | |||||||||
| Walking, min | 2.5b | 5.2a | 2.5b | 7.0a | 3.3b | 0.16 | <0.01 | <0.01 | <0.01 |
| Standing, min | 28.5 | 66.1 | 43.7 | 75.3 | 40.8 | 0.70 | 0.01 | 0.07 | 0.14 |
| Lying, min | 98.0 | 53.4 | 82.1 | 43.4 | 85.0 | 0.77 | 0.03 | 0.04 | 0.12 |
| Foot stamping, n | 1.6 | 12.9 | 5.4 | 28.3 | 31.7 | 0.75 | <0.01 | 0.37 | 0.64 |
a–dLeast square means within a row with differing superscripts differ (P ≤ 0.05).
1Values in the table represent the means of visual analog scale (VAS), leg movement, and vocalizations and behavioral observations.
2CT = sham noncastrated calves; KN = knife-castrated calves; BK = branded and knife-castrated calves; NM = single s.c. injection of lactated ringer’s immediately before procedure; M = single injection of s.c. meloxicam (0.5 mg/kg) immediately before procedure; PRD = procedure effect; MED = medication effect.
3The values correspond to nontransformed means; however, the SEM and the P-values correspond to ANOVA analysis using square root + 1 transformation.
These results demonstrate that surgical castration and hot-iron branding are painful procedures as observed by greater VAS scores and numerically greater vocalizations compared with CT calves, however, branding elicits more vigorous behavioral responses than surgical castration at the time of the procedure. This could be due to the differences in pain, as somatic pain is localized and allows for rapid motor reflexes, whereas visceral pain is poorly localized and leads to muscle contraction and autonomic and emotional responses (Gebhart and Ness, 1991). Similar behavioral results for hot-iron branding have been previously reported in a study comparing hot-iron branding and freeze branding, where hot-iron branded calves vocalized more and had greater exertion forces than freeze or sham calves (Schwartzkopf-Genswein et al., 1997b). Greater VAS scores have also been reported in surgically castrated calves compared with band and control calves (Fell et al., 1986; Meléndez et al., 2017b).
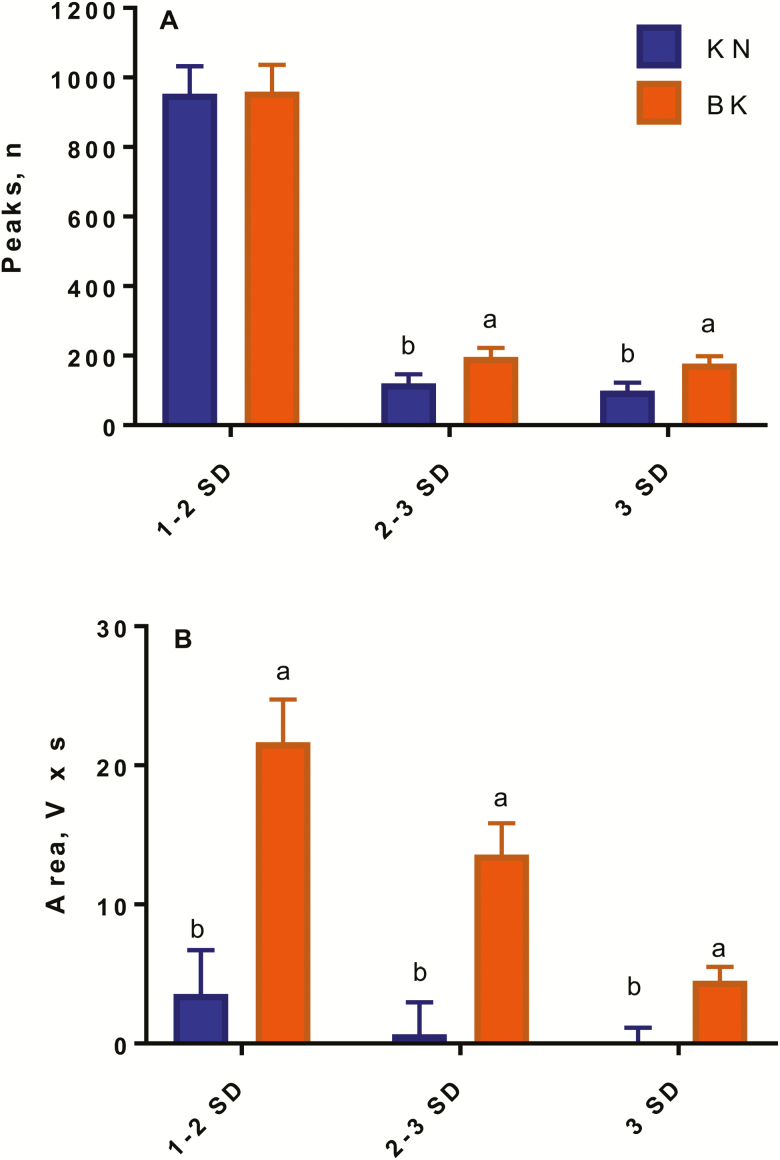
Electronic reactivity measurements.
During branding, a procedure effect (P < 0.01) was observed for number of accelerometer peaks between 2 and 3 SD above and below the mean (baseline of control calves) and greater or lower than 3 SD above or below the mean, where BK calves had a greater number of peaks than KN calves (Fig. 6A). However, no differences (P > 0.10) were observed for number of peaks above and below the mean between 1 and 2 SD at the time of branding. A procedure effect (P < 0.05) was also observed for total area, where BK calves had greater (P < 0.05) total area than KN calves between the mean ± 1 SD, the mean ± 2 SD, and the mean ± 3 SD (Fig. 6B). During castration, no medication or procedure effects (P > 0.10) were observed for number of peaks between 1 and 2 SD, 2 and 3 SD, and greater or lower than 3 SD, and total area between the mean ± 1 SD, mean ± 2 SD, and mean ± 3SD above and below the mean.
Figure 6.
Least square means and SEM for electronic reactivity measurements (A) peaks (number) and (B) area (V × s) during sham branding (KN, n = 24) and hot-iron branding (BK, n = 24) of 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration. a,bLeast square means with differing superscripts differ (P ≤ 0.05).
Movement in the chute has been previously measured during branding (Schwartzkopf-Genswein et al., 1997b) and castration (Moya et al., 2014; Meléndez et al., 2017a) in cattle. However, this was the first time that the portable electronic reactivity movement was used on a tip table to quantify movement at the time of castration and branding. As expected, no differences were observed for accelerometer movement at the time of castration, as both groups of calves were surgically castrated. However, differences were observed for branding, as 1 group was branded with a hot-iron, whereas the other group was sham branded. These results are in agreement with the results observed for VAS scores, indicating that BK calves experienced more pain than KN calves.
Stride length.
No medication or procedure effects (P > 0.10) were observed for stride length immediately after or 180 min after castration. However, a procedure effect (P < 0.01) was observed for stride length, where KN (43 ± 1.1 cm) and BK (43 ± 1.0 cm) calves had greater stride length than CT (40 ± 1.0 cm) calves on days 1, 2, 3, and 7. No medication effect (P > 0.10) was observed for stride length on days 1, 2, 3, and 7.
Similar results were observed by Meléndez et al. (2017b) who reported no differences in stride length immediately after and 120 min after castration in control, band-, and knife castrated 2-mo-old calves. Contrary to our findings, control, band-, and knife-castrated calves at 2 mo of age did not present differences in stride length on days 1, 2, 3, and 5 after castration (Meléndez et al., 2017b). This finding is difficult to explain, as we would expect KN and BK calves to have a shorter stride length than CT calves. Currah et al. (2009) suggested shortening of the stride length as a behavioral indicator of pain associated with surgical castration after observing longer stride lengths in 3-mo-old calves receiving flunixine meglumin and a lidocaine epidural than calves receiving a lidocaine epidural or no medication. Differences between studies could be due to the time of sampling as differences in the previous study were observed 4 and 8 h after castration, whereas in the present study, calves were sampled immediately after and 4 h after castration. In addition, measurements were carried out differently between studies that could explain differences observed in results. Differences included the use of different software for image analysis and lack of grid background at the time of video recording in the current study.
Behavioral observations.
A procedure × medication interaction (P < 0.01) was observed for walking duration (Table 2). The BK-NM and KN-NM calves had greater (P < 0.05) walking duration than CT, KN-M, and BK-M calves 2 to 4 h after treatment. Lying duration had a medication effect (P = 0.04) where M (87 ± 0.4 min) calves had greater (P < 0.05) lying duration than NM (66 ± 0.4 min) calves 2 to 4 h after treatment. A procedure effect (P = 0.03) was also observed for lying duration 2 to 4 h after treatment, the KN (66 ± 0.5 min) and BK (64 ± 0.5 min) calves had lower (P < 0.05) lying durations than CT (97 ± 0.5 min) calves.
A procedure effect (P = 0.01; P < 0.01) was observed for standing and foot stamping, where the KN (55 ± 0.5 min) and BK (58 ± 0.5 min) calves had greater (P < 0.05) standing duration than CT (29 ± 0.5 min) calves and the BK (30 ± 0.5) calves had greater foot stamping than CT (2 ± 0.5) and KN (9 ± 0.5) calves 2 to 4 h after treatment. A procedure × medication × time effect (P = 0.03) was observed for foot stamping (Fig. 3B), where BK-NM calves had greater (P < 0.05) foot stamping than CT-NM, KN-M, and BK-M calves and tended (P = 0.06) to be greater than CT-M calves on day 1 after treatment. On day 2 after treatment, BK-NM calves had greater (P < 0.05) foot stamping than CT, KN-NM, KN-M, and BK-M calves. No differences (P > 0.10) were observed on days 3 and 7 after treatment.
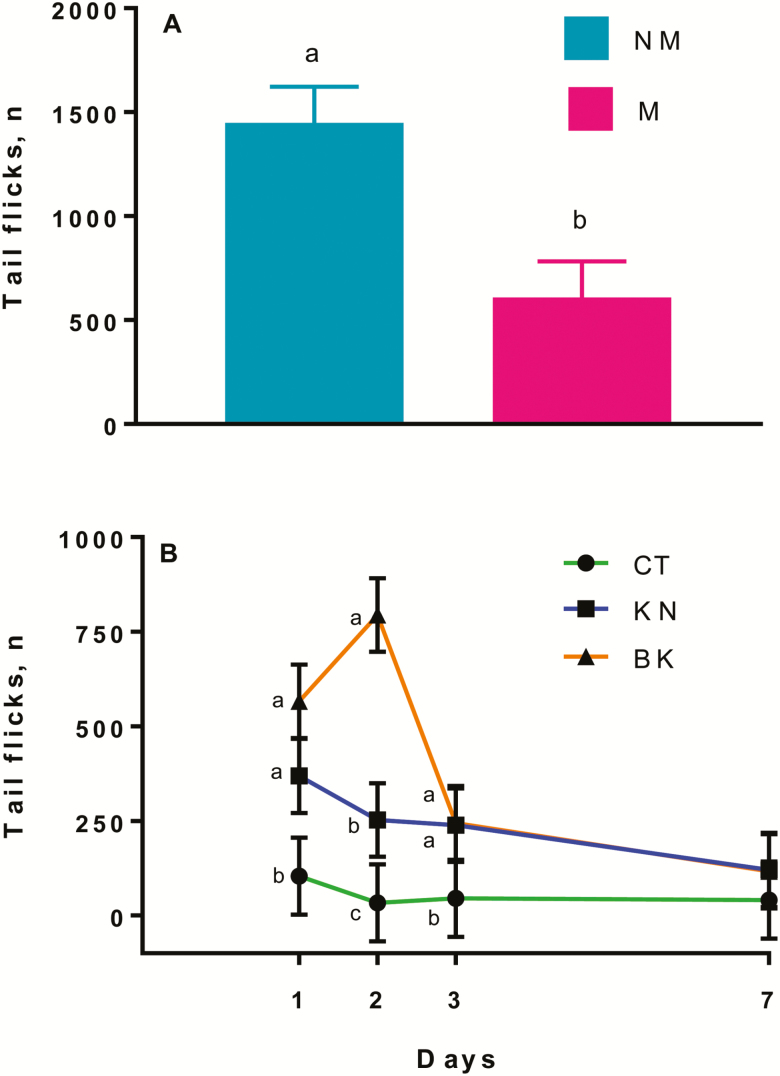
A medication effect (P < 0.01) was observed for tail flicks, the NM calves had greater number of tail flicks than M calves 2 to 4 h after treatment (Fig. 7A). A procedure effect (P < 0.01) was also observed for tail flicks, the KN (1346 ± 3.0), and BK (1711 ± 3.0) calves had a greater (P < 0.05) number of tail flicks than CT (29 ± 3.0) calves 2 to 4 h after treatment. A procedure × time effect (P = 0.01) was observed for tail flicks, where KN and BK calves had a greater (P < 0.05) number of tail flicks than CT calves on days 1 and 3 after treatment, whereas BK calves had the greatest number of tail flicks, followed by KN calves, and then by CT calves on day 2 after castration (Fig. 7B).
Figure 7.
Least square means and SEM for tail flicks (A) 2 to 4 h after castration and (B) on days 1, 2, 3, and 7 of noncastrated (CT, n = 23), knife (KN, n = 24), and branded and knife (BK, n = 24) castrated 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration. a–cLeast square means with differing superscripts differ (P ≤ 0.05).
A procedure effect (P = 0.08) was observed for head turning, BK (24 ± 0.6) calves tended to have greater head turning than CT (3 ± 0.6) calves; however, no differences were observed between both groups and KN (12 ± 0.6) calves 2 to 4 h after treatment. A procedure × medication interaction (P = 0.01) was observed for head turning, where KN-NM calves had greater (P < 0.05) head turns than CT, KN-M, and BK-M calves, but no differences were observed between KN-NM and BK-NM calves on days 1, 2, 3, and 7 after castration (Table 3). Head turning was greater (P < 0.05) in BK-NM calves than CT-NM and KN-M calves, but no differences (P > 0.10) were observed between BK-NM calves and CT-M and BK-M calves. No differences (P > 0.10) were observed between CT, KN-M, and BK-M. A procedure × time tendency (P = 0.06) was observed for head turning (Table 3), where BK (9.7 ± 2.25) calves had greater (P < 0.05) head turns and KN (9.3 ± 2.25) calves tended (P = 0.09) to have greater head turns than CT (5.0 ± 2.36) calves on day 1. The BK (12.1 ± 1.91) and KN (6.0 ± 1.91) calves had greater (P < 0.05) head turns than CT (4.0 ± 2.00) calves on day 2 after castration, whereas no differences (P > 0.10) were observed between treatments on days 3 and 7.
Table 3.
Least square means (± SEM) of behavioral observations on days 1, 2, 3, and 7 and standing and lying behavior on days 0, 1, 2, 3, 4, 5, and 6 of noncastrated (CT, n = 23), knife (KN, n = 24), and branded and knife (BK, n = 24) castrated 2-mo-old Angus crossbred calves with (M, n = 36) or without (NM, n = 35) a single s.c. meloxicam administration
| Treatment (T)1 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CT | KN | BK | P-value | |||||||
| Item | NM | M | NM | M | NM | M | SEM2 | PRD | MED | PRD × MED |
| Behavioral observation | ||||||||||
| Walking, min | 1.8 | 2.2 | 2.0 | 2.6 | 1.8 | 2.0 | 0.10 | 0.88 | 0.24 | 0.89 |
| Standing, min | 29.6 | 28.7 | 33.3 | 29.5 | 35.5 | 34.6 | 0.55 | 0.53 | 0.85 | 0.82 |
| Lying, min | 64.5 | 65.1 | 60.1 | 63.9 | 58.7 | 59.3 | 0.42 | 0.44 | 0.51 | 0.96 |
| Eating, min | 25.5 | 29.1 | 19.7 | 22.3 | 14.5 | 17.6 | 0.47 | 0.01 | 0.16 | 0.91 |
| Head turning, n | 4.1c | 6.5bc | 11.8a | 4.9c | 10.7ab | 6.9bc | 0.26 | 0.08 | 0.08 | 0.01 |
| Lesion licking, n | 0.7 | 0.9 | 1.6 | 0.8 | 1.6 | 0.8 | 0.12 | 0.42 | 0.11 | 0.38 |
| Standing and lying behavior | ||||||||||
| Standing, % | 39.3 | 39.2 | 38.6 | 40.8 | 41.3 | 38.8 | 0.01 | 0.74 | 0.86 | 0.06 |
| Lying, % | 60.7 | 60.8 | 61.4 | 59.2 | 58.7 | 61.2 | 0.01 | 0.74 | 0.86 | 0.06 |
| Standing duration, min | 41.8 | 45.0 | 39.5 | 42.0 | 43.3 | 38.5 | 0.17 | 0.39 | 0.86 | 0.21 |
| Lying duration, min | 59.7 | 62.8 | 58.9 | 58.0 | 60.1 | 56.3 | 0.18 | 0.39 | 0.87 | 0.57 |
| Standing bouts, n | 14.4 | 13.5 | 15.6 | 15.2 | 15.1 | 15.7 | 0.09 | 0.08 | 0.70 | 0.57 |
a–cLeast square means within a row with differing superscripts differ (P ≤ 0.05).
1CT = sham noncastrated calves; KN = knife-castrated calves; BK = branded and knife-castrated calves; NM = single s.c. injection of lactated ringer’s immediately before procedure; M = single injection of s.c. meloxicam (0.5 mg/kg) immediately before procedure; PRD = procedure effect; MED = medication effect.
2The values represented correspond to nontransformed means; however, SEM and P-values correspond to ANOVA using square root + 1 transformed data for behavioral observations.
These results suggest that branding in combination with castration is more painful than surgical castration alone, as seen by a greater number of tail flicks and foot stamps 2 to 4 h and on days 1 and 2 after the procedure. Although not significant, a previous study reported greater number of tail flicks in knife (191) than band (78) and control (86) 2-mo-old calves on days 1, 2, 3, and 5 after castration (Meléndez et al., 2017b). Tail flicks were also greater at the time of hot-iron branding than freeze or sham branding in 320-kg calves (Schwartzkopf-Genswein et al., 1997b). In the present study, meloxicam reduced pain-related behaviors as seen by a reduction in walking, tail flicking, and head turning and an increase in lying duration in M calves compared with NM calves. Similar findings have reported lower tail flick behavior in ketoprofen-treated cows than saline-treated cows on day 1 after the first stage of fistulation surgery (Newby et al., 2014) and lower ear flicks and head shakes in meloxicam-treated calves than saline-treated calves after dehorning in 6- to 12-wk-old dairy calves (Heinrich et al., 2010). Contrary to our findings, Sutherland et al. (2013) did not see differences in tail flicking or time spent foot stamping between castrated, dehorned, and castrated + dehorned 3-mo-old calves either receiving pain relief or no pain relief 3 h after castration. Discrepancies between studies could be due to the difference in painful procedures (dehorning vs. branding), which can elicit different behavioral responses and/or to differences in medication (lidocaine + flunixine meglumin vs. meloxicam). Although no differences were observed for tail flicks and head turns between BK and KN calves, BK calves had numerically greater number of tail flicks and head turns 2 to 4 h after castration, suggesting that BK calves experienced more pain.
No medication or procedure effects (P > 0.10) were observed for eating or lesion licking 2 to 4 h after treatment. A procedure effect (P = 0.01) was observed for eating, where CT (27 ± 0.4 min) calves had greater eating duration than BK (16 ± 0.4 min) calves; however, no differences were observed between both groups and KN (21 ± 0.4 min) calves. Although there were no differences between CT and KN calves, it is likely that greater eating duration leads to greater ADG as CT calves had greater ADG than KN and BK calves. However, values for eating could be different if these were scored for 24 h compared with 4 h. Contrary to our results, castrated, dehorned, and castrated + dehorned calves receiving lidocaine and flunixine meglumine had greater eating times than un-medicated castrated, dehorned, and castrated + dehorned calves (Sutherland et al., 2013). Differences between studies could be due to the added effect of the anesthetic that could temporarily block the pain associated with the procedures and consequently calves would be more likely to eat compared with calves experiencing pain.
Standing and lying behavior.
Standing percentage tended (procedure × medication interaction; P = 0.06) to be greater, whereas lying percentage tended (procedure × medication interaction; P = 0.06) to be lower in BK-NM calves than KN-NM and BK-M calves; however, no differences were observed between these groups and CT and KN-M calves. Lying duration was greater (procedure × time interaction; P < 0.01) in CT calves than KN and BK calves on days 0, 1, and 2 after treatment (Fig. 5C). No differences were observed on days 3, 4, 5, or 6 after treatment (data not shown), suggesting that animals in pain lie for less time than animals that are not in pain. This is in agreement with a previous study where knife-castrated calves had greater standing percentage than band-castrated and control calves 2 to 4 h and on days 1, 2, 3, and 5 after castration (Meléndez et al., 2017b). Holstein calves receiving oral meloxicam lay down for longer periods of time on days 1, 2, 3, and 4 after dehorning in comparison to un-medicated calves (Theurer et al., 2012), whereas i.m. meloxicam-treated calves were less active than un-medicated Holstein calves during the 5 h following dehorning (Heinrich et al., 2010).
A procedure × time effect (P < 0.01) was observed for standing bouts, where KN and BK calves had greater (P < 0.05) standing bouts than CT calves on days 1 and 2, whereas BK calves had greater (P < 0.01) standing bouts than CT calves, and there was a tendency (P = 0.09) for KN calves to have greater standing bouts than CT calves on day 0. No differences (P > 0.10) were observed on days 3, 4, 5, and 6.
Lying and standing bouts are an indicator of restless behavior, which is associated with pain caused by ischemia (Dinniss et al., 1999). A previous study reported a decrease in standing and lying bouts in band-castrated 1-wk-old calves, whereas an increase in standing and lying bouts in 4-mo-old band-castrated calves, but no differences in 2-mo-old band-castrated calves (Meléndez et al., 2017b). It seems that restlessness is not only linked with pain caused by ischemia, but it might be linked with general discomfort as calves that were surgically castrated, and branded + castrated presented greater standing bouts than CT calves.
No medication effects (P > 0.10) were observed for walking, standing, lying, eating, and lesion licking on days 1, 2, 3, and 7 after castration, for standing and lying bouts nor for standing and lying duration on days 0, 1, 2, 3, 4, 5, or 6 (Table 3). No procedure effects (P > 0.10) were observed for walking, standing, lying, and lesion licking on days 1, 2, 3, and 7 after the procedure, neither for standing and lying duration on days 0, 1, 2, 3, 4, 5, and 6 after the procedure. Lack of differences in behavioral and physiological parameters could be due to several reasons such as sample size, high individual variability, lack of sensitivity of parameters collected, or suboptimal sampling time. Although sample size was calculated for salivary cortisol and tail flicks, it is possible that the sample size was too small to observe differences between treatments for other parameters. High individual variability for physiological and behavioral responses could also mask treatment effects. In addition, the parameters collected may not be sensitive to physiological and behavioral changes associated with pain and inadequate sampling times could also be a limiting factor to observe differences between treatments.
CONCLUSION
Overall, the combination of procedures elicited a greater physiological and behavioral response than performing knife castration alone, suggesting that the pain/discomfort experienced is greater. Meloxicam did not have an effect on salivary cortisol, substance P, SAA, PLT, stride length, standing and lying duration, standing and lying bouts, and behavioral observation for eating and lesion licking. However, meloxicam was effective at reducing the haptoglobin response, RBC and WBC counts, N:L ratio, scrotal and rectal temperature, tail flicks, walking and lying behavior (2 to 4 h after procedure), and head turning and foot stamping (1, 2, 3, and 7 d after procedure). No differences were observed between KN-M and BK-M calves for the previously mentioned parameters, suggesting that meloxicam was equally effective at mitigating pain caused by knife castration alone and the combination of knife castration + branding. Meloxicam administered s.c. can be used as a drug to mitigate pain associated with castration and branding. Further research is needed to better understand the nature of pain associated with castration and branding practices and the best protocols to mitigate pain and optimize calf health and well-being.
Footnotes
This is Lethbridge Research and Development Centre contribution # 38717072.
The authors appreciate the invaluable help of Agriculture and Agri-Food Canada research feedlot staff and beef welfare technicians Randy Wilde and Fiona Brown. We are very thankful for the funding provided by Agriculture and Agri-Food Canada and the Beef Cattle Research Council through the Canadian Beef Cattle Industry Science Cluster. We would also like to thank all the students who helped with data collection and behavioral scoring: Jonathan Low, Louise Théron, Andrea Lippa, Nicole Desautels, Katelyn Younjin Park, and Santosh Timsina.
LITERATURE CITED
- Ballou M. A., Sutherland M. A., Brooks T. A., Hulbert L. E., Davis B. L., and Cobb C. J.. 2013. Administration of anesthetic and analgesic prevent the suppression of many leukocyte responses following surgical castration and physical dehorning. Vet. Immunol. Immunopathol. 151:285–293. doi: 10.1016/j.vetimm.2012.11.018 [DOI] [PubMed] [Google Scholar]
- Bretschneider G. 2005. Effects of age and method of castration on performance and stress response of beef male cattle: A review. Livest. Prod. Sci. 97:89–100. doi: 10.1016/j.livprodsci.2005.04.006 [DOI] [Google Scholar]
- Brown A. C., Powell J. G., Kegley E. B., Gadberry M. S., Reynolds J. L., Hughes H. D., Carroll J. A., Burdick Sanchez N. C., Thaxton Y. V., Backes E. A.,. et al. 2015. Effect of castration timing and oral meloxicam administration on growth performance, inflammation, behavior, and carcass quality of beef calves. J. Anim. Sci. 93:2460–2470. doi: 10.2527/jas.2014-8695 [DOI] [PubMed] [Google Scholar]
- Canada Council of Animal Care (CCAC) 2009. CCAC guidelines on: Animal use protocol review CCAC, Ottawa, ON: https://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf (Accessed 18 June 2017). [Google Scholar]
- Ceciliani F., Ceron J. J., Eckersall P. D., and Sauerwein H.. 2012. Acute phase proteins in ruminants. J. Proteomics 75:4207–4231. doi: 10.1016/j.jprot.2012.04.004 [DOI] [PubMed] [Google Scholar]
- Coetzee J. F., Mosher R. A., KuKanich B., Gehring R., Robert B., Reinbold J. B., and White B. J.. 2012. Pharmacokinetics and effect of intravenous meloxicam in weaned Holstein calves following scoop dehorning without local anesthesia. BMC Vet. Res. 8:153. doi: 10.1186/1746-6148-8-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee J. F., Nutsch A. L., Barbur L. A., and Bradburn R. M.. 2010. A survey of castration methods and associated livestock management practices performed by bovine veterinarians in the United States. BMC Vet. Res. 6:12. doi: 10.1186/1746-6148-6-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currah J. M., Hendrick S. H., and Stookey J. M.. 2009. The behavioral assessment and alleviation of pain associated with castration in beef calves treated with flunixin meglumine and caudal lidocaine epidural anesthesia with epinephrine. Can. Vet. J. 50:375–382. [PMC free article] [PubMed] [Google Scholar]
- Dinniss A. S., Stafford K. J., Mellor D. J., Bruce R. A., and Ward R. N.. 1999. The behaviour pattern of lambs after castration using a rubber ring and/or castrating clamp with or without local anaesthetic. N. Z. Vet. J. 47:198–203. doi: 10.1080/00480169.1999.36143 [DOI] [PubMed] [Google Scholar]
- Earley B., and Crowe M. A.. 2002. Effects of ketoprofen alone or in combination with local anesthesia during the castration of bull calves on plasma cortisol, immunological, and inflammatory responses. J. Anim. Sci. 80:1044–1052. doi: 10.2527/2002.8041044x [DOI] [PubMed] [Google Scholar]
- Fell L. R., Wells R., and Shutt D. A.. 1986. Stress in calves castrated surgically or by the application of rubber rings. Aust. Vet. J. 63:16–18. doi: 10.1111/j.1751-0813.1986.tb02864.x [DOI] [PubMed] [Google Scholar]
- Gebhart G. F., and Ness T. J.. 1991. Central mechanisms of visceral pain. Can. J. Physiol. Pharmacol. 69:627–634. doi: 10.1139/y91-093 [DOI] [PubMed] [Google Scholar]
- Heinrich A., Duffield T. F., Lissemore K. D., and Millman S. T.. 2010. The effect of meloxicam on behavior and pain sensitivity of dairy calves following cautery dehorning with a local anesthetic. J. Dairy Sci. 93:2450–2457. doi: 10.3168/jds.2009-2813 [DOI] [PubMed] [Google Scholar]
- Heinrich A., Duffield T. F., Lissemore K. D., Squires E. J., and Millman S. T.. 2009. The impact of meloxicam on postsurgical stress associated with cautery dehorning. J. Dairy Sci. 92:540–547. doi: 10.3168/jds.2008-1424 [DOI] [PubMed] [Google Scholar]
- Hernandez C. E., Thierfelder T., Svennersten-Sjaunja K., Berg C., Orihuela A., and Lidfors L.. 2014. Time lag between peak concentrations of plasma and salivary cortisol following a stressful procedure in dairy cattle. Acta Vet. Scand. 56:61. doi: 10.1186/s13028-014-0061-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J., Miller J., Sauter E., Howes A., Araji A., Gregory T., and Hurst C.. 1977. Bulls steers. II. Palatability and retail acceptance. J. Anim. Sci. 45:699–702. doi: 10.2527/jas1977.454699x [DOI] [Google Scholar]
- Lees P., Landoni M. F., Giraudel J., and Toutain P. L.. 2004. Pharmacodynamics and pharmacokinetics of nonsteroidal anti-inflammatory drugs in species of veterinary interest. J. Vet. Pharmacol. Ther. 27:479–490. doi: 10.1111/j.1365-2885.2004.00617.x [DOI] [PubMed] [Google Scholar]
- Lupoli B., Johansson B., Uvnäs-Moberg K., and Svennersten-Sjaunja K.. 2001. Effect of suckling on the release of oxytocin, prolactin, cortisol, gastrin, cholecystokinin, somatostatin and insulin in dairy cows and their calves. J. Dairy Res. 68:175–187. doi: 10.1017/S0022029901004721 [DOI] [PubMed] [Google Scholar]
- Martin P., and Bateson P.. 2007. Recording methods. In: Measuring behaviour: An introductory guide. 3rd ed Cambridge University Press, Cambridge: p. 48–54. [Google Scholar]
- Meléndez D., Marti S., Pajor E., Moya D., Gellatly D., Janzen E., and Schwartzkopf-Genswein K.. 2017a. Effect of timing of subcutaneous meloxicam administration on indicators of pain after knife castration of weaned calves. J. Anim. Sci. 95:5218–5229. doi: 10.2527/jas2017.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meléndez D., Marti S., Pajor E., Moya D., Heuston C., Gellatly D., Janzen E., and Schwartzkopf-Genswein K.. 2017b. Effect of band and knife castration of beef calves on welfare indicators of pain at three relevant industry ages: I. Acute pain. J. Anim. Sci. 95:4352–4366. doi: 10.2527/jas.2017.1762 [DOI] [PubMed] [Google Scholar]
- Mercadante S., Casuccio A., Agnello A., Pumo S., Kargar J., and Garofalo S.. 1999. Analgesic effects of nonsteroidal anti-inflammatory drugs in cancer pain due to somatic or visceral mechanisms. J. Pain Symptom Manage. 17:351–356. doi: 10.1016/S0885-3924(98)00141-9 [DOI] [PubMed] [Google Scholar]
- Moggy M., Pajor E., Thurston W., Parker S., Greter A., Schwartzkopf-Genswein K., Campbell J., and Windeyer M.. 2017. Management practices associated with pain in cattle on western Canadian cow–calf operations: A mixed methods study. J. Anim. Sci. 95:958–969. doi: 10.2527/jas.2016.0949 [DOI] [PubMed] [Google Scholar]
- Mosher R. A., Wang C., Allen P. S., and Coetzee J. F.. 2013. Comparative effects of castration and dehorning in series or concurrent castration and dehorning procedures on stress responses and production in Holstein calves. J. Anim. Sci. 91:4133–4145. doi: 10.2527/jas.2012-6007 [DOI] [PubMed] [Google Scholar]
- Moya D., González L., Janzen E., Caulkett N., Fireheller E., and Schwartzkopf-Genswein K.. 2014. Effects of castration method and frequency of intramuscular injections of ketoprofen on behavioral and physiological indicators of pain in beef cattle. J. Anim. Sci. 92:1686–1697. doi: 10.2527/jas.2013-7298 [DOI] [PubMed] [Google Scholar]
- Newby N. C., Tucker C. B., Pearl D. L., LeBlanc S. J., Leslie K. E., von Keyserlingk M. A., and Duffield T. F.. 2014. An investigation of the effects of ketoprofen following rumen fistulation surgery in lactating dairy cows. Can. Vet. J. 55:442–448. [PMC free article] [PubMed] [Google Scholar]
- Pang W. Y., Earley B., Sweeney T., and Crowe M. A.. 2006. Effect of carprofen administration during banding or burdizzo castration of bulls on plasma cortisol, in vitro interferon-gamma production, acute-phase proteins, feed intake, and growth. J. Anim. Sci. 84:351–359. doi: 10.2527/2006.842351x [DOI] [PubMed] [Google Scholar]
- Parrott R., Misson B., and Baldwin B.. 1989. Salivary cortisol in pigs following adrenocorticotrophic hormone stimulation: Comparison with plasma levels. Br. Vet. J. 145:362–366. doi: 10.1016/0007-1935(89)90034-1 [DOI] [PubMed] [Google Scholar]
- Petersen H. H., Nielsen J. P., and Heegaard P. M.. 2004. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 35:163–187. doi: 10.1051/vetres:2004002 [DOI] [PubMed] [Google Scholar]
- Repenning P., Ahola J., Callan R., French J., Giles R., Bigler B., Coetzee J., Wulf L., Peel R., Whittier J.,. et al. 2013. Impact of oral meloxicam administration before and after band castration on feedlot performance and behavioral response in weanling beef bulls. J. Anim. Sci. 91:4965–4974. doi: 10.2527/jas.2012-6070 [DOI] [PubMed] [Google Scholar]
- Roberts S., Hughes H., Burdick Sanchez N., Carroll J., Powell J., Hubbell D., and Richeson J.. 2015. Effect of surgical castration with or without oral meloxicam on the acute inflammatory response in yearling beef bulls. J. Anim. Sci. 93:4123–4131. doi: 10.2527/jas.2015-9160 [DOI] [PubMed] [Google Scholar]
- Schwartzkopf-Genswein K., Stookey J., Berg J., Campbell J., Haley D., Pajor E., and McKillop I.. 2012. Code of practice for the care and handling of beef cattle: Review of scientific research on priority issues. National Farm Animal Care Council, Lacombe, AL, Canada. [Google Scholar]
- Schwartzkopf-Genswein K., Stookey J. M., Passillé A. D., and Rushen J.. 1997a. Comparison of hot-iron and freeze branding on cortisol levels and pain sensitivity in beef cattle. Can. J. Anim. Sci. 77:369–374. doi: 10.4141/A96-127 [DOI] [Google Scholar]
- Schwartzkopf-Genswein K. S., Stookey J. M., and Welford R.. 1997b. Behavior of cattle during hot-iron and freeze branding and the effects on subsequent handling ease. J. Anim. Sci. 75:2064–2072. doi: 10.2527/1997.7582064x [DOI] [PubMed] [Google Scholar]
- Smith B. P. 2008. Large animal internal medicine. 4th ed Mosby, St. Louis, MI: p. 265. [Google Scholar]
- Stafford K. J., and Mellor D. J.. 2005. The welfare significance of the castration of cattle: A review. N. Z. Vet. J. 53:271–278. doi: 10.1080/00480169.2005.36560 [DOI] [PubMed] [Google Scholar]
- Sutherland M. A., Ballou M. A., Davis B. L., and Brooks T. A.. 2013. Effect of castration and dehorning singularly or combined on the behavior and physiology of Holstein calves. J. Anim. Sci. 91:935–942. doi: 10.2527/jas.2012-5190 [DOI] [PubMed] [Google Scholar]
- Theurer M. E., White B. J., Coetzee J. F., Edwards L. N., Mosher R. A., and Cull C. A.. 2012. Assessment of behavioral changes associated with oral meloxicam administration at time of dehorning in calves using a remote triangulation device and accelerometers. BMC Vet. Res. 8:48. doi: 10.1186/1746-6148-8-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting S. T., Earley B., Hughes J. M., and Crowe M. A.. 2003. Effect of ketoprofen, lidocaine local anesthesia, and combined xylazine and lidocaine caudal epidural anesthesia during castration of beef cattle on stress responses, immunity, growth, and behavior. J. Anim. Sci. 81:1281–1293. doi: 10.2527/2003.8151281x [DOI] [PubMed] [Google Scholar]
- Tucker C. B., Mintline E. M., Banuelos J., Walker K. A., Hoar B., Varga A., Drake D., and Weary D. M.. 2014. Pain sensitivity and healing of hot-iron cattle brands. J. Anim. Sci. 92:5674–5682. doi: 10.2527/jas.2014-7887 [DOI] [PubMed] [Google Scholar]
- University of British Columbia Animal Welfare Program (UBC AWP) 2013. UBC Animal Welfare Program: SOP-HOBO data loggers. Univ. British Columbia, Vancouver, BC, Canada: p. 1–23. [Google Scholar]
- Uvnäs-Moberg K., Alster P., Petersson M., Sohlström A., and Björkstrand E.. 1998. Postnatal oxytocin injections cause sustained weight gain and increased nociceptive thresholds in male and female rats. Pediatr. Res. 43:344–348. doi: 10.1203/00006450-199803000-00006 [DOI] [PubMed] [Google Scholar]
- Van Engen N. K., Stock M. L., Engelken T., Vann R. C., Wulf L. W., Karriker L. A., Busby W. D., Lakritz J., Carpenter A. J., Bradford B. J.,. et al. 2014. Impact of oral meloxicam on circulating physiological biomarkers of stress and inflammation in beef steers after long-distance transportation. J. Anim. Sci. 92:498–510. doi: 10.2527/jas.2013-6857 [DOI] [PubMed] [Google Scholar]
- Weaver A. D., St Jean G., and Steiner A.. 2008. Castration. In: Bovine surgery and lameness. 2nd ed Blackwell Pub, Oxford, UK: p. 191–197. [Google Scholar]