Abstract
Brown adipocyte lineage commitment and differentiation are under complex regulation. Brown adipocytes are derived from mesenchymal stem cells (MSC). Whether porcine bone marrow-derived MSC (BM-MSC) possess the potential to differentiate into brown adipocytes remains unclear. In the current study, we evaluated the ability of porcine BM-MSC to differentiate into brown adipocytes and browning of differentiated adipocytes. We found that similar to rodent models, bone morphogenetic protein 7 (BMP7) was able to trigger the commitment of BM-MSC to the brown adipocyte lineage by elevating expression of marker genes, nrf-1, tfam, zic1, and pgc-1α (P < 0.05). The expression of brown adipocyte-specific genes, prdm16, dio2, and cidea, was significantly induced (P < 0.05) in BMP7-treated porcine BM-MSC after hormonal induction of adipogenesis. The UCP2 and UCP3 protein levels in BMP7-treated porcine BM-MSC were higher than the control group after hormonal induction of adipogenesis, accompanied by increased mitochondrial DNA copy number and mitochondria-specific gene expression (P < 0.05). Furthermore, acute norepinephrine stimulation potentiated brown adipocyte-specific mRNA expression (P < 0.05) in differentiated adipocytes. Similarly, UCP2 and UCP3 protein levels were increased in differentiated adipocytes upon acute norepinephrine stimulation. In addition, mitochondrial DNA copy number and mitochondria-specific gene expression were also significantly increased (P < 0.05) in differentiated adipocytes after acute norepinephrine exposure. Taken together, these results demonstrate for the first time that porcine BM-MSC are able to commit to the brown adipocyte lineage and differentiate into brown adipocytes. Differentiated adipocytes derived from porcine BM-MSC have the developmental potential to transdifferentiate into brown-like adipocytes upon norepinephrine stimulation.
Keywords: brown adipocytes, browning, mesenchymal stem cells, porcine
INTRODUCTION
White adipose tissue (WAT) and brown adipose tissue (BAT) are specialized organs for energy storage and energy expenditure, respectively. WAT stores excess energy in the form of triacylglycerol and releases free fatty acid and glycerol by lipolysis of stored triacylglycerol when energy is required. In contrast to WAT, BAT generates heat by catabolizing stored triacylglycerol during exposure to cold (Cannon and Nedergaard, 2004). In rodents, white adipocytes and brown adipocytes are both derived from mesenchymal stem cells (MSC; Morganstein et al., 2010, Louveau et al., 2016). Commitment of MSC to the white or brown adipocyte lineage is rigorously regulated by a number of secreted factors, such as bone morphogenetic proteins (BMP; Wang et al., 1993, Tang et al., 2004; Tseng et al., 2008; Huang et al., 2009), fibroblast growth factors (FGF; Fisher et al., 2012), and others (Bordicchia et al., 2012; Boström et al., 2012). It has been demonstrated that mature white adipocytes can transdifferentiate into beige adipocytes (brown-like adipocyte) under cold environmental stimulation or norepinephrine administration (Harms and Seale, 2013, Bartelt and Heeren, 2014). This process is also called browning of white adipocytes.
Currently, evidence about brown adipogenesis is mainly focused on human and rodent models. In the past, histological results showed that BAT was observed in young pigs (Dauncey et al., 1981; Attig et al., 2008). Unlike humans and rodents, the critical marker gene of BAT, uncoupling protein 1 (ucp1), is disrupted and no active protein of ucp1 is detected in pigs (Trayhurn et al., 1989; Berg et al., 2006; Hou et al., 2017). Recently, mutation of the myostatin gene or cold exposure in pigs appears to induce the browning of WAT and the expression of UCP3 protein in the subcutaneous fat was induced (Cai et al., 2017; Lin et al., 2017).
Bone morphogenetic protein 7 (BMP7) plays an important role in the control of murine MSC commitment to the brown adipocyte lineage (Tseng et al., 2008). Administration of β3-adrenergic receptor agonists, such as norepinephrine in mice could trigger the browning of WAT (Cousin et al., 1992; Petrovic et al., 2010). In pigs, previous studies all focused on investigation of the existence of BAT or potential of browning of WAT in vivo. Little is known about whether BMP7 is essential for brown adipocyte differentiation in pigs and whether white adipocytes derived from MSC are able to transdifferentiate into beige adipocytes upon norepinephrine administration.
Therefore, the current studies were designed to examine the potential of brown adipogenesis and browning of white adipocytes in porcine bone marrow-derived MSC (BM-MSC). The results provide valuable information on the regulation of white and brown adipogenesis of MSC in pigs and clarify the existence of brown and beige adipocytes in pigs.
MATERIALS AND METHODS
Cell Culture
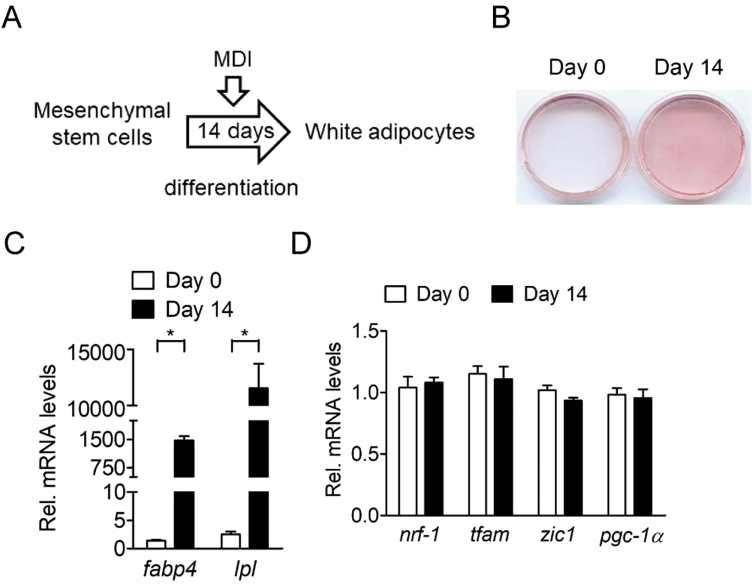
Chemical reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless specified otherwise. The BM-MSC from femur and tibia bones of four 6-mo-old male crossbred pigs (Landrace × Yorkshire × Duroc) were kindly provided by S. C. Wu (National Taiwan University). Porcine BM-MSC were cultured in minimum essential medium (MEM) with 20% FBS (Hyclone, Logan, UT), 2 mM L-glutamine (Invitrogen, Carlsbad, CA), 100 U/mL of penicillin, and 100 µg/mL of streptomycin (Invitrogen) at 37 °C in an atmosphere of 5% CO2. To induce brown preadipocyte commitment, confluent BM-MSC were cultured in growth medium containing 8.3 nM human recombinant BMP7 (Invitrogen) for 3 d (Fig. 1A). For terminal brown adipocyte differentiation, BMP7-treated BM-MSC were induced by MDI treatment (MEM containing 20% FBS, 0.5 mM 3-isobutyl-1-methylxanthine, 5 µM dexamethasone, 20 nM insulin, and 0.125 mM indomethacin) for 2 wk, changing the medium twice per week (Fig. 1A). For induction of browning of differentiated adipocytes, confluent BM-MSC were induced to adipocyte differentiation by MDI treatment for 2 wk, changing the medium twice per week (Fig. 1A). Since these differentiated adipocytes were not stimulated by BMP7, they were defined as white adipocytes in the current study. After adipogenic induction for 2 wk, differentiated adipocytes were treated with MEM containing 1 µM norepinephrine for 6 h. At the end of the experiment, cells on the plates were stained with Oil-Red O reagent to measure the degree of adipocyte differentiation or harvested for extraction of mRNA.
Figure 1.
Adipogenesis of porcine BM-MSC. (A) White adipocyte differentiation, confluent BM-MSC were induced to adipocyte differentiation by MDI treatment (MEM containing 20% FBS, 0.5 mM 3-isobutyl-1-methylxanthine, 5 µM dexamethasone, 20 nM insulin, and 0.125 mM indomethacin) for 2 wk, with medium changes twice per week. (B) Oil-Red O staining of adipocyte differentiation before and after adipogenic induction cocktail (MDI) for 14 d (MDI) in the absence of BMP7 (n = 4). The results shown are representative of an individual experiment. (C) Expression of common adipogenic genes (fabp4 and lpl) in porcine BM-MSC before and after adipogenic induction cocktail for 14 d (MDI) in the absence of BMP7 (n = 4). The bars indicate the means ± SE. (D) Expression of brown adipogenic lineage commitment genes (nrf-1, tfam, zic1, and pgc-1α) in porcine BM-MSC before and after adipogenic induction cocktail for 14 d (MDI) in the absence of BMP7 (n = 4). The bars indicate the means ± SE. *P < 0.05 vs. day 0. BM-MSC = bone marrow-derived mesenchymal stem cell.
Extraction of RNA
Total RNA was extracted from cells using the TRIzol (Invitrogen) according to the manufacturer’s instructions. The quality of the RNA was monitored by examination of the 18S and 28S ribosomal RNA bands after agarose electrophoresis. The RNA was quantified by spectrophotometry at 260 nm and stored at −80 °C.
Quantitative Reverse Transcription-PCR
Total RNA was reverse transcribed into cDNA using Transcriptor Reverse Transcriptase kit (Roche Applied Science, Indianapolis, IN). Quantitative reverse transcriptase-PCR was performed using MiniopticonTM Real-Time PCR Detection System (Bio-Rad, Hercules, CA) and KAPA SYBR FAST qPCR Kit (Kapa Biosystems, Inc., Boston, MA). PCR was performed with 40 cycles at 95 °C for 30 s, 58–60 °C for 60 s, and 72 °C for 30 s. β-actin was used as the internal control gene. The sequence of primers for quantitative reverse transcription-PCR is listed in Table 1. The mRNA expression of each gene was normalized to its β-actin in the same sample. Threshold cycle (Ct) values were obtained and relative gene expression was calculated using the formula (1/2)Ct target genes–Ct β-actin.
Table 1.
Primer sequences for quantitative PCR
| Gene | GenBank accession number | Sequence | |
|---|---|---|---|
| nrf-1 | XM_021079000 | Forward | 5′-GTGGCCACCTACACTGAACA-3′ |
| Reverse | 5′-CCAGATGGGCTGTTACCTCA-3′ | ||
| tfam | XM_001928552 | Forward | 5′-TCATCCACCCTGAGTGGTTTT-3′ |
| Reverse | 5′-GAAGTTCCCTCCACAGCTCAG-3′ | ||
| zic1 | XM_003358599 | Forward | 5′-CAACACAGTCTGTTCGCTGC-3′ |
| Reverse | 5′-TGCCCATTAACCACGTTGGG-3′ | ||
| pgc-1α | AH013165 | Forward | 5′-GTCTGCGGCTATTTGGTGAC-3′ |
| Reverse | 5′-CAGAGGCGGCATCTTTAGAGT-3′ | ||
| fabp4 | HM453202 | Forward | 5′-ACCATAACCTTAGATGGAGGCG-3′ |
| Reverse | 5′-AATTCTGGTAGCCGTGACACC-3′ | ||
| lpl | NM_214286 | Forward | 5′-GCCCTGGCTTTGCTATTGAGAA-3′ |
| Reverse | 5′-TTCACAAACACCACAGAGGACT-3′ | ||
| bmpr1a | XM_005671180 | Forward | 5′-GGGTAAATGGCGTGGTGAGA-3′ |
| Reverse | 5′-CCAAGTATGTTTTCATGGCGCA-3′ | ||
| bmpr1b | XM_021100429 | Forward | 5′-AGCTTCCCTACCATGACCTG-3′ |
| Reverse | 5′-TCTGCCTTAGACACTCGTCG-3′ | ||
| bmpr2 | XM_003133596 | Forward | 5′-ATCCCCTGAGCAGTACCAGT-3′ |
| Reverse | 5′-CGGGTGGGACATCAGGAATTA-3′ | ||
| prdm16 | XM_021095209 | Forward | 5′-TACACGTGCAGGTACTGTGG-3′ |
| Reverse | 5′-GAGGTGTCTGTCCAGGTTGG-3′ | ||
| dio2 | NM_001001626 | Forward | 5′-GGGCAGTACCTGGGGATTCT-3′ |
| Reverse | 5′-CCCCATAAGCTACGTTGGCA-3′ | ||
| cidea | NM_001112696 | Forward | 5′-GTTATCGCCAGCAGAGTGGT-3′ |
| Reverse | 5′-CCGGTGTCCACTTTTGTCCT-3′ | ||
| ucp2 | NM_214289 | Forward | 5′-ATGTGTGAGACCTGACGAAGC-3′ |
| Reverse | 5′-CCTTTCTCCCTGGATCTGC-3′ | ||
| ucp3 | NM_214049 | Forward | 5′-CAACAGGAAGTACAGCGGGA-3′ |
| Reverse | 5′-CACCATCTCGGCACAGTTCA -3′ | ||
| cox7a1 | NM_214411 | Forward | 5′-CTGGTCCGCTCCTTTAGCTC-3′ |
| Reverse | 5′-GGATGTTGTCAGTTGCACCG-3′ | ||
| cox8b | NM_001097500 | Forward | 5′-CACACATCTATGCCAAGCCAG-3′ |
| Reverse | 5′-CTTGTAGTGATCCAGGTGGGA-3′ | ||
| cyc | NM_001129970 | Forward | 5′-CAAACACAAGACTGGTCCAAACC-3′ |
| Reverse | 5′-GATGCCTTTGTTCTTGTTGGCATC-3′ | ||
| β-actin | XM_021086047 | Forward | 5′-GCCAGGTCATCACCATCGG-3′ |
| Reverse | 5′-GTAGAGGTCCTTGCGGATGTC-3′ | ||
| cox-2 | MG009446 | Forward | 5′-GGCTTACCCTTTCCAACTAGG-3′ |
| Reverse | 5′-AGGTGTGATCGTGAAAGTGTAG-3′ | ||
| β-globin | X86791 | Forward | 5′-GGGGTGAAAAGAGCGCAAG-3′ |
| Reverse | 5′-CAGGTTGGTATCCAGGGCTTCA-3′ | ||
Western Blot
Total protein from porcine cells extracted by radioimmunoprecipitation assay buffer with protease and phosphatase inhibitors. The sample was centrifuged at 12,000 rpm for 10 min and supernatant was subjected to western blot. Protein lysate (20 µg) was separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transblotted onto a polyvinylidine fluoride membrane (Perkin Elmer, Norwalk, CT). The UCP2 (ab97931) and UCP3 (ab10985) primary antibodies were purchased from Abcam (Cambridge, MA). β-actin antibody (GTX109639, GeneTex, Irvine, CA) was used for the loading control in the lysates of total protein. The secondary antibody coupled to horseradish peroxidase was used in the chemiluminescence procedure (Immobilon Western, EMD Millipore, Danvers, MA). The western blotting procedure was performed according to the manufacturer’s instruction.
Measurement of Mitochondrial DNA Copy Number
DNA from cells was extracted using phenol/chloroform extraction. Quantitative PCR was performed using MiniopticonTM Real-Time PCR Detection System (Bio-Rad) and KAPA SYBR FAST qPCR Kit (Kapa Biosystems, Inc.). The relative mitochondrial DNA copy number was calculated as a ratio of the abundances of mitochondrial cyclooxygenase-2 (COX-2)/nuclear β-globin. The sequence of primers for measurement of mitochondrial DNA copy number is listed in Table 1.
Measurement of Triglyceride and Glycerol Levels
Triglyceride was extracted from cells using organic solvents and determined using a colorimetric kit (BioVision, Milpitas, CA). Glycerol from culture media was harvested and determined using a colorimetric kit (Cayman Chemical, Ann Arbor, MI).
Statistical Analysis
Results are expressed as means ± SE. All experiments were performed at least three times. Comparisons between two groups were performed using the unpaired t-test. A P value less than 0.05 was considered statistically significant.
RESULTS
BMP7 Potentiates Brown Preadipocyte Commitment of Porcine Bone Marrow-Derived Mesenchymal Stem Cells
It has been demonstrated that brown adipocytes are derived from MSC (Morganstein et al., 2010; Louveau et al., 2016). During brown adipocyte differentiation, BMP7 was able to trigger the commitment of MSC to the brown adipocyte lineage through BMP receptors and activated a full program of brown adipocyte differentiation upon exposure to adipogenic signals (Tseng et al., 2008). As expected, considerable adipocyte differentiation was observed in porcine BM-MSC after adipogenic induction (Fig. 1B), accompanied by increased common adipogenic marker gene expressions, such as fabp4 and lpl (P < 0.05; Fig. 1C). However, the marker gene expression for brown adipogenic lineage commitment was not increased upon hormonal induction of adipogenesis (Fig. 1D). These results demonstrated that brown adipogenesis was not spontaneously induced in porcine BM-MSC in the absence of BMP7 and this model is suitable to examine the potential of brown adipogenesis and browning of white adipocytes in pigs.
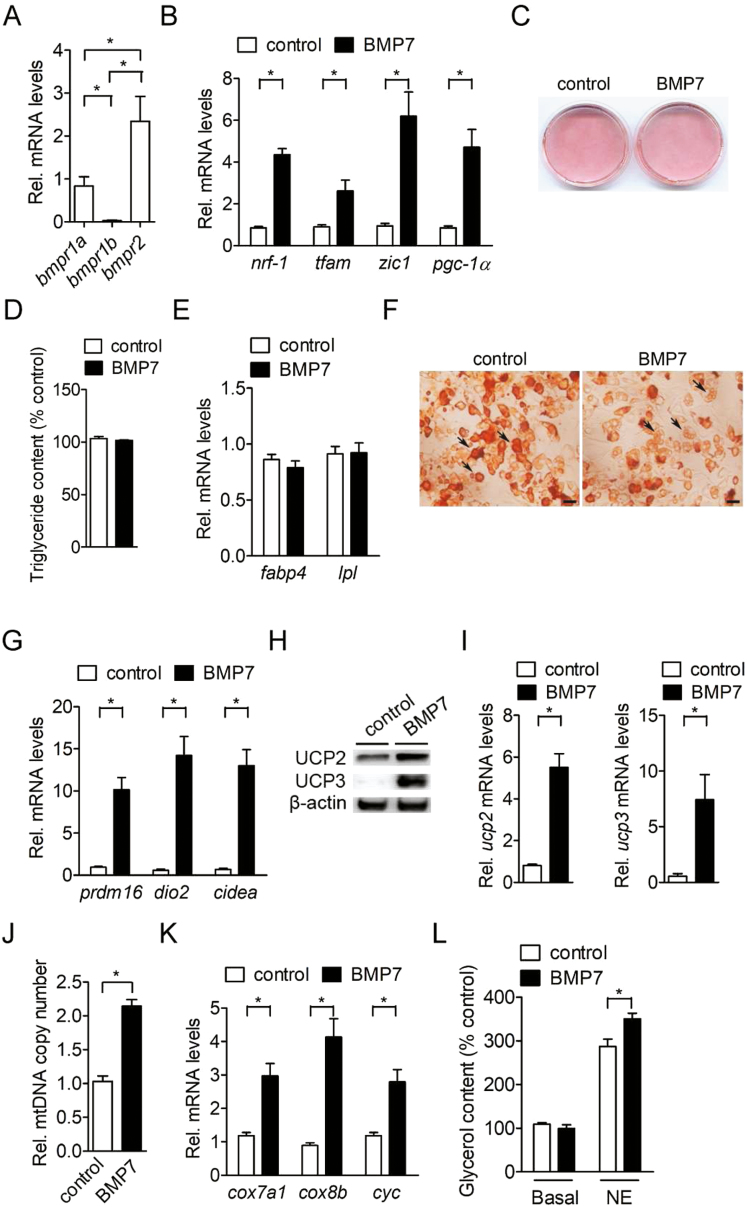
We then investigated whether BMP7 could induce the commitment of BM-MSC into the brown adipocyte lineage. We found that there was differential expression of BMP receptors in porcine BM-MSC (Fig. 2A). The mRNA of bmpr2 was the most highly expressed isoform in porcine BM-MSC (P < 0.05; Fig. 2A). The second most highly expressed isoform of BMP receptors in porcine BM-MSC was bmpr1a (P < 0.05; Fig. 2A). The mRNA of bmpr1b was the least highly expressed among the BMP receptors in porcine BM-MSC (P < 0.05) (Fig. 2A). Furthermore, expression of marker genes for brown adipogenic lineage commitment of porcine BM-MSC, including nrf-1, tfam, zic1, and pgc-1α were significantly induced (P < 0.05) after exposure to BMP7 (Fig. 2B). After adipogenic induction, BMP7 did not further promote adipocyte differentiation (Fig. 2C), intracellular triglyceride levels (Fig. 2D) and induce common adipocyte differentiation gene expression compared with control cells (Fig. 2E). However, the intracellular multilocular lipid droplets were abundant after treatment of brown adipogenic induction compared with unilocular lipid droplets in control group (Fig. 2F). The mRNA level of brown adipocyte-specific genes, including prdm16, dio2, and cidea, was also significantly elevated (P < 0.05) in porcine BM-MSC after adipogenic induction (Fig. 2G). Since uncoupling proteins have been considered to be important markers for brown adipocytes. We then examined the protein level of uncoupling proteins, including UCP2 and UCP3 in BMP7-treated porcine BM-MSC. After adipogenic induction, the UCP2 and UCP3 protein expression were remarkably increased in BMP7-treated porcine BM-MSC compared with adipogenic induction alone (Fig. 2H). Consistently, the mRNA level of uncoupling proteins, including ucp2 and ucp3 in BMP7-treated porcine BM-MSC were increased (P < 0.05) after adipogenic induction (Fig. 2I). Furthermore, the mitochondrial DNA copy number and mitochondria-specific gene expression, including cox7a1, cox8b, and cyc were significantly elevated (P < 0.05) in BMP7-treated porcine BM-MSC compared to control cells (Fig. 2J and K). Norepinephrine-stimulated lipolysis, as indicated by glycerol release, was also increased in BMP7-treated porcine BM-MSC compared to control cells (Fig. 2L). These results indicated that porcine BM-MSC possess the developmental potential to differentiate into brown adipocytes in response to BMP7 stimulation.
Figure 2.
BMP7 promotes brown preadipocyte commitment and terminal differentiation of porcine BM-MSC. (A) Expression of bone morphogenetic protein receptor genes (bmpr1a, bmpr1b, and bmpr2) in confluent porcine BM-MSC (n = 4). The bars indicate the means ± SE. (B) Expression of brown adipogenic lineage commitment genes (nrf-1, tfam, zic1, and pgc-1α) in porcine BM-MSC treated with BMP-7 for 3 d (n = 4). The bars indicate the means ± SE. (C) Oil-Red O staining of porcine BM-MSC after stimulation of differentiation by a brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 4). The results shown are representative of an individual experiment. (D) Quantification of intracellular triglyceride levels in porcine BM-MSC treated with brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 4). (E) Expression of common adipogenic genes (fabp4 and lpl) in porcine BM-MSC treated with brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 4). The bars indicate the means ± SE. (F) Microscographs of Oil-Red O staining of porcine BM-MSC treated with brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 4). Magnification was 60×. Bars indicate a length of 100 µm. Black arrow in control group indicates unilocular lipid droplets. Black arrow in BMP7-treated group indicates multilocular lipid droplets. The results shown are representative of an individual experiment. (G) Expression of brown fat-specific genes (prdm16, dio2, and cidea) in porcine BM-MSC treated with brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 4). The bars indicate the means ± SE. (H) Expression of UCP proteins (UCP2 and UCP3) in porcine BM-MSC treated with brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 3). The results shown are representative of an individual experiment. (I) Expression of UCP gene (ucp2 and ucp3) mRNA in porcine BM-MSC treated with brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 4). The bars indicate the means ± SE. (J) Measurement of mitochondrial DNA copy number in porcine BM-MSC treated with brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 4). The bars indicate the means ± SE. (K) Expression of mitochondria-specific genes (cox7a1, cox8b, and cyc) in porcine BM-MSC treated with brown adipogenic induction cocktail for 14 d (BMP7 + MDI) (n = 4). (L) The extracellular glycerol levels in brown adipogenic induction cocktail (BMP7)-treated porcine BM-MSC in response to 1 µM norepinephrine (NE) treatment for 6 h (n = 4). The bars indicate the means ± SE. (F) *P < 0.05 vs. control. BM-MSC = bone marrow-derived mesenchymal stem cell.
Norepinephrine Promotes Brown Adipocyte-Like Characteristics in Porcine-Differentiated Adipocytes
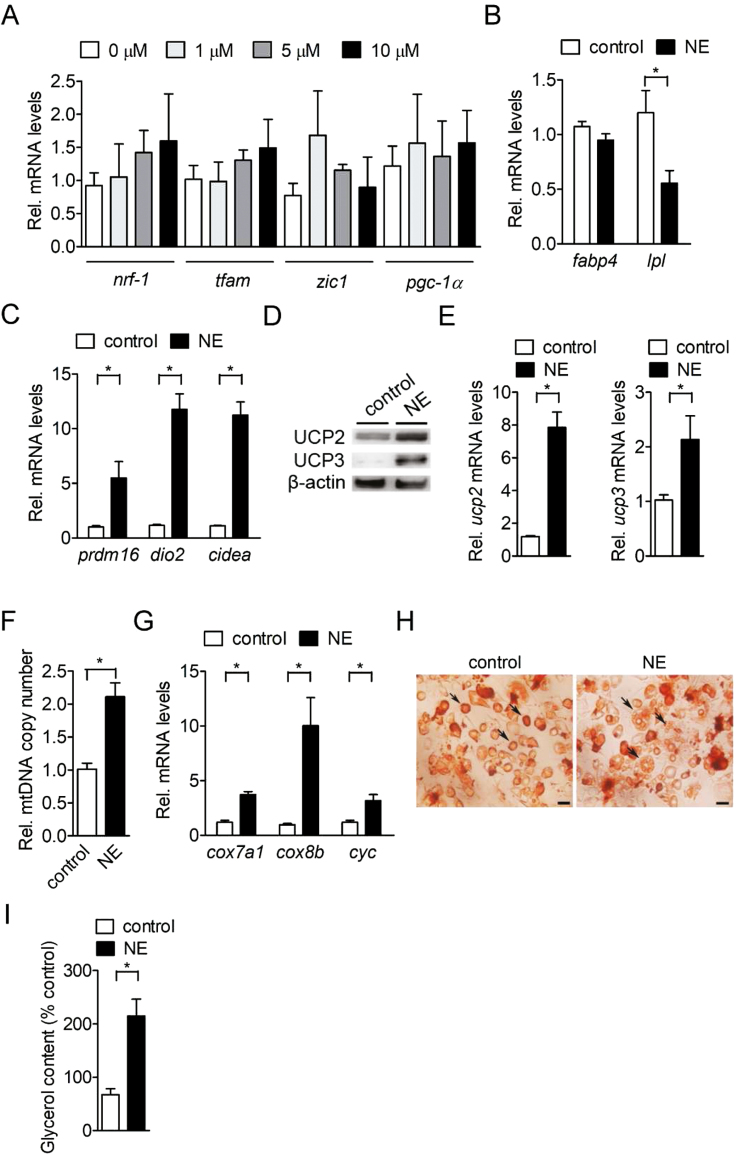
It has been reported that norepinephrine is able to promote the browning of white adipocytes in human and rodent models. Therefore, we next investigated whether norepinephrine also could induce the browning of white adipocytes in a porcine model. Our results showed that no dosage effect of norepinephrine was observed on marker gene expression for brown adipogenic lineage commitment in the porcine BM-MSC before adipogenic induction, indicating that norepinephrine did not play a role in brown adipocyte lineage commitment of porcine BM-MSC (Fig. 3A). The fabp4 mRNA level was not altered in differentiated adipocytes after acute norepinephrine stimulation, while lpl mRNA level in differentiated adipocytes was significantly reduced (P < 0.05; Fig. 3B). However, the brown adipocyte-specific genes were increased (P < 0.05) in differentiated adipocytes in response to acute norepinephrine stimulation (Fig. 3C). Importantly, acute norepinephrine significantly induced the UCP2 and UCP3 protein expression in differentiated adipocytes compared with control cells (Fig. 3D). The mRNA level of uncoupling proteins, including ucp2 and ucp3 in norepinephrine-treated differentiated adipocytes were also increased (P < 0.05; Fig. 3E). Furthermore, acute norepinephrine stimulation in induced adipogenesis of porcine BM-MSC also elevated mitochondrial DNA copy number and mitochondria-specific gene expression compared with control cells (P < 0.05) (Fig. 3F and G). The intracellular multilocular lipid droplets were increased compared with control group after acute norepinephrine stimulation (Fig. 3H), which was accompanied by an increase in extracellular glycerol levels (Fig. 3I). Taken together, these findings demonstrated that porcine adipocytes derived from BM-MSC were able to trigger the white-to-brown conversion through acute norepinephrine stimulation.
Figure 3.
Norepinephrine stimulation induces brown adipocyte-like characteristics in porcine-differentiated adipocytes. (A) Expression of brown adipogenic lineage commitment genes (nrf-1, tfam, zic1, and pgc-1α) in confluent porcine BM-MSC treated with different concentration (0–10 µM) of norepinephrine for 6 h (n = 4). The bars indicate the means ± SE. (B) Expression of common adipogenic genes (fabp4 and lpl) in differentiated adipocytes treated with 1 µM norepinephrine for 6 h (n = 4). The bars indicate the means ± SE. (C) Expression of brown fat-specific genes (prdm16, dio2, and cidea) in differentiated adipocytes treated with 1 µM norepinephrine for 6 h (n = 4). The bars indicate the means ± SE. (D) Expression of UCP proteins (UCP2 and UCP3) in differentiated adipocytes treated with 1 µM norepinephrine for 24 h (n = 3). The results shown are representative of an individual experiment. (E) Expression of UCP gene (ucp2 and ucp3) mRNA in differentiated adipocytes treated with 1 µM norepinephrine for 6 h (n = 4). The bars indicate the means ± SE. (F) Measurement of mitochondrial DNA copy number in differentiated adipocytes treated with 1 µM norepinephrine for 24 h (n = 4). The bars indicate the means ± SE. (G) Expression of mitochondria-specific genes (cox7a1, cox8b, and cyc) in differentiated adipocytes treated with 1 µM norepinephrine for 6 h (n = 4). (H) Microscographs of Oil-Red O staining in differentiated adipocytes treated with 1 µM norepinephrine (NE) for 24 h (n = 4). The results shown are representative of an individual experiment. Magnification was 60×. Bars indicate a length of 100 µm. Black arrow in control group indicates unilocular lipid droplets. Black arrow in NE-treated group indicates multilocular lipid droplets. (I) Measurement of extracellular glycerol levels in differentiated adipocytes treated with 1 µM norepinephrine for 24 h (n = 4). The bars indicate the means ± SE. *P < 0.05 vs. control. BM-MSC = bone marrow-derived mesenchymal stem cell.
DISCUSSION
In this study, we demonstrated for the first time that BMP7 could induce the expression of brown progenitor-specific genes in porcine BM-MSC. Further, the brown adipocyte marker gene mRNA, UCP2 and UCP3 protein expression were increased in BMP7-treated BM-MSC after exposure to adipogenic induction medium. Adipocytes derived from porcine BM-MSC showed brown adipocyte-like mRNA, UCP2 and UCP3 protein expression in response to acute norepinephrine stimulation.
BAT controls temperature homeostasis through heat generation by burning stored triacylglycerol under cold-acclimated conditions (Cannon and Nedergaard, 2004). Brown adipocyte differentiation elevates the expression of mitochondrial fatty acid oxidation genes coincident with an increase in the number of mitochondria (Harms and Seale, 2013). In rodents, it has been demonstrated that BMP7 signaling is required for BAT development (Tseng et al., 2008). Brown adipocytes are derived from MSC and the commitment of MSC to the brown adipocyte lineage is triggered by exposure to BMP7 for 3 d (Tseng et al., 2008, Morganstein et al., 2010). Our results are consistent with previous study (Tseng et al., 2008), in which BMP7 stimulation in MSC increased the marker gene expression for brown adipogenic lineage commitment. We found that treating porcine BM-MSC with BMP7 for 3 d also induced the marker gene expression for brown adipogenic lineage commitment, indicating that porcine BM-MSC were able to commit to the brown adipocyte lineage. Furthermore, based on our gene and UCP protein expression results, we demonstrated that brown adipocyte-specific gene expression is increased in BMP7-treated porcine BM-MSC after adipogenic induction. Present findings are also in line with a previous study performed by Tseng et al. (2008). These results demonstrate that porcine BM-MSC have the potential to undergo brown adipocyte lineage commitment and terminal differentiation under BMP7 stimulation. In rodents, it has been reported that BMP7 stimulates BAT growth and function in vivo (Tseng et al., 2008; Boon et al., 2013). Whether BMP7 could also mediate the recruitment and activation of BAT in pigs remains to be further investigated.
It has been demonstrated that the marker gene of BAT, uncoupling protein 1 (ucp1), is disrupted and not active UCP1 protein is detected in pigs (Berg et al., 2006, Hou et al., 2017). In the present study, the UCP2 and UCP3 protein expression was alternatively increased after brown adipogenic induction or acute norepinephrine stimulation. In rodents, it has been demonstrated UCP2-deficient mice exhibit a whitening phenotype in BAT and impaired thermogenesis in response to cold exposure (Caron et al., 2017). Recent studies have suggested that myostatin-deficiency or cold exposure in pigs results in the browning of WAT and the expression of UCP3 protein in the subcutaneous fat was induced (Cai et al., 2017; Lin et al., 2017). Taken together, these results demonstrate that UCP2 and UCP3 protein may be a substitute under brown adipogenic induction or cold exposure in the absence of UCP1 in pigs. Whether UCP2 and UCP3 proteins contribute to uncoupling activities for heat production and promote oxygen consumption in porcine brown and beige adipocytes derived from MSC remain to be further clarified.
The browning of WAT is regulated by a complex hormonal and environmental interaction accompanied by increased multilocular lipid droplet morphology, high mitochondrial content, and the expression of a core set of brown fat-specific genes (Harms and Seale, 2013; Bartelt and Heeren, 2014). Several hormones and compounds are identified as inducers of browning, such as β3-adrenoceptor agonist and peroxisome proliferator-activated receptor γ agonist (Bonet et al., 2013). It has been reported that acute norepinephrine stimulation of the murine white adipocytes induces the pgc-1α and ucp1 mRNA expression (Petrovic et al., 2010). Here, we also found that treating porcine-differentiated adipocytes with norepinephrine was able to augment the expression of brown adipocyte-specific gene mRNA and UCP protein in porcine adipocytes. The increased mitochondrial biogenesis is also a typical phenotype of browning of WAT in rodents (Harms and Seale, 2013). In the present study, we consistently found that mitochondrial DNA copy number and mitochondria-specific genes were elevated in differentiated adipocytes after acute norepinephrine stimulation. In addition, thiazolidinediones (TZD), peroxisome proliferator-activated receptor γ agonists, trigger the browning process by elevating prdm16 and pgc-1α mRNA levels in white adipocytes of mice (Ohno et al., 2012). Dietary TZD supplementation significantly increased pgc-1α mRNA levels in adipocytes of skeletal muscle tissue and improved growth performance in pigs (Chen et al., 2013). Recently, myostatin-deficiency or cold exposure in pigs was able to induce considerable browning of WAT (Cai et al., 2017; Lin et al., 2017). Reconstitution of murine ucp1 gene in the WAT of pigs induces the browning and improves the thermogenic capacity (Zheng et al., 2017). These results indicate that the potential of browning in pigs might be similar to that in rodents although there are clear metabolic and physiological differences between rodents and pigs. Whether beige cells from porcine MSC possess identical physiological functions remains to be further determined.
It has been demonstrated that white adipocytes and brown adipocytes are both derived from MSC (Morganstein et al., 2010). Specifically, brown adipocyte precursors derive from a Myf5-positive lineage, whereas white adipocyte precursors derive from Myf5-negative lineage (Tews and Wabitsch, 2011; Brestoff and Artis, 2015). The Myf5-negative white adipocytes are able to transdifferentiate into beige adipocytes upon acute NE stimulation (Harms and Seale, 2013; Brestoff and Artis, 2015). In the present study, porcine BM-MSC may contain Myf5-positive and Myf5-negative lineage subpopulation. Since our results show that markers of brown fat formation are increased after 6 h of NE treatment to levels equivalent to 14 d of brown adipocyte differentiation. The findings imply that NE is a potent inducer at initiating the brown adipogenic program than BMP7. In addition, BMP7 may predominantly induce the brown adipogenesis of Myf5-positive porcine BM-MSC, while acute NE treatment triggers the conversion of Myf5-negative white adipocytes into beige adipocytes. However, the percentage of Myf5-positive and Myf5-negative cells in porcine BM-MSC remains to be investigated in future studies.
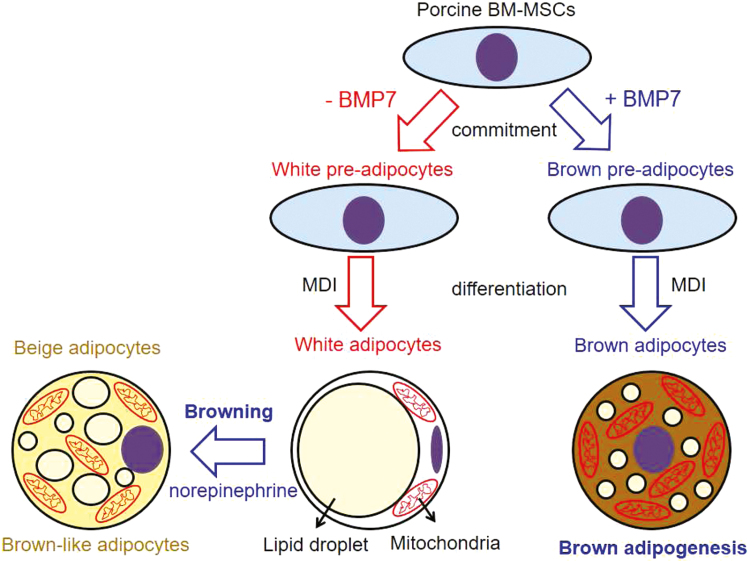
In conclusion, as shown in Fig. 4, we provide evidence that porcine BM-MSC have the potential to differentiate into brown adipocytes under BMP7 stimulation. Differentiated adipocytes derived from porcine BM-MSC could induce a browning program by acute norepinephrine. These findings provide a novel model for examining the brown adipogenic potential in BM-MSC in response to potent feed additives for reducing body fat deposition in pigs.
Figure 4.
Scheme of brown adipogenesis of porcine BM-MSC and browning of white adipocytes. BM-MSC = bone marrow-derived mesenchymal stem cell.
ACKNOWLEDGMENTS
We thank Dr Shinn-Chih Wu (National Taiwan University) for providing porcine mesenchymal stem cells. We thank Dr Shao-Yu Peng (National Pingtung University of Science and Technology) and Dr Felix Shih-Hsiang Hsiao (Tunghai University) for their technical support.
Footnotes
This work was supported by the Ministry of Science and Technology (MOST 105-2313-B-197-002) in Taiwan.
LITERATURE CITED
- Attig L., Djiane J., Gertler A., Rampin O., Larcher T., Boukthir S., Anton P. M., Madec J. Y., Gourdou I., and Abdennebi-Najar L.. 2008. Study of hypothalamic leptin receptor expression in low-birth-weight piglets and effects of leptin supplementation on neonatal growth and development. Am. J. Physiol. Endocrinol. Metab. 295:E1117–E1125. doi: 10.1152/ajpendo.90542.2008 [DOI] [PubMed] [Google Scholar]
- Bartelt A., and Heeren J.. 2014. Adipose tissue browning and metabolic health. Nat. Rev. Endocrinol. 10:24–36. doi: 10.1038/nrendo.2013.204 [DOI] [PubMed] [Google Scholar]
- Berg F., Gustafson U., and Andersson L.. 2006. The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. Plos Genet. 2:e129. doi: 10.1371/journal.pgen.0020129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonet M. L., Oliver P., and Palou A.. 2013. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta. 1831:969–985. doi: 10.1016/j.bbalip.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Boon M. R., van den Berg S. A., Wang Y., van den Bossche J., Karkampouna S., Bauwens M., De Saint-Hubert M., van der Horst G., Vukicevic S., de Winther M. P., et al. 2013. BMP7 activates brown adipose tissue and reduces diet-induced obesity only at subthermoneutrality. Plos One. 8:e74083. doi: 10.1371/journal.pone.0074083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordicchia M., Liu D., Amri E. Z., Ailhaud G., Dessì-Fulgheri P., Zhang C., Takahashi N., Sarzani R. Collins S., 2012. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest. 122:1022–1036. doi: 10.1172/JCI59701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boström P., Wu J., Jedrychowski M. P., Korde A., Ye L., Lo J. C., Rasbach K. A., Boström E. A., Choi J. H., Long J. Z., et al. 2012. A PCG1α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 481:463–468. doi: 10.1038/nature10777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brestoff J. R., and Artis D.. 2015. Immune regulation of metabolic homeostasis in health and disease. Cell. 161:146–160. doi: 10.1016/j.cell.2015.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai C., Qian L., Jiang S., Sun Y., Wang Q., Ma D., Xiao G., Li B., Xie S., Gao T., et al. 2017. Loss-of-function myostatin mutation increases insulin sensitivity and browning of white fat in Meishan pigs. Oncotarget. 8:34911–34922. doi: 10.18632/oncotarget.16822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon B., and Nedergaard J.. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84:277–359. doi: 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- Caron A., Labbé S. M., Carter S., Roy M. C., Lecomte R., Ricquier D., Picard F., and Richard D.. 2017. Loss of UCP2 impairs cold-induced non-shivering thermogenesis by promoting a shift toward glucose utilization in brown adipose tissue. Biochimie. 134:118–126. doi: 10.1016/j.biochi.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Chen X., Feng Y., Yang W. J., Shu G., Jiang Q. Y., and Wang X. Q.. 2013. Effects of dietary thiazolidinedione supplementation on growth performance, intramuscular fat and related genes mRNA abundance in the longissimus dorsi muscle of finishing pigs. Asian-Australas. J. Anim. Sci. 26:1012–1020. doi: 10.5713/ajas.2012.12722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin B., Cinti S., Morroni M., Raimbault S., Ricquier D., Pénicaud L., and Casteilla L.. 1992. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J. Cell Sci. 103:931–942. [DOI] [PubMed] [Google Scholar]
- Dauncey M. J., Wooding F. B., and Ingram D. L.. 1981. Evidence for the presence of brown adipose tissue in the pig. Res. Vet. Sci. 31:76–81. [PubMed] [Google Scholar]
- Fisher F. M., Kleiner S., Douris N., Fox E. C., Mepani R. J., Verdeguer F., Wu J., Kharitonenkov A., Flier J. S., Maratos-Flier E., et al. 2012. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26:271–281. doi: 10.1101/gad.177857.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms M., and Seale P.. 2013. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19:1252–1263. doi: 10.1038/nm.3361 [DOI] [PubMed] [Google Scholar]
- Hou L., Shi J., Cao L., Xu G., Hu C., and Wang C.. 2017. Pig has no uncoupling protein 1. Biochem. Biophys. Res. Commun. 487:795–800. doi: 10.1016/j.bbrc.2017.04.118 [DOI] [PubMed] [Google Scholar]
- Huang H., Song T. J., Li X., Hu L., He Q., Liu M., Lane M. D., and Tang Q. Q.. 2009. BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. U. S. A. 106:12670–12675. doi: 10.1073/pnas.0906266106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Qian L., Lin J., Huang G., Hao N., Wei X., Wang W., and Liang J.. 2017. CD44 regulates prostate cancer proliferation, invasion and migration via PDK1 and PFKFB4. Oncotarget. 8:65143–65151. doi: 10.18632/oncotarget.17821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Cao C., Tao C., Ye R., Dong M., Zheng Q., Wang C., Jiang X., Qin G., Yan C., et al. 2017. Cold adaptation in pigs depends on UCP3 in beige adipocytes. J. Mol. Cell Biol. 9:364–375. doi: 10.1093/jmcb/mjx018 [DOI] [PubMed] [Google Scholar]
- Louveau I., Perruchot M. H., Bonnet M., and Gondret F.. 2016. Invited review: pre- and postnatal adipose tissue development in farm animals: from stem cells to adipocyte physiology. Animal. 10:1839–1847. doi: 10.1017/S1751731116000872 [DOI] [PubMed] [Google Scholar]
- Morganstein D. L., Wu P., Mane M. R., Fisk N. M., White R., and Parker M. G.. 2010. Human fetal mesenchymal stem cells differentiate into brown and white adipocytes: a role for ERRalpha in human UCP1 expression. Cell Res. 20:434–444. doi: 10.1038/cr.2010.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Shinoda K., Spiegelman B. M., and Kajimura S.. 2012. Pparγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15:395–404. doi: 10.1016/j.cmet.2012.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic N., Walden T. B., Shabalina I. G., Timmons J. A., Cannon B., and Nedergaard J.. 2010. Chronic peroxisome proliferator-activated receptor gamma (PPArgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285:7153–7164. doi: 10.1074/jbc.M109.053942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q. Q., Otto T. C., and Lane M. D.. 2004. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. U. S. A. 101:9607–9611. doi: 10.1073/pnas.0403100101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tews D., and Wabitsch M.. 2011. Renaissance of brown adipose tissue. Horm. Res. Paediatr. 75:231–239. doi: 10.1159/000324806 [DOI] [PubMed] [Google Scholar]
- Trayhurn P., Temple N. J., and Van Aerde J.. 1989. Evidence from immunoblotting studies on uncoupling protein that brown adipose tissue is not present in the domestic pig. Can. J. Physiol. Pharmacol. 67:1480–1485. [DOI] [PubMed] [Google Scholar]
- Tseng Y. H., Kokkotou E., Schulz T. J., Huang T. L., Winnay J. N., Taniguchi C. M., Tran T. T., Suzuki R., Espinoza D. O., Yamamoto Y., et al. 2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 454:1000–1004. doi: 10.1038/nature07221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. A., Israel D. I., Kelly S., and Luxenberg D. P.. 1993. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 9:57–71. [DOI] [PubMed] [Google Scholar]
- Zheng Q., Lin J., Huang J., Zhang H., Zhang R., Zhang X., Cao C., Hambly C., Qin G., Yao J., et al. 2017. Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity. Proc. Natl. Acad. Sci. U. S. A. 114:E9474–E9482. doi: 10.1073/pnas.1707853114 [DOI] [PMC free article] [PubMed] [Google Scholar]