Abstract
Two consecutive trials were carried out to study the effects of dietary CP and adding gallic acid (GA) in basal rations on nitrogen (N) metabolism and nitrous oxide (N2O) emissions from the urine of beef cattle. In Trial I, eight Simmental castrated male cattle with initial liveweight of 310.5 ± 21.5 kg were used as experimental animals. Two levels of dietary CP (113.5 and 150.8 g/kg DM) and two levels of GA (0.0 and 15.2 g/kg DM) were used as experimental treatments in a 2 × 2 reversal design. Two cattle received each treatment in each of two experimental periods. Each experimental period lasted 19 d, of which the first 14 d were for adaptation and the last 5 d were for sampling. In Trial II, the urine samples collected from Trial I were used for measuring N2O-N emissions using static incubation technique. Glass jars containing soil were used as the incubation vessels. Three jars were used for each of the urine samples as replicates and two jars without urine samples were used as blanks. The incubation lasted 15 d, and the daily N2O-N emission from each jar was determined using gas chromatography. The results showed that no effects of interactions were found between dietary CP and GA on the N metabolism of beef cattle and the estimated cattle N2O-N emissions (P > 0.05). Increasing dietary CP from 113.5 to 150.8 g/kg DM increased the excretions of total N, urinary N, and urea (P < 0.001), whereas adding GA at 15.2 g/kg DM in ration did not affect these parameters (P > 0.05). Increasing dietary CP from 113.5 to 150.8 g/kg DM increased the estimated cattle urine N2O-N emissions by 36.8% (without adding GA) and 32.3% (adding GA at 15.2 g/kg DM) (P < 0.01), whereas adding GA at 15.2 g/kg DM in ration decreased the estimated cattle urine N2O-N emissions by 28.5% (dietary CP 113.5 g/kg DM) and 30.9% (dietary CP 150.8 g/kg DM) (P < 0.01). The inhibiting effects of GA on decreasing the N2O-N emissions of urine could have been resulted from the effects of GA metabolites including pyrogallol and resorcinol excreted in urine. Feeding cattle with relatively low dietary CP or adding GA in ration is effective to decrease the N2O-N emissions from the urine patches of beef cattle applied to soil.
Keywords: cattle, dietary crude protein, gallic acid, nitrous oxide, urine
INTRODUCTION
Nitrous oxide (N2O) is a potent greenhouse gas with a global warming potential of 265 times of carbon dioxide (CO2) (IPCC, 2013). N2O is also the major factor depleting the ozone sphere of the earth (Ravishankara et al., 2009). Total N2O emissions from global agriculture are estimated to be 4.1 Tg N2O-N/yr and contribute for about 80% of the total anthropogenic emissions of N2O (IPCC, 2013). Hence, it becomes more and more important to mitigate N2O emissions from animal production to protect the environment of the earth.
N2O from the excreta of livestock is mainly produced in the processes of hydrolysis, mineralization, nitrification, and denitrification of the nitrogenous compounds (Wrage et al., 2001). Although no strong relationships were found between the N2O emission and the total nitrogen (N) in the manure of cattle in feedlots (Redding et al., 2015), increasing the N deposition in soil increased the soil N2O emissions (Wang et al., 2014). Hence, decreasing the N excretion from grazing cattle would reduce the N2O emissions from pasture soil.
Cattle are large domestic animals with high intakes of feed and water as well as large output of excreta. The N utilization efficiency of cattle is low and the N excretion is high compared with other species of livestock. It was reported that the ratios of total N excretion/N intake were found to be 83.3% (Hirooka et al., 2007) for Holstein steers and 81.0% to 84.1% for finishing beef heifers (Li et al., 2014), respectively. Although the total N excretion is positively correlated with the dietary CP level in beef cattle (Nennich et al., 2005), the N content of the feces of beef cattle is relatively constant whereas that of the urine is greatly affected by dietary CP (Powell and Rotz, 2015). It was reported that feeding beef cattle (initial liveweight 296 ± 8.1 kg) with a ration of 100 g CP/kg DM had a lower urinary N excretion than fed with rations of 120 g CP/kg DM and 140 CP/kg DM, whereas the N retention and the average daily gain (ADG; 1.3, 1.5 and 1.5 kg/d; N retention 28.4, 28.9, and 28.3 g/d) were not affected (Menezes et al., 2016). Since the percentage of N lost as N2O emission was much greater for urine (0.012 g N2O-N/g N) than for dung (0.001 g N2O-N/g N) (Lessa et al., 2014), lowering the dietary CP level to decrease the total N excretion, especially the urinary N excretion, would effectively mitigate the N2O emissions from the excreta of cattle under grazing conditions.
Tannins are a kind of plant secondary metabolites that are usually classified into hydrolysable tannins (HT) and condensed tannins (CT) (Getachew et al., 2008). Some studies showed that feeding cattle with CT improved the N utilization efficiency and reduced the urinary N excretion (Kronberg and Liebig, 2011; Powell et al., 2011). Adding tannin acid (TA) in the ration of beef cattle also decreased the urinary urea N excretion, the ratios of urea N/urinary N, urinary N/total N excretion at 6.5, 13.0, and 26.0 g/kg DM, and decreased the total urinary N excretion at 26.0 g/kg DM (Yang et al., 2016). Adding TA in ration has the potential to decrease the ratio of urinary N/fecal N and regulate the urinary nitrogenous components and reduce the N2O emissions from the excreta of cattle (Zhao, 2017).
Tannins can interact with feed proteins by forming hydrogen bonds and form tannin–protein complexes. Hence, tannins are often used for protecting feed CP from microbial degradation in the rumen to improve the utilization efficiency of dietary CP. The formation of the tannin–protein complexes was affected by the dietary CP level (Patra and Saxena, 2011). Adding tannins in the ration is beneficial to animal performance when the dietary CP level exceeded the CP requirement of ruminants, but the effects would be detrimental to animal performance when the dietary CP level is close to or below the requirement of animals (Waghorn, 2008). Hence, the effects of tannins for modulating the N metabolism of ruminants and the N2O emissions from excreta of animals could be affected by the interactions between the dietary CP and added tannin.
Gallic acid (GA) is a product of the hydrolysis of specific HT (Reed, 1995). Adding GA to ration decreased the ratio of urinary N/total N excretion at 5.3, 10.5, and 21.1 g/kg DM and decreased the urinary urea N excretion at 21.1 g/kg DM in beef cattle (Wei et al., 2016). Hence, it is hypothesized that adding GA would decrease the urinary N excretion and consequently the N2O emissions from the urine of beef cattle.
The objectives of the research were to 1) investigate the effects of dietary CP level and adding GA in the basal rations on the N excretion and the urinary nitrogenous components of beef cattle and 2) further study the effects of dietary CP level and adding GA on the N2O emissions from the urine of beef cattle.
MATERIALS AND METHODS
All experimental procedures were approved by the Animal Care and Use Committee of China Agricultural University.
Animals and Feeding Management
Eight Simmental castrated male cattle with initial liveweight of 310.5 ± 21.5 kg were used as the experimental animals. The cattle were kept in separate pens with individual feed bunks and were fed with one of the two total mixed rations (TMR; containing dietary CP 113.5 and 150.8 g/kg DM with iso-energy concentrations, 4.94 kg DM/d) (Table 1). The controlled amount of TMR offered to each cattle was calculated based on the ad libitum DM intake from a preliminary trial to avoid any feed refusals. The amount of ration was divided into two equal meals and fed to each cattle at 0700 and 1700, respectively. The cattle had free access to fresh drinking water.
Table 1.
Components and chemical composition of the basal rations
| Items | LCP1 ration | HCP2 ration |
|---|---|---|
| Components, g/kg DM | ||
| Corn silage | 485.0 | 485.0 |
| Corn gluten feed | 103.0 | 103.0 |
| Wheat bran | 92.0 | 85.0 |
| Corn | 278.0 | 209.0 |
| Soybean meal | 12.0 | 88.0 |
| Sodium bicarbonate | 10.0 | 10.0 |
| Sodium chloride | 10.0 | 10.0 |
| Calcium carbonate | 10.0 | 10.0 |
| Chemical composition, g/kg DM | ||
| CP | 113.5 | 150.8 |
| NDF | 432.1 | 431.8 |
| ADF | 253.4 | 259.6 |
| OM | 942.4 | 937.3 |
| EE | 20.0 | 20.6 |
| GE, Mcal/kg DM | 4.41 | 4.42 |
| NEmf3, Mcal/kg DM | 1.44 | 1.45 |
1LCP, 113.5 g CP/kg DM.
2HCP, 150.8 g CP/kg DM.
3NEmf = net energy for maintaining and fattening of beef cattle, calculated based on GE, OM, and NDF according to the Nutrient Requirements and Feeding Standards of Beef Cattle (Feng, 2000).
Treatments and Experimental Design
The rations and two levels of GA (0 and 15.2 g/kg DM; C7H6O5·H2O, purity ≥ 98.5%, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were allocated in a 2 × 2 factorial design with the reversal of experimental treatments (Table 2). The GA was added to the rations and mixed completely before feeding. Each experimental period lasted 19 d, of which the first 14 d were for adaptation and the last 5 d were for sampling.
Table 2.
A 2 × 2 reversal design for two levels of CP and two levels of gallic acid (GA)
| Period 1 | GA, 0.0 g/kg DM | GA, 15.2 g/kg DM | ||
|---|---|---|---|---|
| LCP1 | HCP2 | LCP | HCP | |
| Cattle 1 | Cattle 3 | Cattle 5 | Cattle 7 | |
| Cattle 2 | Cattle 4 | Cattle 6 | Cattle 8 | |
| Period 2 | GA, 15.2 g/kg DM | GA, 0 g/kg DM | ||
| LCP1 | HCP2 | LCP | HCP | |
| Cattle 3 | Cattle 1 | Cattle 7 | Cattle 5 | |
| Cattle 4 | Cattle 2 | Cattle 8 | Cattle 6 | |
1LCP, 113.5 g/kg DM.
2HCP, 150.8 g/kg DM.
Sampling
Liveweights of the cattle were recorded at the beginning and the end of each experimental period before feeding in the morning. During the sampling period, the cattle were watched all the time during 24 h of each day, and the feces and urine were collected completely and immediately during defecation and urination. The feces were collected using plastic buckets, and the urine was collected using rubber funnels, which were harnessed to the steers by rubber band and connected to plastic barrels with polyvinyl chloride pipes.
The weight of the feces collected from each cattle was recorded and mixed well, and 2% of the total feces were sampled and added with sulfuric acid (10%, vol/vol) to keep the pH < 3.0. The volume of the urine collected from each cattle was also recorded and mixed well. Two aliquots of 2% of the total urine were sampled. One aliquot of the urine sample was added with sulfuric acid (10%, vol/vol) to keep the pH < 3.0, and another aliquot was not added with sulfuric acid. The samples of feces and urine added with sulfuric acid were for chemical analysis, and the urine samples without sulfuric acid were for measuring N2O in static incubation. The feed samples were collected daily during the sampling period. All the samples were kept in a freezer at −20 °C.
Static Incubation for Measuring N2O Emissions
The soil for static incubation (sand 53.21%DM, clay 30.02%DM, and silt 16.77%DM; pH 9.01; 0.66 g N/kg soil DM) was collected from a field located in Shandong province, China (37°13′12″N, 118°1′12″E) and screened through a sieve with the pore size of 4 mm.
The modified methods of Liang et al. (2015) were used for static incubation. Glass jars with 14 cm in length, 8.5 cm in internal diameter, and 500 mL in scaled volume were used as the vessels for static incubation. Each jar was packed with 275 g of soil DM, 5 cm in height, and 1.1 g soil DM/cm3 in bulk density. The jars were covered with parafilm (Bemis Company, Inc.) with four small holes to keep the moisture while allow the emission of gas. Before incubation, the jars were kept in a biochemical incubator (SHP-250, Shanghai Peiyin Instrument Co., Ltd., Shanghai, China) at 25 °C for a week for stabilization. Then, 10 mL of each of the urine samples collected from Trial I was added to the surface of the soil in each jar at several different points and the moisture of the soil was adjusted by adding distilled water to yield a water-filled pore space (WFPS) of 60%. The jars were then covered with parafilm, and the initial weight of each jar with soil and urine was recorded and then kept at 25 °C for static incubation.
Thirteen glass jars were used for each of the urine samples as replicates, of which three jars were used for measuring N2O-N emissions daily during the incubation period of 15 d, whereas 10 jars were used for taking soil samples. At the end of incubation on day 15, three of the jars for measuring N2O emissions were also used for soil sampling. Two jars without urine but added with 10-mL distilled water in each jar were used as the blanks.
During incubation, the moisture of the soil in each jar was checked every 3 d and adjusted by adding distilled water based on the weight loss of the jars to keep the WFPS at 60%.
Before gas sampling, two urine samples from cattle were used to verify if the N2O emissions increase with the incubation time in a linear manner. It was found that the N2O emissions were closely correlated to the incubation time at 0, 10, 20, 30, and 40 min (urine sample 1: R2 = 0.9988, P < 0.01, n = 5; urine sample 2: R2 = 0.9979, P < 0.01, n = 5; three replicates were used for each time point). Hence, two time points (0 and 20 min) were used for gas sampling in the trial.
The modified methods of Sanchez-Martín et al. (2017) and Kool et al. (2006) were used for gas sampling. The parafilm was uncovered from each jar and a mini electric fan was used to blow the gas out of the jars for 5 min to ensure that the gas in the jars was replaced by air. Then, the jars were closed by rubber corks fitted with a glass tube with a valve, and immediately, 20-mL gas was sampled using a syringe from each jar. After 20 min, another gas sample was taken from each jar.
At 0, 3, 6, 9, and 12 d of incubation, two jars from each of the urine samples were used daily for soil sampling. The soil samples were extracted to obtain solutions using the method of Mulvaney (1996) for the analysis of NO3−-N and NH4+-N. The soil in each jar was taken out completely and mixed well, then 5 g of the soil (containing water) was taken into a 250-mL conical bottle, then 50 mL of KCl solution (1 mol/L) was added into the bottle and shaken for 30 min. The bottles were left in the lab for 30 min and filtered through N-free filter papers. Then, the pH (pH-KCl) of the solution was measured immediately using a pH meter (PHB-8, Shanghai Yoke Instrument Co., Ltd., Shanghai, China). The solutions were then kept in a freezer at −20 °C for the analysis of NO3−-N and NH4+-N.
Analysis of Soil, Feeds, Feces, and Urine
The contents of DM, ether extract, and ash of feeds were determined according to AOAC (1990) using the methods of Nos. 934.01, 920.39, and 924.05, respectively. The OM was calculated as DM minus ash. The NDF and ADF of feeds were analyzed according to the methods of Van Soest et al. (1991) using Ankom 2000 Fibre Analyser (Ankom Technology Co., Macedon, NY). The GE of the feed samples was analyzed using a Parr 6300 calorimeter (Parr Instrument Company, Moline, IL). The net energy for maintenance and fattening of beef cattle was calculated based on GE, OM, and NDF according to the Nutrient Requirements and Feeding Standards of Beef Cattle (Feng, 2000). The N of soil, feeds, feces, and urine samples were analyzed using the Kjeldahl method (No. 984.13; AOAC, 1990), and the CP content was calculated as N × 6.25. The urinary urea and creatinine were analyzed using colorimetric assay kits (Nanjing Jiancheng Bioengeneering Institute, Nanjing, China) on a spectrophotometer (UV-1801, Beijing Rayleigh Analytica Instruments Co. Ltd., Beijing, China). The urinary hippuric acid was analyzed according to China Hygienic Standard (WS/T 52–1996) on a spectrophotometer (UV-1801, Beijing Rayleigh Analytica Instruments Co. Ltd., Beijing, China). The urinary allantoin and uric acid were analyzed using the methods of Chen and Gomes (1992) on a spectrophotometer. The metabolites of GA, that is pyrogallol and resorcinol of the urine samples, were analyzed using the method of Shahrzad and Bitsch (1998) on HPLC (LC98 I, Beijing Wenfen Analytical Instruments Co. Ltd., Beijing, China; Kromasil C18 column, 5 µm in particle size, 250 mm in length, 4.6 mm in internal diameter) at 280 nm of detection wavelength of and 40 °C of column temperature. The detecting limit of the HPLC for GA, pyrogallol, and resorcinol of urine samples was 2 × 10−3 mmol/L.
Analysis of N2O
The gas samples were analyzed for N2O using gas chromatograph (3420A, Beijing Beifen Tianpu Instrument Technology Co., Beijing, China) equipped with an electron capture detector (ECD) and two Porapak Q columns (2 m in length × 3 mm in internal diameter, Beijing Beifen Tianpu Instrument Technology Co., Beijing, China) at 360 °C of ECD temperature, 85 °C of injector temperature, and 80 °C of column temperature, respectively. Nitrogen gas (purity ≥ 99.99%) was used as the carrier gas at 14.6 mL/min of flow rate.
Determination of Moisture and Analysis of NO3−-N and NH4+-N
The moisture of the soil samples was determined according to AOAC (1990) using the method of No. 934.01. The NH4+-N and the NO3−-N of the solutions extracted from the soil samples were analyzed using the colorimetric method of Hinds and Lowe (1980) and the dual-wavelength ultraviolet spectrophotometry method of Norman et al. (1985), respectively.
Calculations and Statistical Analysis
The N retention of cattle was calculated as follows:
The WFPS was calculated as follows:
where m1 refers to soil water weight, g; m2 refers to soil DM, g; ρb refers to soil bulk density, g/cm3; ρp refers to soil particle density, identified as 2.65 g/cm3.
The N2O-N emission was calculated according to the method of Watanabe et al. (1997):
where F refers to the N2O-N emission, µg N2O-N·kg−1 soil DM·d−1; 273, the Kelvin temperature; T, the incubation temperature, °C; 28, the mass of N in N2O, 28 g/mol; 1,440, the total minutes of 1 d; V, the volume of the jar above the soil, L; dc/dt, the average changing rate of N2O concentration with time, nL·L−1·min−1; 22.4, the molar volume of gas at 273 K, 22.4 L/mol; W, the DM of soil in jars, kg.
The cumulative N2O-N emission of 10-mL urine sample during 15 d of incubation (urine sample N2O-N emission) was calculated as follows:
where F1, F2, … F15 refers to the N2O emissions of days 1, 2, … 15, respectively, µg N2O-N·kg−1 soil DM·d−1.
The N2O-N/urine-N application was calculated as follows:
The total N2O-N emission from each cattle during a day was estimated by the N2O-N emission in jars multiplied by the urine volume excreted during a day with the assumption that the N2O-N emission from the urine of each cattle during a day was positively proportional to the urine volume.
The estimated N2O cumulative emission from each cattle (estimated cattle N2O-N emission) was calculated as follows:
where E1, E2, … E15 refer to the N2O emission of days 1, 2, … 15, respectively, µg N2O-N cattle−1 d−1; V, the daily urine volume of each cattle, milliliter; 10, the volume of urine sample used for static incubation, mL.
Statistical analysis was carried out using SAS 9.2 (SAS Institute, Inc., Cary, NC). The significance of differences between CP and GA was tested using General Linear Model. The model for statistical analysis was as follows:
where yijk refers to the observation from cattle; µ, overall mean; ai, GA treatment (i = 1, 2); bj, CP treatment (j = 1, 2); ai × bj, the interaction effect (i = 1 and 2, j = 1, 2); pl, period effect (l = 1, 2); eijk, residual error. Differences between treatments were considered to be significant at P ≤ 0.05 and considered as a trend toward significance at 0.05 < P < 0.10.
RESULTS
N Excretion, N Retention, and ADG
The results of Trial I (Table 3) showed that no interactions between dietary CP and GA were found on the indices of N metabolism (P > 0.05). Increasing dietary CP level from 113.5 to 150.8 g/kg DM increased the excretions of total N and urinary N, decreased the ratios of fecal N/urinary N, fecal N/total N excretion, fecal N/N intake, increased the ratios of urinary N/total N excretion, urinary N/N intake, and the N retention at two GA levels (P < 0.01). The results also showed that adding GA at 15.2 g/kg DM in ration did not affect the excretions of fecal N, urinary N and total N, and the ratios of fecal N/urinary N, fecal N/total N excretion, urinary N/total N excretion, fecal N/N intake, urinary N/N intake, the N retention/N intake, and the N retention at both dietary CP levels (P > 0.05). No differences were found in ADG of cattle among different treatments (P > 0.05).
Table 3.
Effects of dietary CP and gallic acid (GA) on N excretion and N retention of beef cattle
| Items | Treatments | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| LCP1 | HCP2 | LCPGA3 | HCPGA4 | CP | GA | CP × GA | ||
| DMI, kg | 4.94 | 4.94 | 4.94 | 4.94 | — | — | — | — |
| N intake, g/d | 89.52 | 118.91 | 89.52 | 118.91 | — | — | — | — |
| Fecal N, g/d | 29.72 | 31.30 | 30.99 | 30.19 | 0.671 | 0.666 | 0.943 | 0.206 |
| Urinary N, g/d | 28.61 | 50.21 | 30.30 | 48.98 | 2.772 | <0.001 | 0.899 | 0.434 |
| Total N excretion, g/d | 58.33 | 81.50 | 61.29 | 79.17 | 2.951 | <0.001 | 0.896 | 0.264 |
| Fecal N/urinary N | 1.04 | 0.63 | 1.03 | 0.63 | 0.057 | <0.001 | 0.883 | 0.843 |
| Fecal N/total N excretion, % | 50.80 | 38.39 | 50.61 | 38.32 | 1.720 | <0.001 | 0.891 | 0.954 |
| Urinary N/total N excretion, % | 49.20 | 61.61 | 49.39 | 61.68 | 1.720 | <0.001 | 0.891 | 0.954 |
| Fecal N/N intake, % | 33.20 | 26.31 | 34.62 | 25.39 | 1.237 | <0.001 | 0.804 | 0.217 |
| Urinary N/N intake, % | 31.96 | 42.22 | 33.85 | 41.19 | 1.421 | <0.001 | 0.797 | 0.391 |
| N retention, g/d | 31.19 | 37.41 | 28.22 | 39.74 | 1.731 | 0.003 | 0.896 | 0.264 |
| N retention/N intake, % | 34.84 | 31.46 | 31.53 | 33.42 | 1.226 | 0.736 | 0.766 | 0.250 |
| ADG, kg/d | 0.48 | 0.52 | 0.52 | 0.49 | 0.034 | 0.983 | 0.933 | 0.547 |
1LCP, 113.5 g CP/kg DM.
2HCP, 150.8 g CP/kg DM.
3LCPGA, 113.5 g CP/kg DM + 15.2 g GA/ kg DM.
4HCPGA, 150.8 g CP/kg DM + 15.2 g GA/kg DM.
Urinary Nitrogenous Components
The results of Trial I (Table 4) showed that no effects of interaction were found between GA and CP on the urinary N components. Increasing dietary CP level from 113.5 to 150.8 g/kg DM increased the urinary excretions of urea, allantoin, and the ratio of urea-N/urinary N (P < 0.001) and decreased the ratios of creatinine-N/urinary N and uric acid-N/urinary N (P < 0.001). The results also showed that adding GA at 15.2 g/kg DM in ration decreased the urinary excretion of creatinine (P < 0.05) and the ratio of creatinine-N/urinary N (P < 0.01), increased the urinary excretion of allantoin and the ratio of allantoin-N/urinary N (P < 0.001) while did not affect the urinary excretions of urea, uric acid, and hippuric acid and the ratios of urea-N/urinary N, hippuric acid-N/urinary N, and uric acid-N/urinary N at both CP levels (P > 0.05).
Table 4.
Effects of dietary CP and gallic acid (GA) on urinary components of beef cattle
| Items | Treatments | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| LCP1 | HCP2 | LCPGA3 | HCPGA4 | CP | GA | CP × GA | ||
| Urea, mmol/d | 689.8 | 1,377.8 | 694.4 | 1,277.8 | 86.543 | <0.001 | 0.398 | 0.356 |
| Urea-N/urinary N, % | 67.56 | 76.81 | 63.85 | 73.43 | 1.663 | 0.002 | 0.137 | 0.941 |
| Hippuric acid, mmol/d | 117.3 | 112.3 | 85.28 | 131.0 | 14.530 | 0.444 | 0.790 | 0.334 |
| Hippuric acid-N/urinary N, % | 5.79 | 3.17 | 4.05 | 3.63 | 0.590 | 0.120 | 0.486 | 0.247 |
| Creatinine, mmol/d | 61.78 | 62.93 | 50.10 | 49.26 | 2.801 | 0.960 | 0.002 | 0.744 |
| Creatinine-N/urinary N, % | 9.17 | 5.28 | 7.11 | 4.34 | 0.519 | <0.001 | 0.011 | 0.266 |
| Allantoin, mmol/d | 32.58 | 49.83 | 43.56 | 66.29 | 3.506 | <0.001 | 0.006 | 0.491 |
| Allantoin-N/urinary N, % | 6.38 | 5.53 | 8.12 | 7.64 | 0.346 | 0.268 | 0.007 | 0.752 |
| Uric acid, mmol/d | 6.63 | 7.55 | 6.90 | 7.16 | 0.265 | 0.129 | 0.878 | 0.175 |
| Uric acid-N/urinary N, % | 1.31 | 0.85 | 1.31 | 0.82 | 0.077 | <0.001 | 0.869 | 0.836 |
| GA, mmol/d | ND5 | ND | ND | ND | — | — | — | — |
| Pyrogallol, mmol/d | ND | ND | 9.93 | 10.24 | 1.316 | 0.673 | <0.001 | 0.673 |
| Resorcinol, mmol/d | ND | ND | 29.06 | 47.50 | 2.321 | <0.001 | <0.001 | <0.001 |
1LCP, 113.5 g CP/kg DM.
2HCP, 150.8 g CP/kg DM.
3LCPGA, 113.5 g CP/kg DM + 15.2 g GA/kg DM.
4HCPGA, 150.8 g CP/kg DM + 15.2 g GA/kg DM.
5ND = not detectable when the detecting limit was 2 × 10−3 mmol/L.
Pyrogallol and resorcinol were detected in urine when GA was added at 15.2 g/kg DM, whereas pyrogallol and resorcinol were not detected in urine from cattle fed with rations without adding GA. Increasing dietary CP level from 113.5 to 150.8 g/kg DM increased the urinary excretion of resorcinol (P < 0.001) but did not affect that of pyrogallol (P > 0.05).
N2O Emissions
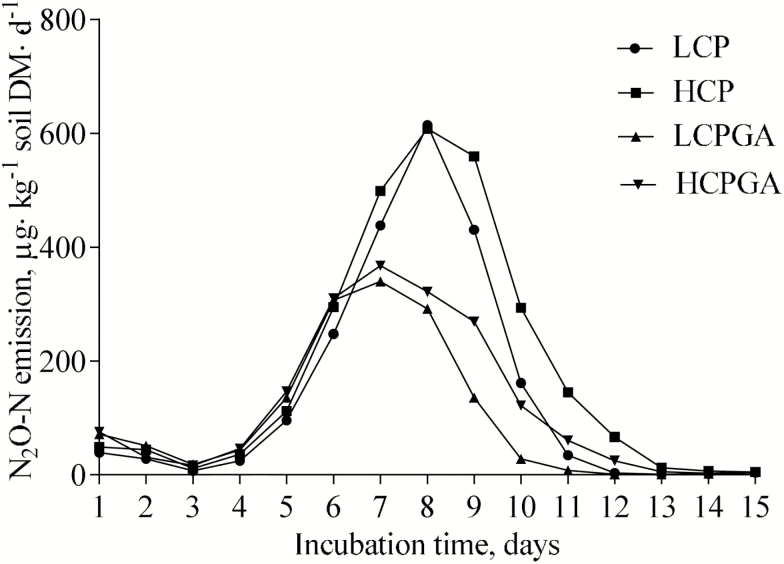
Figure 1 showed that the variation of the daily N2O-N emissions of different treatments during 15 d of static incubation. The N2O-N emissions were low during the first 4 d but increased from day 5 and returned to low levels after day 12 of incubation.
Figure 1.
The N2O-N emissions of urine samples applied to soil in static incubation (LCP, 113.5 g CP/kg DM; HCP, 150.8 g CP/kg DM; LCPGA, 113.5 g CP/kg DM + 15.2 g GA/kg DM; HCPGA, 150.8 g CP/kg DM + 15.2 g GA/kg DM). No effects of interactions were found between dietary CP and GA on the mean value of urine sample N2O-N emissions (P > 0.05). Increasing dietary CP level did not affect the mean value of urine sample N2O-N emissions (P > 0.05), whereas adding GA decreased the parameter (P < 0.05).
Table 5 showed that no effects of interactions between dietary CP level and GA were found on the urine sample N2O-N emissions, the ratio of N2O-N/urine-N application, and the estimated cattle N2O-N emissions on a daily basis (P > 0.05). Increasing the dietary CP from 113.5 to 150.8 g/kg DM did not affect the urine sample N2O-N emissions (P > 0.05), tended to increase the ratio of N2O-N/urine-N application (P = 0.074), and increased the estimated cattle N2O-N emissions (P < 0.05) on daily basis (P < 0.05). Adding GA at 15.2 g/kg DM in rations decreased the urine sample N2O-N emissions (P < 0.05), the ratio of N2O-N/urine-N application (P < 0.05), and the estimated cattle N2O-N emissions (P < 0.01) on daily basis (P < 0.01).
Table 5.
Effect of dietary CP and gallic acid (GA) on N2O-N emissions
| Items | Treatments | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|---|
| LCP1 | HCP2 | LCPGA3 | HCPGA4 | CP | GA | CP × GA | ||
| Urine sample N2O-N emission5, µg·kg−1 soil DM·d−1 | 2,127.8 | 2,717.1 | 1,438.5 | 1,803.7 | 172.56 | 0.143 | 0.025 | 0.715 |
| N2O-N/urine-N application, % | 1.59 | 1.25 | 1.09 | 0.88 | 0.086 | 0.074 | 0.011 | 0.632 |
| Urine volume of each cattle in 1 d, mL | 7,029.5 | 7,733.0 | 7,581.0 | 8,157.0 | 226.28 | 0.185 | 0.303 | 0.890 |
| Estimated cattle N2O-N emission, mg/d | 413.1 | 565.3 | 295.3 | 390.7 | 31.08 | 0.019 | 0.008 | 0.500 |
| Estimated cattle N2O-CO2 equivalent emission6, g/d | 172.0 | 235.4 | 123.0 | 162.7 | — | — | — | — |
The enteric CH4 emission of beef cattle is 179 g CH4·head−1·d−1 (ranging 117 to 240 g CH4·head−1·d−1) (Bell et al., 2016), and the global warming potential of 1 g CH4 is 28 g CO2 equivalent emissions based on a 100-yr timescale (IPCC, 2013), then the enteric CH4-CO2 equivalent emission of beef cattle can be calculated to be 179 × 28 = 5,012 g CO2·head−1·d−1 (ranging 3,276 to 6,720 g CO2·head−1·d−1). The ratios of the estimated cattle urine N2O-CO2 equivalent emission/the enteric cattle CH4-CO2 equivalent emission for treatments LCP and HCP (without adding GA in rations) are 3.43% and 4.70%, respectively.
1LCP, 113.5 g CP/kg DM.
2HCP, 150.8 g CP/kg DM.
3LCPGA, 113.5 g CP/kg DM + 15.2 g GA/kg DM.
4HCPGA, 150.8 g CP/kg DM + 15.2 g GA/kg DM.
5Urine sample N2O-N emission refers to the cumulative emission for the 10-mL urine sample over 15 d.
6The estimated cattle N2O-CO2 equivalent emissions are calculated based on the estimated cattle N2O emission and the global warming potential of N2O (1 g N2O is 265 g CO2 equivalent emissions based on a 100-yr timescale; IPCC, 2013).
Soil pH, NH4+-N, and NO3−-N
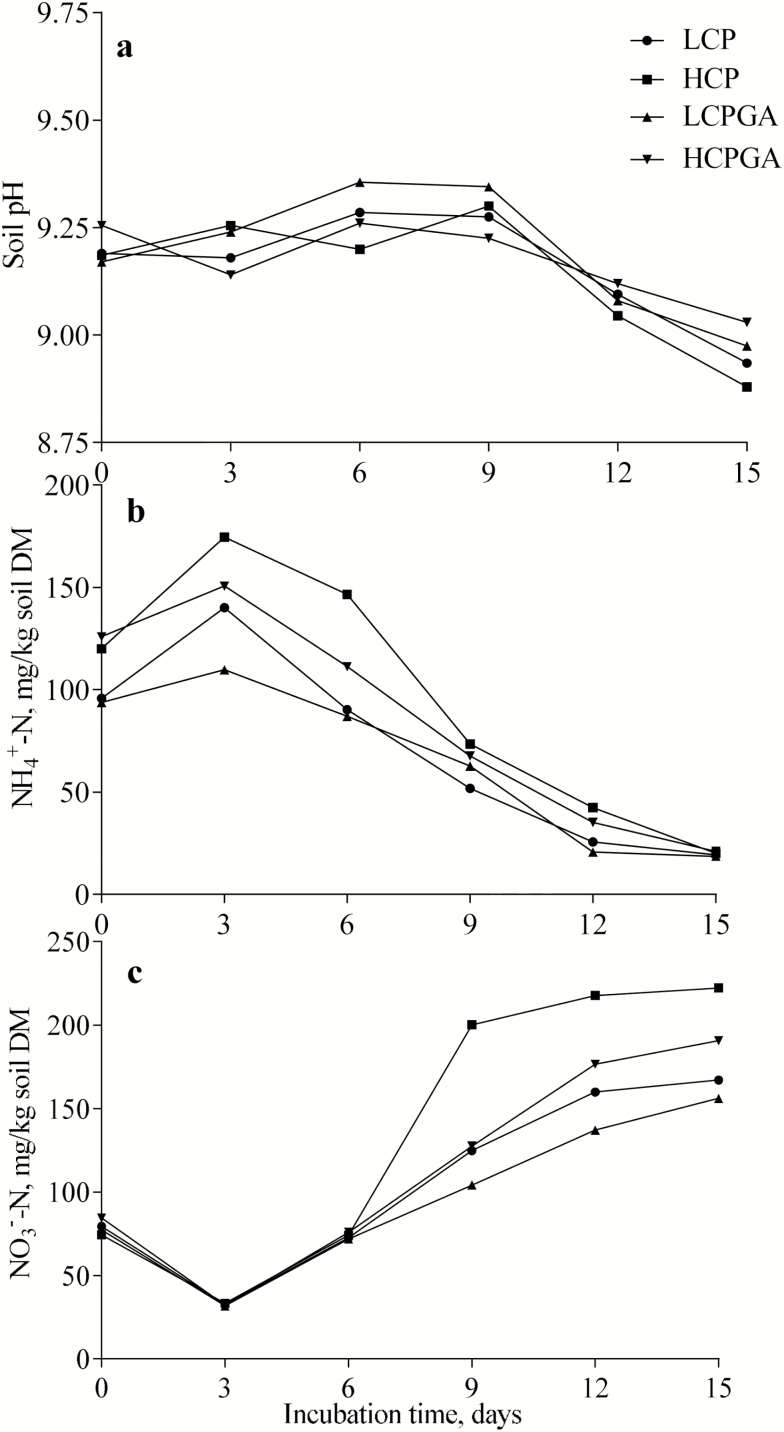
No effects of interactions were found between dietary CP and GA on soil pH-KCl, NH4+-N, and NO3−-N (P > 0.05). Figure 2a showed that increasing dietary CP level from 113.5 to 150.8 g/kg DM did not affect the mean value of pH-KCl of soil applied with urine samples (P > 0.05) whereas adding GA at 15.2 g/kg DM tended to decrease the mean value of pH-KCl (P = 0.063). Figure 2b and c showed that increasing dietary CP from 113.5 to 150.8 g/kg DM increased the mean concentrations of NH4+-N (P < 0.001) and NO3−-N of soil applied with urine samples (P < 0.01), whereas adding GA at 15.2 g/kg DM decreased the mean concentrations of NH4+-N and NO3−-N of soil applied with urine samples (P < 0.05). Figure 2b and c also showed that the NH4+-N concentrations of soil applied with urine samples decreased with incubation time, whereas the NO3−-N concentrations of the soil increased with incubation time.
Figure 2.
The pH-KCl (a), NH4+-N (b), and NO3−-N (c) of soil applied with urine samples (LCP, 113.5 g CP/kg DM; HCP, 150.8 g CP/kg DM; LCPGA, 113.5 g CP/kg DM + 15.2 g GA/kg DM; HCPGA, 150.8 g CP/kg DM + 15.2 g GA/kg DM). No effects of interactions were found between dietary CP and GA on soil pH-KCl, NH4+-N, and NO3−-N (P > 0.05). Increasing dietary CP level did not affect the mean value of soil pH-KCl (P = 0.086), but increased the mean concentrations of soil NH4+-N (P < 0.001) and NO3−-N (P < 0.05). Adding GA tended to increase the mean value of soil pH-KCl (P = 0.086) and decreased the mean concentrations of NH4+-N (P < 0.05) and NO3−-N (P < 0.05).
DISCUSSION
Static Incubation and Factors Affecting N2O Emissions
Static incubation is a routine technique for measuring greenhouse gas emissions from soils (Alves et al., 2012). The advantages of static incubation technique using small vessels (like glass jars used in the present research) over other techniques include simplicity and easily handling. Therefore, static incubation technique is widely used for measuring the N2O emissions of soil samples.
N2O is produced in a series of complex processes mainly including hydrolysis, nitrification, and denitrification of the nitrogenous components. The nitrogenous components of animal excreta applied to soil can be hydrolyzed by the microorganisms in soil to NH4+ (hydrolysis) and subsequently oxidized to NO3− (nitrification), and NO3− can be transformed to N2O (denitrification) (Wrage et al., 2001). These processes are the dominant microbial processes that are responsible for N2O formation. Other processes in soils such as dissimilatory nitrate reduction to ammonia also affect the N2O formation (Zhang et al., 2015). Although the pathways for the N2O production are complex, the total available N and the nitrogenous components of animal excreta are the major factors affecting the N2O production in soil.
The type of soil is one of important factors affecting the N2O emissions. It was reported that clay soil produced much higher N2O than sandy soil (Van Groenigen et al., 2004), and the average N2O emission factors of clay soil are 1.5 times of the sand soil (Lesschen et al., 2011).
N2O emission is also affected by temperature, WFPS, and bulk density of soils. Öquist et al. (2004) reported that there was a significant positive relationship between the soil temperature (0 to 25 °C) and the N2O emissions. Bouwman (1998) reported that the highest amount of N2O was produced when the soil WFPS was kept at 50% to 80%.
The main objectives of the present research were to compare the effects of different treatments on the N2O emissions from the urine of beef cattle, therefore, all the urine samples were incubated under the same conditions including the same soil composition, 25 °C of incubation temperature, and 60% of soil WFPS for static incubation, and different conditions affecting N2O production were not taken into account in the present research. Indeed, the conditions of covering jars with parafilm to keep the moisture were relatively standardized for incubation and did not really simulate the variable field conditions, but the aims of the static incubation (Trial II) in the present research were to compare the N2O-N emissions from the urine samples of beef cattle with different treatments, it is certainly easier to compare the effects of different treatments on the N2O emissions under relatively standardized conditions than the variable field conditions. It is necessary to study the effects of dietary CP and adding GA on the N2O emissions from the urine of beef cattle under field conditions in the future to confirm the lab results.
Under most feeding and management regimes, the urinary N excretion of beef cattle accounts for about 60% to 80% of the total N excretion (Varel, 1997), and the urinary nitrogenous components are more easily utilizable for N2O production than fecal nitrogenous components (Hristov et al., 2013). Therefore, the urine of beef cattle was used for measuring the N2O emissions in the present research.
The curves of the N2O-N emissions of different treatments (Fig. 1) indicated that the hydrolysis of the nitrogenous compounds into NH4+-N should have occurred during incubation and the process had completed in 15 d of incubation under the conditions of the present research. The decrease of the NH4+-N concentration and the increase of the NO3−-N concentration of different treatments with incubation time (Fig. 2) indicated that the nitrification should have occurred in which NH4+-N was transformed into NO3−-N. Simultaneously, the denitrification should have also occurred because of the continuous N2O-N emissions of different treatments even though the amounts of N2O-N emissions varied with incubation time. Other pathways that produce N2O could not be defined because only NH4+-N and NO3−-N of the treatments were analyzed in the present research.
Effects of Dietary CP on N Excretion, Urinary N Components, and N2O Emissions
It was reported that the urinary excretions of N and urea are positively correlated to the total CP intake in heifers (Marini and Van Amburgh, 2005). The results of Trial I showed that the total N excretion and the urinary urea excretion were increased with the dietary CP, which were in agreement with Marini and Van Amburgh (2005). The results of Trial II showed that the concentrations of NO3−-N and NH4+-N of the soil samples (applied with urine samples) and the N2O-N emissions from the urine of beef cattle increased with the dietary CP level. It was calculated that increasing dietary CP level from 113.5 to 150.8 g/kg DM increased the estimated cattle urine N2O-N emissions by 36.8% (without adding GA) and 32.3% (adding GA at 15.2 g/kg DM). Consequently, increasing dietary CP level from 113.5 to 150.8 g/kg DM also increased the estimated cattle N2O-CO2 equivalent emissions of different treatments.
The results were in agreement with the results of Trial I that the urinary excretions of total N and urea were increased with the dietary CP level. Therefore, feeding cattle with relatively lower dietary CP in cattle production would decrease the N2O emission from the urine patches.
Bell et al. (2016) summarized the individual animal data from 17 published experiments that included sheep (n = 288), beef cattle (n = 71), and dairy cows (n = 284) and reported that an average of beef cattle’s total CH4 production was 179 g·head−1·d−1 (ranging 117 to 240 g CH4·head−1·d−1), which was equivalent to 179 × 28 = 5,012 g CO2·head−1·d−1 (ranging 3,276 to 6,720 g CO2·head−1·d−1), calculated according to the CH4 warming potential (1 g CH4 is 28 g CO2 equivalent emissions; IPCC, 2013). The estimated cattle N2O-CO2 equivalent emissions of the present research were calculated to be 172.0 g·head−1·d−1 (113.5 g CP/kg DM, without adding GA in rations) and 235.4 g·head−1·d−1 (150.8 g CP/kg DM, without adding GA in rations), which were 3.43% and 4.70% of the enteric CH4-CO2 equivalent emission of beef cattle (average value 5,012 g CO2·head−1·d−1) calculated according to Bell et al. (2016), respectively. Although the estimated cattle urine N2O-CO2 equivalents are much lower than the enteric CH4-CO2 equivalent of beef cattle, the results of the present research clearly indicated that the N2O emissions from the urine of beef cattle are an important source of greenhouse gas. Furthermore, N2O is the major factor depleting the ozone sphere of the earth (Ravishankara et al., 2009). Hence, mitigating the N2O emissions from the urine of cattle through technical or management approaches is important to protect the global environment.
The urinary N components of cattle mainly consist of urea, allantoin, creatinine, uric acid, and hippuric acid (Bristow et al., 1992). The N percentages of urea, allantoin, uric acid, creatinine, and hippuric acid in total urinary N are 76.7% to 85.2%, 8.3% to 14.1%, 0.6% to 0.8%, 2.0% to 3.2%, and 4.1% to 5.1%, respectively (Kool et al., 2006). The degradation rates of different nitrogenous components in the urine of cattle are in the sequence: urea > allantoin > creatine > creatinine > hippuric acid (Whitehead et al., 1989).
Previous studies showed that urinary hippuric acid has the potential to inhibit the processes of nitrification and denitrification and consequently decrease the N2O production of soil applied with the urine of cattle (Kool et al., 2006; Van Groenigen et al., 2006; Bertram et al., 2009). Therefore, decreasing the excretions of total N and urinary N (especially urea) and increasing the urinary excretion of hippuric acid would decrease the N2O emissions of soil applied with the urine of cattle. In the present research, increasing dietary CP level did not affect the urinary excretions of hippuric acid but increased the ratios of urinary creatinine N/urinary N, uric acid-N/urinary N, and allantoin-N/urinary N. The changes of the ratios of the nitrogenous components should have affected the N2O emissions from the urine of beef cattle applied to soil, but the impacts of these urinary components could have been small because the percentages of creatinine-N, uric acid-N, and allantoin-N are much lower than that of urea-N in total urinary N. Increasing dietary CP level tended to decrease the ratio of N2O-N/urine-N application could be attributed to these impacts to some extent.
In the present research, increasing dietary CP level from 113.5 to 150.8 g/kg DM did not affect the ADG of beef cattle and the ratio of N retention/N intake. The results are in agreement with Menezes et al. (2016) who reported that increasing dietary CP level from 100, 120 to 140 g CP/kg DM did not affect the N retention and the ADG of beef cattle. The results indicated that keeping the dietary CP at relatively lower level would decrease the N2O-N emissions from the urine of beef cattle without affecting the performance of beef cattle.
In the present research, the N retention of beef cattle fed with rations containing 113.5 or 150.8 g CP/kg DM is positive. The results indicated that the dietary CP level had met the CP requirement of the cattle. However, the ADG of the cattle was only around 0.50 kg/d because of the controlled feeding and may not be acceptable in cattle production. Furthermore, each experimental period of Trial I in the present research was only 19 d, which was not long enough to evaluate the performance of the cattle. Long-term feeding trials with beef cattle fed with higher feed intake or ad libitum feeding are necessary to be carried out to verify whether lowering dietary CP level is acceptable for the ADG and for mitigating the N2O emissions from the urine of beef cattle.
Effects of GA on N Excretion, Urinary N Components, and N2O Emissions
In heifers, Koenig and Beauchemin (2017) reported that adding chestnut tree extracts (DM basis, a kind of HT) tannins in ration did not affect the total N excretion, but increased the fecal N excretion and decreased the urinary N excretion. In sheep, Wischer et al. (2014) reported that adding 8.3 g/kg DM chestnut and valonea extracts (both mainly containing HT) in ration increased the fecal N excretion and decreased the urinary N excretion. In beef cattle, Yang et al. (2016) reported that adding tannic acid in ration improved the N retention rate, decreased the urinary urea excretion, and increased the hippuric acid excretion. Also in beef cattle, Wei et al. (2016) reported that adding GA up to 21.1 g/kg DM in ration improved the N retention rate, decreased the urinary urea excretion but did not affect the urinary hippuric acid excretion. In the present trial, adding GA at 15.2 g/kg DM in the rations did not affect the N retention rate and the urinary urea excretion. The results are not in agreement with Wei et al. (2016). The reasons for the diversified results could be that the level of GA added in the present research was lower than the GA level used by Wei et al. (2016).
Although adding GA at 15.2 g/kg DM did not affect the excretions of total N, urinary N, urea, hippuric acid, and uric acid in the present research, adding GA at 15.2 g/kg DM decreased the estimated cattle urine N2O-N emissions. It was calculated that adding GA at 15.2 g/kg DM decreased the estimated cattle urine N2O-N emissions by 28.5% (dietary CP 113.5 g/kg DM) and 30.9% (dietary CP 150.8 g/kg DM), respectively. The results are in agreement with the decreased concentrations of NO3−-N and NH4+-N of the soil applied with urine from the cattle added with GA in the rations. The reasons for the results could be that the metabolites of GA including pyrogallol and resorcinol excreted in urine inhibited the processes of N2O production and decreased the ratio of N2O-N/urine-N application. Gallic acid was not detectable in the urine samples indicated that all of the GA added to the rations should have been metabolized in the body of cattle and excreted in the urine of cattle as the metabolites including pyrogallol and resorcinol. No literatures are available presently to support the inhibitory effect of pyrogallol and resorcinol on NO3−-N, NH4+-N, and N2O. It is necessary to study the mechanisms of the inhibitory effects of pyrogallol and resorcinol on NO3−-N, NH4+-N, and N2O in future trials.
The estimated cattle N2O-CO2 equivalent emissions of the treatments LCPGA and HCPGA were calculated to be 123.0 g·head−1·d−1 and 162.7 g·head−1·d−1, which are lower than that of the treatments LCP and HCP, respectively, in the present research. The results indicate that adding GA decreased the estimated cattle N2O-CO2 equivalent emissions from the urine of beef cattle applied to soil.
Adding GA at 15.2 g/kg DM decreased the urinary excretion of creatinine and increased that of allantoin. These effects should have also impacted the N2O-N emissions from the urine of the cattle applied to soil. However, no literatures are available at present to explain the effects, and it is necessary to be studied in the future.
No significant interactions were found between dietary CP and GA on the N excretion of the beef cattle in the present research. The results indicated that the effects of GA on the N metabolism of beef cattle were not affected by the dietary CP level.
It should be noted that the GA used in the present trial is a pure product and expensive. The aim of the research was to demonstrate the effect of GA on reducing the N2O emissions from the urine of beef cattle applied to soil. Pure GA may not be suitable as a feed additive for cattle production because of high cost, but some by-products of plant sources, such as oak, strawberries, grapes, and bananas, that contain GA could be considered to be utilized as feed additives to obtain similar effects of adding GA.
CONCLUSIONS
Increasing the dietary CP level from 113.5 to 150.8 g/kg DM increased the excretions of total N, urinary N, and urea and consequently the estimated cattle urine N2O-N emissions by 36.8% (without adding GA) and 32.3% (adding GA at 15.2 g/kg DM) when applied to soil. Adding GA at 15.2 g/kg DM in rations did not affect the excretions of total N, urinary N, and urea but decreased the estimated cattle urine N2O-N emissions by 28.5% (dietary CP 113.5 g/kg DM) and 30.9% (dietary CP 150.8 g/kg DM) when applied to soil. The effects of GA on decreasing the N2O-N emissions of cattle urine applied to soil could be resulted from the effects of the GA metabolites including pyrogallol and resorcinol excreted in the urine of beef cattle. Feeding cattle with relatively low dietary CP level or adding GA in ration is effective to decrease the N2O-N emissions from cattle urine when applied to soil. No interactions between the dietary CP level and adding GA were found on the N metabolism of beef cattle and the N2O-N emissions from the urine of cattle applied to soil.
Footnotes
The research was supported by National Natural Science Foundation of China (grant number 31572428).
LITERATURE CITED
- Alves B. J. R., Smith K. A., Flores R. A., Cardoso A. S., Oliveira W. R. D., Jantalia C. P., Urquiaga S., and Boddey R. M.. 2012. Selection of the most suitable sampling time for static chambers for the estimation of daily mean N2O flux from soils. Soil Biol. Biochem. 46:129–135. doi: 10.1016/j.soilbio.2011.11.022 [DOI] [Google Scholar]
- AOAC 1990. Official methods of analysis. 15th ed Assoc. Off. Anal. Chem, Arlington, VA. [Google Scholar]
- Bell M., Eckard R., Moate P., and Yan T.. 2016. Modelling the effect of diet composition on enteric methane emissions across sheep, beef cattle and dairy cows. Animals 6:54. doi: 10.3390/ani6090054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram J. E., Clough T. J., Sherlock R. R., Condron L. M., O’Callaghan M., Wells N. S., and Ray J. L.. 2009. Hippuric acid and benzoic acid inhibition of urine derived N2O emissions from soil. Glob. Chang. Biol. 15:2067–2077. doi: 10.1111/j.1365-2486.2008.01779.x [DOI] [Google Scholar]
- Bouwman A. F. 1998. Nitrogen oxides and tropical agriculture. Nature 392:866–867. doi: 10.1038/31809 [DOI] [Google Scholar]
- Bristow A. W., Whitehead D. C., and Cockburn J. E.. 1992. Nitrogenous constituents in the urine of cattle, sheep and goats. J. Sci. Food Agric. 59:387–394. doi: 10.1002/jsfa.2740590316 [DOI] [Google Scholar]
- Chen X. B., and Gomes M. J.. 1992. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivatives – An overview of the technical details. Rowett Res. Inst, Aberdeen, UK: p. 1–19. doi: 10.1016/j.cvfa.2007.03.003 [DOI] [Google Scholar]
- China Hygienic Standard 1996. WS/T 52–1996. Urine – Determination of hippuric acid – Spectrophotometric method. Standards Press of China, Beijing, China. [Google Scholar]
- Feng Y. L. 2000. The nutrient requirements and feeding standards of beef cattle. China Agric. Univ. Press, Beijing, China. [Google Scholar]
- Getachew G., Pittroff W., Putnam D. H., Dandekar A., Goyal S., and DePeters E. J.. 2008. The influence of addition of gallic acid, tannic acid, or quebracho tannins to alfalfa hay on in vitro rumen fermentation and microbial protein synthesis. Anim. Feed Sci. Technol. 140:444–461. doi: 10.1016/j.anifeedsci.2007.03.011 [DOI] [Google Scholar]
- Hinds A. A., and Lowe L. E.. 1980. Application of the Berthelot reaction to the determination of ammonium-N in soil extracts and soil digests. Commun. Soil Sci. Plant Anal. 11:469–475. doi: 10.1080/00103628009367054 [DOI] [Google Scholar]
- Hirooka H., Liang J. B., and Terada F.. 2007. Development and evaluation of a model for prediction of fecal and urinary nitrogen excretions in cattle. Livest. Sci.107:282–288. doi: 10.1016/j.livsci.2006.12.002 [DOI] [Google Scholar]
- Hristov A. N., Oh J., Lee C., Meinen R., Montes F., Ott T., Firkins J., Rotz A., Dell C., Adesogan C., et al. 2013. Mitigation of greenhouse gas emissions in livestock production – A review of technical options for non-CO2 emission. In: Gerber P. J., Henderson B., and Makkar H. P. S., editors, FAO animal production and health. Food and Agric. Organ. United Nations, Rome, Italy: No. 177, p. 1–206. [Google Scholar]
- IPCC 2013. The physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on climate change. In: Stocker T. F., Qin D., Plattner G. K., Tignor M., Allen S. K., Boschung J., Nauels A., Xia Y., Bex V., and Midgley P. M., editors, Climate change 2013. Cambridge Univ. Press, Cambridge, UK: p. 1–29. [Google Scholar]
- Koenig K. M., and Beauchemin K. A.. 2017. Feeding tannins to reduce nitrogen losses from feedlot cattle fed high protein diets containing wheat distillers grains: Ruminal fermentation, digestibility, and route of nitrogen excretion. J. Anim. Sci. 95(Suppl 4):278–279. doi: 10.2527/asasann.2017.569 [DOI] [Google Scholar]
- Kool D. M., Hoffland E., Hummelink E. W. J., and van Groenigen J. W.. 2006. Increased hippuric acid content of urine can reduce soil N2O fluxes. Soil Biol. Biochem. 38:1021–1027. doi: 10.1016/j.soilbio.2005.08.017 [DOI] [Google Scholar]
- Kronberg S. L., and Liebig M. A.. 2011. Condensed tannin in drinking water reduces greenhouse gas precursor urea in sheep and cattle urine. Rangel. Ecol. Manag. 64:543–547. doi: 10.2111/REM-D-10-00165.1 [DOI] [Google Scholar]
- Lessa A. C. R., Madari B. E., Paredes D. S., Boddey R. M., Urquiaga S., Jantalia C. P., and Alves B. J. R.. 2014. Bovine urine and dung deposited on Brazilian savannah pastures contribute differently to direct and indirect soil nitrous oxide emissions. Agric. Ecosyst. Environ. 190:104–111. doi: 10.1016/j.agee.2014.01.010 [DOI] [Google Scholar]
- Lesschen J. P., Velthof G. L., de Vries W., and Kros J.. 2011. Differentiation of nitrous oxide emission factors for agricultural soils. Environ. Pollut. 159:3215–3222. doi: 10.1016/j.envpol.2011.04.001 [DOI] [PubMed] [Google Scholar]
- Li Y. L., Beauchemin K. A., McAllister T. A., and Yang W. Z.. 2014. Intakes and excretion route of nitrogen, phosphorous and sulfur by finishing beef heifers fed increasing levels of wheat dried distillers grains with solubles to substitute for barley grain and barley silage. Livest. Sci. 170:43–52. doi: 10.1016/j.livsci.2014.09.020 [DOI] [Google Scholar]
- Liang L. L., Eberwein J. R., Allsman L. A., Grantz D. A., and Jenerette G. D.. 2015. Regulation of CO2 and N2O fluxes by coupled carbon and nitrogen availability. Environ. Res. Lett. 10:034008. doi: 10.1088/1748-9326/10/3/034008 [DOI] [Google Scholar]
- Marini J. C., and Van Amburgh M. E.. 2005. Partition of nitrogen excretion in urine and the feces of Holstein replacement heifers. J. Dairy Sci. 88:1778–1784. doi: 10.3168/jds.S0022-0302(05)72852-6 [DOI] [PubMed] [Google Scholar]
- Menezes A. C. B., Valadares Filho S. C., Costa e Silva L. F., Pacheco M. V. C., Pereira J. M. V., Rotta P. P., Zanetti D., Detmann E., Silva F. A. S., Godoi L. A.,. et al. 2016. Does a reduction in dietary crude protein content affect performance, nutrient requirements, nitrogen losses, and methane emissions in finishing Nellore bulls?Agric. Ecosyst. Environ. 223:239–249. doi: 10.1016/j.agee.2016.03.015 [DOI] [Google Scholar]
- Mulvaney R. L. 1996. Nitrogen – Inorganic forms. In: Sparks D. L., Page A. L., Helmke P. A., Loeppert R. H., Soltanpour P. N., Tabatabai M. A., Johnston C. T., and Sumner M. E., editors, Methods of soil analysis. Part 3. Chemical methods. Soil Sci. Soc. Am., Inc, Madison, WI: p. 1123–1184. [Google Scholar]
- Nennich T. D., Harrison J. H., VanWieringen L. M., Meyer D., Heinrichs A. J., Weiss W. P., St-Pierre N. R., Kincaid R. L., Davidson D. L., and Block E.. 2005. Prediction of manure and nutrient excretion from dairy cattle. J. Dairy Sci. 88:3721–3733. doi: 10.3168/jds.S0022-0302(05)73058-7 [DOI] [PubMed] [Google Scholar]
- Norman R. J., Edberg J. C., and Stucki J. W.. 1985. Determination of nitrate in soil extracts by dual-wavelength ultraviolet spectrophotometry. Soil Sci. Soc. Am. J. 49:1182–1185. doi: 10.2136/sssaj1985.03615995004900050022x [DOI] [Google Scholar]
- Öquist M. G., Nilsson M., Sörensson F., Kasimir-Klemedtsson A., Persson T., Weslien P., and Klemedtsson L.. 2004. Nitrous oxide production in a forest soil at low temperatures – Processes and environmental controls. FEMS Microbiol. Ecol. 49:371–378. doi: 10.1016/j.femsec.2004.04.006 [DOI] [PubMed] [Google Scholar]
- Patra A. K., and Saxena J.. 2011. Exploitation of dietary tannins to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 91:24–37. doi: 10.1002/jsfa.4152 [DOI] [PubMed] [Google Scholar]
- Powell J. M., Aguerre M. J., and Wattiaux M. A.. 2011. Dietary crude protein and tannin impact dairy manure chemistry and ammonia emissions from incubated soils. J. Environ. Qual. 40:1767–1774. doi: 10.2134/jeq2011.0085 [DOI] [PubMed] [Google Scholar]
- Powell J. M., and Rotz C. A.. 2015. Measures of nitrogen use efficiency and nitrogen loss from dairy production systems. J. Environ. Qual. 44:336–344. doi: 10.2134/jeq2014.07.0299 [DOI] [PubMed] [Google Scholar]
- Ravishankara A. R., Daniel J. S., and Portmann R. W.. 2009. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 326:123–125. doi: 10.1126/science.1176985 [DOI] [PubMed] [Google Scholar]
- Redding M. R., Devereux J., Phillips F., Lewis R., Naylor T., Kearton T., Hill C. J., and Weidemann S.. 2015. Field measurement of beef pen manure methane and nitrous oxide reveals a surprise for inventory calculations. J. Environ. Qual. 44:720–728. doi: 10.2134/jeq2014.04.0159 [DOI] [PubMed] [Google Scholar]
- Reed J. D. 1995. Nutritional toxicology of tannins and related polyphenols in forage legumes. J. Anim. Sci. 73:1516–1528. doi: 10.2527/1995.7351516x [DOI] [PubMed] [Google Scholar]
- Sanchez-Martín L., Beccaccia A., De Blas C., Sanz-Cobena A., García-Rebollar P., Estellés F., Marsden K. A., Chadwick D. R., and Vallejo A.. 2017. Diet management to effectively abate N2O emissions from surface applied pig slurry. Agric. Ecosyst. Environ. 239:1–11. doi: 10.1016/j.agee.2016.12.007 [DOI] [Google Scholar]
- Shahrzad S., and Bitsch I.. 1998. Determination of gallic acid and its metabolites in human plasma and urine by high-performance liquid chromatography. J. Chromatogr. B Biomed. Sci. Appl. 705:87–95. doi: 10.1016/S0378-4347(97)00487-8 [DOI] [PubMed] [Google Scholar]
- Van Groenigen J. W., Kasper G. J., Velthof G. L., Van Den Pol-Van Dasselaar A., and Kuikman P. J.. 2004. Nitrous oxide emissions from silage maize fields under different mineral nitrogen fertilizer and slurry applications. Plant Soil. 263:101–111. doi: 10.1023/B:PLSO.0000047729.43185.46 [DOI] [Google Scholar]
- Van Groenigen J. M., Palermo V., Kool D. M., and Kuikman P. J.. 2006. Inhibition of denitrification and N2O emission by urine-derived benzoic and hippuric acid. Soil Biol. Biochem. 38:2499–2502. doi: 10.1016/j.soilbio.2006.02.023 [DOI] [Google Scholar]
- Van Soest P. J., Robertson J. B., and Lewis B. A.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74:3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]
- Varel V. H. 1997. Use of urease inhibitors to control nitrogen loss from livestock waste. Bioresour. Technol. 62:11–17. doi: 10.1016/S0960-8524(97)00130-2 [DOI] [Google Scholar]
- Waghorn G. 2008. Beneficial and detrimental effects of dietary condensed tannins for sustainable sheep and goat production – Progress and challenges. Anim. Feed Sci. Technol. 147:116–139. doi: 10.1016/j.anifeedsci.2007.09.013 [DOI] [Google Scholar]
- Wang F., Li J., Wang X., Zhang W., Zou B., Neher D. A., and Li Z.. 2014. Nitrogen and phosphorus addition impact soil N2O emission in a secondary tropical forest of South China. Sci. Rep. 4:5615. doi: 10.1038/srep05615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Osada T., Yoh M., and Tsuruta H.. 1997. N2O and NO emissions from grassland soils after the application of cattle and swine excreta. Nutr. Cycl. Agroecosyst. 49:35–39. doi: 10.1023/a:1009794705731 [DOI] [Google Scholar]
- Wei C., Yang K., Zhao G., Lin S., and Xu Z.. 2016. Effect of dietary supplementation of gallic acid on nitrogen balance, nitrogen excretion pattern and urinary nitrogenous constituents in beef cattle. Arch. Anim. Nutr. 70:416–423. doi: 10.1080/1745039X.2016.1214345 [DOI] [PubMed] [Google Scholar]
- Whitehead D. C., Lockyer D. R., and Raistrick N.. 1989. Volatilization of ammonia from urea applied to soil: Influence of hippuric acid and other constituents of livestock urine. Soil Biol. Biochem. 21:803–808. doi: 10.1016/0038-0717(89)90174-0 [DOI] [Google Scholar]
- Wischer G., Greiling A. M., Boguhn J., Steingass H., Schollenberger M., Hartung K., and Rodehutscord M.. 2014. Effects of long-term supplementation of chestnut and valonea extracts on methane release, digestibility and nitrogen excretion in sheep. Animal 8:938–948. doi: 10.1017/S1751731114000639 [DOI] [PubMed] [Google Scholar]
- Wrage N., Velthof G. L., Van Beusichem M. L., and Oenema O.. 2001. Role of nitrifier denitrification in the production of nitrous oxide. Soil Biol. Biochem. 33:1723–1732. doi: 10.1016/S0038-0717(01)00096-7 [DOI] [Google Scholar]
- Yang K., Wei C., Zhao G., Xu Z., and Lin S.. 2016. Dietary supplementation of tannic acid modulates nitrogen excretion pattern and urinary nitrogenous constituents of beef cattle. Livest. Sci. 191:148–152. doi: 10.1016/j.livsci.2016.07.020 [DOI] [PubMed] [Google Scholar]
- Zhang J., Müller C., and Cai Z.. 2015. Heterotrophic nitrification of organic N and its contribution to nitrous oxide emissions in soils. Soil Biol. Biochem. 84:199–209. doi: 10.1016/j.soilbio.2015.02.028 [DOI] [Google Scholar]
- Zhao G. 2017. Modulation of protein metabolism to mitigate nitrous oxide (N2O) emission from excreta of livestock. Curr. Protein Pept. Sci. 18:525–531. doi: 10.2174/1389203717666160627080423 [DOI] [PubMed] [Google Scholar]