Abstract
Phytogenics have been reported to improve growth performances in farm animals and are thereby considered as potential key solutions for antibiotic-free livestock nutrition. Yet, their effects on meat quality are still not well defined; therefore, the aim of this study was to determine the effects of 5 experimental phytogenic additives (3 dietary and 2 water supplements) on growth and meat quality in broilers. One-day-old broiler chicks (n = 576) were assigned to 48 floor pens and divided into 6 treatments (Control, AV/HGP/16 premix [AVHGP], Superliv concentrate premix [SCP], bacteriostatic herbal growth promotor [BHGP], AV/SSL/12 [AVSSL], and Superliv Gold [SG]) in a complete randomized design (8 pens/treatment with 12 birds/pen, and 96 birds/group). Feed intake and BW were recorded, and birds were processed at 42 d to evaluate carcass traits. Breast muscle tissues were excised to determine stress- and antioxidant-related genes expression. Both AVSSL- and SG-treated broilers produced heavier (P < 0.05) slaughter weights compared with the control-fed broilers, whereas AVSSL supplementation decreased (P < 0.05) fat pad size and increased (P < 0.05) breast weights compared with the control-fed broilers. Although pH and a* values remained unchanged, L* was decreased (P < 0.05) in all treatment and b* was reduced (P < 0.05) in SG when compared with controls. The trained sensory panelists detected more (P < 0.05) green herb flavor in the breast meat from AVHGP than SCP, SG, and control birds. The expression of superoxide dismutase 2, extracellular signal-regulated kinase 1/2, and JNK gene was upregulated in AVHGP and BHGP compared with the control (P < 0.05). Together, these results indicated that phytogenic additives might improve meat quality of broilers through modulation of stress- and antioxidant-related pathways.
Keywords: antioxidation, gene expression, meat quality, phytogenics, sensory
INTRODUCTION
Broiler production in the United States, and worldwide, is more efficient and cost-effective now than 20 yr ago because of advances in genetic selection and on-farm housing/environmental and nutritional management. Today, a broiler averaging over 2.80 kg of BW can be sent to market in 42 d, whereas, in 1995, a bird was sent to market at the same age averaged only 2 kg (National Chicken Council, 2017). High-yielding, fast-growing feed-efficient broilers are beneficial to help meeting the growing demands for poultry product, but may be to the detriment of meat quality. With rapid growth rate, metabolic muscle myopathies have developed, including woody breast and white striping, as well as issues with water-holding capacity, tenderness, and fat oxidation (Wu et al., 1994; Smith et al., 2002; Lyon et al., 2004). There is, therefore, a critical need for novel effective strategies (nutrition, management, and/or selection) to prevent, or reduce, the incidence of these metabolic disorders and improve meat quality.
Restriction on the use of direct-feed antibiotics in many countries has fueled the interest in alternative products like phytogenics (a group of natural products) that have been the focus of several studies in recent years. These plant-derived products have been reported to have several beneficial effects from antimicrobial and antioxidant activities to improvement of gut health and growth performance (Bazargani-Gilani et al., 2014; Olnood et al., 2015; Yang et al., 2015). For instance, oregano and other herbal-based products have been included in the diet to help alleviate coccidiosis issues and improve the gut health of the birds (Mohiti-Asli and Ghanaatparast-Rashti, 2015). A recent field trial study has shown that phytogenic (Superliv)-supplemented diet increase BW and feed conversion ratio (FCR) in broilers (Amitav et al., 2013, 2015; Sudhir et al., 2016). However, whether these phytogenic additives improve meat quality remains unknown.
The present study aimed, therefore, to determine the effects of 3 phytogenic feed additives and 2 phytogenic water additives, including AV/HGP/16, Superliv concentrate premix, AGP premix (bacteriostatic herbal growth promotor with essential oils), AV/SSL/12, and Superliv Gold, respectively, on the carcass parameters and meat quality traits in commercial modern broilers.
MATERIALS AND METHODS
The present study was conducted in accordance with the recommendations in the guide for the care and use of laboratory animals of the National Institutes of Health and the protocol was approved by the University of Arkansas Animal Care and Use Committee (protocol no. 16084).
Animals, Diets, and Experimental Design
A total of 576-d-old male broiler chicks (Cobb 500 by-products, Cobb-Vantress, Inc., Siloam Spring, AR) with an average BW of 41 ± 0.4 g were allotted randomly to 48 pens (12 birds/pen), and 8 pens were assigned randomly to 1 of 6 treatments: basal starter, grower, and finisher diet (CTRL; Table 1), basal diet supplemented with 0.55 g/kg of diet of either a bacteriostatic herbal growth promotor with essential oils (BHGP), AV/HGP/16 premix (AVHGP), or Superliv concentrate premix (SCP); or fed CTRL diet with either AV/SSL/12 (AVSSL), and Superliv Gold (SG) added to water at 2, 4, and 7 mL/100 birds/d during the starter, grower, and finisher phases, respectively. The composition of these phytogenic additives are proprietary to Ayurvet Ltd. (Kaushambi, Ghaziabad, India), but are apolyherbal formulations of prestandardized and tested herbs. Birds had ad libitum access to feed (Choretime feeders; Georgia Poultry, Newton Grove, NC) and clean water (Ziggity water system, Georgia Poultry, Newton Grove, NC) at all times, and ambient temperature was reduced gradually from 32 to 25 °C during the first 21 d of the trial, whereas relative humidity (RH, ~20%) and a light:dark cycle of 20-h light:4-h dark were maintained throughout the 42-d feeding trial.
Table 1.
Composition of basal diets
| Ingredient, % | Starter (0 to14 d) |
Grower (15 to 28 d) |
Finisher (29 to 42 d) |
|---|---|---|---|
| Corn | 59.36 | 64.22 | 66.48 |
| Soybean meal | 32.85 | 27.70 | 25.25 |
| MBM, 50% | 2.50 | 2.50 | 2.50 |
| Poultry oil | 2.01 | 2.65 | 3.14 |
| Sodium chloride | 0.38 | 0.31 | 0.31 |
| Sodium bicarbonate | 0.00 | 0.05 | 0.05 |
| Limestone | 0.80 | 0.74 | 0.70 |
| Dicalcium phosphate | 1.13 | 1.00 | 0.85 |
| Vitamin premix1 | 0.10 | 0.10 | 0.10 |
| Mineral premix2 | 0.10 | 0.10 | 0.10 |
| Choline chloride | 0.10 | 0.10 | 0.10 |
| Selenium premix 0.06% | 0.02 | 0.02 | 0.02 |
| Santoquin | 0.02 | 0.02 | 0.02 |
| L-Lys HCl | 0.17 | 0.14 | 0.10 |
| DL-Met | 0.30 | 0.24 | 0.21 |
| L-Thr | 0.11 | 0.06 | 0.05 |
| Copper chloride | 0.02 | 0.02 | . |
| Xylanase | 0.01 | 0.01 | 0.01 |
| Phytase | 0.02 | 0.02 | 0.02 |
1Premix supplied 3,181.5 IU vitamin A; 2,272.7 IU vitamin D3; 22.73 vitamin E; 0.005 mg vitamin B12; 0.62 mg menadione; 2.73 mg riboflavin; 4.09 mg d-panthothenic acid; 15.91 mg niacin; 0.36 mg folic acid; 1.14 mg pyridoxine; 0.64 mg thiamine; and 0.034 mg biotin per kg of diet.
2Premix supplied 55-mg Ca, 100-mg Mn, 27-mg Mg, 100-mg Zn, 50-mg Fe, 10-mg Cu, and 1-mg I per kg of diet.
Birds were processed at 42 d of age using a commercial inline system at the University of Arkansas Pilot Processing Plant (Fayetteville, AR). Birds were electrically stunned (11 V, 11 mA for 11 s), exsanguinated, scaled at 53.8 °C for 2 min, and picked using a commercial, inline defeatherer (Foodcraft Model 3; Baker international, MI, USA). After manual evisceration and rinsing, carcasses were prechilled at 12 °C for 15 min and chilled for 90 min at 1 °C in immersion chilling tanks with manual agitation at 15-min regular intervals. Slaughter weight, prechill, and postchill carcasses, as well as abdominal fat weights were recorded, and following a 4-h chill at 4 °C, breasts and tenders were removed and weighed before breast fillets were stored overnight in Ziploc bags (17.7 × 12.7 cm, SC Johnson Inc., Racine, WI) at 4 ºC. At the end of the 24-h chilling period, each breast fillet was removed from bags, blotted dry on paper towels, and reweighed to calculate drip loss according to the procedure of Woelfel et al. (2002). Drip loss was calculated as the difference between initial and final weights and expressed as a percentage of the initial breast fillet weight. Following drip loss measurement, instrumental (L*, a*, and b*) color was measured on the ventral side of the right breast fillet from 3 readings with a Minolta colorimeter (CR-400; Konica Minolta Sensing Inc., Sakai Osaka, Japan; size 102 (W) × 217 (H) × 63 (D) mm) using illuminant D65 and a 2.54-cm aperture. In addition, ultimate muscle pH was measured with a temperature-compensating pH meter (Testo 205; Testo Inc., West Chester, PA) inserted into the cranial region of the right breast lobe with averaged 3 measurements per each sample.
Thiobarbituric Acid-Reactive Substances
Thiobarbituric acid-reactive substances (TBARS) were determined according to the procedure of Witte et al. (1970) as modified by Kuntapanit (1978). Briefly, right breast fillet from 3 birds/pen were cut into equal-dimensional sections, packaged in Whirlpak bags (7.5 × 18.5 cm; Sigma-Aldrich, St. Louis, MO), stored at 4 °C, and samples from each bird were removed at 1-, 4-, 7-, and 10-d postrocessing, and subsequently frozen at −80 °C until analysis. Samples were tempered at −20 °C for 24 h before knife-minced, and 2 g of mince breast fillet were mixed with 8 mL of 50 mM phosphate buffer (pH 7.0) and 2 mL of 30% trichloroacetic acid, homogenized (VWR 250 Homogenizer; VWR, Radnor, PA) for 20 to 30 s, and the homogenate was filtered through Whatman filter paper (grade 4; Sigma-Adrich, St. Louis, MO). A 2-mL aliquot of filtrate was mixed with 2 mL of 0.02 M 2-thiobarbuturic acid, placed in a 100 °C water bath for 20 min, and subsequently placed into an ice-water bath for 15 min. Absorbance read at 533 nm on a spectrophotometer (Synergy HT multi-mode micro plate reader, BioTek, Winooski, VT) was multiplied by 12.21 to calculate mg of maldonaldehyde (MDA)/kg of breast meat.
Sensory Panel
Frozen breast fillets were thawed overnight at 4 °C before being cooked in a convection oven preheated to 176 °C on aluminum-lined and covered pans with raised wire racks to an internal endpoint temperature of 76 °C monitored with a handled digital thermometer. Cooked fillets were cut into 2.54 × 2.54 cm × fillet thickness cubes. Duplicate samples were served warm to a 9-person professional meat descriptive panel (University of Arkansas Food Science Department’s Sensory Service Center, Fayetteville, AR) in 2 sessions. The descriptive panel was initially trained according to the Spectrum method (Meilgaard et al., 2007), and panelists had more than 7,500 h of sensory experience, especially with poultry and other meat products. A presampling orientation session was performed to refine particular sensory attribute definitions similar to that described by Lyon and Lyon (1998) and Cavitt et al. (2004). Each panelist evaluated samples presented to them in a random order, and evaluated aromas (cooked chicken, brothy, chicken fat, blood serum/metallic, green herb, wet feathers, and barnyard), basic tastes (salt, sweet, sour, and bitter), flavors (cooked chicken, brothy, chicken fat, organy, blood serum, metallic, green herb, cardboard, wet feathers, and barnyard), feeling factors (metallic and astringency), and texture (moisture release). All sensory attributes and texture characteristics were scored to the nearest 0.5 on a scale ranging from 0 (least intense) to 15 (most intense).
RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
Total RNA were extracted from chicken left-beast muscle tissues by Trizol reagent (Life Technologies, Grand Island, NY) according to manufacturer’s recommendations. After DNAse treatment and purification, total RNA concentrations were determined for each sample by Take 3 Micro-Volume Plate using Synergy HT multimode microplate reader (BioTek,Winooski, VT), and RNA integrity and quality were assessed by both OD260/OD280 nm absorption ratio (>1.9) and by using 1% agarose gel electrophoresis. For cDNA synthesis, total RNA (1 µg) was reverse transcribed using qScript cDNA Synthesis Kit (Quanta Biosciences, Gaithersburg, MD) in a 20-µL total reaction. The reverse transcription reaction was performed at 42 °C for 30 min followed by an incubation at 85 °C for 5 min. Real-time quantitative PCR (Applied Biosystems 7500 Real-Time PCR system) was performed using 5 µL of 10×-diluted cDNA, 0.5 µM of each forward and reverse specific primer, and SYBR Green Master Mix (ThermoFisher Scientific, Rockford, IL) in a total 20-µL reaction as previously described by Lassiter et al. (2015) and Flees et al. (2017). Oligonucleotide primers specific for chicken superoxide dismutase 1 and 2 (SOD1/2), glutathione peroxidase 1 (GPX1), heat shock protein 70 (HSP-70), extracellular signal-regulated kinase 1 and 2 (ERK1/2), c-jun N-terminal kinase 1 (JNK1), nuclear factor erythroid 2-related factor (NRF2), and r18S as housekeeping gene were summarized in Table 2. Relative expressions of target genes were determined by the 2−∆∆Ct method (Schmittgen and Livak, 2008). Samples extracted from CTRL-fed broilers were used as a calibrator.
Table 2.
Oligonucleotide primers for real-time qPCR
| Gene1 | Accession number2 | Primer sequence (5′ → 3′) | Orientation | Product size (bp) |
|---|---|---|---|---|
| SOD1 | NM_205064 | TGGCTTCCATGTGCATGAAT | Forward | 58 |
| AGCACCTGCGCTGGTACAC | Reverse | |||
| SOD2 | NM_204211 | GCTGGAGCCCCACATCAGT | Forward | 61 |
| GGTGGCGTGGTGTTTGCT | Reverse | |||
| GPX1 | NM_001277853 | TCCCCTGCAACCAATTCG | Forward | 57 |
| AGCGCAGGATCTCCTCGTT | Reverse | |||
| HSP70 | JO2579 | GGGAGAGGGTTGGGCTAGAG | Forward | 55 |
| TTGCCTCCTGCCCAATCA | Reverse | |||
| ERK1 | NM_204150 | CGGACCATGATCACACAGGAT | Forward | 63 |
| CAGGAGCCCTGTACCAACGT | Reverse | |||
| ERK2 | AY033635 | CGGACCATGATCACACAGGAT | Forward | 63 |
| CAGGAGCCCTGTACCAACGT | Reverse | |||
| JNK1 | NM_205095 | GCCGATGATCAGCCAGGAT | Forward | 62 |
| GGCCCAATGGAAGCAAGAG | Reverse | |||
| r18S | AF173612 | TCCCCTCCCGTTACTTGGAT | Forward | 60 |
| GCGCTCGTCGGCATGTA | Reverse | |||
| NRF2 | NM_001030646 | GGCCAACGTCCGAAGTGAT | Forward | 55 |
| CCATGACACCCGCTGCTT | Reverse |
1ERK1 and ERK2 = extracellular signal-regulated kinase 1 and 2, respectively; GPX1 = glutathione peroxidase 1; HSP70 = heat shock protein 70; JNK1 = c-jun N-terminal kinase 1; SOD1 and SOD2 = superoxide dismutase 1 and 2, respectively; r18S = ribosomal 18S; NRF2 = nuclear factor (erythroid-derived 2)-like 2.
2Accession number refers to Genbank (NCBI).
Total Antioxidant Activity
Antioxidant properties of the phytogenic additives were determined by measuring radical-scavenging ability using the stable free radical 2,2-diphenyl-1-picrylhydrazyl (DPPH) colorimetric assay as previously described by Loizzo et al. (2010). Briefly, phytogenic extracts were diluted 20:1 using methanol and centrifuged (6037 g) for 5 min. In a 48-well plate, 0.2 mL of sample supernatant was added to 0.2 mL DPPH (0.1 mM in methanol). After shaking for 30 min in dark conditions, plates were read in microplate reader (BioTek Instruments, Winooski, VT) at 517 nm. All samples and blanks were read in triplicate, and scavenging effect (%) was determined using the following formula:
Statistical Analysis
Data were analyzed as a completely randomized design, with pen as the experimental unit. The ANOVA was generated using the mixed models procedure of SAS (SAS Institute, Inc., Cary, NC), with phytogenic supplement as the lone fixed effect in the model. In the ANOVA of the sensory data, panelist nested within session was included as a random effect in the model. Least square means were computed and separated with the pairwise t-tests (PDIFF option of SAS) when a significant (P < 0.05) F-test was noted. Gene expression and TBARS data were analyzed by 1- and 2-way ANOVA, respectively, and means for gene expression and TBARS were compared using Student–Newman–Keuls (SNK) and Tukey’s multiple comparison tests, respectively, in GraphPad Prism (Version 7.0, Graph Pad Software, La Jolla, CA).
RESULTS
Productive Data and Carcass Characteristics
Birds drinking water supplemented with AVSSL and SG had heavier (P < 0.05) slaughter weights than all other birds, and produced heavier (P < 0.05) hot (HCW) and chilled (CCOG) carcass weights than birds consuming diets supplemented with AVHGP, SCP, and BHGP (Table 3). Supplementing broiler water with AVSSL and SG resulted in decreased (P < 0.05) fat pad percentages and increased (P < 0.05) breast weights compared with the untreated (control) birds. The other additives (AVHGP, SCP, and BHGP) did not elicit any significant changes in fat pad size and breast weights compared with the control group (Table 3).
Table 3.
Productive and carcass characteritistics
| Parameters1 | Experimental groups2 | |||||
|---|---|---|---|---|---|---|
| C | AVHGP | SCP | BHGP | AVSSL | SG | |
| SW (g) | 2263.7 ± 34b | 2214.8 ± 32.1b | 2228.9 ± 36.4b | 2243.9 ± 34b | 2383.8 ± 34a | 2361.8 ± 34a |
| HCW (g) | 1630.5 ± 26.2ab | 1581.3 ± 24.7b | 1604.3 ± 28b | 1607.4 ± 26.2b | 1701.7 ± 26.2a | 1691.5 ± 26.2a |
| CCWG (g) | 1672.5 ± 26.8ab | 1615.5 ± 25.3b | 1642.2 ± 28.6b | 1642.1 ± 26.8b | 1748.2 ± 26.8a | 1741.9 ± 26.8a |
| Fat Pad (%) | 2.01 ± 0.05ab | 2.11 ± 0.05a | 2.09 ± 0.05a | 1.91 ± 0.05bc | 1.8 ± 0.05c | 1.91 ± .05bc |
| Breast (g) | 422.9 ± 9.6bc | 409.4 ± 9.1c | 419.5 ± 10.3bc | 422.2 ± 9.6bc | 451.7 ± 9.6a | 445.2 ± 9.6ab |
| Breast (%) | 25.21 ± 0.22 | 25.27 ± 0.21 | 25.4 ± 0.24 | 25.62 ± 0.23 | 25.73 ± .23 | 25.43 ± 0.23 |
Values shown are means ± SEM (n = 80).
1CCWG = chilled carcass without giblets; HCW = hot carcass weight; SW = slaughter weight.
2AVHGP = AV/HGP/16; AVSSL = AV/SSL/12; BHGP = bacteriostatic herbal growth promoter with essential oils; C = control; SCP = Superliv Concentrate Premix; SG = Superliv Gold. Different letters within a row indicate a significant difference at P < 0.05.
Meat Color and pH
Breast pH after 24-h postmortem did not differ (P > 0.05) among the treatment groups; however, fillets from AVSSL-supplemented birds had the greatest (P < 0.05) drip loss percentages, whereas drip losses were greater (P < 0.05) in fillets of birds fed CTRL, AVHGP, and SCP than either broilers eating BHGP-supplemented diets or drinking SG-supplemented water (Table 4).
Table 4.
Effects of phytogenic supplementation on meat color and water holding capacity
| Parameters1 | Experimental groups2 | |||||
|---|---|---|---|---|---|---|
| C | AVHGP | SCP | BHGP | AVSSL | SG | |
| pH | 5.73 ± 0.01 | 5.73 ± 0.01 | 5.73 ± 0.01 | 5.71 ± 0.01 | 5.75 ± 0.01 | 5.73 ± 0.01 |
| L* | 53.34 ± 0.30a | 52.71 ± 0.25a | 51.69 ± 0.2b | 51.88 ± 0.2b | 51.23 ± 0.2b | 52.20 ± 0.3b |
| a* | 4.14 ± 0.08 | 4.13 ± 0.09 | 4.04 ± 0.06 | 4.16 ± 0.07 | 4.27 ± 0.08 | 3.96 ± 0.07 |
| b* | 1.26 ± 0.10a | 1.40 ± 0.10a | 1.05 ± 0.10a | 1.1 ± 0.10a | 1.11 ± 0.10a | 1.62 ± 0.10b |
| Drip loss | 1.43 ± 0.14a | 1.41 ± 0.10a | 1.45 ± 0.10a | 0.97 ± 0.08b | 2.14 ± 0.15c | 0.86 ± 0.08bd |
Values shown are means ± SEM (n = 80).
1a* = redness; b* = yellowness; L* = lightness.
2AVHGP = AV/HGP/16; AVSSL = AV/SSL/12; BHGP = bacteriostatic herbal growth promoter with essential oils; C = control; SCP = Superliv Concentrate Premix; SG = Superliv Gold. Different letters within a row indicate a significant difference at P < 0.05.
Even though redness (a*) values of breast fillets did not differ (P > 0.05) among experimental groups, fillets from CTRL- and AVHGP-fed birds were lighter (greater L* values, P < 0.05) in color than fillets from broilers supplemented with SCP, BHGP, AVSSL, and SG (Table 4). In addition, fillets from birds drinking SG had greater (P < 0.05) b* values (indicative of a more yellow color) than breast fillets from all other experimental groups (Table 4).
TBARS and Total Antioxidant Activity
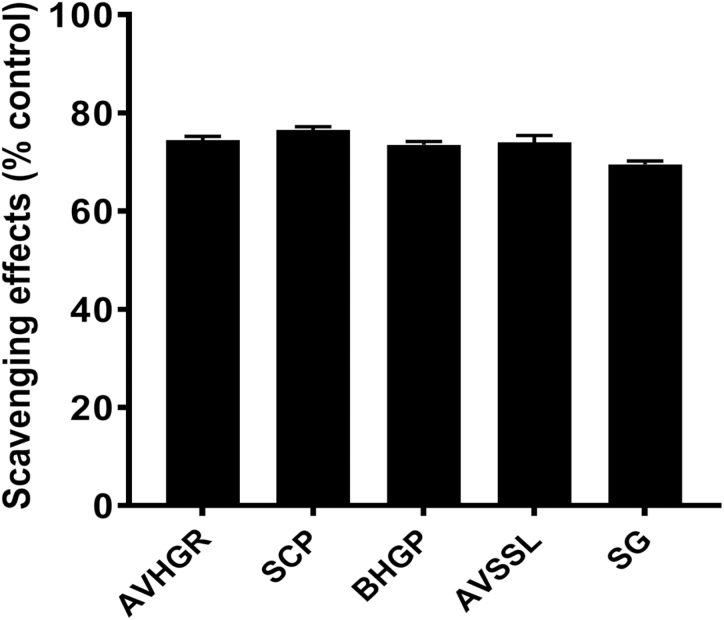
The DPPH analysis demonstrated that all phytogenic additives used in this study had a greater total antioxidant activity compared with the CTRL-fed broilers (Figure 1). Across all treatment groups, TBARS values were greater (P < 0.01) after 4, 7, and 10 d of refrigerated storage than initial (day 0) TBARS values, and there was a tendency (P = 0.07) for TBARS values to be reduced 37.0%, 28.0%, 13.3%, and 19.2% in breast samples from birds supplemented with SC, AVSSL, BHGP, and SCP, respectively, whereas diets supplemented with AVHGP had 62.0% greater TBARS values, compared with CTRL-fed broilers (results not presented in graphic or tabular form).
Figure 1.
Antioxidant properties of phytogenic additives determined by stable free radical DPPH assay. C = control; AVHGP = AV/HGP/16; AVSSL/12 = AVSSL; SCP = Superliv Concentrate Premix; BHGP = AGP premix: bacteriostatic herbal growth promoter with essential oils; SG = Superliv Gold.
Meat Aroma, Flavor, Taste, and Texture
Descriptive panelists did not detect (P > 0.05) any difference in aromas (cooking chicken, brothy, blood serum metallic, green herb, wet feather, or barnyard; Table 5) and basic tastes (salt, sweet, sour, and bitter; Table 6) of breast fillets among treatment groups. Panelists noted greater (P < 0.05) green herb flavor intensity in fillets from birds supplemented with AVHGP than those consuming CTRL- and SCP-diets, as well as birds drinking SG-supplemented water; otherwise, phytogenic supplementation had no effects (P > 0.05) on the intensity of cooked chicken, brothy, chicken fat, organy, blood serum, metallic, cardboard, wet feather, or barnyard flavors (Table 7). Although panelists failed to detect differences in metallic- (P > 0.05) and astringency- (P > 0.05) feeling factors among samples, panelists indicated that moisture release intensity was greater (P < 0.05) in fillets from birds fed CTRL, AVHGP, and BHGP than those drinking SG-supplemented water (Table 8).
Table 5.
Effect of phytogenic supplementation on aromas of broiler breast fillets
| AROMA | Experimental groups1 | |||||
|---|---|---|---|---|---|---|
| C | AVHGP | SCP | BHGP | AVSSL | SG | |
| Cooked chicken | 4.9 ± 0.19 | 4.9 ± 0.19 | 4.9 ± 0.19 | 4.8 ± 0.19 | 4.9 ± 0.19 | 4.9 ± 0.19 |
| Brothy | 2.0 ± 0.71 | 1.8 ± 0.71 | 2.0 ± 0.71 | 1.8 ± 0.71 | 2.0 ± 0.71 | 1.8 ± 0.71 |
| Blood serum metallic | 3.0 ± 0.25 | 2.9 ± 0.25 | 3.0 ± 0.25 | 3.0 ± 0.25 | 3.0 ± 0.25 | 3.1 ± 0.25 |
| Green herb | 0.0 ± 0.49 | 0.2 ± 0.49 | 0.0 ± 0.49 | 0.2 ± 0.49 | 0.4 ± 0.49 | 0.3 ± 0.49 |
| Wet feather | 1.5 ± 0.77 | 1.5 ± 0.77 | 0.9 ± 0.77 | 1.6 ± 0.77 | 1.2 ± 0.77 | 1.1 ± 0.77 |
| Barnyard | 0.4 ± 0.06 | 0.4 ± 0.06 | 0.4 ± 0.06 | 0.4 ± 0.06 | 0.4 ± 0.06 | 0.4 ± 0.06 |
Values shown are means ± SEM (n = 94).
1AVHGP = AV/HGP/16; AVSSL = AV/SSL/12; BHGP = bacteriostatic herbal growth promoter with essential oils; C = control; SCP = Superliv Concentrate Premix; SG = Superliv Gold.
Table 6.
Effects of phytogenic supplementation on basic taste of broiler breast fillets
| Basic taste | Experimental groups1 | |||||
|---|---|---|---|---|---|---|
| C | AVHGP | SCP | BHGP | AVSSL | SG | |
| Salt | 2.6 ± 0.24 | 2.7 ± 0.24 | 2.7 ± 0.24 | 2.6 ± 0.24 | 2.7 ± 0.24 | 2.7 ± 0.24 |
| Sweet | 0.2 ± 0.07 | 0.1 ± 0.07 | 0.1 ± 0.07 | 0.1 ± 0.07 | 0.1 ± 0.07 | 0.1 ± 0.07 |
| Sour | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.6 ± 0.2 |
| Bitter | 0.5 ± 0.21 | 0.5 ± 0.21 | 0.5 ± 0.21 | 0.6 ± 0.21 | 0.6 ± 0.21 | 0.4 ± 0.21 |
Values shown are means ± SEM (n = 94).
1AVHGP = AV/HGP/16; AVSSL = AV/SSL/12; BHGP = bacteriostatic herbal growth promoter with essential oils; C = control; SCP = Superliv Concentrate Premix; SG = Superliv Gold.
Table 7.
Effects of phytogenic supplementation on flavor of broiler breast fillets
| Flavor | Experimental groups1 | |||||
|---|---|---|---|---|---|---|
| C | AVHGP | SCP | BHGP | AVSSL | SG | |
| Cooked chicken | 6.1 ± 0.21 | 6.0 ± 0.21 | 6.0 ± 0.21 | 5.9 ± 0.21 | 6.1 ± 0.21 | 6.1 ± 0.21 |
| Brothy | 1.7 ± 0.56 | 1.8 ± 0.56 | 1.4 ± 0.56 | 1.4 ± 0.56 | 1.7 ± 0.56 | 1.7 ± 0.56 |
| Chicken fat | 0.0 ± 0.33 | 0.2 ± 0.33 | 0.0 ± 0.33 | 0.0 ± 0.33 | 0.2 ± 0.33 | 0.2 ± 0.33 |
| Organy | 0.8 ± 0.57 | 0.6 ± 0.57 | 0.7 ± 0.57 | 0.9 ± 0.57 | 1.0 ± 0.57 | 1.1 ± 0.57 |
| Blood serum | 2.8 ± 0.42 | 2.7 ± 0.42 | 2.7 ± 0.42 | 2.6 ± 0.42 | 2.7 ± 0.42 | 2.8 ± 0.42 |
| Metallic | 1.5 ± 0.36 | 1.3 ± 0.36 | 1.7 ± 0.36 | 1.4 ± 0.36 | 1.6 ± 0.36 | 1.2 ± 0.36 |
| Green herb | 0.0 ± 0.54b | 0.7 ± 0.54a | 0.0 ± 0.54b | 0.5 ± 0.54ab | 0.5 ± 0.54ab | 0.2 ± 0.54b |
| Cardboard | 1.2 ± 0.43 | 1.2 ± 0.43 | 1.1 ± 0.43 | 1.0 ± 0.43 | 1.2 ± 0.43 | 1.1 ± 0.43 |
| Wet feather | 0.9 ± 0.5 | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.4 ± 0.5 | 0.9 ± 0.5 | 0.9 ± 0.5 |
| Barnyard | 0.4 ± 0.03 | 0.4 ± 0.03 | 0.4 ± 0.03 | 0.4 ± 0.03 | 0.4 ± 0.03 | 0.4 ± 0.03 |
Values shown are means ± SEM (n=94).
1AVHGP = AV/HGP/16; AVSSL = AV/SSL/12; BHGP = bacteriostatic herbal growth promoter with essential oils; C = control; SCP = Superliv Concentrate Premix; SG = Superliv Gold. Different letters within a row indicate a significant difference at P < 0.05.
Table 8.
Effects of phytogenic supplementation on texture of broiler breast fillets
| Texture | Experimental groups1 | |||||
|---|---|---|---|---|---|---|
| C | AVHGP | SCP | BHGP | AVSSL | SG | |
| Metallic FF | 2.9 ± 0.45 | 2.9 ± 0.45 | 3.1 ± 0.45 | 2.9 ± 0.45 | 3.0 ± 0.45 | 3.0 ± 0.45 |
| Astringency FF | 6.7 ± 0.29 | 6.6 ± 0.29 | 6.7 ± 0.29 | 6.7 ± 0.29 | 6.7 ± 0.29 | 6.8 ± 0.29 |
| Moisture release | 1.1 ± 0.36a | 1.2 ± 0.36a | 0.9 ± 0.36ab | 1.2 ± 0.36a | 0.9 ± 0.36ab | 0.7 ± 0.36b |
Values shown are means ± SEM (n = 94).
1AVHGP = AV/HGP/16; AVSSL = AV/SSL/12; BHGP = bacteriostatic herbal growth promoter with essential oils; C = control; SCP = Superliv Concentrate Premix; SG = Superliv Gold. Different letters within a row indicate a significant difference at P < 0.05.
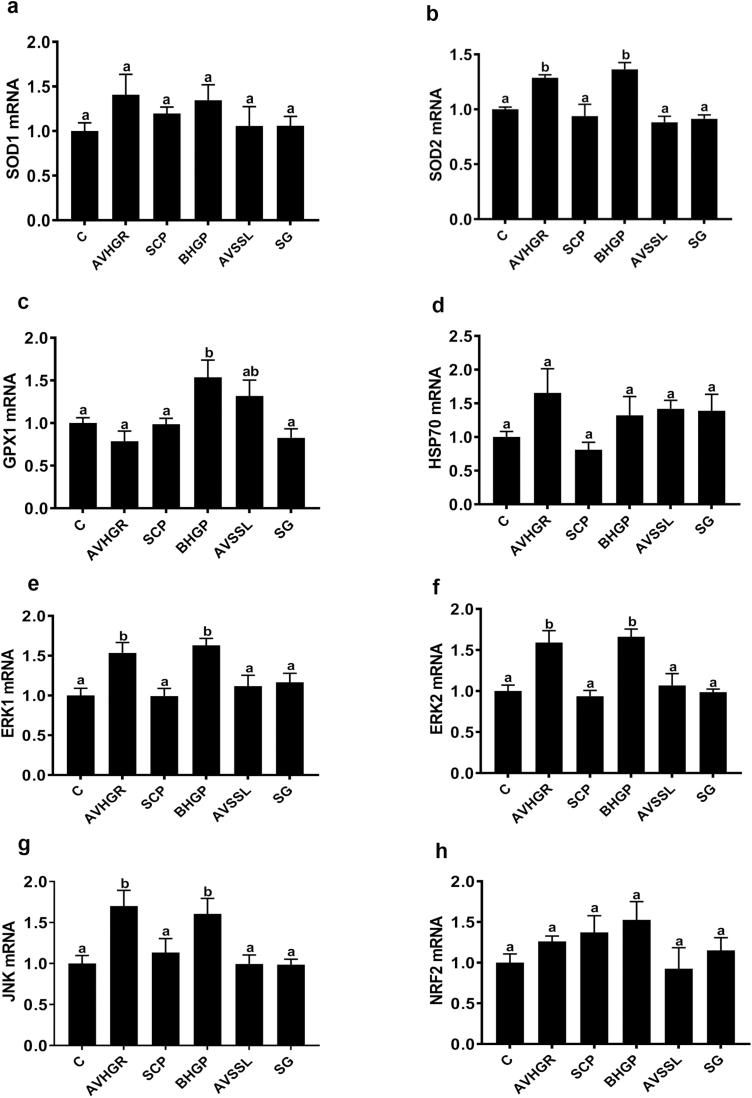
Gene Expression
Feeding diets supplemented with AVHGP and BHGP upregulated (P < 0.05) the muscle expression of SOD2, ERK1, ERK2, and JNK compared with the CTRL-fed birds (Figure 2b and e–g, respectively). However, the GPX1 mRNA abundance was increased (P < 0.05) in breast muscle of BHGP-fed birds compared with the CTRL-fed group (Figure 2c), but the expression of SOD1 (Figure 2a), HSP70 (Figure 2d), and NRF2 (Figure 2h) did not differ (P > 0.05) among the treatment groups.
Figure 2.
Effect of phytogenic additives on the expression of stress- and antioxidant-related genes in broiler breast muscle. SOD1 (a), SOD2 (b), GPX1 (c), HSP70 (d), ERK1 (e), ERK2 (f), JNK (g), and NRF2 (h). mRNA abundances were measured by qPCR. Data are presented as mean ± SEM (n = 6/group). Different letters indicate significant difference at P < 0.05. C = control; AVHGP = AV/HGP/16; AVSSL/12 = AVSSL; SCP = Superliv Concentrate Premix; BHGP = AGP premix: bacteriostatic herbal growth promoter with essential oils; SG = Superliv Gold.
DISCUSSION
This study examined the effects of 5 phytogenic additives (3 were supplemented in feed and 2 in water) on carcass characteristics and meat quality in modern broiler chickens. Interestingly, supplementing AVSSL and SG in the drinking water, but not feeding phytogenic-supplemented diets, improved BW as well as HCW and CCOG. Beside a high probable difference in active ingredient content between the 2 categories of additives that was not divulged during, or since the experiment, and represents a limitation in this study, it is plausible that this observed difference in growth performance might be due to higher water intake and consequently higher phytogenic additive intake. In fact, water intake of broilers is approximately 2 to 3 times as much as feed intake (Williams, 1996; Feddes et al., 2002), which may result in higher levels of phytogenic additive intake and, consequently, a higher amount of active ingredients reaching the intestinal tract of the birds when compared with feed-phytogenic additives. Furthermore, it is possible that water-phytogenic additive had a higher absorption rate, which is supported by the modulation of gut segment morphology (villi high, crypt depth, and muscle thickness; results not shown).
In addition, both water (AVSSL and SG) phytogenic additives improved breast muscle mass and reduced fad pad size which suggests an enhancement of muscle protein synthesis and reduction of fat deposition through inhibition of lipogenesis and/or increase of lipolysis process.
Herbs and spices are traditionally defined as any part of a plant that is used in the diet for their aromatic properties with no or low nutritional value (Smith and Winder, 1996). However, recent studies have demonstrated that natural herbal additives contain various phytochemicals and can possess powerful antioxidant activity (Park and Yoo, 1999; Dragland et al., 2003; Liu et al., 2006). Several culinary and medicinal herbs are important sources of dietary antioxidants (Dragland et al., 2003). In agreement with these studies, the DPPH assay showed that both water- and feed-phytogenic additives exhibited greater radical scavenging effects and total antioxidant activities when compared with the CTRL-fed diet. Antioxidant-rich herbs have the potential to protect against oxidative damage by reducing lipid peroxidation and improve the quality of a product (Wood and Enser, 1997). This is evidenced in the present study by the reduction in TBARS, a byproduct of lipid peroxidation, at day 1 postmortem in breasts from phytogenic-fed birds compared with the untreated group.
At molecular levels, the first line antioxidant defense comprises 3 key enzymes, namely, SOD, catalase (CAT), and GPX. These enzymes play an indispensable role in catalyzing and detoxifying of superoxide and hydroxyl radicals and, thereby, in the antioxidant protective capacity of any biological system (Lü et al., 2010). In this study, although all the studied phytogenic additives exhibited a high total antioxidant activity, only AVHGP and BHGP upregulated the expression of SOD2, but not SOD1, gene. According to Rosen et al. (1993), the metalloenzyme SOD1 requires Cu and Zn as metal cofactors, whereas SOD2 needs Mn. This suggested that these 2 additives might contain high levels of Mn or active ingredient(s) that specifically and directly trigger SOD2 transcription and/or its metal cofactor. Research has revealed that the induction of detoxifying enzyme activities occurs at the transcriptional levels and is regulated by a cis-acting element that is present in the promoter region defined as an antioxidant response element (ARE; Rushmore and Pickett, 1990). In agreement with previous studies (Yu et al., 2000), the upregulation of the expression of mitogen-activated protein kinase (MAPK)-related genes (ERK1/2 and JNK1) in the present study indicates that AVHGP and BHGP phytogenic additives might activate SOD2 transcription via induction of MAPK and ARE. Although MAPK are well-known to be associated with stress conditions (Harrison et al., 2004; Darling and Cook, 2014), emerging studies have shown that MAPK cascades also regulate a diverse class of “normal” biological processes from gene expression to cell proliferation and differentiation (Robinson and Cobb, 1997; Schaeffer and Weber, 1999). The unchanged levels of HSP70 mRNA—the gold standard of stress marker—between all the groups indicated that the induction of MAPK expression was unlikely due to stress in the present experimental conditions; however, exactly how MAPK were activated in this study and what are the upstream mediators remain to be identified.
As lipid oxidation has been reported to cause rancidity and deterioration in flavor and color of meat (Suryanti et al., 2014), it is imperative to determine the effect of phytogenic supplementation on meat organoleptic quality (pH, color, and sensory parameters). The magnitude of the range of pH, L*, a*, and b* values was similar to that reported in previous studies (Van Laack et al., 2000; Woelfel et al., 2002; Janisch et al., 2011), indicating that these values were in the normal range. Interestingly, all the phytogenic additives except AVHGP reduced the breast lightness (L* values). Although the underlying mechanisms are not known, it is possible that these additives affected the pigment and myoglobin content in broiler breast muscles. The relative amounts of myoglobin in the breast muscle depend on the oxygen availability, myoglobin autoxidation rate, and reducing capacity (Ledward, 1992). On the other hand, the oxygen availability depends on the oxygen partial pressure, penetration depth, and oxygen consumption rate of the muscle (Ledward, 1992). It was hypothesized that the phytogenic additives might modulate the thickness of different myoglobin layers in the breast muscle, which, in turn, affect the depth of light penetration and L* values. It is also conceivable that during postmortem glycolysis, the supplemented phytogenic additives modulate the sarcoplasmic protein denaturation and precipitation on the myofibrils, thereby affecting light scattering and penetration, which merits further in-depth investigations.
Many herbs and their extracts have been added to a variety of food and diets to improve their sensory characteristics. Contrary to expectation, professional descriptive panelists did not discern any difference in aroma and basic tastes among treatment groups. However, they identified a more intense green herb flavor in breast fillets from AVHGP-fed than CTRL-fed birds. This could be of particular interest for consumers preferring a “natural” meat flavor.
Taken together, these results demonstrated that water-phytogenic additives are more efficient in improving growth performance than the feed-additives. Although further in-depth studies are warranted, phytogenic supplementation appears to enhance meat quality probably through modulation of MAPK- and antioxidant-related genes.
Footnotes
This work was supported by grant from Ayurvet Ltd. (to S.D.). Ayurvet Ltd. had no role in conducting the research, generating the data, interpreting the results, or writing the manuscript.
AUTHOR CONTRIBUTIONS
J.F., E.S.G., and S.D. performed the live bird trial; S.D. purchased the reagents; S.O., J.F., E.S.G., F.L.Y., C.M.O., M.K., N.A., and S.D. assisted in the processing of the trail and collection of meat quality data; J.F. and S.D. conducted gene expression analysis and statistical analysis; D.A. and S.L. measured antioxidant activity; and S.D. wrote the manuscript.
Conflict of interest statement. None declared.
LITERATURE CITED
- Amitav B., Adarsh C., Shivi M., and Ravikanth K.. 2015. Effect of supplementation of superliv liquid on the performance of commercial broilers in salimpur poultry farm of Mathura. Int. J. Adv. Res. 3:539–543. [Google Scholar]
- Amitav B., Satish K. G., Vinold K., Debashish R. K., and Shivi M.. 2013. Effects of superliv concentrate on the growth, immunocompetence traits and nutrient retention of commercial broilers during extreme winter. Int. J. Poult. Sci. 12: 51–54. [Google Scholar]
- Bazargani-Gilani B., Tajik H., and Aliakbarlu J.. 2014. Physicochemical and antioxidative characteristics of Iranian pomegranate (Punica granatum L. Cv. Rabbab-e-neyriz) juice and comparison of its antioxidative activity with zataria multiflora boiss essential oil. Vet. Res. Forum 5:313–318. [PMC free article] [PubMed] [Google Scholar]
- Cavitt L., Youm G., Meullenet J., Owens C., and Xiong R.. 2004. Prediction of poultry meat tenderness using razor blade shear, Allo‐Kramer shear, and sarcomere length. J. Food Sci. 69:SNQ11–SNQ15. [Google Scholar]
- Darling N. J., and Cook S. J.. 2014. The role of MAPK signalling pathways in the response to endoplasmic reticulum stress. Biochim. Biophys. Acta 1843:2150–2163. doi: 10.1016/j.bbamcr.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Dragland S., Senoo H., Wake K., Holte K., and Blomhoff R.. 2003. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 133:1286–1290. doi: 10.1093/jn/133.5.1286 [DOI] [PubMed] [Google Scholar]
- Feddes J. J., Emmanuel E. J., and Zuidhoft M. J.. 2002. Broiler performance, body weight variance, feed and water intake, and carcass quality at different stocking densities. Poult. Sci. 81:774–779. doi: 10.1093/ps/81.6.774 [DOI] [PubMed] [Google Scholar]
- Flees J., Rajaei-Sharifabadi H., Greene E., Beer L., Hargis B. M., Ellestad L., Porter T., Donoghue A., Bottje W. G., and Dridi S.. 2017. Effect of morinda citrifolia (noni)-enriched diet on hepatic heat shock protein and lipid metabolism-related genes in heat stressed broiler chickens. Front. Physiol. 8:919. doi: 10.3389/fphys.2017.00919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison J. C., Zyla T. R., Bardes E. S., and Lew D. J.. 2004. Stress-specific activation mechanisms for the “cell integrity” MAPK pathway. J. Biol. Chem. 279:2616–2622. doi: 10.1074/jbc.M306110200 [DOI] [PubMed] [Google Scholar]
- Janisch S., Krischek C., and Wicke M.. 2011. Color values and other meat quality characteristics of breast muscles collected from 3 broiler genetic lines slaughtered at 2 ages. Poult. Sci. 90:1774–1781. doi: 10.3382/ps.2010-01073 [DOI] [PubMed] [Google Scholar]
- Kuntapanit C. 1978. Beef muscle and adipose lipid deterioration as affected by nutritional regime, vacuum aging, display and carcass conditioning. Doctoral Diss. Kansas State University, Manhattan, KS. [Google Scholar]
- Lassiter K., Greene E., Piekarski A., Faulkner O. B., Hargis B. M., Bottje W., and Dridi S.. 2015. Orexin system is expressed in avian muscle cells and regulates mitochondrial dynamics. Am. J. Physiol. Regul. Integr. Comp. Physiol. 308:R173–R187. doi: 10.1152/ajpregu.00394.2014 [DOI] [PubMed] [Google Scholar]
- Meilgaard M., Civile G. V., and Carr B. T.. 2007. Sensory evaluation techniques. 4th ed CRC press, Florida, USA: p. 1–464. [Google Scholar]
- Ledward D. A. 1992. Colour of raw and cooked meat. In: Johnson D. E., Knight M., and Ledward D. A., editors, The chemistry of muscle based foods. Royal Society of Chemistry, Cambridge, UK: p. 128–144. [Google Scholar]
- Liu B., Li W., Chang Y., Dong W., and Ni L.. 2006. Extraction of berberine from rhizome of coptis chinensis franch using supercritical fluid extraction. J. Pharm. Biomed. Anal. 41:1056–1060. doi: 10.1016/j.jpba.2006.01.034 [DOI] [PubMed] [Google Scholar]
- Loizzo M. R., Tundis R., Chandrika U. G., Abeysekera A. M., Menichini F., and Frega N. G.. 2010. Antioxidant and antibacterial activities on foodborne pathogens of artocarpus heterophyllus lam. (Moraceae) leaves extracts. J. Food Sci. 75:M291–M295. doi: 10.1111/j.1750-3841.2010.01614.x [DOI] [PubMed] [Google Scholar]
- Lü J. M., Lin P. H., Yao Q., and Chen C.. 2010. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J. Cell. Mol. Med. 14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon B. G., and Lyon C. E.. 1998. Assessment of three devices used in shear tests of cooked breast meat. Poult. Sci. 77:1585–1590. doi: 10.1093/ps/77.10.1585 [DOI] [PubMed] [Google Scholar]
- Lyon B. G., Smith D. P., Lyon C. E., and Savage E. M.. 2004. Effects of diet and feed withdrawal on the sensory descriptive and instrumental profiles of broiler breast fillets. Poult. Sci. 83:275–281. doi: 10.1093/ps/83.2.275 [DOI] [PubMed] [Google Scholar]
- Meilgaard M. C., Civille G. V., and Carr B. T.. 2007. Sensory evaluation techniques. 4th ed CRC Press, Boca Raton, FL. [Google Scholar]
- Mohiti-Asli M., and Ghanaatparast-Rashti M.. 2015. Dietary oregano essential oil alleviates experimentally induced coccidiosis in broilers. Prev. Vet. Med. 120:195–202. doi: 10.1016/j.prevetmed.2015.03.014 [DOI] [PubMed] [Google Scholar]
- National Chicken Council 2017. Per capita consumption of poultry and livestock, 1965 to estimated 2018, in pounds http://www.nationalchickencouncil.org/about-the-industry/statistics/per-capita-consumption-of-poultry-and-livestock-1965-to-estimated-2012-in-pounds/ (accessed May 2018).
- Olnood C. G., Beski S. S. M., Choct M., and Iji P. A.. 2015. Novel probiotics: their effects on growth performance, gut development, microbial community and activity of broiler chickens. Anim. Nutr. 1:184–191. doi: 10.1016/j.aninu.2015.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., and Yoo S.. 1999. Effects of supplementation of Chinese medicine refuse on performance and physiology in broiler chicks. Korean J. Poult. Sci. 26:195–201. [Google Scholar]
- Robinson M. J., and Cobb M. H.. 1997. Mitogen-activated protein kinase pathways. Curr. Opin. Cell Biol. 9:180–186. [DOI] [PubMed] [Google Scholar]
- Rosen D. R., Siddique T., Patterson D., Figlewicz D. A., Sapp P., Hentati A., Donaldson D., Goto J., O’Regan J. P., and Deng H. X.. 1993. Mutations in cu/zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature 362:59–62. doi: 10.1038/362059a0 [DOI] [PubMed] [Google Scholar]
- Rushmore T. H., and Pickett C. B.. 1990. Transcriptional regulation of the rat glutathione S-transferase ya subunit gene. Characterization of a xenobiotic-responsive element controlling inducible expression by phenolic antioxidants. J. Biol. Chem. 265:14648–14653. [PubMed] [Google Scholar]
- Schaeffer H. J., and Weber M. J.. 1999. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol. Cell. Biol. 19:2435–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen T. D., and Livak K. J.. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3:1101–1108. [DOI] [PubMed] [Google Scholar]
- Smith D. P., Lyon C. E., and Lyon B. G.. 2002. The effect of age, dietary carbohydrate source, and feed withdrawal on broiler breast fillet color. Poult. Sci. 81:1584–1588. doi: 10.1093/ps/81.10.1584 [DOI] [PubMed] [Google Scholar]
- Smith R. J., and Winder M. L.. 1996. Medicinal garden. In: Ober, R, editor, The national herb garden guidebook. The Herb Society of America, Springfield, VA: p. 61–71. [Google Scholar]
- Sudhir K. R., Ramjee G., and Sarju N.. 2016. Effect of superliv on feed consumption, feed conversion efficiency and body weight gain in commercial broiler. Indian Res. J. Ext. Edu. 16:60–64. [Google Scholar]
- Suryanti U., Bintoro V., Atmomarsono U., Pramono Y., and Legowo A.. 2014. Antioxidant activity of Indonesian endogenous duck meat marinated in ginger (zingiber officinale roscoe) extract. Int. J. Poult. Sci. 13:102. [Google Scholar]
- Van Laack R. L., Liu C. H., Smith M. O., and Loveday H. D.. 2000. Characteristics of pale, soft, exudative broiler breast meat. Poult. Sci. 79:1057–1061. doi: 10.1093/ps/79.7.1057 [DOI] [PubMed] [Google Scholar]
- Williams R. B. 1996. The ratio of the water and food consumption of chickens and its significance in the chemotherapy of coccidiosis. Vet. Res. Commun. 20:437–447. [DOI] [PubMed] [Google Scholar]
- Witte V. C., Krause G. F., and Bailey M. E.. 1970. A new extraction method for determining 2‐thiobarbituric acid values of pork and beef during storage. J. Food Sci. 35:582–585. [Google Scholar]
- Woelfel R. L., Owens C. M., Hirschler E. M., Martinez-Dawson R., and Sams A. R.. 2002. The characterization and incidence of pale, soft, and exudative broiler meat in a commercial processing plant. Poult. Sci. 81:579–584. doi: 10.1093/ps/81.4.579 [DOI] [PubMed] [Google Scholar]
- Wood J. D., and Enser M.. 1997. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br. J. Nutr. 78 (Suppl 1):S49–S60. [DOI] [PubMed] [Google Scholar]
- Wu W., Jerome D., and Nagaraj R.. 1994. Increased redness in turkey breast muscle induced by fusarial culture materials. Poult. Sci. 73:331–335. doi: 10.3382/ps.0730331 [DOI] [PubMed] [Google Scholar]
- Yang C., Chowdhury M. A., Huo Y., and Gong J.. 2015. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens 4:137–156. doi: 10.3390/pathogens4010137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R., Chen C., Mo Y. Y., Hebbar V., Owuor E. D., Tan T. H., and Kong A. N.. 2000. Activation of mitogen-activated protein kinase pathways induces antioxidant response element-mediated gene expression via a Nrf2-dependent mechanism. J. Biol. Chem. 275:39907–39913. doi: 10.1074/jbc.M004037200 [DOI] [PubMed] [Google Scholar]