Abstract
Brown/beige adipocytes dissipate energy as heat. We previously showed that brown/beige adipocytes are present in white adipose tissue (WAT) of fattening cattle. The present study examined the effect of vitamin A restriction on mRNA expression of brown/beige adipocyte-related genes. In Japan, fattening cattle are conventionally fed a vitamin A-restricted diet to improve beef marbling. Twelve Japanese Black steers aged 10 mo were fed control feed (n = 6) or vitamin A-restricted feed (n = 6) for 20 mo. Subcutaneous WAT (scWAT) and mesenteric WAT (mesWAT) were collected, and mRNA expression levels of molecules related to the function of brown/beige adipocytes (Ucp1, Cidea, Dio2, Cox7a, and Cox8b) as well as transcriptional regulators related to brown/beige adipogenesis (Zfp516, Nfia, Prdm16, and Pgc-1α) were evaluated. The vitamin A restriction significantly increased or tended to increase expression levels of Cidea and Pgc-1α in scWAT, and Cidea, Dio2, and Nfia in mesWAT. Previous studies revealed that the bone morphogenetic protein (Bmp) pathway was responsible for commitment of mesenchymal stem cells to brown/beige adipocyte-lineage cells. The vitamin A restriction increased expression of Bmp7 and some Bmp receptors in WAT. The interrelationship between gene expression levels indicated that expression levels of Nfia, Prdm16, and Pgc-1α were closely related to those of genes related to the function of brown/beige adipocytes in scWAT. Also, expression levels of Nfia, Prdm16, and Pgc-1α were highly correlated with those of Alk3 in scWAT. In summary, the present results suggest that the vitamin A restriction increases the number or activity of brown/beige adipocytes through regulatory expression of transcriptional regulators to induce brown/beige adipogenesis, especially in scWAT of fattening cattle, which may be governed by the Bmp pathway.
Keywords: beige adipocytes, brown adipocytes, fattening cattle, vitamin A
INTRODUCTION
Brown/beige adipocytes dissipate chemical energy in the form of heat against cold exposure (Cannon and Nedergaard, 2004; Kajimura and Saito, 2014). The thermogenic function of brown/beige adipocytes results from the elevated cellular respiration that is largely uncoupled from ATP synthesis (Cannon and Nedergaard, 2004; Kajimura and Saito, 2014). The uncoupling occurs through uncoupling protein 1 (Ucp1) that is a proton channel located at the inner mitochondrial membrane and predominantly expressed in brown/beige adipocytes (Cannon and Nedergaard, 2004; Kajimura et al., 2015). Since the presence of functional brown/beige adipocytes was verified in adult humans (Cypess et al., 2009; van Marken Lichtenbelt et al., 2009; Saito et al., 2009; Virtanen et al., 2009), interest is directed to the cells as a potential target for prevention and therapeutic agents for human obesity. Extensive studies explored factors involved in differentiation of brown/beige preadipocytes, origin of beige adipocytes, and activation of brown/beige adipocytes (Nedergaard and Cannon, 2010; Inagaki et al., 2016; Wang and Seale, 2016). Currently, several transcription factors and transcription coregulators such as Zfp516, nuclear factor IA (Nfia), PR domain containing 16 (Prdm16), and peroxisome proliferator activated receptor γ (Pparγ) coactivator-1α (Pgc-1α) have been suggested to be involved in differentiation of brown/beige preadipocytes (Puigserver et al., 1998; Seale et al., 2008; Dempersmier et al., 2015; Hiraike et al., 2017).
We previously revealed the presence of brown/beige adipocytes in white adipose tissue (WAT) of fattening cattle (Asano et al., 2013). Unlike in humans, the activation of brown/beige adipocytes is not preferable in fattening cattle, because it leads to a decrease in fattening efficiency that is a ratio of body weight gain to feed intake because of increasing energy loss as heat. We also showed that feed ingredients affected expression level of not only Ucp1 but also the other thermogenesis-related genes that were expressed higher in brown/beige adipocytes than in white adipocytes (Seale et al., 2007; Wu et al., 2012) in fat depots of fattening cattle (Asano et al., 2013; Kanamori et al., 2014). Expression levels of these brown/beige adipocyte-selective genes were generally higher in subcutaneous WAT (scWAT) of cattle fed a high concentrate diet than those fed a high roughage diet (Asano et al., 2013). We also suggested that vitamin A restriction increased expression levels of the brown/beige adipocyte-selective genes in mesenteric WAT (mesWAT, Kanamori et al., 2014) by comparison between feeding a total mixed ration (TMR) rich in β-carotene as provitamin A and feeding a vitamin A-restricted diet consisting of high concentrate without supplying vitamin A and roughage low in β-carotene (Yamada et al., 2013). There may be a criticism in interpretation of the study on comparison of the TMR and the vitamin A-restricted diet, because feed composition other than vitamin A was also different between the 2 diets. For example, vitamin E (α-tocopherol) content was much more in the TMR than in the high concentrate and roughage (Yamada et al., 2013). Therefore, the previous results may be induced not only by difference in vitamin A status. The present study evaluated effects of dietary vitamin A restriction using the same basal diet on expression levels of the brown/beige adipocyte-selective genes in fat depots of fattening cattle. We also examined expression levels of molecules involved in brown/beige adipogenesis and evaluated the relationship between expression levels of the genes.
MATERIALS AND METHODS
Animal care and experiments were approved by the Animal Care Committee, Kyoto University (27–42 and 28–42). All animal experiments were conducted in accordance with the approved guidelines.
Cattle and Feeds
Twelve Japanese Black steers aged 10 mo were used. They were allocated to the control group (n = 6) or the vitamin A-restriction group (n = 6) by initial body weight. The steers were raised in a stall covered with sawdust in 2 pens for cattle in the Kyoto University Livestock Farm; 3 control cattle and 3 cattle fed the vitamin A-restricted diet were raised in a pen. The steers were fed roughage and concentrate on an ad libitum basis for 20 mo; diet was individually given by use of self-locking stanchions. Wheat straw was basically fed as roughage for the early period of fattening, i.e., from 10 to 21 mo of age, whereas rice straw was basically given for the late period of fattening (from 22 to 30 mo of age). Ingredients of the concentrate are shown in Table 1. Vitamin A was supplemented as retinyl acetate (Cow Health A+, Marubeni Nisshin Feed, Tokyo, Japan) to the concentrate for the control feed to meet Japanese Feeding Standard for Beef Cattle (Japan Livestock Industry Association, 2008). They were allowed free access to drinking water and a mineral block (KNZ Ammonium Chloride Lick, Akzo Nobel Functional Chemicals, Arnhem, Netherlands). Daily intake of feeds was individually measured, and average daily intake of feeds, metabolizable energy (ME), and crude protein (CP) was calculated (Japan Livestock Industry Association, 2009). Body weight was measured every month, and daily gain and feed efficiency were calculated. After a fattening period of 20 mo, the steers were commercially slaughtered. The inguinal scWAT and mesWAT were obtained immediately after slaughter.
Table 1.
Ingredients of basal concentrate (g/kg as fed)
| Early period1 | Latter period2 | |
|---|---|---|
| Flaked barley | 340 | 380 |
| Flaked corn | 280 | 320 |
| Wheat bran | 140 | 140 |
| Rice bran | 60 | 90 |
| Soybean meal | 160 | 50 |
| CaCO3 | 10 | 10 |
| NaCl | 10 | 10 |
| Total | 1000 | 1000 |
1Basal concentrate for early period was fed to cattle aged from 10 to 21 mo.
2Basal concentrate for latter period was fed to cattle aged from 22 to 30 mo.
Plasma Retinol Concentration
Blood was obtained from 30-mo-old steers by venipuncture. Plasma samples were obtained from blood by centrifugation at 1,600 × g at 4 °C for 15 min and kept at −80 °C for later analyses of retinol concentration. Plasma retinol concentration was measured by HPLC with a UV detector.
Carcass Characteristics
After the carcasses were cooled, they were dissected at the sixth and seventh rib according to the Japanese meat grading system (JMGA, 1988). Detailed methods were as described in the study by Irie et al. (2011).
RNA Isolation and Reverse Transcription- Quantitative PCR
Total RNA isolation from WAT and subsequent cDNA synthesis were conducted as described by Kida et al. (2018). The cDNA that was reverse-transcribed from 5, 10, or 20 ng of total RNA was used as a template for reverse transcription-quantitative PCR (RT-qPCR) as described previously (Kanamori et al., 2017). The oligonucleotide primers for cell death-inducing DFFA-like effector a (Cidea), cytochrome c oxidase (Cox) 7a, Cox8b, iodothyronine deiodinase 2 (Dio2), hypoxanthine phosphoribosyltransferase 1 (Hprt1), Pgc-1α, Prdm16, and Ucp1 were described in the study by Asano et al. (2013), and those for activin βB (ActβB), activin receptor type 2a (Actr2a), activin receptor-like kinase (Alk) 2, Alk3, bone morphogenetic protein (Bmp) 4, Bmp7, and Bmp receptor type 2 (Bmpr2) were described previously (Qiao et al., 2015). The other PCR primers are shown in Table 2. The cycle of threshold (Ct) value was determined, and the abundance of gene transcripts was analyzed using the ΔΔCt method using Hprt1 as the corrected gene (Duran et al., 2005). The expression in the control group in scWAT depot was set to 1.
Table 2.
Oligonucleotide PCR primers for reverse transcription-quantitative PCR
| Oligonucleotide | ||
|---|---|---|
| 5′-primer | 3′-primer | |
| Aldh1a1 | 5′-ggagaaactctgtgaggtggaag-3′ | 5′-tcttacagccttcactgctttgtc-3′ |
| Nfia | 5′-catcaccgacccgtcattaca-3′ | 5′-gggtgacgtcggaaaatgaag-3′ |
| Pparγ | 5′-ctgtcattattctcagtggagacc-3′ | 5′-cagcagattgtcttgtatgtcctc-3′ |
| Rarα | 5′-agcggctatagcacgccatcc-3′ | 5′-tgggcacaatctcttcagaactgc-3’ |
| Rarγ | 5′-aggagatggcctctctgtcggt-3′ | 5′-gaagcacggcttgtacacccg-3′ |
| Rxrα | 5′-cagtactgccgctaccagaa-3′ | 5′-tctccactggcatgtcctcgtt-3′ |
| Rxrγ | 5′-ggtcatgggcatgaagagggaag-3′ | 5′-catggccactgttggcacactct-3′ |
| Zfp516 | 5′-gatgaggctgccgatgagagcgg-3′ | 5′-aacggtgtggctttgctcctgctgtg-3′ |
Statistical Analyses
Data are expressed as mean ± SE. As for daily feed intake, daily gain, and feed efficiency, the effect of vitamin A restriction, pen, and their interaction was evaluated by two-way ANOVA. Because the effect of pen and interaction was not significant, data from each pen were pooled, and difference between the control group and the vitamin A-restricted group in each WAT was examined using the unpaired t-test. Data on gene expression were log-transformed to provide an approximation of a normal distribution before analysis. Differences between the control group and the vitamin A-restricted group in each WAT were examined using the unpaired t-test. Differences between the expression levels in scWAT and the expression levels in mesWAT in each vitamin A status were examined by paired t-test. The reciprocal relationship of the expression levels of genes was investigated by means of Pearson’s correlation coefficient. Differences of P < 0.05 were considered significant.
RESULTS
The vitamin A restriction did not affect daily feed intake, daily gain, and feed efficiency (Table 3). Plasma concentrations of retinol, a major vitamin A in circulation, were significantly lower in fattening cattle fed a vitamin A-restricted diet (Table 4). Dietary vitamin A is frequently restricted in fattening cattle to improve beef marbling, but plasma concentration of retinol should not be below 120 μg/liter to prevent severe vitamin A deficiency (Oka, 1996). Therefore, taken the results of daily gain and feed efficiency together, the present results suggest that the restriction of dietary vitamin A was not severe. Carcass characteristics indicated no difference in carcass weight, but there was an increase in dressing percentage and ribeye area in the vitamin A-restricted group (Table 4). In addition, marbling score was significantly increased by feeding a vitamin A-restricted diet, resulting in higher quality grade.
Table 3.
Effect of dietary vitamin A restriction on daily feed intake and daily gain of Japanese Black fattening cattle (mean ± SE)
| Item | Control | Vit A-res1 |
|---|---|---|
| Feed intake, kg/d | ||
| Early period | ||
| Roughage | 1.32 ± 0.17 | 1.35 ± 0.12 |
| Concentrate | 6.98 ± 0.05 | 6.95 ± 0.12 |
| Latter period | ||
| Roughage | 1.12 ± 0.08 | 1.09 ± 0.05 |
| Concentrate | 8.00 ± 0.45 | 7.65 ± 0.33 |
| ME intake, MJ/d | ||
| Early period | ||
| Roughage | 7.30 ± 0.96 | 7.42 ± 0.65 |
| Concentrate | 84.34 ± 0.65 | 83.97 ± 1.46 |
| Total | 91.64 ± 1.58 | 91.39 ± 1.80 |
| Latter period | ||
| Roughage | 6.07 ± 0.44 | 5.96 ± 0.28 |
| Concentrate | 96.06 ± 5.38 | 91.92 ± 3.94 |
| Total | 102.13 ± 5.63 | 97.87 ± 4.03 |
| CP intake, kg/d | ||
| Early period | ||
| Roughage | 0.01 ± 0.001 | 0.01 ± 0.0004 |
| Concentrate | 1.12 ± 0.01 | 1.11 ± 0.02 |
| Total | 1.12 ± 0.01 | 1.12 ± 0.02 |
| Latter period | ||
| Roughage | 0.01 ± 0.0004 | 0.01 ± 0.0003 |
| Concentrate | 0.98 ± 0.05 | 0.94 ± 0.04 |
| Total | 0.98 ± 0.06 | 0.94 ± 0.04 |
| Daily gain, kg/d | ||
| Early period | 0.96 ± 0.03 | 1.01 ± 0.03 |
| Latter period | 0.58 ± 0.06 | 0.52 ± 0.03 |
| Total | 0.79 ± 0.04 | 0.80 ± 0.03 |
| Feed efficiency, g/MJ | ||
| Early period | 10.43 ± 0.27 | 11.10 ± 0.23 |
| Latter period | 5.63 ± 0.29 | 5.31 ± 0.18 |
| Total | 8.24 ± 0.20 | 8.48 ± 0.17 |
n = 6.
1Vitamin A-restricted.
Table 4.
Effect of dietary vitamin A restriction on plasma concentration of retinol and carcass characteristics of Japanese Black fattening cattle (mean ± SE)
| Item | Control | Vit A-res1 |
|---|---|---|
| Plasma retinol, μg/liter | 349.3 ± 19.2 | 176.0 ± 16.9** |
| Carcass weight, kg | 508.7 ± 15.9 | 518.5 ± 22.2 |
| Dressing percentage | 72.4 ± 0.5 | 75.1 ± 0.7** |
| Quality grade2 | 2.7 ± 0.2 | 3.3 ± 0.2* |
| Fat thickness, cm | 2.4 ± 0.3 | 2.1 ± 0.3 |
| Rib thickness, cm | 7.4 ± 0.3 | 7.8 ± 0.3 |
| Ribeye area, cm | 50.3 ± 3.4 | 67.7 ± 3.6** |
| Marbling score3 | 3.5 ± 0.2 | 5.5 ± 0.5** |
| Color score4 | 4.3 ± 0.2 | 4.7 ± 0.2 |
n = 6.
1Vitamin A-restricted.
2Quality grade: 1 = inferior; 5 = excellent.
3Marbling score: 1 = lowest; 12 = highest.
4Color score: 1 = pale; 12 = dark.
*P < 0.05; **P < 0.01.
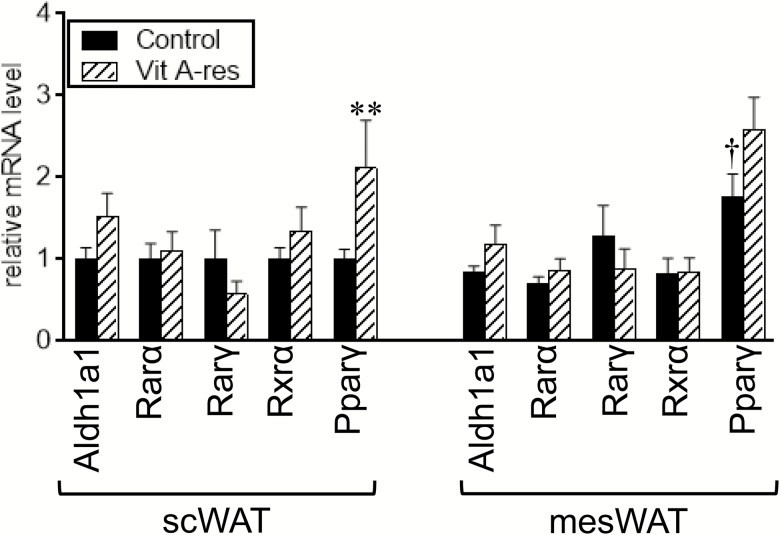
All-trans-retinoic acid, an activated form of retinol, can be produced from retinol by 2 sequential steps: retinol is converted to retinal, followed by further oxidation to retinoic acid. The second oxidation was catalyzed by the aldehyde dehydrogenase 1 family, member A1 (Aldh1a1). We first examined the effect of dietary vitamin A restriction on expression levels of Aldh1a1 and receptors for retinoic acid in WAT of fattening cattle (Figure 1). The vitamin A restriction affected neither expression of Aldh1a1 nor that of retinoic acid receptor (Rar), Rarα, and Rarγ. Rar heterodimerizes with retinoid X receptor (Rxr) and transactivates the target genes (Chambon, 1996). The vitamin A restriction did not affect the expression level of Rxrα in WAT. Rxr also binds to Pparγ, which enables it to function as a transcription factor of Pparγ (Tontonoz et al., 1994). Expression level of Pparγ was significantly increased by feeding with a vitamin A-restricted diet in scWAT but not in mesWAT. Expression of Pparγ tended to be higher in mesWAT than in scWAT, when the control diet was given (P = 0.07).
Figure 1.
Expression of aldehyde dehydrogenase 1 family, member A1 (Aldh1a1), receptors for retinoic acid, and peroxisome proliferator activated receptor γ (Pparγ) in fat depots of fattening cattle. Japanese Black steers were fed control diet or vitamin A-restricted diet for 20 mo. Expression of Aldh1a1, receptors for retinoic acid, and Pparγ in subcutaneous (sc) white adipose tissue (WAT) and mesenteric WAT (mesWAT) were examined by reverse transcription-quantitative PCR (RT-qPCR) analyses. Mean ± SE (n = 6). **P < 0.05 vs. control group in the same WAT. †P < 0.05 and ††P < 0.10 vs. scWAT in the dietary group.
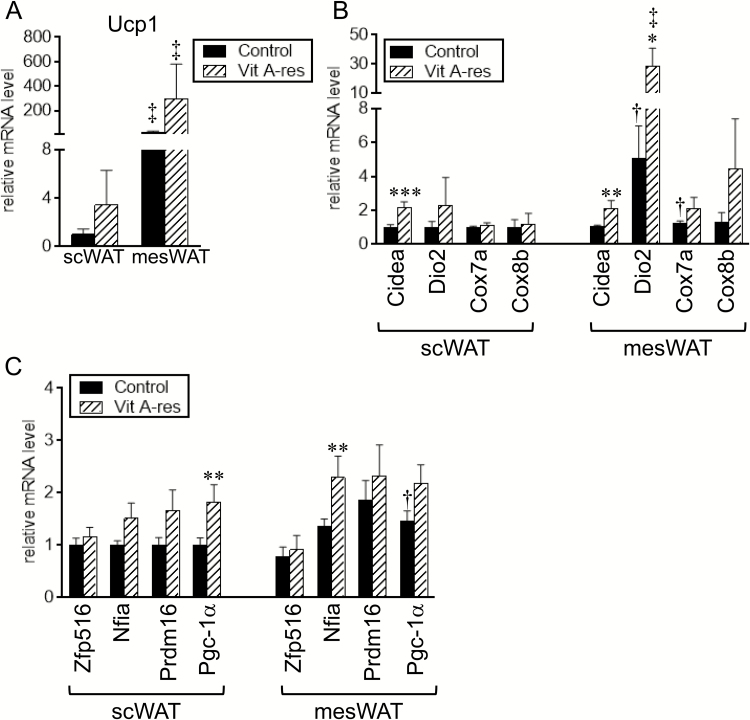
Expression of Ucp1 was numerically but not significantly increased in scWAT and mesWAT of fattening cattle fed the vitamin A-restricted diet (Figure 2A). One cattle fed the vitamin A-restricted diet exhibited extremely higher expression of Ucp1 in scWAT and mesWAT. The daily gain, feed efficiency during the latter period, carcass weight, fat thickness, and rib thickness of the cattle were lower than the lower limit of 95% CI of these parameters in the other cattle; the daily gain, carcass weight, fat thickness, and rib thickness in the cattle with extremely higher expression of Ucp1 and 95% CI in the other cattle were as follows: daily gain during the early period: 0.94 kg/d and 0.94 to 1.04 kg/d; daily gain during the early period: 0.42 kg/d and 0.49 to 0.64 kg/d; daily gain during the total period: 0.71 kg/d and 0.75 to 0.86 kg/d; feed efficiency during the latter period: 4.70 g/MJ and 5.17 to 5.92 g/MJ; carcass weight: 429.0 kg and 495.4 to 547.2 kg; fat thickness: 1.4 cm and 1.8 to 2.8 cm; and rib thickness: 7.1 cm and 7.2 to 8.1 cm, respectively. With regard to the other molecules related to the function of brown/beige adipocytes, the expression level of Cidea in both fat depots was significantly higher in the vitamin A-restricted group than in the control group (Figure 2B). In addition, Dio2 expression in mesWAT tended to be higher in the vitamin A-restricted group than in the control group (P = 0.09). Expression of Dio2 in scWAT and Cox7a and Cox8b in mesWAT was also numerically increased by the vitamin A restriction (Figure 2B). Expression levels of Ucp1 and Dio2 were significantly or tended to be higher in mesWAT than in scWAT, irrespective of diet (Ucp1: P < 0.001 in the control group and P = 0.007 in the vitamin A-restricted group; Dio2: P = 0.08 in the control group and P = 0.01 in the vitamin A-restricted group). In addition, Cox7a expression in mesWAT of the control group tended to be higher than that in scWAT (P = 0.06).
Figure 2.
Expression of brown/beige adipocyte-selective genes in fat depots of fattening cattle. Japanese Black steers were fed control diet or vitamin A-restricted diet for 20 mo. Expression of uncoupling protein 1 (Ucp1) (A), molecules related to function of brown/beige adipocytes (B), and transcriptional regulators related to brown/beige adipogenesis (C) in subcutaneous (sc) white adipose tissue (WAT) and mesenteric WAT (mesWAT) was examined by reverse transcription-quantitative PCR (RT-qPCR) analyses. Mean ± SE (n = 6). *P < 0.10, **P < 0.05, and ***P < 0.01 vs. control group in the same WAT. †P < 0.10 and ‡P < 0.01 vs. scWAT in the dietary group.
We also examined expression levels of transcription factors and transcriptional coactivators, which are involved in brown/beige adipogenesis and highly expressed in brown/beige adipocytes (Seale et al., 2007, 2008; Dempersmier et al., 2015; Hiraike et al., 2017) (Figure 2C). The expression levels of Pgc-1α in scWAT and Nfia in mesWAT were significantly increased by feeding with the vitamin A-restricted diet. Furthermore, expression of Pgc-1α in the control group tended to be higher in mesWAT than in scWAT (P = 0.09).
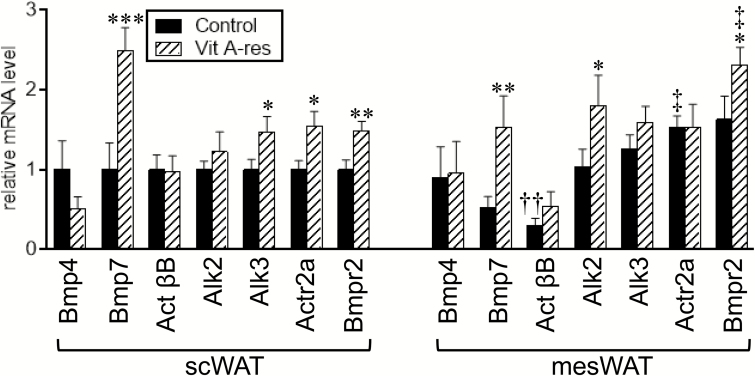
The Bmp pathway is known to commit mesenchymal stem cells to brown/beige adipocyte-lineage cells (Tseng et al., 2008; Xue et al., 2014). Expression of molecules involved in the Bmp pathway is shown in Figure 3; Bmp transmits its signal through complex formation with type I receptor (Alk2, 3, and 6) and type II receptor (Actr2a and b, and Bmpr2) (Miyazono et al., 2010). In addition, activin B, a homodimer of ActβB, signals via Alk2 and Actr2a (Kanamori et al., 2016). In bovine WAT, significant expression of Alk6 and Actr2b was not detected (data not shown). In scWAT, the vitamin A-restricted diet significantly increased expression of Bmp7 and Bmpr2, and tended to increase Alk3 (P = 0.08) and Actr2a (P = 0.05) expressions. Similar to the results, expression of some components related to the Bmp signaling was numerically increased in mesWAT of fattening cattle fed the vitamin A-restricted diet; Bmp7 expression was significantly increased by the vitamin A-restricted diet, and expression of Alk2 (P = 0.08) and Bmpr2 (P = 0.07) tended to be increased. ActβB expression in mesWAT of the control group was significantly lower than in scWAT of the vitamin A-restricted group. In contrast, Actr2a expression in the control group and Bmpr2 expression in the vitamin A-restricted group were significantly higher in mesWAT than in scWAT.
Figure 3.
Expression of genes involved in the bone morphogenetic protein (Bmp) signaling in fat depots of fattening cattle. Japanese Black steers were fed control diet or vitamin A-restricted diet for 20 mo. Expression of genes to elicit Bmp signaling, i.e., ligand (Bmp4, Bmp7, and activin βB (ActβB)), type I receptor for Bmp (activin receptor-like kinase (Alk) 2 and Alk3), and type II receptor for Bmp (activin receptor type 2a (Actr2a) and Bmp receptor type 2 (Bmpr2)), in subcutaneous (sc) white adipose tissue (WAT) and mesenteric WAT (mesWAT) was examined by reverse transcription-quantitative PCR (RT-qPCR) analyses. Mean ± SEM (n = 6). *P < 0.10, **P < 0.05, and ***P < 0.01 vs. control group in the same WAT. ††P < 0.05 and ‡P < 0.01 vs. scWAT in the dietary group.
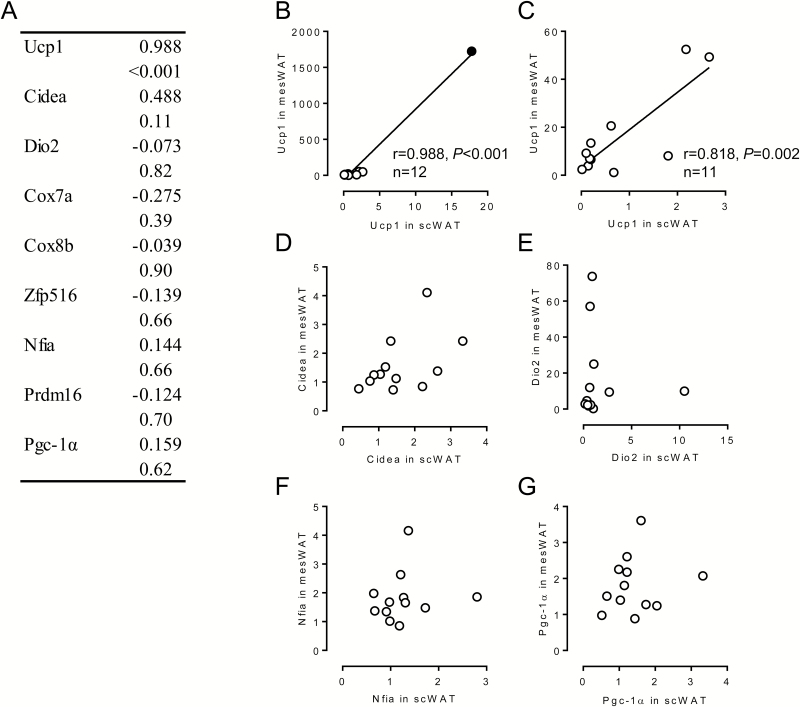
We next evaluated the reciprocal relationship of expression levels of molecules related to the function of brown/beige adipocytes as well as transcriptional regulators related to brown/beige adipogenesis between scWAT and mesWAT by Pearson’s correlation analysis (Figure 4). As for Ucp1 expression, expression levels in scWAT were highly correlated with those in mesWAT (Figure 4A and B). Even when the data exhibiting relatively higher expression of Ucp1 in both fat depots shown as a filled circle in Figure 4B are omitted, the correlation of Ucp1 expressions was significant (Figure 4C). However, expressions of the other molecules related to brown/beige adipocyte function and brown/beige adipogenesis were not interrelated in scWAT and in mesWAT (Figure 4A); some relationships are shown in Figure 4D–G. Although Cidea expression was increased by the vitamin A-restricted diet in both fat depots, the expression levels in scWAT were not related to those in mesWAT (Figure 4D). These results suggest that the vitamin A restriction similarly affected Ucp1 expression in scWAT and mesWAT in a head of cattle. On the contrary, the regulatory expression levels of the other molecules in scWAT and mesWAT varied among individuals.
Figure 4.
Relationship of expression levels of brown/beige adipocyte-selective genes between in subcutaneous (sc) white adipose tissue (WAT) and mesenteric WAT (mesWAT) of fattening cattle. Japanese Black steers were fed control diet or vitamin A-restricted diet for 20 mo. (A) Reciprocal relationship of expression levels of brown/beige adipocyte-selective genes between scWAT and mesWAT was evaluated. Upper: correlation coefficient. Lower: P value. (B)–(G) Relationship between the indicated genes was shown as a scatterplot. (C) Relationship of uncoupling protein (Ucp1) expression deleted the sample shown as closed circle in (B) is shown.
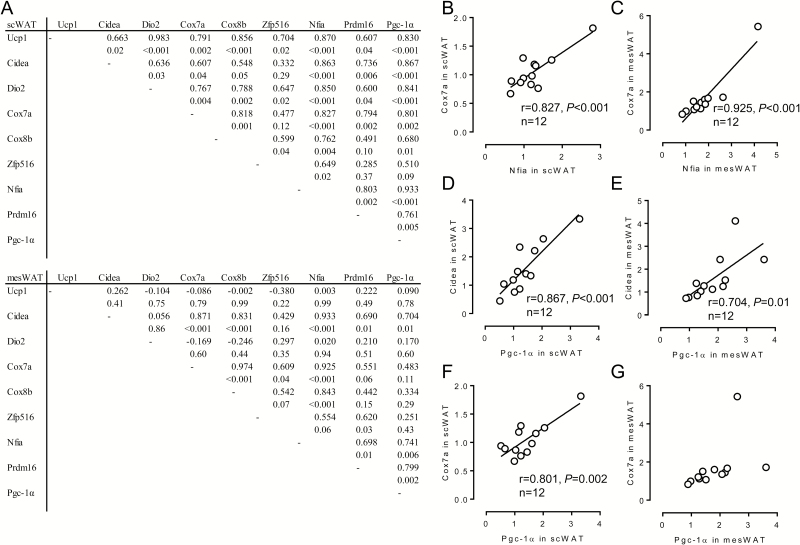
Expression levels of the brown/beige-adipocyte-selective genes other than Zfp516 were reciprocally related especially in scWAT (Figure 5A–C); twenty-six relationships within 28 combinations, except for the relationships between Cidea and Cox8b (P = 0.05) and between Prdm16 and Cox8b (P = 0.10), were significant. Similar interrelationships between the brown/beige adipocyte-selective genes were detected also in mesWAT, although the relationship was relatively weak; eleven relationships were statistically significant (Figure 5A). Some relationships exhibited higher correlations both in scWAT and in mesWAT (Figure 5B–E), and others showed significant relationship limited in scWAT (Figure 5F and G).
Figure 5.
Relationship between expression levels of molecules related to function of brown/beige adipocytes as well as transcriptional regulators related to brown/beige adipogenesis in each fat depot of fattening cattle. Japanese Black steers were fed control diet or vitamin A-restricted diet for 20 mo. (A) Reciprocal relationship between expression levels of molecules related to the function of brown/beige adipocytes as well as transcriptional regulators related to brown/beige adipogenesis in each fat depot was evaluated. Upper: correlation coefficient. Lower: P value. (B)–(G) Relationship between the indicated genes was shown as a scatterplot.
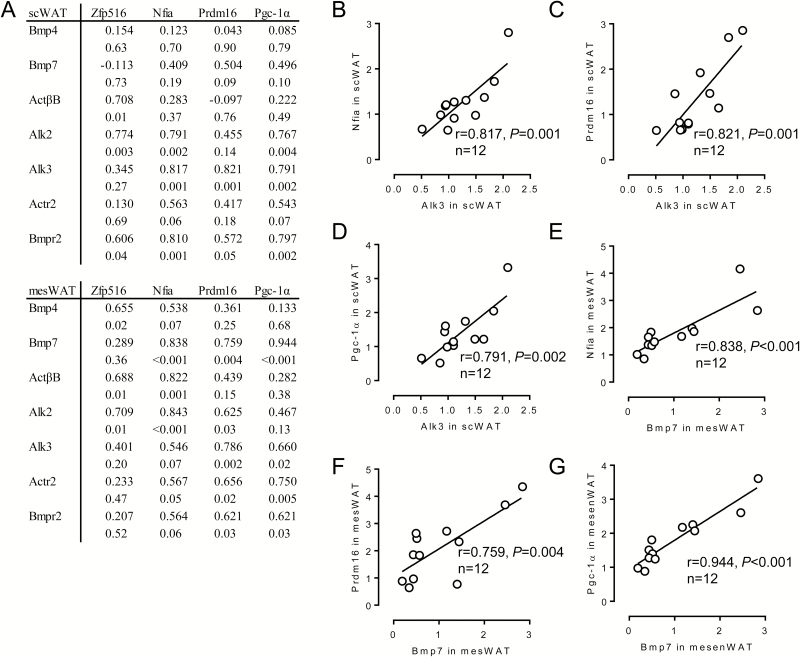
We further evaluated the relationship between expression levels of components of the Bmp signaling and expression levels of transcriptional regulators (Figure 6). In scWAT, expression levels of Alk3 or Bmpr2 were significantly correlated with those in Nfia, Prdm16, or Pgc-1α (Figure 6A–D). In addition, Alk2 expression was significantly related to Nfia or Pgc-1α expression. Similarly, significant relationships between the expression levels of some Bmp receptors and those of Nfia, Prdm16, or Pgc-1α were detected in mesWAT (Figure 6A). In addition, Bmp7 expression was correlated with that of the transcriptional regulators in mesWAT (Figure 6A and E–G).
Figure 6.
Relationship between expression levels of bone morphogenetic protein (Bmp) signal components and those of transcriptional regulators related to brown/beige adipogenesis in each fat depot of fattening cattle. Japanese Black steers were fed control diet or vitamin A-restricted diet for 20 mo. (A) Reciprocal relationship between expression levels of Bmp signal components and those of transcriptional regulators related to brown/beige adipogenesis in each fat depot was evaluated. Upper: correlation coefficient. Lower: P value. (B)–(G) Relationship between the indicated genes was shown as a scatterplot.
DISCUSSION
We previously reported that mRNA expression levels of molecules related to the function of brown/beige adipocytes were higher in mesWAT of fattening cattle fed a diet consisting of high concentrate without supplying vitamin A and roughage low in β-carotene than in that of fattening cattle fed a TMR rich in β-carotene (Kanamori et al., 2014). The present study was designed to evaluate simple effects of vitamin A restriction; the diet with or without vitamin A supplement was used. Our results indicated that expression levels of some function-related brown/beige adipocyte-selective genes were numerically increased in not only mesWAT but also scWAT of vitamin A-restricted fattening cattle; the results are partly consistent with the previous results (Kanamori et al., 2014). Although the vitamin A restriction affected mRNA expression levels of the brown/beige adipocyte-selective molecules in both fat depots, the relationship between the expression levels in scWAT and those in mesWAT was not correlated, except for Ucp1. These results suggest that vitamin A status affects differentiation or activation of brown/beige (pre)adipocytes in scWAT or mesWAT, or in both WAT, and that the effect of vitamin A restriction on mRNA expressions in WAT varied between individuals. Considering that the activation of brown/beige adipocytes dissipates energy as heat, the current results imply that the vitamin A restriction potentially leads to decreased fattening efficiency in fattening cattle. In addition, the present study showed that one cattle showing extremely higher expression of Ucp1 in scWAT and mesWAT exhibited relative lean, which was suggested by daily gain, feed efficiency during the latter period of fattening, and carcass characteristics. These results imply that higher Ucp1 expression in WAT leads to decreased weight gain and fat accumulation through stimulating energy expenditure in fattening cattle.
Aldh1a1 has been shown to be involved in regulation of brown/beige adipogenesis (Kiefer et al., 2012; Wang et al., 2017). Targeted disruption of Aldh1a1 expression induced brown/beige adipocyte-selective genes and emergence of Ucp1-positive adipocytes in WAT of mice. Retinal increased expression of Ucp1 in adipocytes, which was mediated by Rarα (Kiefer et al., 2012). In view of comparable expression of Aldh1a1 in WAT of fattening cattle irrespective of vitamin A status, expression of brown/beige adipocyte-selective genes in bovine WAT is unlikely to be modulated through regulatory expression of Aldh1a1.
Previous studies have shown that retinoic acid increased expression of Ucp1 in murine adipocytes (Alvarez et al., 1995; Rabelo et al., 1996; Murholm et al., 2013), which could be suggested as a direct effect of retinoic acid on Ucp1 transcription. Consistent with the results, Bonet et al. (2000) observed that feeding with a vitamin A-deficient diet tended to decrease expression of Ucp1 in brown adipose tissue of mice. However, retinoic acid inhibited or had no effect on human adipocyte cell line and primary human white adipocytes (Murholm et al., 2013). The species-dependent effect may result from the difference in nucleotide sequence of retinoic acid response elements on the Ucp1 promoter between rat and human, and the corresponding sequence of bovine Ucp1 was divergent from rat Ucp1 (Kanamori et al., 2014). Thus, the vitamin A restriction might not decrease but rather increase Ucp1 expression in WAT of fattening cattle.
The present results showed that expression levels of some brown/beige adipocyte-selective genes such as Ucp1, Dio2, Cox7a, Nfia, and Prdm16 were significantly or tended to be higher in mesWAT, a visceral WAT, of fattening cattle than in scWAT. These results are conceptually distinct from the evidence in mice; expression levels of brown/beige adipocyte-selective genes were generally higher in scWAT than in visceral WAT (Barbatelli et al., 2010; Seale et al., 2011). In addition, more beige adipocytes were emerged in response to cold exposure in scWAT than in visceral WAT (Barbatelli et al., 2010). These results together with the present results indicate that there may be species differences in the presence of beige adipocytes. The mechanism underlying emergence of beige adipocytes is possibly different between cattle and mice.
Several transcriptional regulators such as Zfp516, Nfia, Prdm16, and Pgc-1α centrally regulate differentiation of brown/beige preadipocytes (Puigserver et al., 1998; Seale et al., 2008; Dempersmier et al., 2015; Hiraike et al., 2017). These transcriptional regulators stimulate brown/beige adipogenesis through the mutual interrelationship; Zfp516 interacted with Prdm16, and stimulated transcription of Ucp1 and Pgc-1α (Dempersmier et al., 2015). In addition, transgenic mice with overexpression of Zfp516 in adipocytes exhibited Ucp1-positive adipocytes in WAT (Dempersmier et al., 2015). Forced expression of Nfia stimulated lipid accumulation and expression of Ucp1 and Pgc-1α in C2C12 myogenic cells, and knockdown of the Nifa gene in brown preadipocytes decreased expression of Ucp1 and Pgc-1α in differentiated adipocytes (Hiraike et al., 2017). Forced expression of Prdm16 in adipocytes induced expression of brown/beige adipocyte-selective molecules in fat depots (Seale et al., 2008, 2011). Also, Prdm16 directly bound to Pgc-1α to increase its transcriptional activities (Seale et al., 2007). Pgc-1α stimulated mitochondria biogenesis (Kajimura et al., 2010; Ma et al., 2015) as well as expression of molecules related to the function of brown/beige adipocytes (Fisher et al., 2012, Seale, 2015). The present study revealed that expression levels of the transcriptional regulators other than Zfp516 were closely related to those of genes related to the function of brown/beige adipocytes in scWAT. It is possible that emergence of brown/beige adipocytes in scWAT of fattening cattle is governed by Nfia, Prdm16, or Pgc-1α.
The Bmp pathway regulates commitment of mesenchymal stem cells into a variety of cells (Varga and Wrana, 2005) including brown/beige preadipocytes (Tseng et al., 2008; Qian et al., 2013; Xue et al., 2014). For example, treatment with Bmp4 or Bmp7 committed C3H10T1/2, a mesenchymal stem cell line, to brown preadipocytes (Tseng et al., 2008; Xue et al., 2014). The strength of Bmp signaling is often regulated by the expression levels of the components of Bmp signaling including Bmp receptors (Miyazono, 2000; Murakami et al., 2009). The present study indicated that the vitamin A restriction significantly increased or tended to increase expression of Bmp7 and Bmpr2 in both fat depots, Alk3 and Actr2a in scWAT, and Alk2 in mesWAT. The results are partly consistent with the results of a study using TMR as described previously (Qiao et al., 2015); feeding the vitamin A-restricted diet significantly increased or tended to increase expression of Bmp4, ActβB, Alk2, Alk3, Actr2a, and Bmpr2 in mesWAT. The current study also showed that expression levels of Alk3 in scWAT were positively correlated with those of transcriptional regulators other than Zfp516 to induce brown/beige adipogenesis in scWAT. In addition, expression levels of Bmp7 in mesWAT were correlated with those of Nfia, Prdm16, and Pgc-1α in mesWAT. Thus, the vitamin A restriction possibly modulated Bmp signaling through alteration of expression levels of Bmp signal components in WAT, which controls expression levels of the transcriptional regulators and subsequent brown/beige adipogenesis.
Previous studies using rodents also revealed that dietary vitamin A level affected Pparγ expression in WAT (Ribot et al., 2001; Redonnet et al., 2008), although the effects were not consistent. Ribot et al. (2001) showed that vitamin A deficiency increased expression of Pparγ in epididymal WAT of mice but not in scWAT. In contrast, high vitamin A diet also increased Pparγ expression in scWAT of rats (Redonnet et al., 2008). The current results revealed that the vitamin A-restricted diet increased the expression level of Pparγ in scWAT but not mesWAT of fattening cattle. Prdm16 and Pgc-1α formed a complex with Pparγ, and the complex formation enhanced differentiation of brown preadipocytes and expression of the function-related genes in brown adipocytes (Puigserver et al., 1998; Seale et al., 2008). Furthermore, Nfia and Pparγ co-localized at the enhancer regions of brown/beige adipocyte-selective genes (Hiraike et al., 2017). These results indicate that the vitamin A restriction may also stimulate emergence of brown/beige adipocytes through increase in Pparγ expression.
The present study examined expression of molecules involved in commitment to and differentiation of brown/beige preadipocytes, and function of brown/beige adipocytes in WAT of fattening cattle fed a vitamin A-restricted diet, and evaluated the relationships of expression levels between the molecules. The present results imply that dietary vitamin A level modulates expression levels of Bmp signal components, which is the first event to induce emergence of brown/beige adipocytes in WAT of fattening cattle. Future studies are needed to clarify regulatory expression of Bmp signal components by vitamin A as well as transcriptional regulators by the Bmp pathway in detail. Brown adipocytes and beige adipocytes exhibited specific molecular signature (Wu et al., 2012; Sharp et al., 2012). Thus, the discrimination of brown adipocytes from beige adipocytes cannot be determined by anatomical location alone. In fact, brown adipose tissue, which mainly consists of Ucp1-positive adipocytes, is not formed in adult humans, but Ucp1-positive adipocytes that exhibited molecular signature resembling mouse brown adipocytes as well as mouse beige adipocytes were present in human neck fat (Cypess et al., 2013). Therefore, it should be clarified whether Ucp1-positive adipocytes in bovine WAT are brown adipocytes or beige adipocytes through molecular signature.
Conflict of interest statement. None declared.
Footnotes
This work was supported by the JSPS KAKENHI (26292137 to T.M.).
LITERATURE CITED
- Alvarez R., de Andrés J., Yubero P., Viñas O., Mampel T., Iglesias R., Giralt M., and Villarroya F.. 1995. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J. Biol. Chem. 270:5666–5673. doi: 10.1074/jbc.270.10.5666 [DOI] [PubMed] [Google Scholar]
- Asano H., Yamada T., Hashimoto O., Umemoto T., Sato R., Ohwatari S., Kanamori Y., Terachi T., Funaba M., and Matsui T.. 2013. Diet-induced changes in ucp1 expression in bovine adipose tissues. Gen. Comp. Endocrinol. 184:87–92. doi: 10.1016/j.ygcen.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Barbatelli G., Murano I., Madsen L., Hao Q., Jimenez M., Kristiansen K., Giacobino J. P., De Matteis R., and Cinti S.. 2010. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298:E1244–E1253. doi: 10.1152/ajpendo.00600.2009 [DOI] [PubMed] [Google Scholar]
- Bonet M. L., Oliver J., Picó C., Felipe F., Ribot J., Cinti S., and Palou A.. 2000. Opposite effects of feeding a vitamin A-deficient diet and retinoic acid treatment on brown adipose tissue uncoupling protein 1 (UCP1), UCP2 and leptin expression. J. Endocrinol. 166:511–517. doi: 10.1677/joe.0.1660511 [DOI] [PubMed] [Google Scholar]
- Cannon B., and Nedergaard J.. 2004. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84:277–359. doi: 10.1152/physrev.00015.2003 [DOI] [PubMed] [Google Scholar]
- Chambon P. 1996. A decade of molecular biology of retinoic acid receptors. Faseb J. 10:940–954. doi: 10.1096/fasebj.10.9.8801176 [DOI] [PubMed] [Google Scholar]
- Cypess A. M., Lehman S., Williams G., Tal I., Rodman D., Goldfine A. B., Kuo F. C., Palmer E. L., Tseng Y. H., Doria A.,. et al. 2009. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360:1509–1517. doi: 10.1056/NEJMoa0810780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cypess A. M., White A. P., Vernochet C., Schulz T. J., Xue R., Sass C. A., Huang T. L., Roberts-Toler C., Weiner L. S., Sze C.,. et al. 2013. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19:635–639. doi: 10.1038/nm.3112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempersmier J., Sambeat A., Gulyaeva O., Paul S. M., Hudak C. S., Raposo H. F., Kwan H. Y., Kang C., Wong R. H., and Sul H. S.. 2015. Cold-inducible zfp516 activates UCP1 transcription to promote browning of white fat and development of brown fat. Mol. Cell 57:235–246. doi: 10.1016/j.molcel.2014.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran E. M., Shapshak P., Worley J., Minagar A., Ziegler F., Haliko S., Moleon-Borodowsky I., and Haslett P. A.. 2005. Presenilin-1 detection in brain neurons and FOXp3 in peripheral blood mononuclear cells: normalizer gene selection for real time reverse transcriptase PCR using the deltadeltact method. Front. Biosci. 10:2955–2965. doi: 10.2741/1751 [DOI] [PubMed] [Google Scholar]
- Fisher F. M., Kleiner S., Douris N., Fox E. C., Mepani R. J., Verdeguer F., Wu J., Kharitonenkov A., Flier J. S., Maratos-Flier E.,. et al. 2012. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26:271–281. doi: 10.1101/gad.177857.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraike Y., Waki H., Yu J., Nakamura M., Miyake K., Nagano G., Nakaki R., Suzuki K., Kobayashi H., Yamamoto S.,. et al. 2017. NFIA co-localizes with pparγ and transcriptionally controls the brown fat gene program. Nat. Cell Biol. 19:1081–1092. doi: 10.1038/ncb3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki T., Sakai J., and Kajimura S.. 2016. Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol. 17:480–495. doi: 10.1038/nrm.2016.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irie M., Kouda M., and Matono H.. 2011. Effect of ursodeoxycholic acid supplementation on growth, carcass characteristics, and meat quality of wagyu heifers (Japanese black cattle). J. Anim. Sci. 89:4221–4226. doi: 10.2527/jas.2011-4211 [DOI] [PubMed] [Google Scholar]
- Japan Livestock Industry Association. 2008. Japanese feeding standard for beef cattle. Natl. Agric. Feed Res. Organ. NARO, Tokyo. [Google Scholar]
- Japan Livestock Industry Association. 2009. Standard tables of feed composition in Japan. Natl. Agric. Feed Res. Organ. NARO, Tokyo. [Google Scholar]
- JMGA (Japan Meat Grading Association) 1988. New beef carcass grading standards. Jpn. Meat Grading Assoc, Tokyo. [Google Scholar]
- Kajimura S., and Saito M.. 2014. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu. Rev. Physiol. 76:225–249. doi: 10.1146/annurev-physiol-021113-170252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S., Seale P., and Spiegelman B. M.. 2010. Transcriptional control of brown fat development. Cell Metab. 11:257–262. doi: 10.1016/j.cmet.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S., Spiegelman B. M., and Seale P.. 2015. Brown and beige fat: physiological roles beyond heat generation. Cell Metab. 22:546–559. doi: 10.1016/j.cmet.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori Y., Murakami M., Sugiyama M., Hashimoto O., Matsui T., and Funaba M.. 2017. Interleukin-1β (IL-1β) transcriptionally activates hepcidin by inducing CCAAT enhancer-binding protein δ (C/ebpδ) expression in hepatocytes. J. Biol. Chem. 292:10275–10287. doi: 10.1074/jbc.M116.770974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori Y., Sugiyama M., Hashimoto O., Murakami M., Matsui T., and Funaba M.. 2016. Regulation of hepcidin expression by inflammation-induced activin B. Sci. Rep. 6:38702. doi: 10.1038/srep38702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori Y., Yamada T., Asano H., Kida R., Qiao Y., Abd Eldaim M. A., Tomonaga S., Matsui T., and Funaba M.. 2014. Effects of vitamin a status on expression of UCP1 and brown/beige adipocyte-related genes in white adipose tissues of beef cattle. J. Vet. Med. Sci. 76:1261–1265. doi: 10.1292/jvms.14-0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kida R., Noguchi T., Murakami M., Hashimoto O., Kawada T., Matsui T., and Funaba M.. 2018. Supra-pharmacological concentration of capsaicin stimulates brown adipogenesis through induction of endoplasmic reticulum stress. Sci. Rep. 8:845. doi: 10.1038/s41598-018-19223-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer F. W., Vernochet C., O’Brien P., Spoerl S., Brown J. D., Nallamshetty S., Zeyda M., Stulnig T. M., Cohen D. E., Kahn C. R.,. et al. 2012. Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat. Med. 18:918–925. doi: 10.1038/nm.2757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X., Lee P., Chisholm D. J., and James D. E.. 2015. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front Endocrinol. (Lausanne) 6:1. doi: 10.3389/fendo.2015.00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Marken Lichtenbelt W. D., Vanhommerig J. W., Smulders N. M., Drossaerts J. M., Kemerink G. J., Bouvy N. D., Schrauwen P., and Teule G. J.. 2009. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360:1500–1508. doi: 10.1056/NEJMoa0808718 [DOI] [PubMed] [Google Scholar]
- Miyazono K. 2000. Positive and negative regulation of TGF-beta signaling. J. Cell Sci. 113:1101–1109. [DOI] [PubMed] [Google Scholar]
- Miyazono K., Kamiya Y., and Morikawa M.. 2010. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 147:35–51. doi: 10.1093/jb/mvp148 [DOI] [PubMed] [Google Scholar]
- Murakami M., Kawachi H., Ogawa K., Nishino Y., and Funaba M.. 2009. Receptor expression modulates the specificity of transforming growth factor-beta signaling pathways. Genes Cells 14:469–482. doi: 10.1111/j.1365-2443.2009.01283.x [DOI] [PubMed] [Google Scholar]
- Murholm M., Isidor M. S., Basse A. L., Winther S., Sørensen C., Skovgaard-Petersen J., Nielsen M. M., Hansen A. S., Quistorff B., and Hansen J. B.. 2013. Retinoic acid has different effects on UCP1 expression in mouse and human adipocytes. BMC Cell Biol. 14:41. doi: 10.1186/1471-2121-14-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard J., and Cannon B.. 2010. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 11:268–272. doi: 10.1016/j.cmet.2010.03.007 [DOI] [PubMed] [Google Scholar]
- Oka A. 1996. Effects of vitamin A on beef quality and body weight of Japanese black steers (in Japanese). J. Anim. Genet. 24:31–36. [Google Scholar]
- Puigserver P., Wu Z., Park C. W., Graves R., Wright M., and Spiegelman B. M.. 1998. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92:829–839. doi: 10.1016/S0092-8674(00)81410-5 [DOI] [PubMed] [Google Scholar]
- Qian S. W., Tang Y., Li X., Liu Y., Zhang Y. Y., Huang H. Y., Xue R. D., Yu H. Y., Guo L., Gao H. D.,. et al. 2013. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl. Acad. Sci. USA. 110:E798–E807. doi: 10.1073/pnas.1215236110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y., Yamada T., Kanamori Y., Kida R., Shigematsu M., Fujimoto Y., Tomonaga S., Matsui T., and Funaba M.. 2015. Regulatory expression of components in the BMP pathway in white adipose tissues of cattle. Livest. Sci. 174:144–149. doi: 10.1016/j.livsci.2015.02.008 [DOI] [Google Scholar]
- Rabelo R., Reyes C., Schifman A., and Silva J. E.. 1996. A complex retinoic acid response element in the uncoupling protein gene defines a novel role for retinoids in thermogenesis. Endocrinology 137:3488–3496. doi: 10.1210/endo.137.8.8754778 [DOI] [PubMed] [Google Scholar]
- Redonnet A., Ferrand C., Bairras C., Higueret P., Noël-Suberville C., Cassand P., and Atgié C.. 2008. Synergic effect of vitamin A and high-fat diet in adipose tissue development and nuclear receptor expression in young rats. Br. J. Nutr. 100:722–730. doi: 10.1017/S0007114508967568 [DOI] [PubMed] [Google Scholar]
- Ribot J., Felipe F., Bonet M. L., and Palou A.. 2001. Changes of adiposity in response to vitamin A status correlate with changes of PPAR gamma 2 expression. Obes. Res. 9:500–509. doi: 10.1038/oby.2001.65 [DOI] [PubMed] [Google Scholar]
- Saito M., Okamatsu-Ogura Y., Matsushita M., Watanabe K., Yoneshiro T., Nio-Kobayashi J., Iwanaga T., Miyagawa M., Kameya T., Nakada K.,. et al. 2009. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58:1526–1531. doi: 10.2337/db09-0530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P. 2015. Transcriptional regulatory circuits controlling brown fat development and activation. Diabetes 64:2369–2375. doi: 10.2337/db15-0203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Bjork B., Yang W., Kajimura S., Chin S., Kuang S., Scimè A., Devarakonda S., Conroe H. M., Erdjument-Bromage H.,. et al. 2008. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454:961–967. doi: 10.1038/nature07182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Conroe H. M., Estall J., Kajimura S., Frontini A., Ishibashi J., Cohen P., Cinti S., and Spiegelman B. M.. 2011. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121:96–105. doi: 10.1172/JCI44271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P., Kajimura S., Yang W., Chin S., Rohas L. M., Uldry M., Tavernier G., Langin D., and Spiegelman B. M.. 2007. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6:38–54. doi: 10.1016/j.cmet.2007.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp L. Z., Shinoda K., Ohno H., Scheel D. W., Tomoda E., Ruiz L., Hu H., Wang L., Pavlova Z., Gilsanz V.,. et al. 2012. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS One 7:e49452. doi: 10.1371/journal.pone.0049452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P., Hu E., Graves R. A., Budavari A. I., and Spiegelman B. M.. 1994. mPPAR gamma 2: tissue-specific regulator of an adipocyte enhancer. Genes Dev. 8:1224–1234. doi: 10.1101/gad.8.10.1224 [DOI] [PubMed] [Google Scholar]
- Tseng Y. H., Kokkotou E., Schulz T. J., Huang T. L., Winnay J. N., Taniguchi C. M., Tran T. T., Suzuki R., Espinoza D. O., Yamamoto Y.,. et al. 2008. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454:1000–1004. doi: 10.1038/nature07221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A. C., and Wrana J. L.. 2005. The disparate role of BMP in stem cell biology. Oncogene 24:5713–5721. doi: 10.1038/sj.onc.1208919 [DOI] [PubMed] [Google Scholar]
- Virtanen K. A., Lidell M. E., Orava J., Heglind M., Westergren R., Niemi T., Taittonen M., Laine J., Savisto N. J., Enerbäck S.,. et al. 2009. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360:1518–1525. doi: 10.1056/NEJMoa0808949 [DOI] [PubMed] [Google Scholar]
- Wang W., and Seale P.. 2016. Control of brown and beige fat development. Nat. Rev. Mol. Cell Biol. 17:691–702. doi: 10.1038/nrm.2016.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Wang Z., de Avila J. M., Zhu M. J., Zhang F., Gomez N. A., Zhao L., Tian Q., Zhao J., Maricelli J.,. et al. 2017. Moderate alcohol intake induces thermogenic brown/beige adipocyte formation via elevating retinoic acid signaling. Faseb J. 31:4612–4622. doi: 10.1096/fj.201700396R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Boström P., Sparks L. M., Ye L., Choi J. H., Giang A. H., Khandekar M., Virtanen K. A., Nuutila P., Schaart G.,. et al. 2012. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376. doi: 10.1016/j.cell.2012.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue R., Wan Y., Zhang S., Zhang Q., Ye H., and Li Y.. 2014. Role of bone morphogenetic protein 4 in the differentiation of brown fat-like adipocytes. Am. J. Physiol. Endocrinol. Metab. 306:E363–E372. doi: 10.1152/ajpendo.00119.2013 [DOI] [PubMed] [Google Scholar]
- Yamada T., Higuchi M., and Nakanishi N.. 2013. Plasma 8-isoprostane concentrations and adipogenic and adipokine gene expression patterns in subcutaneous and mesenteric adipose tissues of fattening wagyu cattle. J. Vet. Med. Sci. 75:1021–1027. doi: 10.1292/jvms.13-0071 [DOI] [PubMed] [Google Scholar]