Abstract
Maintenance of mixed grass–legume pastures for stand longevity and improved animal utilization is a challenge in warm-season climates. The goal of this study was to assess grazing management on stand persistence, forage intake, and N balance of beef heifers grazing mixed pastures of Brachiaria brizantha and Arachis pintoi. A 2-yr experiment was carried out in Brazil, where four grazing management were assessed: rest period interrupted at 90%, 95%, and 100% of light interception (LI) and a fixed rest period of 42 d (90LI, 95LI, 100LI, and 42D, respectively). The LI were taken at 50 points at ground level and at 5 points above the canopy for each paddock using a canopy analyzer. For all treatments, the postgrazing stubble height was 15 cm. Botanical composition and canopy structure characteristics such as canopy height, forage mass, and vertical distribution of the morphological composition were evaluated pre- and post-grazing. Forage chemical composition, intake, and microbial synthesis were also determined. A randomized complete block design was used, considering the season of the year as a repeated measure over time. Grazing management and season were considered fixed, while block and year were considered random effects. In the summer, legume mass accounted for 19% of the canopy at 100LI, which was less than other treatments (a mean of 30%). The 100LI treatment had a greater grass stem mass compared with other treatments. In terms of vertical distribution for 100LI, 38.6% of the stem mass was above the stubble height, greater than the 5.7% for other treatments. The canopy structure limited NDF intake (P = 0.007) at 100LI (1.02% of BW/d), whereas 42D, 90LI, and 95LI treatments had NDF intake close to 1.2% of BW/d. The intake of digestible OM (P = 0.007) and the ratio of CP/digestible OM (P < 0.001) were less at 100LI in relation to the other treatments. The production of microbial N (P < 0.001) and efficiency of microbial synthesis (P = 0.023) were greater at 95LI and 90LI, followed by 42D and less at 100LI. Overall, the range from 90% to 95% of LI is the recommendation to interrupt the rest period, since this strategy enhanced community stability, forage intake, and nutritional value of the diet. Under on-farm conditions, brachiaria grass and forage peanut pastures should be managed at a range height of 24 to 30 cm.
Keywords: Arachis pintoi, forage intake, legume, mixed pasture, palisadegrass
INTRODUCTION
Grasses from the Brachiaria genus are the most important forage source for grazing ruminants in warm climates (Jank et al., 2014). Forage peanut (Arachis pintoi) is a warm-season legume with potential for mixtures with grasses (Tamele et al., 2017). The association of grass and legume provides complementarity for the benefit of both grazing livestock and ecosystem function (Muir et al., 2011). However, it is a challenge for farmers to maintain long-term persistence of mixed pastures.
Defoliation management determines the balance and contribution of each component in the stand (Tamele et al., 2017) and forage intake by grazing animals, since the canopy structure is a nonnutritional factor (Poppi et al., 1987). Defoliation may change the botanical composition and vertical distribution of the stand, which influences long-term sward persistence (Black et al., 2009). Short rest periods (i.e., 90% of light interception; LI) in mixed pastures promote greater legume population (Pereira et al., 2017). Conversely, long periods of rest, sufficient for the canopy to intercept 100% of LI potentially limit intake rates due to the greater stem elongation (Geremia et al., 2014).
These factors, along with defoliation intensity, modulate the selectivity of grazing animals and the nutritive value of the diet. Diets with greater proportions of legume tend to have greater nutritive value than grass monocultures (Muir et al., 2014). To date, there is no established strategy to manage brachiaria grass and forage peanut pastures. Thus, we hypothesized that the grazing management in mixtures determines the level of light competition between species, modulating the canopy stability; a canopy structure with reduced presence of stems will result in greater forage intake with better nutritive value. Hence, the objective of this study was to assess four grazing management based on LI or a fixed interval between grazing on mixed Brachiaria brizantha and A. pintoi pasture canopy characteristics, forage intake, and N use efficiency using growing cattle.
MATERIALS AND METHODS
The experimental procedures of this study were approved by the Ethics and Animal Welfare Committee of the Federal University of Lavras (protocol number 062/2013).
Experimental Site
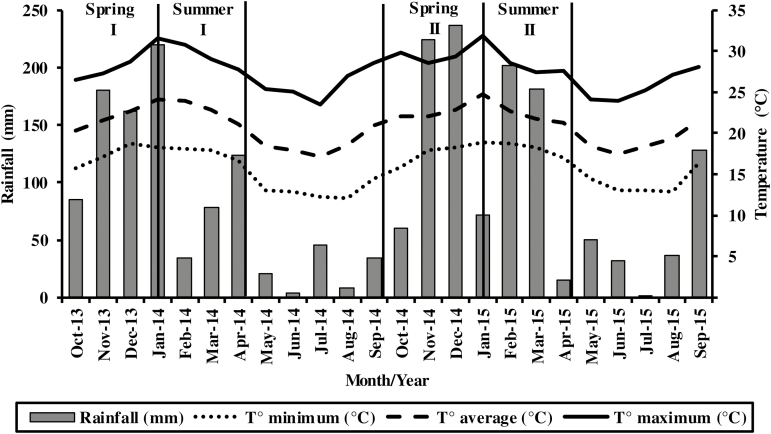
The study was carried out at the Experimental Farm of the Federal University of Lavras, Brazil (21°14′S, 45°00′W; 918 m above sea level). This area has a subtropical humid mesothermal climate with dry winters (Köppen-Geiger climate classification: Cwa; Sá Júnior et al., 2012). Meteorological data were obtained from a weather station located 1,000 m from the experimental area (Fig. 1).
Figure 1.
Monthly temperatures (°C) and rainfall (mm) in Lavras, Brazil, during the experimental period (seasons of the year).
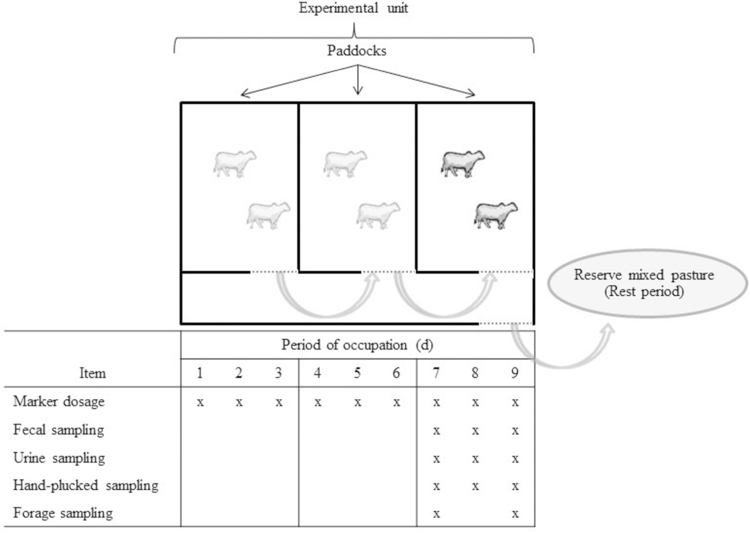
The pasture used was established in December 2006 by joint seeding of brachiaria grass (B. brizantha Stapf. A. Rich. cv. Marandu) with forage peanut (A. pintoi Krapov. & W.C. Greg. cv. BRS Mandobi). The seeding rates were 7.6 and 7.2 kg/ha of pure live seed for brachiaria grass and forage peanut, respectively. The pasture was divided into 12 experimental units. Each experimental unit was divided into three paddocks of 220 m2 (Fig. 2). In October 2012, grazing management treatments were imposed. These paddocks were grazed for 1 yr (adaptation period) and after that, the assessments were started. A 2-yr experimental period (from 2013 to 2015) was adopted, from October to January (rainy spring) and from January to April (rainy summer; Fig. 1). Assessments were not performed from May to September for both years due to the climatic conditions (dry winter).
Figure 2.
The method used to manage heifers in the experimental unit and collect fecal, urine, and forage samples. The experimental unit was divided into three paddocks with 3-d occupation period in each one. Marker dosage was performed over 9-d period and fecal, urine, hand-plucked, and forage samples were collected in the last paddock only. A reserve mixed pasture of brachiaria grass and forage peanut was used to keep animals during rest periods.
The soil characteristics were determined using the methods recommended by Embrapa (1997). The soil of the experimental site at the start of the study (November 2012) were pH = 7.0, OM = 4.93%, P = 2.3 mg/dm3, K+ = 64 mg/dm3, Ca2+ = 3.4 cmolc/dm3, Mg2+ = 1.1 cmolc/dm3, H + Al = 2.9 cmolc/dm3, Al3+ = 0.0 cmolc/dm3, and cation exchange capacity = 7.56 cmolc/dm3. In early spring during each year of the trial, all experimental units were grazed to reach a stubble height of 15 cm. Afterward, fertilizers were applied as single superphosphate, potassium chloride, and micronutrients. Applications (kg/ha) corresponded to 40 of P2O5, 53 of K2O, 38 of Ca, 1.7 of S, 0.5 of B, 0.25 of Cu, 0.6 of Mn, 0.03 of Mo, and 2.7 of Zn.
Treatments and Experimental Management
Four grazing management strategies were assessed: grazing rest period interruption when the canopy reached an LI of 1) 90% (90LI), 2) 95% (95LI), 3) 100% (100LI), and 4) rotational stocking every 42 d (42D). The rest period length was determined as a function of each respective treatment. A minimum of two Tabapuã heifers (260 ± 43 kg) were used to graze paddocks to the target stubble height. Additional put-and-take heifers were used to manage forage to a target stubble height of 15 cm during a 3-d period. Stocking density and stocking rate (animals/ha) were calculated dividing the number of heifers by paddock area and the number of heifers by the estimated total area used to complete a grazing cycle, respectively (Allen et al., 2011).
Experimental Evaluations
Canopy structure.
A LAI 2200 canopy analyzer (LI-COR, 2009, Lincoln, NE) was used to measure LI (%). Readings were taken at 50 points at ground level and at 5 points above the canopy for each experimental unit. The average canopy height (CH) was measured using a sward stick (Barthram, 1985) at 50 random points. Forage mass was sampled by using three frames of 1 × 0.5 m (pre- and post-grazing) per paddock, once for each grazing cycle (Fig. 2). After harvesting the forage, botanical and morphological separations were performed. The grass samples were separated into stem (stem + sheath), leaf (leaf blade), and dead material. Legume samples were separated into stem and leaf (stipule + petiole + leaflet). Forage samples were oven-dried at 55 °C for 72 h to a constant weight. Grass mass (kg/ha) was considered whole-plant without dead material. Legume mass (kg/ha) was considered the leaf plus stem mass. Total forage mass (kg/ha) was the sum of grass and legume components in the above ground canopy. Forage removal rate (%) was calculated by the difference between the pregrazing and postgrazing masses divided by the pregrazing mass.
The forage mass was evaluated in four strata of the CH (% of CH). The first stratum, close to the ground, represented 0% to 25%, the second stratum 25% to 50%, the third stratum 50% to 75%, and the fourth stratum (at the top of the canopy) 75% to 100% of the CH (Tamele et al., 2017). For this, the canopy structure was evaluated using an inclined point quadrat with 120 data points per plot (Warren Wilson, 1960). To achieve the forage mass of each stratum (% of mass per stratum), the frequency of each botanical and morphological component was multiplied by the respective mass in the four canopy strata.
Nutritive value and forage intake.
Hand-plucked forage samples (three samples per paddock) were collected for forage nutritive value analysis (De Vries, 1995). A composite sample of the 3-d occupation period of each species was collected for each experimental unit (Fig. 2). The samples were oven-dried at 55 °C for 72 h, weighed, and ground in a Cyclotec mill (Tecator, Herndon, VA) to pass a 1-mm screen.
The DM of each sample was obtained by oven drying at 100 °C for 18 h (method 934.01; AOAC, 2000). The ash concentration was determined by 2-h incineration process in a 600 °C muffle furnace (method 942.05; AOAC, 2000). The CP concentration was obtained based on the N concentration (CP = total N × 6.25), which was determined using the Kjeldahl procedure (method 920.87; AOAC, 2000). The ether extract was analyzed according to the method 920.39 (AOAC, 2000). The ash-free NDF and ADF were determined sequentially by autoclave method at 105 °C for 60 min (Pell and Schofield, 1993). The lignin was analyzed according to the method 973.18D (AOAC, 2000). The in vitro DM digestibility (IVDMD) was determined by using the DAISYII method at 48 h (Ankom Technology Corp., Fairport, NY; Holden, 1999). Rumen fluid was collected before feeding from two cannulated heifers fed a diet that consisted of mixed pasture of brachiaria grass and forage peanut. The neutral detergent insoluble nitrogen (NDIN), acid detergent insoluble nitrogen (ADIN), and NPN were estimated using the method described by Licitra et al. (1996). The calculation of protein fractions (A, B1 + B2, B3, and C) were estimated according to Tylutki et al. (2008), where the fraction A (NPN) was determined by the difference between the quantity of N insoluble in trichloroacetic acid solution and the total sample N. The fraction B3 was determined by the difference between NDIN and ADIN (fraction C). The fraction B1 + B2 was determined by the difference between the quantity of total N and the other fractions. Condensed tannin was determined by use extraction using methanol, acetone and ascorbic acid solution, and the Fe reagent and n-butanol-HCl were added to the tannin extract, which was then heated at 95 °C (Porter et al.,1986). The absorbance of tannin extract solution was measured at 550 nm.
Spot fecal samples were collected once a day and a composite sample was performed for each animal for the 3-d occupation period (Fig. 2). On the sampling days, the heifers were brought over from the paddocks to a barn to collect feces directly from the rectum. Fecal production was estimated using titanium dioxide as external marker (Titgemeyer et al., 2001) during nine consecutive days, six for adaptation, and three for collection. The first and the last day of collection corresponded to the first and last day of the occupation period. The titanium dioxide was dosed daily in the amount of 10 g/animal, daily. Fecal samples were oven-dried at 55 °C for 72 h to determine DM concentration, and air equilibrated, weighed, and ground in a Cyclotec mill (Tecator) to pass a 1-mm screen. The fecal samples were analyzed for titanium dioxide concentration according to Myers et al. (2004).
The proportion of grass and legume in fecal samples was estimated through of the ratio of natural 12C and 13C isotopes (Eq. 1).
| (1) |
where Cleg (%) is the proportion of carbon from legume in the fecal sample, δ13CG, δ13CL, δ13CS are the values of 13C abundance of the feces from animals fed on grass, legume, and the experimental diet, respectively. To estimate the intake of grass and legume (% BW/d), the total fecal excretion was divided by their indigestibility. (Jones et al., 1979; Macedo et al., 2010). The intake of N (g/d), OM, CP, and NDF (% BW/d) were also estimated.
Nitrogen utilization.
Digestible OM was calculated based on the intake of OM and excretion of OM in the feces. The ratio of CP/digestible OM was calculated based on intake of digestible OM and CP (g/kg). Fecal N excretion (g of N/d) was assessed by the concentration of N in the feces (method 920.87; AOAC, 2000) in relation to the fecal production.
Microbial N synthesis (g of N/d) was estimated by using the technique of the purine derivatives in urine (Chen and Gomes, 1992). The spot sampling was used to assess the excretion of urinary nitrogenous compounds (Valadares et al., 1999). Spot urine samples were obtained by vulval stimulation at the same time as fecal sample collection. A 45 mL aliquot was taken and 5 mL of 20% sulfuric acid (H2SO4) was added. A 3-d composite sample was collected and stored in a plastic flask at −20 °C. Urine creatinine concentration was determined using a commercial kit (Creatinine K, Labtest, Lagoa Santa, Brazil). Urine volume was estimated using creatinine concentration as a marker and assuming a daily creatinine output according to Eq. 2 (Costa e Silva et al. 2012).
| (2) |
where UV (L/d) is daily total urinary production, SBW (kg) is shrunk body weight, UCc (g/L) is urine creatinine concentration. Allantoin was analyzed as described by Chen and Gomes (1992). Uric acid was determined using a commercial kit (Uric acid monoreagent, Bioclin, Belo Horizonte, Brazil). Excretion of allantoin and uric acid were estimated multiplying by their concentrations in urine by the daily urinary volume. Excretion of the purine derivatives in urine was calculated by the sum of the allantoin and uric acid excretions (mmol/d). The daily purine absorption (Pa) and the production of ruminal microbial N (g/d) were calculated using the Eq. 3 and 4, respectively (Chen and Gomes, 1992).
| (3) |
| (4) |
where Pa (mmol/d) is purine absorbed, PDe (mmol/d) is purine derivatives excreted (uric acid and allantoin), SBW (kg) is shrunk body weight, and NMIC (g of N/d) is ruminal production of microbial nitrogen.
Efficiency of microbial synthesis in the rumen (g microbial N/kg of digestible OM) was calculated by dividing the production of ruminal microbial N by the digestible OM intake (kg/d). Urinary N excretion (g of N/d) was determined by its N concentration relative to urinary volume (method 920.87; AOAC, 2000).
Statistical analyses.
The experimental design was randomized complete blocks with four treatments (grazing management), three replications, and repeated measurements over time (seasons of the year). Data were analyzed using the mixed models method (Littell et al., 2000), performed by the MIXED procedure of SAS (SAS Institute, Cary NC). The effects of grazing management and seasons were considered fixed and the effect of block and year as random effect. The Akaike information criterion was used to choose the best (co)variance structure (Akaike, 1974). All variance components were estimated using the restricted maximum likelihood method. The treatment averages were estimated using the LSMEANS statement and compared using Student’s t-test with P ≤ 0.05. The statistical model for data analysis was as follows:
where Yijkz = value observed in the ith block of the jth GM of the kth season of the zth year; μ = overall average; Bi = random effect associated with the ith block, i = 1, 2, 3; GMj = fixed effect associated with jth grazing management, j = 1, 2, 3, 4; γij = random error associated with the ith block in the jth GM. Yk = random effect associated with zth year, z = 1, 2; Sz = fixed effect associated with kth season, k = 1, 2; (GM × S)jz = fixed effect of interaction jth GM with the kth season. εijkz = random error associated with the ith block, the jth GM, the kth season, and the zth year.
The effect of strata was included in statistical model to run canopy strata data. Their interactions were also considered in the model.
RESULTS
Canopy Structure
Pregrazing canopy characteristics are reported in Table 1. The length of the rest periods were 42, 43, 51, and 89 d for 42D, 90LI, 95LI, and 100LI, respectively. The LI were 90.2%, 94.9%, and 97.9% for 90LI, 95LI, and 100LI, respectively. The LI for 42D was 87.5%, similar to the 90LI treatment. On average, the CH before grazing (P < 0.001) were 38.4 cm, 29.6 cm, 24.5 cm, and 21.2 cm for 100LI, 95LI, 90LI, and 42D, respectively. Stocking density (P = 0.001) and stocking rate (P = 0.020) were greater at 100LI, lesser at 42D and intermediate at 90LI and 95LI. Forage mass (P < 0.001), grass (P < 0.001), and grass–leaf (P < 0.001) masses were greater at 100LI, and progressively declined at 95LI, 90LI, and 42D. The grass stem mass (P < 0.001) was greater at 100LI, followed by the 95LI, 90LI, and 42D. The mass of dead material (data not shown) did not vary with grazing management (P = 0.146) nor with seasons of the year (P = 0.547), with an average of 2,332 kg/ha.
Table 1.
Pregrazing canopy of brachiaria grass-forage peanut pastures as influenced by grazing management
| Grazing management (GM)1 | P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 42D | 90LI | 95LI | 100LI | SEM | GM | S2 | GM × S |
| Rest period, d | 42 | 43 | 51 | 89 | 3.8 | — | — | — |
| Light interception, % | 87.5 | 90.2 | 94.9 | 97.9 | 0.6 | — | — | — |
| Stocking density, animal/ha | 73.7c | 79.5bc | 91.5b | 141a | 5.7 | 0.001 | 0.020 | 0.237 |
| Stocking rate, animal/ha | 4.5c | 5.6b | 6.2ab | 6.9a | 0.4 | 0.020 | 0.002 | 0.377 |
| Canopy height, cm | 21.2d | 24.5c | 29.6b | 38.4a | 2.1 | <0.001 | 0.097 | 0.395 |
| Forage mass, kg/ha | 4,388d | 5,158c | 5,755b | 6,606a | 469 | <0.001 | 0.011 | 0.438 |
| Grass mass, kg/ha | 3,074d | 3,661c | 4,227b | 5,145a | 190 | <0.001 | 0.019 | 0.998 |
| Grass–leaf mass, kg/ha | 1,915d | 2,351c | 2,608b | 2,944a | 161 | <0.001 | 0.901 | 0.963 |
| Grass stem mass, kg/ha | 1,159c | 1,310bc | 1,619b | 2,201a | 162 | <0.001 | <0.001 | 0.980 |
| Legume mass, kg/ha | ||||||||
| Spring | 1,134Ab | 1,293Aab | 1,430Aab | 1,682Aa | 371 | 0.668 | 0.263 | 0.024 |
| Summer | 1,493Aab | 1,701Aa | 1,625Aa | 1,240Bb | 357 | |||
| Legume leaf mass, kg/ha | ||||||||
| Spring | 568Ab | 626Ab | 691Aab | 894Aa | 137 | 0.508 | 0.682 | 0.008 |
| Summer | 659Aab | 723Aa | 756Aa | 551Bb | 127 | |||
a–dLeast squares means within a row with different superscripts lowercase differ (P ≤ 0.05).
A,BLeast squares means within a column differing superscripts uppercase differ (P ≤ 0.05).
142D = rest period of 42 d; 90IL = 90% light interception; 95LI = 95% light interception, 100LI = 100% light interception.
2S = seasons. Spring (from October to January) and summer (from January to April).
There was an interaction between grazing management and seasons of the year on legume (P = 0.024) and legume leaf masses (P = 0.008). In the spring, there was greater legume and legume leaf mass at 100LI, less at 42D, and both treatments did not differ from 90LI and 95LI. In the summer, legume and legume leaf mass were greater at 90LI and 95LI compared to 100LI. The mean legume stem mass was 766 kg/ha, which was not affected by grazing management (P = 0.641) nor by the seasons of the year (P = 0.054; data not shown). The forage mass (P = 0.011), grass mass (P = 0.019), and grass stem mass and (P < 0.001) were greater in the summer, averaging 5,777, 4,262, and 1,813 kg/ha, respectively. In the spring, these variables averaged of 5,200, 3,863, and 1,331 kg/ha, respectively. Grass–leaf mass did not vary between seasons (P = 0.901).
Postgrazing canopy characteristics are shown in Table 2. The mean stubble height was 15.1 cm across treatments. The mean LI was 72.8%, which was not affected by grazing management (P = 0.392) nor by seasons of the year (P = 0.579). Forage (P = 0.015) and legume masses (P = 0.006) were less at 100LI in relation to the 42D and 90LI post-grazing. Grass mass was not influenced by grazing management (P = 0.854). The grass–leaf mass (P < 0.001) and legume leaf mass (P < 0.001) were less at 100LI, intermediate at 95LI and 90LI, and greater at 42D. Grass stem mass (P < 0.001) was greater at 100LI, intermediate at 95LI and 90LI, and less at 42D. The legume stem mass (P = 0.048) was greater at 90LI and less at 100LI, and both treatments did not differ from 42D and 95LI. The forage (P = 0.011), grass (P = 0.002), and grass stem (P < 0.001) masses were greater in the summer, averaging 2,621, 1,993, and 1,306 kg/ha, respectively, in relation to spring, with an average of 2,275, 1,664, and 1,019 kg/ha, respectively. The other variables of the canopy structure did not vary as a function of the seasons. Forage (P < 0.001), grass (P < 0.001), and legume removal rates (P = 0.002) were greater at 100LI and progressively reduced at 95LI, 90LI, and 42D.
Table 2.
Postgrazing canopy of brachiaria grass-forage peanut pastures as influenced by grazing management
| Grazing management (GM)1 | P value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 42D | 90LI | 95LI | 100LI | SEM | GM | S2 | GM × S |
| Light interception, % | 74.3 | 74.2 | 72.8 | 70.0 | 3.3 | 0.392 | 0.579 | 0.140 |
| Canopy height, cm | 14.7 | 15.0 | 15.4 | 15.4 | 0.4 | — | — | — |
| Forage mass, kg/ha | 2,582a | 2,603a | 2,436ab | 2,170b | 441 | 0.015 | 0.011 | 0.901 |
| Grass mass, kg/ha | 1,853 | 1,811 | 1,850 | 1,798 | 252 | 0.854 | 0.002 | 0.339 |
| Grass–leaf mass, kg/ha | 936a | 771b | 651b | 326c | 162 | <0.001 | 0.667 | 0.293 |
| Grass stem mass, kg/ha | 923c | 1,046b | 1,205b | 1,477a | 119 | <0.001 | <0.001 | 0.223 |
| Legume mass, kg/ha | 733a | 795a | 589ab | 376b | 206 | 0.006 | 0.883 | 0.533 |
| Legume leaf mass, kg/ha | 237a | 197b | 136b | 66.7c | 43 | <0.001 | 0.114 | 0.178 |
| Legume stem mass, kg/ha | 497ab | 599a | 455ab | 311b | 171 | 0.048 | 0.463 | 0.495 |
| Forage removal rate, % | 41.2d | 49.5c | 57.7b | 67.2a | 5.0 | <0.001 | 0.794 | 0.469 |
| Grass removal rate, % | 39.7c | 50.5b | 56.2b | 65.0a | 4.8 | <0.001 | 0.645 | 0.255 |
| Legume removal rate, % | 44.2c | 46.9c | 61.4b | 74.3a | 7.7 | 0.002 | 0.065 | 0.103 |
a–dLeast squares means within a row with different superscripts lowercase differ (P ≤ 0.05).
142D = grazing performed every 42 d; 90IL = 90% light interception; 95LI = 95% light interception, 100LI = 100% light interception.
2S = seasons of the year.
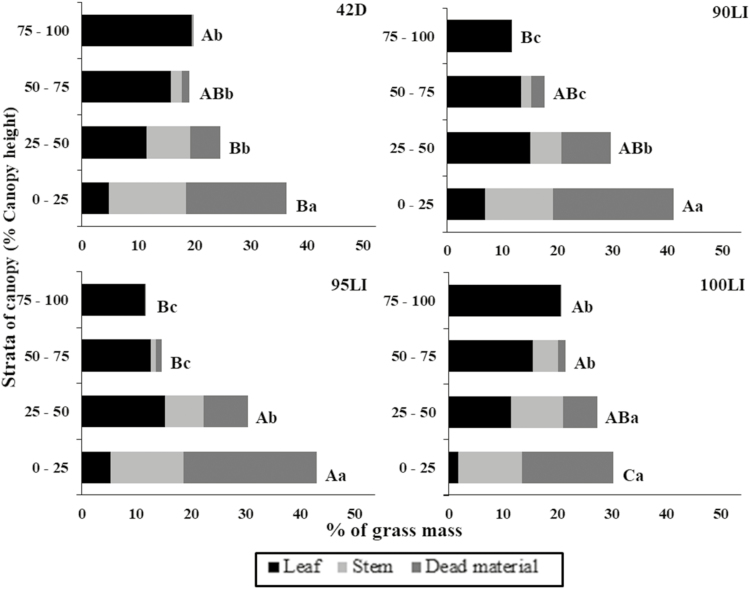
There was an interaction between the grazing management and the canopy strata on the grass mass distribution (P < 0.001; Fig. 3). For all treatments, there was a greater grass mass at the base of the canopy, especially dead material and stem. Conversely, the proportion of forage was less at the top of the canopy, represented predominantly by leaves. The difference in the percentage of grass mass from the lowest stratum to the upper stratum was less at 100LI, followed by 42D, and greater at 90LI and 95LI (on average 9.6, 16.6, 31.3, and 29.4%).
Figure 3.
Proportion of morphological components of grass mass in strata of brachiaria grass-forage peanut pastures as influenced by grazing management. 90LI = light interception of 90%, 95LI = light interception of 95%, 100LI = light interception of 100%, 42D = rest period of 42 days. A–CStrata with different letters differ significantly (P ≤ 0.05). a–cBars with different letters differ significantly (P ≤ 0.05).
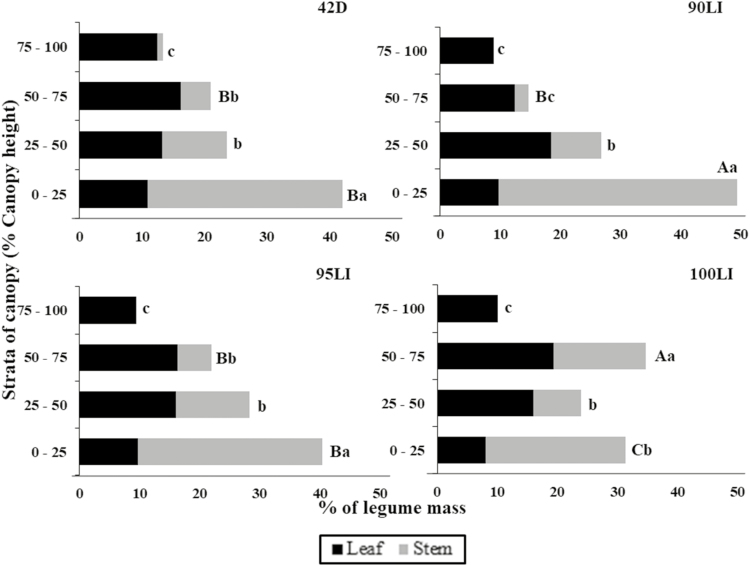
There was an interaction between the grazing management and the canopy strata on the legume mass distribution (P < 0.001; Fig. 4). The greatest percentage of legume mass in the lower stratum was found at 42D, 90LI, and 95LI. The proportion of legume mass progressively decreased in the upper strata for these treatments. The greatest percentage of legume mass occurred in stratum from 50% to 75% of CH for 100LI, followed by strata from 0% to 25% and from 25% to 50%.
Figure 4.
Proportion of morphological components of legume mass in strata of brachiaria grass-forage peanut pastures as influenced by grazing management. 90LI = light interception of 90%, 95LI = light interception of 95%, 100LI = light interception of 100%, 42D = rest period of 42 days. A–CStrata with different letters differ significantly (P ≤ 0.05). a–cBars with different letters differ significantly (P ≤ 0.05).
Nutritive Value and Forage Intake
The nutritive value of the brachiaria grass and forage peanut are shown in Table 3. Regarding the chemical composition of brachiaria grass, the CP concentration (P < 0.001) was less at 100LI compared to the other treatments. There was no variation of the B1 + B2 (P = 0.378) and C fractions (P = 0.728) as a function of the grazing management. The A fraction (P = 0.046) was greater at 42D and less at 95LI and 100LI. There was a greater proportion of B3 fraction (P = 0.033) at 100LI compared to 42D and 90LI. The NDF concentrations (P < 0.001) were greater at 95LI and 100LI than 42D and 90LI. The ADF (P = 0.025) and lignin concentrations (P = 0.002) were greater at 100LI compared to the other grazing management. The IVDMD (P < 0.001) was greater at 42D and 90LI in relation to the other treatments. The condensed tannin (P = 0.153) and ether extract concentrations (P = 0.443) did not vary with grazing management. The IVDMD of grass (P = 0.030) was greater in the spring compared to the summer. The NDF concentrations (P = 0.034) were less in the spring than in the summer. The other parameters were not affected by the seasons of the year.
Table 3.
Nutritive value of brachiaria grass-forage peanut pastures as influenced by grazing management and seasons of the year
| Grazing management (GM)1 | Seasons (S)2 | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | 42D | 90LI | 95LI | 100LI | Spring | Summer | SEM | GM | S | GM × S |
| Brachiaria grass | ||||||||||
| CP, % DM | 10.3a | 10.6a | 9.94a | 7.41b | 9.44 | 9.70 | 0.59 | <0.001 | 0.467 | 0.297 |
| A fraction, % CP | 30.7a | 25.2ab | 22.4b | 18.7b | 25.7 | 22.9 | 4.70 | 0.046 | 0.371 | 0.938 |
| B1 + B2 fraction, % CP | 30.3 | 34.8 | 34.1 | 30.5 | 30.5 | 34.2 | 3.56 | 0.378 | 0.320 | 0.789 |
| B3 fraction, % CP | 32.9b | 33.7b | 37.8ab | 44.0a | 37.7 | 36.5 | 3.43 | 0.033 | 0.537 | 0.246 |
| C fraction, % CP | 6.09 | 6.34 | 5.75 | 6.79 | 6.10 | 6.39 | 0.94 | 0.728 | 0.617 | 0.686 |
| NDF, % DM | 58.7b | 58.3b | 61.5a | 61.7a | 59.5 | 60.6 | 0.99 | <0.001 | 0.034 | 0.053 |
| ADF, % DM | 26.5b | 27.4b | 27.0b | 29.1a | 27.5 | 27.5 | 1.28 | 0.025 | 0.972 | 0.388 |
| Lignin, % DM | 2.56b | 2.76b | 2.56b | 3.41a | 2.79 | 2.86 | 0.18 | 0.002 | 0.412 | 0.062 |
| Ether extract, % DM | 1.84 | 1.78 | 2.02 | 1.51 | 1.80 | 1.78 | 0.30 | 0.443 | 0.930 | 0.450 |
| IVDMD, % | 55.2a | 54.0a | 51.5b | 51.0b | 53.8 | 52.0 | 1.59 | <0.001 | 0.030 | 0.913 |
| Condensed tannin, % DM | 0.089 | 0.069 | 0.048 | 0.073 | 0.082 | 0.057 | 0.02 | 0.153 | 0.194 | 0.465 |
| Dorage peanut | ||||||||||
| CP, % DM | 19.9a | 19.2ab | 18.7b | 16.7c | 18.9 | 18.3 | 0.72 | <0.001 | 0.334 | 0.606 |
| A fraction, % CP | 28.2a | 24.0ab | 19.4b | 14.0c | 23.5 | 19.7 | 4.61 | 0.010 | 0.168 | 0.915 |
| B1 + B2 fraction, % CP | 19.3 | 18.8 | 20.8 | 20.4 | 18.6 | 20.7 | 1.78 | 0.456 | 0.246 | 0.921 |
| B3 fraction, % CP | 45.8c | 49.7bc | 51.6ab | 56.8a | 50.3 | 51.6 | 5.18 | 0.005 | 0.610 | 0.778 |
| C fraction, % CP | 6.69c | 7.47bc | 8.28ab | 8.82a | 7.61 | 8.02 | 3.45 | 0.035 | 0.389 | 0.266 |
| NDF, % DM | 38.9b | 38.2b | 43.0a | 42.6a | 41.1 | 40.2 | 2.40 | <0.001 | 0.318 | 0.145 |
| ADF, % DM | 20.6 | 20.0 | 21.0 | 22.3 | 21.4 | 20.5 | 2.35 | 0.104 | 0.104 | 0.163 |
| Lignin, % DM | 3.97 | 4.14 | 4.30 | 4.68 | 4.14 | 4.41 | 0.47 | 0.361 | 0.256 | 0.984 |
| Ether extract, % DM | 1.83 | 1.89 | 1.73 | 1.80 | 1.81 | 1.82 | 0.21 | 0.881 | 0.942 | 0.404 |
| IVDMD, % | 68.2a | 68.7a | 68.0a | 65.5b | 67.6 | 67.7 | 1.52 | 0.001 | 0.852 | 0.084 |
| Condensed tannin, % DM | 1.95b | 1.68c | 1.96b | 2.10a | 2.13 | 1.73 | 0.31 | 0.001 | 0.006 | 0.105 |
a–cLeast squares means within a row with different superscripts lowercase differ (P ≤ 0.05).
142D = grazing performed every 42 d; 90IL = 90% light interception; 95LI = 95% light interception and 100LI = 100% light interception.
2Spring (from October to January) and Summer (from January to April).
With respect to the forage peanut, the CP (P < 0.001) and A fraction and (P = 0.010) concentrations were greater at 42D and 90LI and decreased progressively from 95LI to 100LI. The B3 (P = 0.005) and C fractions (P = 0.035) were greater at 100IL and less at 42D and 90LI. The B1 + B2 fraction did not vary with grazing management (P = 0.456). The NDF concentrations (P < 0.001) were greater at 100LI and 95LI compared to the other grazing management. The ADF (P = 0.104), lignin (P = 0.361) and ether extract concentrations (P = 0.881) were not affected by the treatments. The IVDMD (P = 0.001) was greater at 42D, 90LI, and 95LI than 100LI treatments. The concentration of condensed tannins (P = 0.001) was greater at 100LI, intermediate at 42D and 95LI, and less at 90LI. The concentration of condensed tannin (P = 0.006) was 23.1% greater in the spring compared to the summer.
The results related to the forage intake are shown in Table 4. The DM (P < 0.001), OM (P = 0.001), and CP intake (P < 0.001) of the total forage and the legume were less at 100LI in relation to the other treatments. The NDF intake of total forage (P = 0.007) was greater at 95LI, intermediate at 90LI and 42D, and less at 100LI. The NDF intake of legume (P = 0.006) was less at 100LI compared to the other grazing management. The CP intake of grass was less at 100LI than in the other treatments (P < 0.001). The DM (P = 0.294), OM (P = 0.343), and NDF intake of grass (P = 0.119) did not vary among grazing management. There was greater intake of DM, OM, NDF, and CP of total forage and legume in the spring compared to the summer. Intake of DM, OM, NDF, and CP of grass did not vary among seasons.
Table 4.
Forage intake of heifers grazing brachiaria grass-forage peanut pastures as influenced by grazing management and seasons of the year
| Grazing management (GM)1 | Seasons (S)2 | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item | 42D | 90LI | 95LI | 100LI | Spring | Summer | SEM | GM | S | GM × S |
| Forage total intake, % BW/d | 2.17a | 2.27a | 2.32a | 1.73b | 2.28 | 1.95 | 0.309 | <0.001 | 0.007 | 0.119 |
| Grass intake, %BW/d | 1.53 | 1.53 | 1.65 | 1.45 | 1.60 | 1.48 | 0.456 | 0.294 | 0.252 | 0.559 |
| Legume intake, %BW/d | 0.64a | 0.74a | 0.67a | 0.28b | 0.69 | 0.48 | 0.165 | 0.001 | <0.001 | 0.060 |
| OM total intake, % BW/d | 1.98a | 2.07a | 2.12a | 1.58b | 2.09 | 1.78 | 0.125 | 0.001 | 0.007 | 0.129 |
| OM grass intake, % BW/d | 1.39 | 1.39 | 1.50 | 1.32 | 1.46 | 1.34 | 0.101 | 0.343 | 0.252 | 0.578 |
| OM legume intake, % BW/d | 0.59a | 0.68a | 0.62a | 0.26b | 0.63 | 0.44 | 0.151 | 0.001 | 0.001 | 0.063 |
| CP total intake, % BW/d | 0.29a | 0.30a | 0.29a | 0.15b | 0.28 | 0.23 | 0.024 | <0.001 | 0.002 | 0.070 |
| CP grass intake, % BW/d | 0.16a | 0.16a | 0.17a | 0.10b | 0.15 | 0.14 | 0.047 | <0.001 | 0.491 | 0.425 |
| CP legume intake, % BW/d | 0.13a | 0.14a | 0.12a | 0.05b | 0.13 | 0.09 | 0.028 | <0.001 | <0.001 | 0.092 |
| NDF total intake, % BW/d | 1.16b | 1.18b | 1.31a | 1.02c | 1.23 | 1.10 | 0.080 | 0.007 | 0.045 | 0.265 |
| NDF grass intake, % BW/d | 0.91 | 0.89 | 1.01 | 0.90 | 0.95 | 0.91 | 0.063 | 0.119 | 0.507 | 0.619 |
| NDF legume intake, % BW/d | 0.25a | 0.29a | 0.30a | 0.12b | 0.28 | 0.21 | 0.081 | 0.006 | 0.002 | 0.130 |
a–cLeast squares means within a row with different superscripts lowercase differ (P ≤ 0.05).
142D = grazing performed every 42 d; 90IL = 90% light interception; 95LI = 95% light interception, 100LI = 100% light interception.
2Spring (from October to January) and summer (from January to April).
Nitrogen Utilization
Forage intake and N use efficiency data are presented in Table 5. The intake of digestible OM (P = 0.007), N (P < 0.001), and CP/digestible OM ratio (P < 0.001) were less at 100LI in relation to the other treatments. The production of microbial N (P < 0.001) and efficiency of microbial synthesis (P = 0.023) in the rumen were greater at 95LI and 90LI, followed by 42D and lesser at 100LI. The urinary N excretion (P = 0.009) was greater at 42D and 90LI, and lowest at 100LI. The fecal N excretion did not vary among treatments (P = 0.113). The fecal N excretion (P = 0.005) was greater in the spring than in the summer. The efficiency of microbial synthesis (P = 0.008) in the rumen was less in the spring compared to the summer. The N and digestible OM intake, CP/digestible OM rate, production of microbial N, and urine N excretion were not affected by the seasons (P > 0.05).
Table 5.
Nitrogen utilization by heifers grazing brachiaria grass-forage peanut pastures as influenced by grazing management and seasons of the year
| Grazing management (GM)2 | Seasons (S)3 | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Item1 | 42D | 90LI | 95LI | 100LI | Spring | Summer | SEM | GM | S | GM × S |
| DOMI, kg/d | 3.6a | 3.7a | 3.6a | 2.7b | 3.5 | 3.3 | 1.35 | 0.007 | 0.542 | 0.121 |
| N intake, g/d | 114a | 119a | 115a | 63.7b | 105 | 101 | 30.0 | <0.001 | 0.471 | 0.075 |
| CP/DOM, g/kg | 198a | 202a | 198a | 150b | 190 | 189 | 17.5 | <0.001 | 0.511 | 0.443 |
| NMIC, g of N/d | 53.0b | 59.9ab | 67.0a | 36.5c | 50.8 | 57.4 | 4.45 | <0.001 | 0.306 | 0.116 |
| EMS, g of NMIC/kg DOM | 14.7b | 16.3ab | 18.4a | 13.7c | 14.7 | 17.2 | 1.25 | 0.023 | 0.008 | 0.096 |
| UNE, g of N/d | 42.4a | 38.8ab | 29.5bc | 21.3c | 33.3 | 32.7 | 5.78 | 0.009 | 0.884 | 0.204 |
| FNE, g of N/d | 33.2 | 34.5 | 37.1 | 30.7 | 37.3 | 30.5 | 3.04 | 0.113 | 0.005 | 0.410 |
a–cLeast squares means within a row with different superscripts lowercase differ (P ≤ 0.05).
1DOMI = digestible OM intake, CP/DOM = CP:digestible OM ratio, NMIC = ruminal production of microbial nitrogen, EMS = efficiency of microbial synthesis, UNE = urinary nitrogen excretion, NFE = fecal nitrogen excretion.
242D = grazing performed every 42 d; 90IL = 90% light interception; 95LI = 95% light interception, 100LI = 100% light interception.
3Spring (from October to January) and Summer (from January to April).
DISCUSSION
Regions with Cw climate type (Köppen-Geiger climate classification) are found in several countries worldwide (Peel et al., 2007). Thus, farmers and extension workers located in these regions may use mixed pastures with brachiaria grass and forage peanut in their farming systems, especially during the spring and summer, when the climatic conditions are favorable. During winter, it is common for forage peanut to lose leaves; however, in the beginning of the rainy season (spring), there are high growth rates due to the high rate of leaf appearance and elongation in this specie (Pereira et al., 2017).
In this study, all experimental units were grazed to reach a stubble height of 15 cm in early spring. Thus, all experimental units had the same initial regrowth conditions. The 100LI management showed a high legume mass with a botanical composition similar to the other treatments, averaging 31.3, 29.6, 28.5, and 27.3% of the forage mass for 42D, 90LI, 95LI, and 100LI, respectively. Although the proportions of legume were similar, there was a greater contribution of legume mass in the intermediate strata of the canopy from 25% to 75% height for 100LI (Fig. 3), corresponding to 9.6 to 28.8 cm. This response may occur due to the intensification of competition for light after canopy reach 95% LI. Light is not a limiting resource in the beginning of the rest period in intermittent stocking. However, when LI is greater than 95% the competition for light is intensified (Carnevalli et al., 2006; Silveira et al., 2016). In mixed pastures of brachiaria grass and forage peanut, the grass causes shading of the legume, which occupies the strata close to the ground (Tamele et al., 2017). When light becomes a limiting resource, the shading of the stratum close to the soil leads to upright growth of the stolons (Pereira et al., 2017). This response reduces the contact of nodes with the soil, reducing the stolon population and hence the contribution of forage peanut in the aboveground sward. Besides reducing the contact of nodes with soil, grazing management based on 100LI also provide greater animal access to the legume. For this reason, the legume removal rate was more intense at 100LI in relation to the other treatments (Table 2). Hence, the forage peanut had less legume mass at 100LI in the pre-grazing during the summer (Table 1), which can be explained by the low legume residue. In the summer, 100LI canopies had only 19% of legume mass, less than the 29.7% found in the other treatments. Based on the above, 100LI negatively affects the stability of the mixture.
Besides the effects on the botanical composition, grazing management influences forage intake by animals (Carnevalli et al., 2006; Silveira et al., 2013). Tropical grasses in monoculture managed for long rest periods are characterized by intense stem elongation and increase of stem contribution in upper strata of the canopy (Silveira et al., 2016). As stem shear force increases (Barrett et al., 2001; Baumont et al., 2004; Gregorini et al., 2011), contribution in the upper strata of the canopy can limit intake (Hodgson, 1990; Benvenutti et al., 2006). In the present study, 100LI had greater grass and stem masses (Table 1), and greater stem contribution above the stubble height (Fig. 3). On average, 38.6% of the stem grass mass was above the stubble height at 100LI, greater than the 5.7% in other grazing management treatments. Thus, even with similar grass–leaf proportions in pre-grazing among the grazing management (on average 61.3% of the grass mass), the 100LI had a greater percentage of stem compared to the other treatments above the target stubble height.
Postgrazing differences in canopy structure were more evident as the rest period increased (Table 2). In order to maintain the same stubble height in all treatments, different defoliation intensities were adopted (an average of 30.7, 38.8, 48.0, and 59.9% of the CH at 42D, 90LI, 95LI, and 100LI, respectively). The pregrazing and postgrazing canopy structures at 100LI may explain the lesser forage intake in relation to other grazing management (Silveira et al., 2013; Silveira et al., 2016). In forage diets, the NDF concentration determines the intake (Baumont et al., 2004). On average, NDF intake has been from 1.2% to 1.5% of BW (Mertens, 1987). The 42DF, 90LI, and 95LI treatments had NDF intake close to 1.2% of BW (Table 4), indicating that other factors limited forage intake at 100LI (1.02% of BW), such as nonnutritional factors (Poppi et al., 1987; Fonseca et al., 2012; Euclides et al., 2017). Canopy structural characteristics of tropical forages are relatively more important than nutritional factors in terms of herbage intake regulation due to stem elongation during vegetative development and its contribution to herbage bulk density (Da Silva and Carvalho, 2005; Geremia et al., 2014). Besides the lesser total forage intake at 100LI, the legume intake was 59.2% less compared to other grazing management. It promoted a reduced intake of N and digestible OM (Table 5).
Regarding nutritive value, in both forages the A fraction of the protein reduced as the B3 fraction increased with longer rest periods (Table 3). Due to this variation in nutritional value, the ratio between CP and digestible OM was less in 100LI (150 g of CP/kg of digestible OM) in relation to the other treatments (on average 199 g of CP/kg of digestible OM; Table 5). The reduction of the nutritive value of the forage occurs due to the advancement of the maturity (Santos et al., 2009; Oliveira et al., 2011). Low ratio between CP and digestible OM has a negative effect on forage intake, efficiency of microbial synthesis, and N use (Poppi and McLennan, 1995; Detmann et al., 2014). This explains the low efficiency of microbial synthesis in 100LI and especially the low efficiency of N use, since 81.6% of the ingested N was excreted, greater than the average 61.9 % of the other grazing management (Table 5).
There was a greater grass mass in the summer due to seedhead emergence (from February), negatively affecting the canopy structure and hence the forage intake by animals (Table 4). The greater proportion of stem influenced the animal’s selectivity and intake, especially of the legume component.
In the spring, the concentration of condensed tannins of forage peanut was 23% greater than in the summer (Table 3). As legume intake was greater in spring, the concentration of condensed tannins in the diet was 44.1% greater in the spring compared to the summer. Moderate concentrations of condensed tannins in the diet may reduce the efficiency of microbial synthesis due to the tannin-protein complex formation, reducing ammonia concentrations in the rumen and increasing amount of metabolizable protein (Barry and McNabb, 1999; Mezzomo et al., 2011). Thus, the effect of condensed tannins may explain the lower efficiency of microbial synthesis in the spring.
In conclusion, long rest periods, such as those obtained in 100LI, should be avoided because they reduce plant community stability, forage intake, and nutritional value of the diet. The other treatments studied allowed stability of the mixed pasture and canopy structure, which optimized forage intake and efficiency of N use by heifers. Thus, 95% LI is the maximum limit to interrupt the regrowth, being possible to work within the range from 90% to 95% LI. In on-farm conditions, we recommend that the input height of mixed pastures for brachiaria grass and forage peanut should be from 24 to 30 cm, with a stubble height of 15 cm. Further studies should focus on seed production of forage peanut on a large scale and its economic and environmental impacts on grazing systems, once propagation through stolons and N input are considered current issues for adopting mixed pastures.
Footnotes
The authors thank the members of NEFOR (Brazilian Forage Team) for their contributions during the field trial setup. This study was funded by the Foundation for Research Support of the State of Minas Gerais (FAPEMIG) and National Council for Scientific and Technological Development (CNPq—9). The author RMB gratefully acknowledges the research fellowships from CNPq and the Rio de Janeiro State Research Foundation (FAPERJ).
REFERENCES
- Akaike H. 1974. A new look at the statistical model identification. IEEE Trans. Automat. Contr. 19:716–723. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- Allen V. G., Batello C., Berretta E. J., Hodgson J., Kothmann M., Li X., McIvor J., Milne J., Morris C., Peeters A.,. et al. 2011. An international terminology for grazing lands and grazing animals. Grass Forage Sci. 66:2–28. doi: 10.1111/j.1365-2494.2010.00780.x [DOI] [Google Scholar]
- AOAC.. 2000. Official methods of analysis. 17th ed Assoc. O. Anal. Chem, Arlington, VA. [Google Scholar]
- Barrett P. D., Laidlaw A. S., Mayne C. S., and Christie H.. 2001. Pattern of herbage intake rate and bite dimensions of rotationally grazed dairy cows as sward height declines. Grass Forage Sci. 56:362–373. doi: 10.1046/j.1365-2494.2001.00286.x [DOI] [Google Scholar]
- Barry T. N., and McNabb W. C.. 1999. The implications of condensed tannins on the nutritive value of temperate forages fed to ruminants. Br. J. Nutr. 81:263–272. doi:10.1017/S0007114599000501 [PubMed] [Google Scholar]
- Barthram G. T. 1985. Experimental techniques: the HFRO sward stick. In: Alcock M. M., editor, Biennial report of the hill farming research organization. Hill Farming Research Organization, Midlothian, p. 29–30. [Google Scholar]
- Baumont R., Cohen-Salmon D., Prache S., and Sauvant D.. 2004. A mechanistic model of intake and grazing behaviour in sheep integrating sward architecture and animal decisions. Anim. Feed Sci. Technol. 112:5–28. doi: 10.1016/j.anifeedsci.2003.10.005 [DOI] [Google Scholar]
- Benvenutti M. A., Gordon I. J., and Poppi D. P.. 2006. The effect of the density and physical properties of grass stems on the foraging behaviour and instantaneous intake rate by cattle grazing an artificial reproductive tropical sward. Grass Forage Sci. 61:272–281. doi: 10.1111/j.1365-2494.2006.00531.x [DOI] [Google Scholar]
- Black A. D., Laidlaw A. S., Moot D. J. and O’Kiely P.. 2009. Comparative growth and management of white and red clovers. Irish J. Agr. Food Res. 48:149–166. [Google Scholar]
- Carnevalli R. A., Da Silva S. C., Bueno A. A. O., Uebele M. C., Bueno F. O., Hodgson J., Silva G. N., and Morais J. P. G.. 2006. Herbage production and grazing losses in Panicum maximum cv. Mombaca under four grazing managements. Trop. Grasslands. 40:165–176. [Google Scholar]
- Chen X. B., and Gomes M. J.. 1992. Estimation of microbial protein supply to sheep and cattle based on urinary excretion of purine derivatives - an overview of the technical details. Rowett Research Institute/International Feed Research Unit, Aberdeen. [Google Scholar]
- Da Silva S. C., Carvalho P. C. F.. 2005. Foraging behaviour and intake in the favourable tropics/sub-tropics. In: McGilloway D. A., editor, Grassland: a global resource. Wageningen Academic Publishers, Department of Agriculture, Fisheries and Food, Ireland, p. 81–95. [Google Scholar]
- Detmann E., Valente E. E. L., Batista E. D. and Huhtanen P.. 2014. An evaluation of the performance and efficiency of nitrogen utilization in cattle fed tropical grass pastures with supplementation. Livest. Sci. 162:141–153. doi: 10.1016/j.livsci.2014.01.029 [DOI] [Google Scholar]
- De Vries M. F. W. 1995. Estimating forage intake and quality in grazing cattle: a reconsiderarion of the hand-plucking method. J. Range Manage. 48:370–375. doi:10.2307/4002491 [Google Scholar]
- EMBRAPA.. 1997. Manual de métodos de análise de solos. 2nd ed Centro Nacional de Pesquisa de Solos, Rio de Janeiro, Brazil. [Google Scholar]
- Euclides V. P. B., Carpejani G. C., Montagner D. B., Nascimento Junior D., Barbosa R. A., and Difante G. S.. 2017. Maintaining post-grazing sward height of Panicum maximum (cv. Mombaça) at 50 cm led to higher animal performance compared with post-grazing height of 30 cm. Grass Forage Sci. 73:174–182. doi: 10.1111/gfs.12292 [DOI] [Google Scholar]
- Costa e Silva L. F., Valadares Filho S. C., Chizzotti M. L., Rotta P. P., Prados L. F., Valadares R. F. D., Zanetti D., and Braga J. M. S.. 2012. Short communication: creatinine excretion and relationship with body weight of Nellore cattle. Revista Brasileira de Zootecnia. 41:807–810. doi: 10.1590/S1516-35982012000300046 [DOI] [Google Scholar]
- Fonseca L., Mezzalira J. C., Bremm C., Filho R. S. A., Gonda H. L., and de F. Carvalho P. C.. 2012. Management targets for maximising the short-term herbage intake rate of cattle grazing in Sorghum bicolor. Livest. Sci. 145:205–211. doi: 10.1016/j.livsci.2012.02.003 [DOI] [Google Scholar]
- Geremia E. V., Pereira L. E. T., Paiva A. J., Oliveira L. P., and Silva S. C.. 2014. Intake rate and nutritive value of elephant grass cv. Napier subjected to strategies of rotational stocking management. Trop. Grasslands-Forrajes Trop. 2:51–52. doi: 10.17138/TGFT(2)51-52 [DOI] [Google Scholar]
- Gregorini P., Gunter S. A., Bowman M. T., Caldwell J. D., Masino C. A., Coblentz W. K., and Beck P. A.. 2011. Effect of herbage depletion on short-term foraging dynamics and diet quality of steers grazing wheat pastures. J. Anim. Sci. 89:3824–3830. doi: 10.2527/jas.2010-3725 [DOI] [PubMed] [Google Scholar]
- Hodgson J. 1990. Grazing management–science into practice. Longman Scientific & Technical, Essex, England, p 203. [Google Scholar]
- Holden L. A. 1999. Comparison of methods of in vitro dry matter digestibility for ten feeds. J. Dairy Sci. 82:1791–1794. doi: 10.3168/jds.S0022-0302(99)75409-3 [DOI] [PubMed] [Google Scholar]
- Jank L., Barrios S. C., Do Valle C. B., Simeão R. M., and Alves G. F.. 2014. The value of improved pastures to Brazilian beef production. Crop Pasture Sci. 65:1132–1137. doi: 10.1071/CP13319 [DOI] [Google Scholar]
- Jones R. J., Ludlow M. M., Troughton J. H. and Blunt C. G.. 1979. Estimation of the proportion of C3 and C4 plant species in the diet of animals from the ratio of natural 12C and 13C isotopes in the faeces. J. Agr. Sci. 92:91–100. doi: 10.1017/S0021859600060536 [DOI] [Google Scholar]
- Licitra G., Hernández T. M., and Van Soest P. J.. 1996. Standarization of procedures for nitrogen fractionation of rumiant feeds. Anim. Feed Sci. Technol. 57:347–358. doi: 10.1016/0377-8401(95)00837-3 [DOI] [Google Scholar]
- Littell R. C., Pendergast J., and Natarajan R.. 2000. Modelling covariance structure in the analysis of repeated measures data. Stat. Med. 19:1793–1819. doi:10.1002/1097-0258(20000715)19:13<1793::AID-SIM482>3.0.CO;2-Q [DOI] [PubMed] [Google Scholar]
- Macedo R., Tarré R. M., Ferreira E., Rezende C. P., Pereira J. M., Cadish G., Rouws J. R. C., Alves B. J. R. and Boddey R. M.. 2010. Forage intake and botanical composition of feed for cattle fed Brachiaria/legume mixtures. Sci. Agric. 67:384–392. doi: 10.1590/S0103-90162010000400002 [DOI] [Google Scholar]
- Mertens D. R. 1987. Predicting intake and digestibility using mathematical models of ruminal function. J. Anim. Sci. 64:1548–1558. doi:10.2527/jas1987.6451548x [DOI] [PubMed] [Google Scholar]
- Mezzomo R., Paulino P. V. R., Detmann E., Valadares Filho S. C., Paulino M. F., Monnerat J. P. I. S., Duarte M. S., Silva L. H. P. and Moura L. S.. 2011. Influence of condensed tannin on intake, digestibility, and efficiency of protein utilization in beef steers fed high concentrate diet. Livest. Sci. 141:1–11. doi: 10.1016/j.livsci.2011.04.004 [DOI] [Google Scholar]
- Muir J. P., Pitman W. D., Dubeux J. C. Jr, and Foster J. L.. 2014. The future of warm-season, tropical and subtropical forage legumes in sustainable pastures and rangelands. Afr. J. Range Forage Sci. 31:187–198. doi: 10.2989/10220119.2014.884165 [DOI] [Google Scholar]
- Muir J. P., Pitman W. D., and Foster J. L.. 2011. Sustainable, low-input, warm-season, grass-legume grassland mixtures: mission (nearly) impossible?Grass Forage Sci. 66:301–315. doi: 10.1111/j.1365-2494.2011.00806.x [DOI] [Google Scholar]
- Myers W. D., Ludden P. A., Nayigihugu V., and Hess B. W.. 2004. Technical note: a procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. doi:10.2527/2004.821179x [DOI] [PubMed] [Google Scholar]
- Oliveira M. A., Pereira O. G., Ribeiro K. G., Santos M. E. R., Chizzotti F. H. M. and Cecon P. R.. 2011. Produção e valor nutritivo do capim-coastcross sob doses de nitrogênio e idades de rebrotação. Arq. Bras. Med. Vet. Zootec. 63:694–703. doi: 10.1590/S0102-09352011000300022 [DOI] [Google Scholar]
- Pell A. N., and Schofield P.. 1993. Computerized monitoring of gas production to measure forage digestion in vitro. J. Dairy Sci. 76:1063–1073. doi: 10.3168/jds.S0022-0302(93)77435-4 [DOI] [PubMed] [Google Scholar]
- Peel M. C., Finlayson B. L., and McMahon T. A.. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. Discuss. 4:439–473. doi: 10.5194/hess-11-1633-2007 [DOI] [Google Scholar]
- Pereira J. C., Gomes F. K., Oliveira M. D. B. L., Lara M. A. S., Bernardes T. F., and Casagrande D. R.. 2017. Defoliation management affects morphogenetic and structural characteristics of mixed pastures of Brachiaria grass and forage peanut. African J. Range Forage Sci. 34:13–19. doi: 10.2989/10220119.2017.1315960 [DOI] [Google Scholar]
- Poppi D. P., Hugues J. P. and L’Huillier P. J.. 1987. Intake of pasture by grazing ruminants. In: Nicol A. M., editor, Feeding livestock on pasture. Society of Animal Production, New Zealand, p. 55–63. [Google Scholar]
- Poppi D. P., and McLennan S. R.. 1995. Protein and energy utilization by ruminants at pasture. J. Anim. Sci. 73:278–290. doi:10.2527/1995.731278x [DOI] [PubMed] [Google Scholar]
- Porter L. J., Hrstich L. N., and Chan B. G.. 1986. The conversion of proanthocyanidins and prodelphinidins to cyanidin and delpinidin. Phytochem. 25:223. doi: 10.1016/S0031-9422(00)94533-3 [DOI] [Google Scholar]
- Santos M. E. R., Fonseca D. M., Balbino E. M., dos J. P. I., Monnerat S. and Silva S. P.. 2009. Capim-braquiária diferido e adubado com nitrogênio: produção e características da forragem. Rev. Bras. Zootec. 38:650–656. doi: 10.1590/S1516-3598200900040009 [DOI] [Google Scholar]
- Sá Júnior A. de, Carvalho L. G., Silva F. F., and Alves M. C.. 2012. Application of the Köppen classification for climatic zoning in the state of Minas Gerais, Brazil. Theor. Appl. Climatol. 108:1–7. doi: 10.1007/s00704-011-0507-8 [DOI] [Google Scholar]
- Silveira M. C. T., Da Silva S. C., de Souza Júnior S. J., Barbero L. M., Rodrigues C. S., Limão V. A., Pena K. S., and Nascimento Júnior D.. 2013. Herbage accumulation and grazing losses on Mulato grass subjected to strategies of rotational stocking management. Scientia Agricola. 70:242–249. doi: 10.1590/S0103-90162013000400004 [DOI] [Google Scholar]
- Silveira M. C. T., Nascimento Júnior D., Rodrigues C. S., Pena K. S., de Souza Júnior S. J., Barbero L. M., Limão V. A., Euclides V. P. B., and Da Silva S. C.. 2016. Forage sward structure of Mulato grass (Brachiaria hybrid ssp.) subjected to rotational stocking strategies. Aust. J. Crop Sci. 10:864–873. doi: 10.21475/ajcs.2016.10.06.p7568 [DOI] [Google Scholar]
- Tamele O. H., Lopes de Sá O. A. A., Bernardes T. F., Lara M. A. S. and Casagrande D. R.. 2017. Optimal defoliation management of Brachiaria grass–forage peanut for balanced pasture establishment. Grass Forage Sci. 00:1–10. doi: 10.1111/gfs.12332 [DOI] [Google Scholar]
- Titgemeyer E. C., Armendariz C. K., Bindel D. J., Greenwood R. H., and Löest C. A.. 2001. Evaluation of titanium dioxide as a digestibility marker for cattle. J. Anim. Sci. 79:1059–1063. doi:10.2527/2001.7941059x [DOI] [PubMed] [Google Scholar]
- Tylutki T. P., Fox D. G., Durbal V. M., Tedeschi L. O., Russell J. B., Van Amburgh M. E., Overton T. R., Chase L. E., and Pell A. N.. 2008. Cornell net carbohydrate and protein system: a model for precision feeding of dairy cattle. Anim. Feed Sci. Technol. 143:174–202. doi: 10.1016/j.anifeedsci.2007.05.010 [DOI] [Google Scholar]
- Valadares R. F., Broderick G. A., Valadares Filho S. C., and Clayton M. K.. 1999. Effect of replacing alfalfa silage with high moisture corn on ruminal protein synthesis estimated from excretion of total purine derivatives. J. Dairy Sci. 82:2686–2696. doi:10.3168/jds.S0022-0302(99)75525-6 [DOI] [PubMed] [Google Scholar]
- Warren Wilson J. 1960. Inclined point quadrats. New Phytol. 59:1–7. doi: 10.1111/j.1469-8137.1960.tb06195.x [DOI] [Google Scholar]