Abstract
Thirty-six newly weaned, crossbred beef steers (323 ± 12 kg; SD) from a single-source were used in a 56-d study to examine the effects of a Saccharomyces cerevisiae fermentation product (SCFP; Original XPC, Diamond V, Cedar Rapids, IA) on total tract nutrient digestibility and response to a vaccination challenge. Twelve days after arrival, steers were blocked by body weight (BW) and randomly assigned to treatments: SCFP at 0 (CON), 14 (SCFP14), or 28 (SCFP28) g·steer−1·d−1. Steers were fed via bunks that measured individual intake and received ear tags (CowManager, Select Sires, Plain City, OH) that recorded rumination and activity. BWs were collected on days 1, 0, 14, 28, 42, 55, and 56. Titanium dioxide was fed as an indigestible marker to all steers from days 12 to 27, followed by consecutive day fecal samples, for determination of total tract nutrient digestibility. On day 34, steers received a Mannheimia haemolytica vaccination (One Shot, Zoetis, Kalamazoo, MI) to induce an acute phase protein response. Blood was collected from all steers on day 34 (prior to vaccination) and 3, 6, 9, 11, and 14 d post-vaccination. Data were analyzed using Proc Mixed of SAS (experimental unit = steer; n = 12 per treatment); the model included the fixed effect of treatment and block and the random effect of steer. Blood measures, ear tag, and dry matter intake (DMI) data for the 15-d vaccination period were analyzed as repeated measures. Contrast statements (CON vs. SCFP14; SCFP14 vs. SCFP28) were used to compare treatment means. Digestibility of dry matter (DM) and organic matter was greater for SCFP14 vs. SCFP28 (P ≤ 0.03). Steers fed SCFP14 exhibited greater crude protein digestibility compared with CON (P < 0.01). Steers fed SCFP14 exhibited greater DMI for 15 d post-vaccination (P = 0.02) and greater average daily gain from days 28 to 56 (P = 0.05) vs. SCFP28-fed steers. Post-vaccination, steers fed SCFP14 spent less time ruminating per kg of DM, neutral detergent fiber (NDF), and physically effective NDF consumed than CON or SCFP28 (P ≤ 0.07). Serum IL-8 and haptoglobin concentrations tended to be lesser for steers fed SCFP14 vs. SCFP28 (P ≤ 0.08). Ceruloplasmin concentrations were lesser on day 14 post-vaccination for steers fed SCFP14 vs. CON or SCFP28 (treatment × d; P = 0.004); there were no differences on other sampling days due to treatment. Although no overall performance benefit was noted, steers fed SCFP14 responded better to a vaccination challenge vs. SCFP28-fed steers.
Keywords: newly weaned beef cattle, Saccharomyces cerevisiae fermentation product, vaccination challenge
INTRODUCTION
The feedlot receiving period poses unique challenges as calves experience a variety of stressors during this time, including recent weaning, transit, commingling, and vaccination. In the face of these challenges, a calf must consume enough feed to remain healthy and productive. It is well established that the stress of feedlot receiving decreases dry matter intake (DMI; Hutcheson and Cole, 1986). The stress of receiving may also cause an acute inflammatory response, leading to immune system activation. Although proper immune system activation is vital for the health of the animal, prolonged stimulation can be detrimental, resulting in skeletal muscle catabolism to provide the immune system with amino acids and energy (Gifford et al., 2012). Therefore, research of nutritional strategies to modulate the negative consequences of receiving stress is warranted.
Saccharomyces cerevisiae fermentation products (SCFP) are produced through the anaerobic fermentation of yeast, which results in the production of beneficial metabolites, such as B vitamins, amino acids, nucleotides, lipids, and organic acids. These products have been shown to stabilize the rumen environment (Harrison et al., 1988), increase ruminal and total tract crude protein (CP) digestibility (Wiedmeier et al., 1987; Yoon and Stern, 1996), and reduce inflammatory markers (Evans et al., 2012; Li et al., 2016). Although variable effects of SCFP on receiving period performance have been observed, the effect of these products on the acute phase protein (APP) response in receiving cattle has not been studied. It was hypothesized that supplementation of SCFP would increase total tract nutrient digestibility by newly weaned beef steers and would positively modulate the inflammatory response following a vaccination challenge. To test this hypothesis, a 56-d study was designed to determine the effects of increasing doses of SCFP on growth performance, nutrient digestibility, and the APP response following a Mannheimia haemolytica vaccination of beef steers.
MATERIALS AND METHODS
Animals and Experimental Design
All procedures and protocols for this experiment were approved by the Iowa State University Animal Care and Use Committee (7-15-8060-B). The 36 steers (323 ± 12 kg; SD) utilized in the present study were from the same cohort and received in the same manner as the steers utilized by Deters et al. (2018). Five days after arrival, steers were sorted into pens with bunks capable of measuring individual steer intake (Dahlke et al., 2008) where they were acclimated for 7 d prior to trial initiation. On day 0, all steers were boostered against clostridial infections (Vision 7, Merck, Madison, NJ), boostered against Bovine Viral Diarrhea Virus (Vista Once, Merck), and implanted (Component E-S: 200-mg progesterone USP, 20-mg estradiol benzoate, and 29-mg tylosin tartrate; Elanco Animal Health Greenfield, IN). All steers received ear tags (Cow Manager, Select Sires, Plain City, Ohio), validated for use in feedlot cattle (Wolfger et al., 2015), that recorded real time ear surface temperature, rumination, and physically active minutes. Steers were blocked by body weight (BW) collected on day 1 and assigned to dietary treatments: SCFP (Diamond V, Cedar Rapids, IA) at 0 (CON), 14 (SCFP14), or 28 (SCFP28) g·steer−1·d−1. The current recommended dose for receiving cattle is 14 g·steer−1·d−1. Steers were fed a corn silage based receiving diet (Table 1) and treatments were delivered as described by Deters et al. (2018). Back calculated SCFP intakes were 14.6 and 29.2 g·steer−1·d−1, for SCFP14 and SCFP28, respectively.
Table 1.
Ingredient composition of receiving diet
| DM, % | 58.2 |
|---|---|
| Ingredient, % DM basis | |
| Corn silage | 27 |
| WCGF1 | 20 |
| Dry-rolled corn | 20 |
| DDGS2 | 23.022 |
| Chopped grass hay | 8 |
| Limestone | 1.52 |
| Salt | 0.31 |
| Bovatec913 | 0.023 |
| Vitamin A premix4 | 0.11 |
| Trace mineral premix5 | 0.024 |
| Analyzed composition6, % | |
| Crude protein | 15.9 |
| NDF | 31.8 |
| Ether extract | 5.4 |
1Wet corn gluten feed (ADM, Cedar Rapids, IA).
2Dried distillers grains with solubles; carrier for microingredients and Saccharomyces cerevisiae fermentation product (Original XPC, Diamond V, Cedar Rapids, IA).
3Provided 300 mg lasalocid·steer−1·d−1 (Zoetis, Florham Park, NJ).
4Contained 4,400,000 IU/kg Vitamin A premix.
5Provided per kilogram of diet DM: 10 mg of Cu (copper sulfate), 30 mg of Zn (zinc sulfate), 20 mg of Mn (manganese sulfate), 0.5 mg of I (calcium iodate), 0.1 mg of Se (sodium selenite), and 0.1 mg of Co (cobalt carbonate).
6Based on total mixed ration composite analysis from Dairyland, Inc., Arcadia, WI.
Steers were weighed prior to feeding on days 1, 0, 14, 28, 42, 55, and 56. Average daily gain (ADG) was calculated from days 0 to 28, 28 to 56, and 0 to 56. Weekly total mixed ration (TMR) samples were collected and dried in a forced-air oven for 48 h at 70 °C to determine diet dry matter (DM). Composites of weekly TMR samples were analyzed by a commercial laboratory (Dairyland, Inc., Arcadia, WI) via wet chemistry procedures for CP (method 990.03; AOAC, 1996), neutral detergent fiber (NDF; method 2002.04; AOAC, 2005), and ether extract (method 920.39; AOAC, 1996). Steer DMI was determined using bunks that monitored the electronic ID of each steer and assigned feed disappearance to each individual animal. Feed efficiency (gain:feed; G:F) was calculated for each period from steer DMI and weight gain. Steers were monitored daily for signs of morbidity. If visual symptoms (i.e., nasal discharge and cough) were observed and rectal temperature was ≥39 °C, the animal was treated with tulathromycin (Draxxin, Zoetis, Parsippany, NJ) by trained personnel.
Total Tract Nutrient Digestibility
To determine total tract nutrient digestibility, titanium dioxide (TiO2) was included as an indigestible marker in the diet of all steers from days 12 to 27. Titanium dioxide was included in the diet as part of a premix to achieve intakes of 10 g·steer−1·d−1 and percent inclusion of the premix was adjusted weekly based on projected average treatment group DMI for the following week; back calculated TiO2 intakes were 10.9, 11.0, and 10.8 g for CON, SCFP14, and SCFP28, respectively. Fecal grab samples were collected prior to feeding on days 26 and 27. Samples of feces and weekly TMR samples from the TiO2 feeding period were dried and ground to pass through a 2-mm screen in a Retsch ZM 100 mill (Retsch GmbH, Haan, Germany) and analyzed for DM, organic matter (OM), NDF, acid detergent fiber (ADF), CP, and using methods described by Russell et al. (2016a). Nutrient and TiO2 concentrations were analyzed separately for consecutive day fecal samples and the average was used for final digestibility calculations; the CV between days was ≤15% for digestibility of all nutrients.
Sample Collection and Analytical Procedures
On day 34, all steers received a modified live M. haemolytica vaccination (One Shot, Zoetis) to induce an inflammatory response (Arthington et al., 2013). Blood was collected from the jugular vein from all steers on day 34 prior to vaccination (time 0) and days 3, 6, 9, 11, and 14 post-vaccination into vacuum tubes (potassium EDTA, No. 367844, 368381; serum, No. 366430; Becton Dickinson, Franklin Lakes, NJ). Blood was transported to the laboratory on ice. Whole blood was sent to Iowa State University Clinical Pathology Laboratory (Ames, IA) for complete blood count and leukocyte differentiation. Blood collected for plasma analysis was immediately centrifuged at 1,200 × g for 20 min at 4 °C. Plasma was then removed, aliquoted into microcentrifuge tubes, and stored at −80 °C until further analysis of ceruloplasmin and trace minerals. Ceruloplasmin concentrations were determined using methods described by Demetriou et al. (1974); the intra- and inter-assay CV were 2.6% and 5.5%, respectively. Plasma for trace mineral analysis was prepared with a 1:7 (vol/vol) dilution of plasma in 5% trace metal grade nitric acid. Samples were vortexed and centrifuged at 1,200 × g for 10 min at 4 °C. The supernatant was collected and analyzed for Cu, Fe, and Zn concentrations using inductively coupled plasma optical emission spectrometry (Optima 7000 DV, PerkinElmer, Waltham, MA). A UTAK serum standard, SMx (UTAK Laboratories, Inc., Valencia, CA) was prepared using the same method and included in all analyses to verify instrument accuracy. Blood collected for serum analysis was allowed to clot at room temperature for 90 min prior to centrifugation, removal, and storage. Serum was sent to Kansas State University Diagnostic Laboratory (Manhattan, KS) for haptoglobin analysis using the method described by Smith et al. (1998). Serum interleukin-8 (IL-8) concentrations were analyzed using a commercially available ELISA kit shown to cross-react with bovine samples (Quantikine ELISA, Human CXCL8/IL-8; R & D Biosystems, Minneapolis, MN; catalog number D8000C) and serum amyloid A (SAA) concentrations were analyzed using a commercially available ELISA kit validated for use in bovine (Multispecies SAA ELISA kit; Tridelta Development Ltd., Kildare, Ireland; catalog number TP802). The intra- and inter-assay CV, respectively, were 2.4% and 8.0% for IL-8, and 5.8% and 6.5% for SAA.
CowManager data were compiled in Excel; nonactive and rumination minutes were subtotaled by day while ear surface temperature was averaged by day. Physically effective NDF (peNDF) was determined as follows. After thawing for at least 48 h, weekly CON TMR samples were composited to weigh approximately 200 g (as-fed). The composite was then separated using a Penn State Particle Separator Box and the as-fed weight of the sample in each tier was recorded. The percent of sample in each tier was calculated by dividing the weight of sample in each tier by the total sample weight. Samples from each tier were dried at 70 °C for 48 h prior to grinding. Ground samples were then dried at 105 °C for 24 h in a forced-air oven and NDF was determined using an ANKOM200 Fiber Analyzer (ANKOM Technology, Macedon, NY). The percent of peNDF in each tier was calculated by multiplying the NDF% of sample in the respective tier, the percentage of sample in that tier, and the percent effectiveness of that tier (100% for the first tier, 60% for the second tier, 20% for the third tier, and 0% for the fourth tier; G. Dahlke, Iowa State University, personal communication). Total peNDF% of the composite was determined by adding the percent of peNDF in each tier. The average peNDF of the CON TMR for the vaccination period was 10%.
Statistical Analysis
Growth performance, digestibility, blood, and ear tag data were analyzed as a randomized complete block design using Proc MIXED of SAS 9.4 (SAS Inst., Inc., Cary, NC). Steer was the experimental unit for all analyses (n = 12 per treatment). The model included the fixed effects of treatment and block and the random effect of steer. Blood measures, ear tag data, and DMI for the 15-d vaccination period were analyzed as repeated measures using the repeated effect of day. The treatment × day effects for activity, rumination, and temperature did not show a discernable pattern and were determined to be a result of daily variation; therefore, only treatment effects are discussed. The average DMI and ear tag data for the 7 d prior to vaccination were used as a covariate in the analysis of vaccination period DMI, rumination, activity, and ear surface temperature; values from day 34 (prior to vaccination) were used as covariates in analysis of blood measures. The autoregressive (AR1) covariance structure was used for all repeated measures analyses based on lowest Akaike’s information criterion. Contrast statements (CON vs. SCFP14; SCFP14 vs. SCFP28) were used to compare treatment effects and data are reported as least square means ± SEM. Data were checked for normality and homogeneity of variance using the Shapiro–Wilks test. White blood cell, neutrophil, eosinophil, monocyte, IL-8, SAA, haptoglobin, ceruloplasmin, and rumination min/kg DM, NDF, and peNDF data were log transformed to fit the assumption of normality and the back transformed means and SEM are presented. Outliers were determined using Cook’s D statistic, and removed if Cook’s D > 0.5. Morbidity data were analyzed using Proc GLIMMIX with steer as the experimental unit, the fixed effect of treatment, a logit link function, and binary distribution. Pearson correlations between DMI and APP were determined using Proc CORR of SAS. Significance was declared at P ≤ 0.05 and tendencies 0.05 < P ≤ 0.10.
RESULTS
Feedlot Performance and Total Tract Nutrient Digestibility
Growth performance and morbidity data are presented in Table 2. Steers fed SCFP at 14 g/d tended to exhibit lesser G:F from days 0 to 28 vs. CON-fed steers (P = 0.10) and exhibited greater ADG from days 28 to 56 vs. SCFP28-fed steers (P = 0.05). There was no effect of treatment on final BW or overall (days 0 to 56) DMI, ADG, or G:F (P ≥ 0.19). There was no effect of treatment on percentage of steers treated for respiratory illness (P ≥ 0.33). Digestibility data are presented in Table 3. DMI for the TiO2 feeding period (days 12 to 27) and total tract digestibility of NDF and ADF were not affected by treatment (P ≥ 0.33). Total tract digestibility of DM and OM was greater for SCFP14-fed steers vs. SCFP28-fed steers (P ≤ 0.03). Steers fed SCFP14 had greater total tract digestibility of CP than CON-fed steers (P ≤ 0.01) but did not differ from SCFP28-fed steers (P = 0.74).
Table 2.
Effect of increased inclusions of a Saccharomyces cerevisiae fermentation product (SCFP) on performance and health of newly weaned beef steers
| Treatment1 | SEM | P-value | ||||
|---|---|---|---|---|---|---|
| CON n = 12 steers |
SCFP14 n = 12 steers |
SCFP28 n = 12 steers |
CON vs. SCFP14 |
SCFP14 vs. SCFP28 | ||
| Initial body weight, kg | 322 | 324 | 322 | 3.5 | 0.62 | 0.65 |
| Final body weight, kg | 428 | 430 | 419 | 5.9 | 0.80 | 0.20 |
| Dry matter intake, kg/d | ||||||
| Days 0 to 28 | 8.8 | 8.7 | 8.1 | 0.36 | 0.90 | 0.25 |
| Days 28 to 56 | 10.4 | 10.5 | 10.1 | 0.41 | 0.78 | 0.46 |
| Days 0 to 56 | 9.6 | 9.6 | 9.1 | 0.35 | 0.92 | 0.30 |
| Average daily gain, kg/d | ||||||
| Days 0 to 28 | 2.19 | 1.94 | 1.96 | 0.116 | 0.14 | 0.88 |
| Days 28 to 56 | 1.60 | 1.84 | 1.50 | 0.116 | 0.16 | 0.05 |
| Days 0 to 56 | 1.89 | 1.89 | 1.73 | 0.082 | 0.96 | 0.19 |
| Gain:Feed | ||||||
| Days 0 to 28 | 0.256 | 0.221 | 0.244 | 0.014 | 0.10 | 0.28 |
| Days 28 to 56 | 0.154 | 0.176 | 0.152 | 0.013 | 0.23 | 0.18 |
| Days 0 to 56 | 0.200 | 0.197 | 0.193 | 0.010 | 0.81 | 0.79 |
| Treated2, % | 16.7 | 8.3 | 25.0 | – | 0.57 | 0.33 |
1CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
2Percentage of steers treated for respiratory illness.
Table 3.
Effect of increased inclusion of a Saccharomyces cerevisiae fermentation product (SCFP) on total tract nutrient digestibility by newly weaned beef steers
| Treatment1 | SEM2 | P-value | ||||
|---|---|---|---|---|---|---|
| CON | SCFP14 | SCFP28 | CON vs. SCFP14 |
SCFP14 vs. SCFP28 |
||
| DMI, kg/d3 | 9.7 | 9.6 | 8.9 | 0.46 | 0.85 | 0.33 |
| DM, % | 64.7 | 66.2 | 63.2 | 0.90 | 0.24 | 0.02 |
| OM, % | 67.1 | 68.4 | 65.2 | 0.97 | 0.36 | 0.03 |
| NDF, % | 61.2 | 61.5 | 60.5 | 0.87 | 0.80 | 0.39 |
| ADF, % | 58.6 | 57.9 | 57.3 | 1.13 | 0.64 | 0.72 |
| CP, % | 60.0 | 63.8 | 64.0 | 0.61 | <0.01 | 0.74 |
1CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
2Highest SEM of any treatment reported.
3DMI for titanium feeding period (days 12 to 27) used as a covariate in analysis of all nutrient digestibility data.
Blood Measures and Behavior
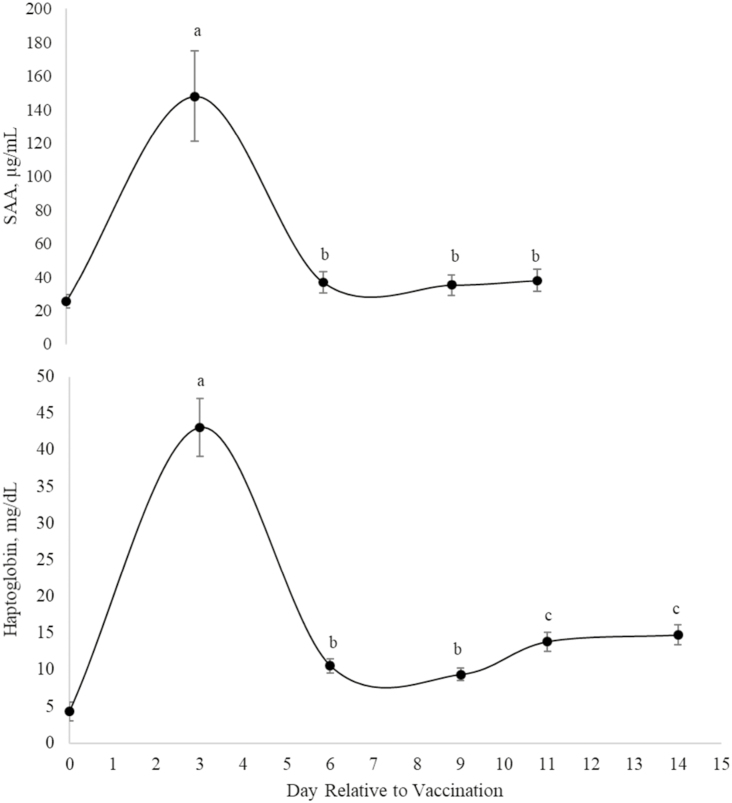
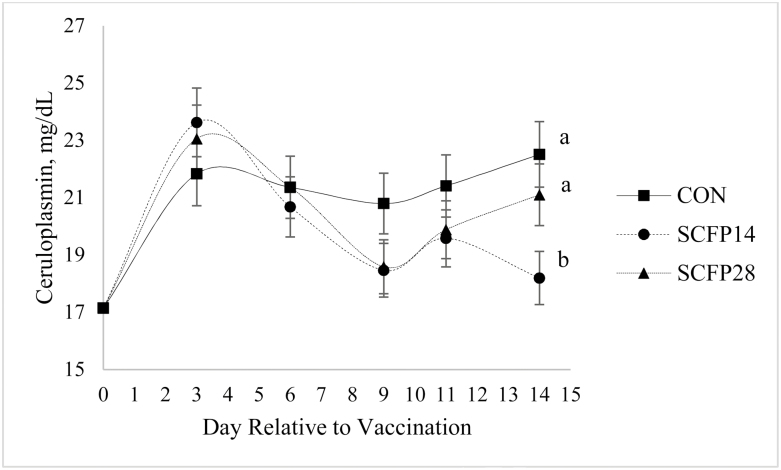
Based on repeated measures analysis of blood collected 3, 6, 9, 11, and 14 d post-vaccination, there was no effect of treatment × day or treatment on any of the whole blood components measured (Table 4; P ≥ 0.11) or on plasma copper (1.02 mg/kg), iron (1.91 mg/kg), and zinc (1.15 mg/kg) concentrations (data not shown; P ≥ 0.24). Day effects for whole blood components and serum IL-8 concentrations were a result of daily variation and did not result in a recognizable pattern. Steers fed SCFP14 tended to have lesser concentrations of serum IL-8 (Table 4; P = 0.08) and haptoglobin (P = 0.07) post-vaccination than steers fed SCFP28. Day effects (Figure 1; P < 0.01) were observed for serum haptoglobin and SAA where concentrations peaked 3 d post-vaccination. Plasma ceruloplasmin data were affected by treatment × day (P = 0.004; Figure 2), with steers fed SCFP14 having lesser concentrations on day 14 post-vaccination vs. CON and SCFP28-fed steers; there was no effect of treatment on any other sampling day. Ceruloplasmin and haptoglobin concentrations were negatively correlated (r = −0.34 and −0.23, respectively) with DMI post-vaccination (P < 0.01).
Table 4.
Effect of increased inclusions of a Saccharomyces cerevisiae fermentation product (SCFP) on blood components, serum IL-8, and serum haptoglobin of newly weaned beef steers following a Mannheimia haemolytica vaccination1
| Treatment2 | SEM | P-value | |||||
|---|---|---|---|---|---|---|---|
| CON | SCFP14 | SCFP28 | CON vs. SCFP14 | SCFP14 vs. SCFP28 | Day4 | ||
| Blood component5 | |||||||
| WBC, 103/µL | 12.1 | 12.1 | 11.9 | 0.30 | 0.94 | 0.62 | 0.01 |
| RBC, 106/µL | 9.4 | 9.4 | 9.4 | 0.07 | 0.81 | 0.99 | 0.07 |
| Hemoglobin, g/dL | 13.4 | 13.3 | 13.4 | 0.11 | 0.45 | 0.50 | 0.01 |
| Hematocrit, % | 37.2 | 36.9 | 37.4 | 0.34 | 0.52 | 0.26 | 0.01 |
| Platelets, 103/µL | 499 | 575 | 531 | 34.3 | 0.11 | 0.34 | 0.36 |
| Neutrophils, 103/µL | 4.0 | 4.0 | 4.0 | 0.22 | 0.94 | 0.94 | 0.01 |
| Eosinophils, 103/µL | 0.2 | 0.2 | 0.2 | 0.02 | 0.86 | 0.25 | 0.06 |
| Basophils, 103/µL | 0.1 | 0.1 | 0.1 | 0.01 | 0.59 | 0.17 | 0.03 |
| Lymphocytes, 103/µL | 6.9 | 7.0 | 6.8 | 0.19 | 0.63 | 0.55 | 0.01 |
| Monocytes, 103/µL | 0.7 | 0.7 | 0.7 | 0.02 | 0.83 | 0.90 | 0.01 |
| Serum | |||||||
| IL-8, pg/mL | 255 | 218 | 295 | 35.4 | 0.35 | 0.08 | 0.01 |
| Haptoglobin, mg/dL | 14.7 | 13.9 | 17.9 | 1.71 | 0.67 | 0.07 | 0.01 |
1Vaccination given on day 34. Blood samples from days 3, 6, 9, 11, and 14 post vaccination. Data from blood samples taken prior to vaccination used as a covariate in analysis.
2CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
3Highest SEM of any treatment reported.
4Based on repeated measures analysis; treatment × day P ≥ 0.28 for all variables.
5WBC = white blood cells; RBC = red blood cells; WBC, neutrophils, eosinophils, monocytes, IL-8, and haptoglobin natural log transformed; back-transformed means and SEM presented.
Figure 1.
Effect of day relative to Mannheimia haemolytica vaccination given on day 34 on serum haptoglobin and serum amyloid A (SAA) concentrations of newly weaned beef steers (day P < 0.01; different superscripts indicate difference between days P ≤ 0.05). Data from blood samples taken prior to vaccination used as a covariate in analysis (day 0 values based on excel means). Natural log transformed; back-transformed means and SEM presented.
Figure 2.
Effect of a Saccharomyces cerevisiae fermentation product (SCFP) and day on plasma ceruloplasmin concentrations of newly weaned beef steers relative to a Mannheimia haemolytica vaccination given on day 34 (treatment × day P = 0.004). Data from blood samples taken prior to vaccination used as a covariate in analysis. Unlike superscripts differ (P ≤ 0.05) within day of sampling. Natural log transformed; back-transformed means and SEM presented. CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
For the 15 d post-vaccination, SCFP14-fed steers exhibited greater DMI than SCFP28-fed steers (Table 5; P = 0.02), whereas SCFP14 vs. CON did not differ (P = 0.15). Physically active min/d did not differ due to treatment (P ≥ 0.11). Although total rumination min/d did not differ due to treatment (P ≥ 0.14), SCFP14-fed steers spent less time ruminating per kg DM, NDF, and peNDF vs. steers fed CON (P ≤ 0.07) or SCFP28 (P = 0.01). Steers fed SCFP14 had lesser ear surface temperature than CON (P = 0.02), whereas SCFP14 vs. SCFP28 did not differ (P = 0.70).
Table 5.
Effect of increased inclusion of a Saccharomyces cerevisiae fermentation product (SCFP) on newly weaned beef steer behavior and ear surface temperature during the Mannheimia haemolytica vaccination period1
| Treatment2 | SEM | P-value | ||||||
|---|---|---|---|---|---|---|---|---|
| CON | SCFP14 | SCFP28 | CON vs. SCFP14 | SCFP14 vs. SCFP28 | Day | Trt × Day | ||
| DMI, kg/d | 10.0 | 10.5 | 9.6 | 0.26 | 0.15 | 0.02 | 0.08 | 0.99 |
| Active, min/d | 79 | 77 | 81 | 1.8 | 0.41 | 0.11 | <0.01 | 0.02 |
| Ruminating, min/d | 596 | 612 | 607 | 9.2 | 0.23 | 0.69 | <0.01 | <0.01 |
| Min/kg DM3 | 62 | 57 | 64 | 1.9 | 0.07 | 0.01 | <0.01 | 0.95 |
| Min/kg NDF3 | 160 | 150 | 165 | 4.9 | 0.05 | 0.01 | <0.01 | 0.95 |
| Min/kg peNDF3,4 | 612 | 565 | 633 | 18.8 | 0.07 | 0.01 | <0.01 | 0.95 |
| Average Temp, °C3 | 24.8 | 24.5 | 24.5 | 0.13 | 0.03 | 0.99 | <0.01 | 0.08 |
1Vaccination given on d 34. Average DMI, activity, rumination, and temperature from 1 wk prior to vaccination used as a covariate in analysis.
2CON = Original XPC (Diamond V, Cedar Rapids, IA) at 0 g·steer−1·d−1; SCFP14 = Original XPC at 14 g·steer−1·d−1; SCFP28 = Original XPC at 28 g·steer−1·d−1.
3Natural log transformed; back-transformed means and SEM presented.
4peNDF = physically effective NDF.
DISCUSSION
Nutritional management of newly received feedlot cattle is important because of the variety of stressors cattle undergo prior to and upon arrival at the feedlot. Physical and psychological stressors encountered during feedlot receiving, including vaccination and transportation, have been shown to elicit an inflammatory response in beef cattle (Marques et al., 2012; Arthington et al., 2013). Regardless of source, the inflammatory response begins with release of proinflammatory cytokines and chemokines such as IL-8. The metabolic priorities of the liver are altered to prioritize the synthesis and secretion of APP, including SAA, haptoglobin, and ceruloplasmin, all well-accepted markers of the APP response in cattle (Eckersall and Conner, 1988). Although the APP response is vital to the health of the animal, prolonged stimulation can lead to skeletal muscle and adipose tissue catabolism to supply the immune system with AA and energy (Gifford et al., 2012). A negative correlation has been observed between circulating APP and receiving period DMI and ADG (Araujo et al., 2010). Therefore, nutritional strategies such as SCFP that have the potential to augment the inflammatory response are vital to optimize cattle health and performance during feedlot receiving. SCFP decreased subacute ruminal acidosis-associated inflammation when fed to dairy cows at 14 g/d (Li et al., 2016); however, the anti-inflammatory effects of SCFP have not previously been studied in feedlot cattle.
To assess the effect of SCFP on inflammation, a M. haemolytica vaccination was used to elicit an APP response. APP concentrations (SAA, haptoglobin, and ceruloplasmin) peaked 3 d post-vaccination, providing evidence that vaccination resulted in an inflammatory response. A similar response in ceruloplasmin concentrations following a M. haemolytica vaccination was observed by Arthington et al. (2013), with concentrations reaching peak 3 d post-vaccination and then decreasing to prevaccination concentrations by day 15. In the current study, ceruloplasmin concentrations were greater on day 14 post-vaccination for CON and SCFP-28 fed steers compared with SCFP14-fed steers. Steers fed SCFP28 also had greater IL-8 and haptoglobin concentrations post-vaccination compared with SCFP14-fed steers.
Inflammation can increase the production of reactive oxygen species (ROS) via increased cellular metabolism and neutrophil oxidative burst (Babior, 1984; Kowaltowski et al., 2009). Although ROS are a product of normal cellular metabolism, in excess they can damage cellular components like lipids, proteins, and DNA. Energy is required to repair this damage and maintain cellular antioxidant capacity, therefore limiting energy available for growth and potentially making the animal less efficient (Russell et al., 2016b). The connection between inflammation and oxidative stress, ROS, results in a vicious cycle that has negative impacts on animal health and performance. Although markers of oxidative stress were not measured in the current study, a companion study measured the effects of SCFP in receiving cattle diets on antioxidant defense (Deters et al., 2018). Steers fed SCFP at 14 g·steer−1·d−1 exhibited greater antioxidant defense compared with steers fed SCFP at 0 or 28 g·steer−1·d−1. It is possible that lesser ceruloplasmin concentrations observed for SCFP14-fed steers in the current study were a result of lesser ROS to positively feedback on the inflammatory response. Evidence for this hypothesis has been observed in vitro where the lowest dose of Original XP, approximately 4 times less concentrated than the SCFP used in the current study, provided the greatest reduction in ROS formation and demonstrated anti-inflammatory capacity (Jensen et al., 2008). SCFP contain components of yeast cell wall components (β-glucans and mannan-oligosaccharides), cell solubles, vitamins, proteins, lipids, and organic acids (Jensen et al., 2008). β-Gucans stimulate phagocytosis by leukocytes, increasing the production of ROS, inflammatory mediators, and cytokines (Williams et al., 1996). Therefore, a greater dose of SCFP may have led to a proinflammatory state in the current study.
Similar to Araujo et al. (2010), concentrations of ceruloplasmin and haptoglobin were negatively correlated with DMI in the present study. In addition to lesser APP, SCFP14-fed steers had numerically greater DMI (10.5 kg/d) during the M. haemolytica vaccination period (days 34 to 48) when compared with CON (10.0 kg/d) and greater DMI during this period when compared with SCFP28-fed steers (9.6 kg/d). The M. haemolytica vaccination given on day 34 of the trial likely negatively influenced steer performance from days 28 to 56. The numerically greater ADG for SCFP14 (1.84 kg/d) vs. CON-fed steers (1.60 kg/d) during this time suggests that the performance benefits of SCFP are greatest during times of stress. In agreement with this, Cole et al. (1992) observed calves fed a diet containing Original XP at 0.75% had greater DMI and tended to maintain heavier weights following an intranasal challenge with infectious bovine rhinotracheitis virus. Belknap et al. (2007) observed an increase in final BW (1.3%) and ADG (8.4%) in ranch-weaned calves fed SCFP at 14 g·steer−1·d−1 indicating that low stress receiving cattle may still benefit from SCFP supplementation.
Performance responses to yeast culture supplementation have been variable; however, a meta-analysis examining the effects of SCFP during the receiving phase reported that supplementation increased ADG and G:F by 5.8% and 2%, respectively (Wagner et al., 2016). In the current study, ADG and G:F from days 28 to 56 were numerically increased by 15% and 14.3%, respectively, for SCFP14 vs. CON-fed steers. No effects of SCFP on overall receiving period performance were noted in a larger performance trial conducted using steers from the same cohort as those used in the current study (Deters et al., 2018). A possible explanation for the lack of overall performance response in these trials could be that steers were allowed to rest for 7 to 12 d upon arrival at Iowa State University before starting on treatment diets. As SCFP may stabilize the rumen environment, it may have been beneficial to start supplementation immediately upon arrival to better understand the impact of SCFP on newly received cattle performance.
Post-vaccination activity, rumination, and ear surface temperature were evaluated using accelerometers (CowManager) attached to steers’ electronic ID tags. CowManager tags were originally designed for use in dairy cattle and limited research has been conducted using this technology in beef cattle. This study utilized CowManager tags, previously validated for use in feedlot cattle (Wolfger et al., 2015), to assess changes in behavior relative to vaccination. Regardless of treatment, steers in the current study ruminated approximately 10 h/d, approximately 5 h more per day than Gentry et al. (2017) observed with the use of rumination collars. This disparity is likely due to diet composition as steers in the current study were fed a corn silage–based receiving diet, whereas steers utilized by Gentry et al. (2017) were fed a steam-flaked corn–based finishing diet. Indeed, Gentry et al. (2017) observed increased rumination min/d with increased inclusion of ground corn stalks. To account for dietary components likely to influence rumination, rumination minutes were divided by kg of DM, NDF, and peNDF consumed. Similar to the work of Gentry et al. (2017), the rumination minutes per kilogram of peNDF accounted for the majority of the total rumination minutes in a day.
Although total rumination min/d were not different across treatments during the 15-d vaccination period, steers fed XCP14 spent less time ruminating per kg DM, NDF, and peNDF than CON or SCFP28-fed steers. Rumination time is primarily dictated by fiber content and physical form of the diet. As both of these factors were similar among treatments, it is unclear how SCFP influenced rumination time. One consideration is that rumination time may be affected by inflammation. Borderas et al. (2008) observed that calves spent less time ruminating after a lipopolysaccharide injection compared with calves that received a saline injection. Soriani et al. (2012) observed increased rumination time in dairy cows with mild inflammation while cows with subclinical or clinical health disorders had decreased rumination time. Both of these studies reported total rumination minutes per hour or day, so it is difficult to determine whether these differences were an artifact of inflammation or simply differences in DMI. It is possible that ruminal fiber digestion could have implications for rumination time. Growth of ruminal bacteria that digest fiber and utilize lactate have been shown to be stimulated by Original XP (Callaway and Martin, 1997); however, only total tract digestibility was measured in the current study, so it cannot be determined whether ruminal fiber digestion was improved with SCFP supplementation. The greater CP digestibility observed for SCFP14-fed steers in the current study could be due to stimulation of proteolytic bacteria in the rumen. Wiedmeier et al. (1987) also saw improved CP digestibility in dairy cows due to yeast culture supplementation.
In conclusion, newly received beef cattle are susceptible to poor performance and increased incidence of illness upon arrival at the feedlot due to a variety of factors including increased inflammation and oxidative stress as a result of physical and psychological stress. When fed at 14 g·steer−1·d−1, SCFP decreased markers of inflammation and improved feedlot performance following a vaccination challenge compared to steers fed SCFP at 28 g·steer−1·d−1. However, further dose titration studies are necessary to determine the optimal dose of SCFP supplementation.
Footnotes
This study was partially supported by Diamond V (Cedar Rapids, IA).
LITERATURE CITED
- Araujo D. B., Cooke R. F., Hansen G. R., Staples C. R., and Arthington J. D.. 2010. Effects of rumen-protected polyunsaturated fatty acid supplementation on performance and physiological responses of growing cattle after transportation and feedlot entry. J. Anim. Sci. 88:4120–4132. doi: 10.2527/jas.2009-2684 [DOI] [PubMed] [Google Scholar]
- Arthington J. D., Cooke R. F., Maddock T. D., Araujo D. B., Moriel P., Dilorenzo N., and Lamb G. C.. 2013. Effects of vaccination on the acute-phase protein response and measures of performance in growing beef calves. J. Anim. Sci. 91:1831–1837. doi: 10.2527/jas.2012-5724 [DOI] [PubMed] [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 1996. Official methods of analysis, 16th ed AOAC Int, Rockville, MD, USA. [Google Scholar]
- Association of Official Analytical Chemists (AOAC) 2005. Official methods of analysis, 18th ed AOAC Int, Rockville, MD, USA. [Google Scholar]
- Babior B. M. 1984. The respiratory burst of phagocytes. J. Clin. Invest. 73:599–601. doi: 10.1172/JCI111249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belknap C. R., Scott R. R., and Forcherio J. C.. 2007. Effect of yeast culture on 28-day performance of newly weaned, low-stress beef calves. J. Anim. Sci. 85(Suppl. 1):551. [Google Scholar]
- Borderas T. F., De Passillé A. M., and Rushen J.. 2008. Behavior of dairy calves after a low dose of bacterial endotoxin. J. Anim. Sci. 86:2920–2927. doi: 10.2527/jas.2008-0926 [DOI] [PubMed] [Google Scholar]
- Callaway E. S., and Martin S. A.. 1997. Effects of a Saccharomyces cerevisiae culture on ruminal bacteria that utilize lactate and digest cellulose. J. Dairy Sci. 80:2035–2044. doi: 10.3168/jds.S0022-0302(97)76148-4 [DOI] [PubMed] [Google Scholar]
- Cole N. A., Purdy C. W., and Hutcheson D. P.. 1992. Influence of yeast culture on feeder calves and lambs. J. Anim. Sci. 70:1682–1690. doi: 10.2527/1992.7061682x [DOI] [PubMed] [Google Scholar]
- Dahlke G., Strohbehn D. R., Ingle C., and Beedle P.. 2008. A feed intake monitoring system for cattle. Iowa State University Animal Industry Report: AS 654, ASL R2279. [accessed June 26, 2018]. https://lib.dr.iastate.edu/ans_air/vol654/iss1/28/. [Google Scholar]
- Demetriou J. A., Drewes P. A., and Gin J. B.. 1974. Ceruoloplasmin. In Cannon, D. C. and Winkelman J. W., editors, Clinical chemistry. Harper and Row, Hagerstown, MD; p. 857–864. [Google Scholar]
- Deters E. L., Stokes R. S., Genther-Schroeder O. N., and Hansen S. L.. 2018. Effects of SCFP in receiving diets of newly weaned beef steers. I: Growth performance and antioxidant defense. J. Anim. Sci. sky246. doi: 10.1093/jas/sky246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckersall P. D., and Conner J. G.. 1988. Bovine and canine acute phase proteins. Vet. Res. Commun. 12:169–178. [DOI] [PubMed] [Google Scholar]
- Evans M., Reeves S., and Robinson L. E.. 2012. A dried yeast fermentate prevents and reduces inflammation in two separate experimental immune models. Evid. Based. Complement. Alternat. Med. 2012:973041. doi: 10.1155/2012/973041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry W. W., Weiss C. P., Meredith C. M., McCollum F. T., and Jennings J. S.. 2017. Effects of roughage inclusion and particle size on performance and rumination behavior of finishing beef steers. J. Anim. Sci. 94:4759–4770. doi: 10.2527/jas2016-0734 [DOI] [PubMed] [Google Scholar]
- Gifford C. A., Holland B. P., Mills R. L., Maxwell C. L., Farney J. K., Terrill S. J., Step D. L., Richards C. J., Burciaga Robles L. O., and Krehbiel C. R.. 2012. Growth and development symposium: impacts of inflammation on cattle growth and carcass merit. J. Anim. Sci. 90:1438–1451. doi: 10.2527/jas.2011-4846 [DOI] [PubMed] [Google Scholar]
- Harrison G. A., Hemken R. W., Dawson K. A., Harmon R. J., and Barker K. B.. 1988. Influence of addition of yeast culture supplement to diets of lactating cows on ruminal fermentation and microbial populations. J. Dairy Sci. 71:2967–2975. doi: 10.3168/jds.S0022-0302(88)79894-X [DOI] [PubMed] [Google Scholar]
- Hutcheson D. P., and Cole N. A.. 1986. Management of transit-stress syndrom in cattle: nutritional and environmental effects. J. Anim. Sci. 62:555–560. [Google Scholar]
- Jensen G. S., Patterson K. M., and Yoon I.. 2008. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp. Immunol. Microbiol. Infect. Dis. 31:487–500. doi: 10.1016/j.cimid.2007.08.005 [DOI] [PubMed] [Google Scholar]
- Kowaltowski A. J., de Souza-Pinto N. C., Castilho R. F., and Vercesi A. E.. 2009. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 47:333–343. doi: 10.1016/j.freeradbiomed.2009.05.004 [DOI] [PubMed] [Google Scholar]
- Li S., Yoon I., Scott M., Khafipour E., and Plaizier J. C.. 2016. Impact of Saccharomyces cerevisiae fermentation product and subacute ruminal acidosis on production, inflammation, and fermentation in the rumen and hindgut of dairy cows. Anim. Feed Sci. Technol. 211:50–60. doi: 10.1016/j.anifeedsci.2015.10.010 [DOI] [Google Scholar]
- Marques R. S., Cooke R. F., Francisco C. L., and Bohnert D. W.. 2012. Effects of twenty-four hour transport or twenty-four hour feed and water deprivation on physiologic and performance responses of feeder cattle. J. Anim. Sci. 90:5040–5046. doi: 10.2527/jas.2012-5425 [DOI] [PubMed] [Google Scholar]
- Russell J. R., Minton N. O., Sexten W. J., Kerley M. S., Hansen S. L.,. and the National Program for Genetic Improvement of Feed Efficiency in Beef Cattle. 2016a. Influence of feed efficiency classification on diet digestibility and growth performance of beef steers. J. Anim. Sci. 94:1610–1619. doi: 10.2527/jas2015-9949 [DOI] [PubMed] [Google Scholar]
- Russell J. R., Sexten W. J., Kerley M. S., and Hansen S. L.. 2016b. Relationship between antioxidant capacity, oxidative stress, and feed efficiency in beef steers. J. Anim. Sci. 94:2942–2953. doi: 10.2527/jas.2016-0271 [DOI] [PubMed] [Google Scholar]
- Smith J. E., Chavey P. S., and Andrews G. A.. 1998. Semiautomatic and robotic methods for determining serum haptoglobin levels. Vet. Clin. Pathol. 27:11–14. [DOI] [PubMed] [Google Scholar]
- Soriani N., Trevisi E., and Calamari L.. 2012. Relationships between rumination time, metabolic conditions, and health status in dairy cows during the transition period. J. Anim. Sci. 90:4544–4554. doi: 10.2527/jas.2012-5064 [DOI] [PubMed] [Google Scholar]
- Wagner J. J., Engle T. E., Belknap C. R., and Dorton K. L.. 2016. Meta-analysis examining the effects of Saccharomyces cerevisiae fermentation products on feedlot performance and carcass traits. Prof. Anim. Sci. 32:172–182. doi: 10.15232/pas.2015-01438 [DOI] [Google Scholar]
- Wiedmeier R. D., Arambel M. J., and Walters J. L.. 1987. Effect of yeast culture and aspergillus oryzae fermentation extract on ruminal characteristics and nutrient digestibility. J. Dairy Sci. 70:2063–2068. doi: 10.3168/jds.S0022-0302(87)80254-0 [DOI] [PubMed] [Google Scholar]
- Williams D. L., Mueller A., and Browder W.. 1996. Glucan-based macrophage stimulators. Clin. Immunother. 5:392–399. [Google Scholar]
- Wolfger B., Timsit E., Pajor E. A., Cook N., Barkema H. W., and Orsel K.. 2015. Technical note: accuracy of an ear tag-attached accelerometer to monitor rumination and feeding behavior in feedlot cattle. J. Anim. Sci. doi: 10.2527/jas.2014-8802 [DOI] [PubMed] [Google Scholar]
- Yoon I. K., and Stern M. D.. 1996. Effects of saccharomyces cerevisiae and aspergillus oryzae cultures on ruminal fermentation in dairy cows. J. Dairy Sci. 79:411–417. doi: 10.3168/jds.S0022-0302(96)76380-4 [DOI] [PubMed] [Google Scholar]