Abstract
To determine the effects of repeated trace mineral injections on heifer development and reproductive performance, commercial Angus heifers (n = 290; 199 ± 34.3 kg; 221 ± 22 d of age) were utilized in a completely randomized design. Heifers were stratified by body weight (BW) and were administered an injectable trace mineral (MM; Multimin 90) or saline (CON) given subcutaneously, post-weaning at 221, 319, 401, and 521 ± 22 d of age. Throughout development, heifers grazed endophyte-infected fescue, red clover pastures and were supplemented with corn distillers grains (2.7 kg per heifer per day) and given access to free choice inorganic minerals. Heifer BW and body condition scores (BCS) were collected at trial initiation and 4- to 7-wk intervals thereafter. Hair coat scores (HCS) and respiration rates (n = 30 heifers per treatment) were collected at 269, 310, and 361 ± 22 d of age. Blood and liver samples were collected at trial initiation and estrous synchronization from 30 heifers per treatment to determine trace mineral status. At 319, 372, and 421 ± 22 d of age, antral follicle count and ovarian size were determined via ultrasonography. Two blood samples from all heifers were collected 10 d apart, concurrent with ultrasound dates, for cyclicity determination. Estrous synchronization was initiated, and reproductive tract scores (RTS) were collected at 421 ± 22 d of age, and heifers were bred via artificial insemination (AI) at 430 ± 22 d of age. Heifer BW, BCS, and HCS did not differ (P ≥ 0.12) throughout development, except at 268 ± 22 d of age when BCS was greater (P = 0.03) for MM than CON heifers. Respiratory rates were greater (P = 0.05) for MM than CON heifers at 269 ± 22 d of age but did not differ (P ≥ 0.66) at 310 and 361 ± 22 d of age. Plasma Mn and Zn concentrations did not differ (P ≥ 0.54). However, MM heifers had greater (P ≤ 0.01) plasma and liver concentrations of Cu and Se compared to CON. Interestingly, MM decreased (P = 0.02) liver Zn concentrations compared to CON, and there was no difference (P = 0.60) in liver Mn. Antral follicle count and ovarian size did not differ (P ≥ 0.51) due to treatment. Throughout development, number of heifers cycling was lesser (P < 0.01) for MM than CON heifers. However, there was no difference (P ≥ 0.19) in RTS, AI pregnancy rates, or overall pregnancy rates. Supplementing an injectable trace mineral increased heifer Cu and Se status; however, no effect was noted on ovarian development or pregnancy rates.
Keywords: antral follicle count, copper, injectable trace mineral, reproduction, selenium, zinc
INTRODUCTION
Trace minerals such as copper, manganese, selenium, and zinc play a critical role in numerous biochemical processes and are key components of a ruminant animal’s health and productivity (Suttle, 2010). An injectable platform offers a unique way to supplement trace minerals in a manner that may circumvent the gastrointestinal tract, thus avoiding antagonists and competition for absorption at the intestinal level (Pogge et al., 2012). Once trace minerals are injected into the animal, the minerals circulate throughout the body and incorporate into cells as needed, while the remaining mineral is transported to the liver where it is either excreted or bound to proteins for long-term storage (Suttle, 2010). Increased mineral status of an animal could be of particular importance at times when biological needs are increased, including when animals are growing or breeding. Additionally, injectable trace minerals offer an advantage compared to traditional oral supplement methods in that they provide a targeted delivery of a specific amount of trace minerals to individual animals. This injection eliminates the variability associated with fluctuation in voluntary intake noted among cattle provided free choice mineral (Arthington and Swenson, 2004).
Multimin 90 (Multimin USA, Fort Collins, CO) is an injectable trace mineral that is labeled for administration every 90 d in heifers, and in particular, the 4 wk prior to breeding. Recently, literature has focused on the administration of an injectable trace mineral pre-breeding to both heifers and cows with inconsistent results noted (Vanegas et al., 2004; Brasche, 2015; Gonzalez-Maldonado et al., 2017; Stokes et al., 2017). Moreover, the effects of utilizing an injectable trace mineral every 90 d on heifer development have yet to be reported. Therefore, the objective of this experiment was to evaluate the effects of repeated trace mineral injections on heifer growth, mineral status, and reproductive development and performance.
MATERIALS AND METHODS
All experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Illinois (IACUC #16046) and followed the guidelines recommended in the Guide for the Care and Use of Agricultural Animal in Agricultural Research and Teaching (FASS, 2010).
Animals and Experimental Design
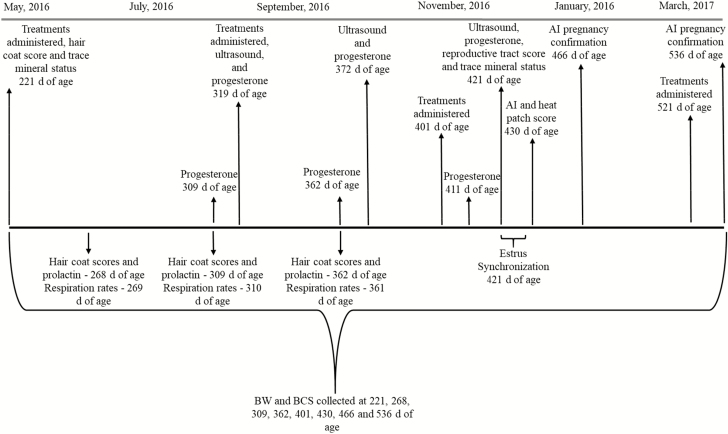
Two hundred and ninety fall-born, commercial Angus heifers (199 ± 34.3 kg) were utilized in a stratified randomized design to determine the effects of repeated trace mineral injections on heifer development and reproductive performance. Heifers were stratified at 221 ± 22 d of age by body weight (BW) and body condition score (BCS) and assigned 1 of 2 treatments: 1) an injectable trace mineral (MM; Multimin 90; Multimin USA, Fort Collins, CO) or 2) a sterilized physiological saline injection (CON; Fig. 1). The Multimin 90 contained 60 mg/mL of zinc, 10 mg/mL of manganese, 5 mg/mL of selenium, and 15 mg/mL of copper. Injections were administered subcutaneously and at label dose. Specifically, administration occurred post-weaning at 221 and 319 ± 22 d of age at a rate of 1 mL/45 kg BW and at 401 and 521 ± 22 d of age at a rate of 1 mL/68 kg BW. Heifers were housed at the Dixon Springs Agricultural Center in Simpson, IL and grazed endophyte-infected fescue (Festuca arundinacea; 81% endophyte-infected tillers) and red clover (Trifolium pretense) pastures (8.7% ash; Fig. 2) and were supplemented with corn distillers grains (2.7 kg per heifer per day; 35.1% neutral detergent fiber (NDF), 11.2% acid detergent fiber (ADF), 13.32% fat, and 30.3% crude protein (CP). Heifers were also given access to free choice inorganic trace minerals (Renaissance Nutrition, Roaring Springs, PA; 0.24% S, 21.37% Ca as calcium carbonate, 2.99% P as monocalcium phosphate, 24.5% salt, 9.35% Na, 5.84% Mg as magnesium oxide, 0.06% K, 2,214 mg/kg Fe as iron oxide, 2,000 mg/kg Mn as manganous oxide, 2,500 mg/kg Zn as zinc oxide, 1,500 mg/kg Cu as copper sulfate, 27 mg/kg Co as cobalt carbonate, 36 mg/kg I, 26 mg/kg Se as sodium selenite, 110,179 IU/kg vitamin A, 3,084 IU/kg vitamin D, 545 IU/kg vitamin E, and 1,179 mg/kg of chlortetracycline). Throughout the experiment heifers consumed 97.6 g per heifer per day of free choice mineral, with a targeted consumption of 85 g per heifer per day. One heifer from the CON treatment was removed from trial at 289 ± 22 d of age for a chronic respiratory infection. At approximately 360 d of age, heifers across all pastures were observed attempting to suckle each other. One heifer from the MM treatment was removed at 359 ± 22 d of age for a mastitis infection, and an additional 8 heifers (3 CON and 5 MM) were removed at 383 ± 22 d of age for mastitis infections or poor performance. An additional heifer from the MM treatment was identified as a freemartin and removed at the time of breeding.
Figure 1.
Experimental time line.
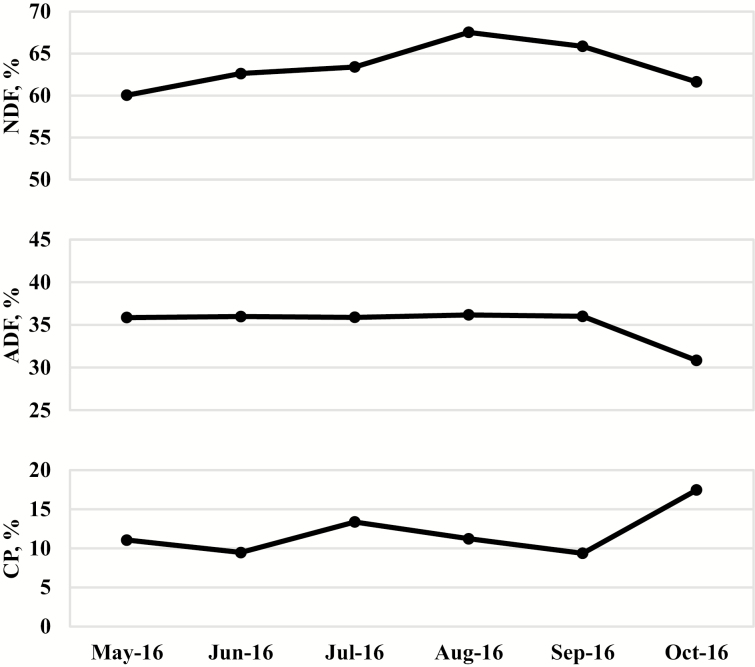
Figure 2.
Forage quality [percentage neutral detergent fiber (NDF), acid detergent fiber (ADF), and crude protein (CP)] of endophyte-infected fescue (Festuca arundinacea) and red clover (Trifolium pretense) pastures from May 2016 to October 2016. Samples were collected as cattle rotated pastures and were composited on a monthly basis.
Sample Collection and Analytical Procedures
Heifer BW and BCS [emaciated = 1; obese = 9; as described by Wagner et al. (1988)] were collected at trial initiation (221 ± 22 d of age), 268, 309, 362, 401, 430 ± 22 d of age, artificial insemination (AI) pregnancy confirmation (466 ± 22 d of age), and final pregnancy confirmation (536 ± 22 d of age). Hair coat scores (HCS; 1 to 5, in which 1 = slick and 5 = unshed) were also recorded at trial initiation and 268, 309, and 362 ± 22 d of age from the same farm technician. Sixty heifers (30 per treatment) that were most similar to average initial (221 ± 22 d of age) BW and BCS were selected for additional sampling and were utilized throughout all observation points. Respiration rates were collected from these 60 heifers at 269, 310, and 361 ± 22 d of age (June 22, 2016, August 2, 2016, and September 22, 2016, respectively).
For nutrient composition analysis, samples of distillers grains were collected monthly and composited and dried at 55° C for a minimum of 3 d and ground through a 1 mm screen using a Wiley mill (Arthur H. Thomas, Philadelphia, PA). Forage samples were collected at grazing height from pastures on a monthly basis and composited and dried similarly. Ground distillers samples were analyzed for CP (Leco TruMac, LECO Corporation, St. Joseph, MI), NDF and ADF using an Ankom 200 Fiber Analyzer (Ankom Technology, Macedon, NY), and crude fat using an Ankom XT10 fat extractor (Ankom Technology, Macedon, NY). Ground forage samples were also analyzed for NDF, ADF, and CP using the same analytical procedures previously described. Total ergot alkaloid analysis of forages was conducted in a commercial laboratory (Agrinostics Limited, Co., Watkinsville, GA).
Blood and Liver Sampling and Analysis
Blood samples were collected from all heifers at trial initiation and 268, 309, and 362 ± 22 d of age (June 21, 2016, August 1, 2016, and September 23, 2016, respectively) for prolactin analysis. Blood was collected via jugular venipuncture into a 10-mL serum blood collection vacuum tube (Becton, Dickinson, and Co., Franklin Lakes, NJ). Blood was allowed to clot for 2 h at room temperature before being centrifuged at 1,300 × g for 20 min at 5 °C. Serum was stored at −20 °C for subsequent prolactin analysis. Serum was analyzed for prolactin analysis via a radioimmunoassay as described by Bernard et al. (1993) at the University of Tennessee (Knoxville, TN). The intra- and inter-assay CV for all prolactin analysis were 6.06% and 5.29%, respectively, and the sensitivity across assays was 0.05 ng/mL.
Liver and blood samples were collected from the same predetermined 60 heifers for trace mineral determination at the initiation of the trial and at breeding (422 ± 22 d of age). Liver biopsy samples were collected using the method of Engle and Spears (2000) with the modification that all heifers were given an intradermal 5 mL injection of Lidocaine Injectable-2% (MWI, Boise, ID) at the site of the biopsy. Biopsy samples were transported to the laboratory on ice where they were frozen at −20 °C until further analysis. Blood was collected at the time of liver biopsy via jugular venipuncture into K2EDTA vacuum tubes (10 mL; Becton, Dickinson, and Co., Franklin Lakes, NJ). Blood was centrifuged at 1,300 × g for 20 min at 5 °C and plasma was stored at −20 °C for subsequent trace mineral analysis. Liver samples for Cu, Mn, Se, and Zn analysis were sent to a commercial lab where they were subjected to nitric acid digestion and inductively coupled plasma spectroscopy analyses for complete minerals (method 975.03: AOAC, 1988; The Ohio State University, Service Testing and Research Lab, Wooster, OH). Plasma samples were sent to Michigan State University Diagnostic Center for Population and Animal Health (East Lansing, MI) and concentrations of Cu, Mn, Se, and Zn were analyzed using an Agilent 7500ce Inductively Coupled Plasma Mass Spectrometer (ICP/MS; Agilent Technologies Inc., Santa Clara, CA) via procedures described previously (Wahlen et al., 2005).
Reproductive Development
Antral follicle counts and ovarian length and width were measured from the 60 predetermined heifers via transrectal ultrasonography (Ibex Portable Ultrasound, variable MHz linear array transducer, E.I. Medical Imaging, Loveland, CO) at 319, 372, and 421 ± 22 d of age (August 11, 2016, October 3, 2016, and November 21, 2016, respectively). The number and location of all antral follicles ≥3 mm in diameter were recorded for each ovary. Two blood samples from all 290 heifers were collected 10 d apart to determine percent of heifers cycling at approximately 10 (309 and 319 ± 22 d of age), 12 (362 and 372 ± 22 d of age), and 14 (411 and 421 ± 22 d of age) mo of age, concurrent with ultrasound dates. Samples were collected via jugular venipuncture in 10 mL K2EDTA vacuum tubes (Becton, Dickinson, and Co., Franklin Lakes, NJ) and immediately placed on ice. Samples were centrifuged at 1,300 × g for 20 min at 5 °C, and plasma was stored at −20 °C until analyzed. Heifers were considered cycling when a single plasma sample contained ≥2 ng/mL of progesterone, or when both samples collected 10 d apart contained ≥1 ng/mL of progesterone as previously described by Gunn et al. (2015). Plasma progesterone concentration was analyzed using a chemiluminescent enzyme immunoassay (Immulite 1000; Siemens Medical Solutions Diagnostics, Los Angeles, CA) validated by Reis et al. (2015). The average intra-assay CV for all progesterone samples was 3.6% and the sensitivity across assays was 0.2 ng/mL.
Estrous Synchronization and Breeding
At 421 ± 22 d of age reproductive tract scores (RTS; 1 = immature to 5 = luteal phase; Anderson et al., 1991) were assigned to all heifers. Concurrently, heifers were enrolled in a 7-d CO-Synch + controlled internal drug release (CIDR) insert and timed-AI protocol. At protocol initiation, heifers received an intravaginal progesterone insert (CIDR; Pfizer Animal Health, New York, NY) and were administered 100 µg of gonadotropin-releasing hormone (GnRH) (Factrel; Zoetis, Parsippany, NJ). Seven days later, the CIDR was removed, and heifers were administered 25-mg dose of prostaglandin (Lutalyse; Pfizer Animal Health). Fifty-four hours following CIDR removal heifers were bred via timed-AI and administered 100 µg of GnRH (Cystorelin; Merial, Duluth, GA). Both sire and AI technician were stratified across treatments. Heat detection patches (Estrotect Heat Detectors; Rockway Inc., Spring Valley, WI) were placed on all heifers at the time of CIDR removal and visually scored from 0 to 3 (0 = not activated, 1 = partially activated, 2 = fully activated, and 3 = missing) at time of AI. Immediately following AI heifers were combined into 2 groups with an equal representation of each treatment and placed on pasture. Ten days following AI, heifers were placed with 8 bulls (which passed breeding soundness exams; 4 bulls per group) for a 96-d breeding season. At 466 ± 22 d of age, AI conception rates were collected by a trained technician via ultrasonography (Aloka 500 instrument, Hitachi Aloka Medical America, Inc., Wallingford, CT; 7.5 MHz general purpose transducer array). Overall pregnancy rates were determined at 536 ± 22 d of age by a trained technician via rectal palpation or ultrasonography (Aloka 500 instrument, Hitachi Aloka Medical America, Inc., Wallingford, CT; 7.5 MHz general purpose transducer array).
Statistical Analysis
Body weight, BCS, HCS, respiration rates, prolactin, antral follicle counts, ovarian length and width, and plasma and liver mineral data were analyzed as a completely randomized design using the MIXED procedures of SAS (SAS Inst. Inc., Cary, NC). All binary data, including progesterone, distribution of RTS, distribution of heat patch scores, AI conception, and overall pregnancy rates were analyzed using the GLIMMIX procedure of SAS. The model included the fixed effect of treatment and group. The model for cyclicity, RTS, heat patch score, AI conception rates, and overall pregnancy rates included age as a covariate. Body weight, BCS, HCS, respiration rates, prolactin, progesterone, antral follicle counts, and ovarian length and width were analyzed as repeated measures with the fixed effects of treatment, group, day, and the interaction between treatment and time. For all variables, the unstructured covariance structure was utilized, as it resulted in the smallest Akaike information criterion. Day was the repeated effect for all repeated measures and age at time of ultrasound was utilized as a covariate for antral follicle counts and ovarian length and width. Liver and plasma trace mineral values determined at d 0 were used as a covariate in the analysis of trace mineral status at breeding. Initial liver Mn and Cu and plasma Se were significant (P ≤ 0.05) when utilized as a covariate; however, to maintain consistency across the model, all initial liver and plasma values were included as covariates. Animal served as the experimental unit for all analyses. Treatment effects were considered significant at P ≤ 0.05 and tendencies were noted at 0.05 < P ≤ 0.10. Means reported in tables are least squares means ± SEM.
RESULTS AND DISCUSSION
Heifer Performance
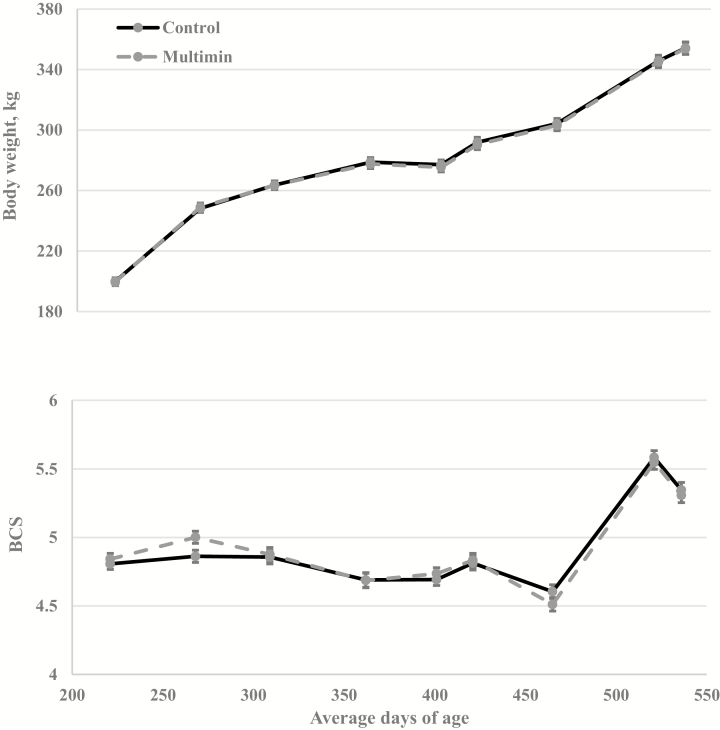
Heifer BW and BCS did not differ (P ≥ 0.27; Fig. 3) throughout development. However, results across literature have been inconsistent regarding the effects of an injectable trace mineral on cattle BW and BCS. Mundell et al. (2012) supplemented cows grazing native range with an injectable trace mineral 30 d prior to fixed-time AI and noted no difference in BW and BCS regardless of treatment. Similarly, Gadberry and Baldridge (2013) administered Angus cows an injectable trace mineral prior to calving and again prior to breeding and noted no effect on cow BW or BCS. Other work utilizing weaned heifers noted an increase in average daily gain (ADG) over 177 d when heifers were administered an injectable trace mineral (Arthington et al., 2014). Ultimately, differences in time of administration of treatment, differences in breeds, and differences in nutrient availability make drawing comparisons across these experiments challenging.
Figure 3.
Effect of an injectable trace mineral (Multimin 90) on heifer BW and BCS. Control cattle received a sterilized saline solution, and Multimin 90 (Multimin) cattle received injectable trace mineral at approximately 90-d intervals. For BW, treatment by day was not significant (P = 0.58), the main effects of treatment and day were (P = 0.90) and (P < 0.01), respectively. For BCS, treatment by day was not significant (P = 0.27), the main effects of treatment and day were (P = 0.83) and (P < 0.01), respectively.
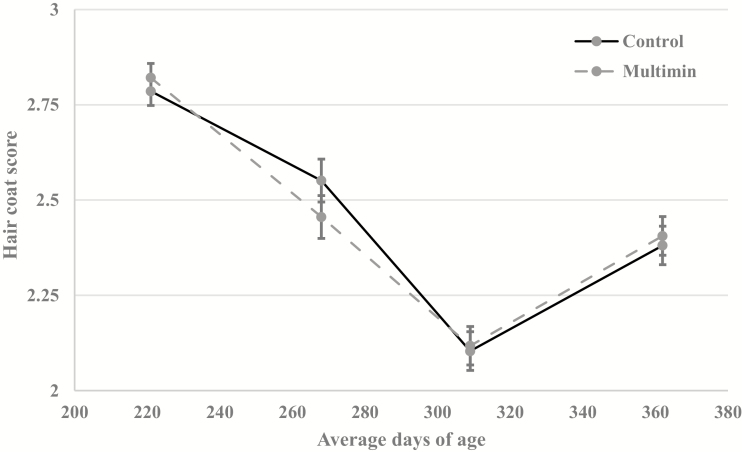
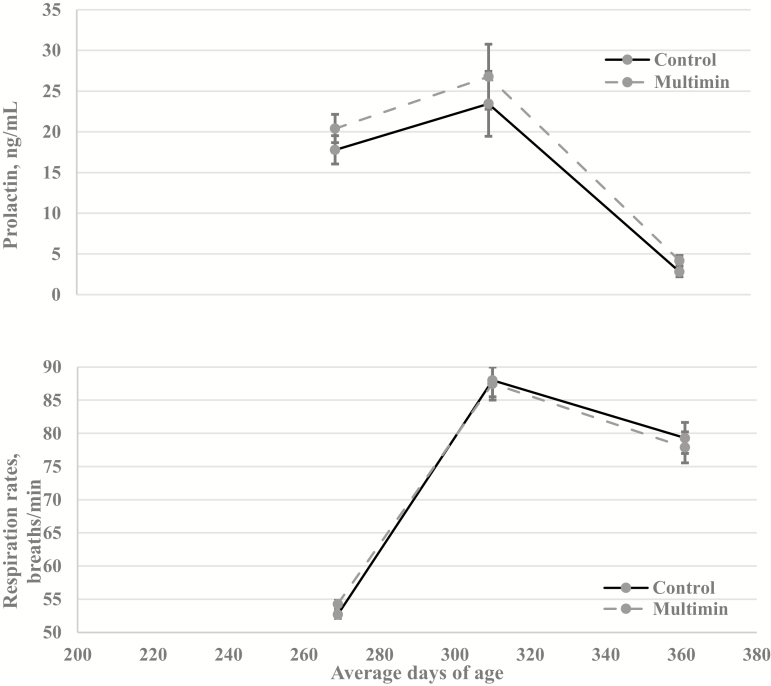
Forage samples for ergot alkaloid concentrations were collected monthly, with the lowest concentrations noted in July and peak concentrations occurring in May, September, and October (May = 2088 µg/kg; June = 921 µg/kg; July = 330 µg/kg; August = 787 µg/kg; September = 2275 µg/kg). Heifer HCS (Fig. 4), respiration rates and prolactin concentrations (Fig. 5) did not differ (P ≥ 0.30) between treatments throughout the experiment. However, there was an effect of day (P < 0.01) on prolactin concentrations, likely driven by all heifers having decreased serum prolactin concentrations at 362 ± 22 d of age. This decrease in serum prolactin coincided with a substantial increase in pasture total ergot alkaloid concentration (1192 µg/kg). Similarly, Gadberry and Baldbridge (2013) also noted no difference in HCS when supplementing calves with an injectable trace mineral 130 d after birth. However, these calves were not grazing endophyte-infected fescue. Previous research has noted that cattle grazing endophyte-infected tall fescue had decreased plasma and liver Cu concentrations (Stoszek et al. 1979; Saker et al., 1998). Both Saker et al (1998) and Stoszek et al. (1979) reported that cattle grazing endophyte-infected fescue had plasma Cu concentrations below 0.5 mg/kg and Stoszek et al. (1979) reported liver Cu concentrations below 50 mg/kg. These plasma and liver Cu concentrations would classify these grazing cattle Cu deficient according to Kincaid (2000). In the current study, MM heifers had greater (P ≤ 0.01; Table 1) plasma and liver concentrations of Cu compared to CON. Stoszek et al. (1979) also noted increases in both plasma and liver Cu, with Cu status changing from deficient to adequate levels, when beef heifers grazing tall fescue were supplemented with either 400 or 1,000 mg/Cu as CuSO4 compared to steers that received no Cu supplementation; however, no level of Cu supplementation affected heifer ADG even when Cu status was improved. Stoszek et al. (1979) did not evaluate respiration, HCS, and prolactin levels. Saker et al. (1998) noted similar increases in Cu plasma concentrations when steers grazing tall fescue received a Cu oxide bolus; however, performance parameters were not measured. While it is well-established endophyte-infected fescue impacts Cu status of animals, it is still unknown whether additional Cu supplementation may improve resistance to fescue toxicity in cattle. More research is needed to clarify the relationship between endophyte-infected fescue and Cu supplementation and the effect this may have on fescue toxicity symptoms in cattle.
Figure 4.
Effect of an injectable trace mineral (Multimin 90) on heifer hair coat score (1 to 5, in which 1 = slick and 5 = unshed). Control cattle received a sterilized saline solution, and Multimin 90 (Multimin) cattle received injectable trace mineral at approximately 90 d intervals. Treatment by day was not significant (P = 0.58), and the main effects of treatment and day were (P = 0.90) and (P < 0.01), respectively.
Figure 5.
Effect of an injectable trace mineral (Multimin 90) on heifer (n = 60; 30 heifers per treatment) prolactin concentrations and respiration rates (breaths per minute). Control cattle received a sterilized saline solution, and Multimin 90 (Multimin) cattle received injectable trace mineral at approximately 90-d intervals. For serum prolactin concentration, treatment by day was not significant (P = 0.86), and the main effects of treatment and day were (P = 0.30) and (P < 0.01), respectively. For respiration rates, treatment by day was not significant (P = 0.61), and the main effects of treatment and day were (P = 0.94) and (P < 0.01), respectively.
Table 1.
Influence of an injectable trace mineral supplementation on heifer mineral status
| Treatment1 | SEM | P-value | ||
|---|---|---|---|---|
| Item | Control | MM | ||
| Plasma mineral | ||||
| Initial2 | ||||
| Cu, mg/L | 0.80 | 0.80 | – | – |
| Mn, µg/L | 1.64 | 2.33 | – | – |
| Se, µg/L | 57.1 | 60.5 | – | – |
| Zn, mg/L | 0.95 | 0.98 | – | – |
| Breeding3 | ||||
| Cu, mg/L | 0.71 | 0.85 | 0.037 | 0.01 |
| Mn, µg/L | 1.64 | 1.73 | 0.100 | 0.54 |
| Se, µg/L | 65.8 | 75.1 | 1.42 | <0.01 |
| Zn, mg/L | 0.94 | 0.93 | 0.037 | 0.94 |
| Liver mineral, mg/kg | ||||
| Initial2 | ||||
| Cu | 167.3 | 221.5 | – | – |
| Mn | 11.02 | 11.53 | – | – |
| Se | 0.70 | 0.63 | – | – |
| Zn | 115.9 | 113.8 | – | – |
| Breeding3 | ||||
| Cu | 108.0 | 175.8 | 10.06 | <0.01 |
| Mn | 9.85 | 9.98 | 0.167 | 0.60 |
| Se | 0.98 | 1.53 | 0.143 | <0.01 |
| Zn | 110.5 | 103.8 | 1.91 | 0.02 |
1Control cattle received a sterilized saline solution, and Multimin 90 (MM) cattle received injectable trace mineral at approximately 90-d intervals.
2Initial values served as covariates in analysis for respective parameters at subsequent sampling dates.
3Values determined at day 0 were used as a covariate in the analysis of trace mineral status at breeding.
Plasma and liver Mn concentrations did not differ (P ≥ 0.54) between MM and CON heifers. Differences in storage and metabolism of Mn may explain why no differences were noted. Manganese that is stored in the liver is often not indicative of dietary intake (Kincaid, 2000) and other authors have reported minimal (Hansen et al., 2006; Pogge et al., 2012) to no (Arthington et al., 2014) response in liver and plasma Mn based on Mn supplementation. Multimin heifers had greater (P ≤ 0.01) plasma and liver concentrations of Se compared to CON. Even though all heifers were receiving free choice trace mineral, based on liver Se concentrations, all heifers were considered marginally Se deficient at the initiation of the trail (0.70 and 0.63 ± 0.091 mg/kg for CON and MM, respectively) using recommended classification by Kincaid (2000). By the time of breeding CON heifers were still considered to be marginally Se deficient based on liver Se concentrations (0.98 ± 0.143 mg/kg); however, MM heifers had reached an adequate Se status (1.53 ± 0.143 mg/kg). Because all heifers were grazing similar pasture and provided the same free choice trace mineral, this difference in Se status is likely attributed to the trace mineral injection.
Plasma Zn did not differ (P = 0.94) between CON and MM heifers; however, liver Zn concentrations were greater (P = 0.02) for CON than MM heifers. The literature has been inconsistent regarding the effects of an injectable trace mineral on liver Zn concentrations. Work by Brasche (2015) reported no effect of an injectable trace mineral (Multimin 90) when mineral status was measured 24 d post injection on liver Zn concentrations in Angus crossbred beef heifers, and Pogge et al. (2012) reported an increase in liver Zn concentration when steers were supplemented with an injectable trace mineral. Both plasma and liver Zn concentrations may be variable based on immune status and age (Kincaid, 2000), suggesting these may not be accurate indicators of Zn status.
Reproductive Development and Performance
To our knowledge, no other study has compared the effects of an injectable trace mineral source on ovarian morphology and follicular development in beef heifers. For all measures of heifer ovarian morphology, treatment by day was not significant (P ≥ 0.45; Table 2) and day was significant (P ≤ 0.01). No differences due to treatment (P ≥ 0.51) were detected in antral follicle count and ovarian size. This is similar to results by Lamb et al. (2008) who noted no effect of additional mineral supplementation on number of follicles, number of corpora lutea, or number of unovulated follicles in Angus heifers. It is important to note heifers utilized by Lamb et al. (2008) were supplemented with 1 of 3 free choice mineral treatments: no additional mineral, an organic mineral, or inorganic form of both macro and micro minerals. Heifers in that study did not receive an injectable trace mineral and mineral status was not assessed in the experiment (Lamb et al., 2008), thus making it a challenge to draw conclusions across these experiments. Other work has assessed the effects of providing Holstein cows post-calving an inorganic or organic form of Cu, Mn, Co, and Zn in a concentrate-based pelleted premix and noted no effects on first-wave follicular dynamics, luteal measures, or embryo quality (Hackbart et al., 2010). Gonzalez-Maldonado et al. (2017) supplemented over conditioned dairy cows with an injectable trace mineral prior to estrus synchronization and noted no differences in follicle population or diameter of preovulatory follicles when measured at the time of CIDR removal. As multiple mechanisms are responsible for mediating the effects of fertility, follicular growth, and reproductive performance, more research is needed to determine the specific role trace minerals may play in these functions.
Table 2.
Influence of injectable trace mineral supplementation on heifer ovarian morphology
| Treatment1 | SEM | P-value2 | ||
|---|---|---|---|---|
| Item | Control | MM | ||
| Left antral follicles3 | 0.92 | |||
| 10 mo | 6.9 | 7.1 | 0.49 | |
| 12 mo | 8.2 | 8.8 | 0.50 | |
| 14 mo | 9.4 | 8.8 | 0.49 | |
| Right antral follicles3 | 0.51 | |||
| 10 mo | 7.0 | 7.5 | 0.55 | |
| 12 mo | 9.0 | 9.1 | 0.55 | |
| 14 mo | 8.5 | 8.8 | 0.55 | |
| Total antral follicles3 | 0.96 | |||
| 10 mo | 13.9 | 14.1 | 0.91 | |
| 12 mo | 17.0 | 17.3 | 0.91 | |
| 14 mo | 17.8 | 17.1 | 0.91 | |
| Average ovarian length3, mm | 0.89 | |||
| 10 mo | 22.4 | 22.1 | 0.58 | |
| 12 mo | 23.0 | 23.1 | 0.58 | |
| 14 mo | 25.8 | 25.8 | 0.58 | |
| Average ovarian height3, mm | 0.71 | |||
| 10 mo | 12.6 | 12.9 | 1.01 | |
| 12 mo | 13.0 | 13.1 | 0.47 | |
| 14 mo | 13.7 | 14.0 | 0.98 | |
1Control cattle received a sterilized saline solution, and Multimin 90 (MM) cattle received injectable trace mineral at approximately 90-d intervals.
2For all measures of heifer ovarian morphology treatment by day was not significant (P ≥ 0.45) and day was significant (P ≤ 0.01). Therefore, only the overall effect of treatment is reported.
3Measurements include total number of antral follicles observed via ultrasonography at approximately 10 (319 ± 22 d of age), 12 (372 ± 22 d of age), and 14 (421 ± 22 d of age) mo of age
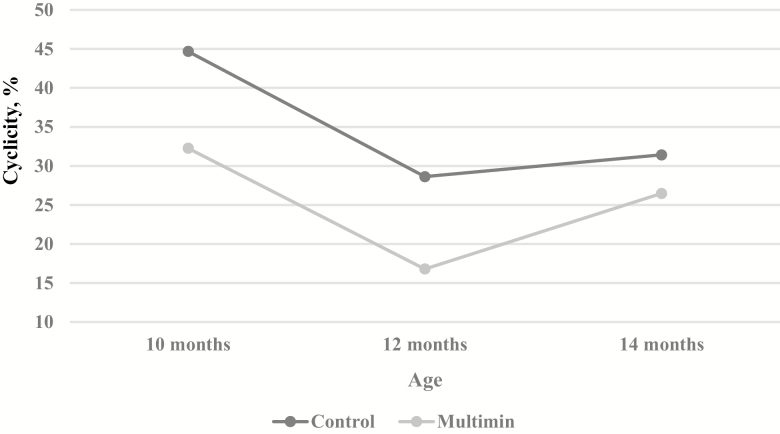
Copper, Mn, Se, and Zn are vital for ruminant health and nutrition, and deficiencies in trace minerals have resulted in the suppression of estrus, reduced conception rates, and overall decreased reproductive performance (Hidiroglou, 1979; Sunde, 1997; Suttle, 2010). Interestingly though, there was not a treatment by day interaction on percent of heifers cycling, there was a main effect of both treatment (P < 0.01; Fig. 6) and day (P < 0.01) with fewer MM heifers cycling compared to CON heifers. It is unknown if additional trace mineral supplementation is driving this overall decrease in cyclicity noted in the MM heifers, or if heifers were randomly assigned to the MM treatment that were predisposed to reach puberty at a later time point. Minimal research has been conducted to determine the role trace minerals may play in the attainment of puberty in beef heifers. Grings et al. (1999) noted no effect on age of puberty when supplementing dietary Cu, Zn, and Mn to beef heifers. However, Grings et al. (1999) also did not report a difference in plasma Cu concentrations and they did not collect liver mineral data, making drawing conclusion regarding the role trace minerals may play in cyclicity in beef heifers challenging. Additionally, utilizing blood measures such as plasma or serum for trace mineral status determination may provide little insight as often times homeostatic mechanisms can limit changes in circulating concentrations of trace minerals until reserves become substantially depleted (Miller, 1975).
Figure 6.
Effect of an injectable trace mineral (Multimin 90) on percent of heifers reaching cyclicity. Control cattle received a sterilized saline solution, and Multimin 90 (Multimin) cattle received injectable trace mineral at approximately 90-d intervals. Cyclicity was defined as when 1 blood sample contained ≥2 ng/mL of progesterone or when both samples contained ≥1 ng/mL of progesterone. Measurements taken at approximately 10 (309 and 319 ± 22 d of age), 12 (362 and 372 ± 22 d of age), and 14 (411 and 421 ± 22 d of age) mo of age. Treatment by day was not significant (P = 0.45), and the main effects of treatment and day were (P < 0.01) and (P < 0.01), respectively.
Both treatments had the greatest number of cycling heifers at 10 mo of age and exhibited a decrease in number of cycling heifers at 12 mo of age and only a slight increase in number of heifers cycling at 14 mo of age. While unexpected, the lesser percent of heifers cycling at 12 mo of age does coincide with the marked decrease in BCS and prolactin concentration and an increase in respiration rates at 12 mo of age, suggesting heifers may have been in a negative energy balance and environmentally stressed prior to breeding. Ultimately, further research is needed to elucidate why MM-treated heifers exhibited an overall decrease in the percentage of heifers attaining cyclicity.
Foreshadowed by the difference in number of heifers obtaining cyclicity, there was a tendency (P = 0.09; Table 3) for MM heifers to have an increased number of inactivated heat patches. However, there were no differences (P ≥ 0.38) between treatments in the number of partially or fully active heat patches. There was also no effect (P ≥ 0.19) of treatment on RTS distributions. Assuming that heifers receiving a RTS of a 4 or 5 would be cycling and ready to breed, RTS did closely coincide with the number of heifers cycling at 14 mo of age. Thirty-six percent of CON heifers and 31% of MM heifers would classify as cycling based on RTS and according to progesterone data 31% and 26% of CON and MM heifers, respectively, were cycling at the time of breeding. Despite the differences noted in number of cycling heifers and the tendency for heat patch scores to be different, there were no differences in (P ≥ 0.36) both AI conception and overall pregnancy rate. These results were consistent with Stokes et al. (2017) who reported in 2 out of 3 experiments no difference in AI or overall pregnancy rates when beef heifers were supplemented an injectable trace mineral (Multimin 90) prior to estrous synchronization. However, the effects of an injectable trace mineral on reproductive performance have been largely inconsistent across the literature. In an additional experiment, Stokes et al. (2017) reported a tendency for heifers administered an injectable trace mineral (Multimin 90) prior to breeding to have increase AI conception rates. Despite this difference in AI conception, no difference was noted in overall pregnancy rates (Stokes et al., 2017). Trace mineral status was not assessed in any of these experiments making it challenging to determine if these effects on reproductive performance were due to differences in mineral status.
Table 3.
Influence of injectable trace mineral supplementation on heifer reproductive performance and pregnancy diagnosis
| Treatment1 | SEM | P-value | ||
|---|---|---|---|---|
| Item | Control | MM | ||
| Reproductive tract score2, % | ||||
| 1 | – | – | – | – |
| 2 | 19 | 26 | – | 0.19 |
| 3 | 34 | 36 | – | 0.73 |
| 4 | 18 | 17 | – | 0.83 |
| 5 | 18 | 14 | – | 0.33 |
| Heat patch score3, % | ||||
| 0 | 44 | 54 | – | 0.09 |
| 1 | 28 | 24 | – | 0.42 |
| 2 | 24 | 20 | – | 0.38 |
| 3 | – | – | – | – |
| AI pregnancy rate, % | 30 | 37 | – | 0.36 |
| Overall pregnancy rate, % | 75 | 74 | – | 0.78 |
1Control cattle received a sterilized saline solution, and Multimin 90 (MM) cattle received injectable trace mineral at approximately 90-d intervals.
2Reproductive tract scores were determined at the time of estrus synchronization initiation from 0 to 5 (1 = immature to 5 = luteal phase).
3Heat patches were visually scored at time of breeding from 0 to 3 (0 = not activated, 1 = partially activated, 2 = fully activated, 3 = missing).
Other authors have also noted inconsistent results throughout the literature when assessing the effects of an injectable trace mineral on heifer reproductive performance. Brasche (2015) noted no differences in AI pregnancy rates when Angus crossbred heifers (n = 109) were supplemented with an injectable trace mineral (Multimin 90) 30 d prior to breeding; however, there was a tendency for overall pregnancy rates to be increased (92.7% = injectable trace mineral; 83.3% = control). Liver mineral status was assessed 170 d post injection and there was no difference in Cu, Se, or Zn liver concentrations for these heifers. However, there was a tendency for liver Mn concentration to be greater for control (11.5 mg/kg) than heifers supplemented with an injectable trace mineral (10.5 mg/kg). In a subsequent experiment by Brasche (2015) no difference was noted in AI or overall pregnancy rates when Angus crossbred heifers (n = 112) were supplemented with an injectable trace mineral (Multimin 90) 30 d prior to breeding. Heifers receiving the injectable trace mineral also had increased liver Cu (371 and 292 mg/kg for trace mineral and control supplemented cattle, respectively) and Se (2.3 and 1.5 mg/kg for trace mineral and control supplemented cattle, respectively) compared to their control counterparts 24 d following the injection (Brasche, 2015). However, both liver Cu and Se status were considered adequate for these control heifers according to Kincaid (2000), and thus the lack of differences in reproductive performance was not remarkable.
Vanegas et al. (2004) conducted 2 experiments administering either 1 or 2 doses of an injectable trace mineral (Multimin 90) to dairy cows approximately 3 wk pre-calving and noted that 1 dose provided no differences in first-service conception rates. Two doses administered 60 d apart significantly decreased the first-service conception rate compared to controls. These dairy cows were also being supplemented with a basal diet containing 0.3 mg/kg of dietary Se and 20 mg/kg of Cu. The NRC (2001) requirements for these cattle were estimated to be 0.3 mg/kg of Se and 11 mg/kg of Cu. However, no liver or blood samples were collected for evaluation of trace mineral status that may help explain these results. The inconsistency noted across literature could be driven by multiple factors including time of administration, breed, previous mineral status, and other nutrient, breeding, or environmental factors.
Both the AI and overall pregnancy rates reported in this experiment were lesser than pregnancy rates of heifers in this herd from previous years. It is important to note that a substantial amount of heifers were retained as replacements for this experiment that in a normal setting would have been removed from the herd. This may have negatively impacted the reproductive success of the whole group as under normal management conditions the younger, lighter weight heifers that are less likely to conceive, would have been removed. As a component of having younger, light weight heifers, these heifers averaged 302 kg at the time of breeding, only 54% of their dams average mature BW of 556 kg. The Nutrient Requirements of Beef Cattle (2016) suggests Bos taurus heifers attain puberty at approximately 60% of mature weight. However, a review by Funston et al. (2012) reported that heifers targeted to 55% of mature weight had similar calf production rates as heifers developed to heavier target weight of 60 or 65%. However, cyclicity and pregnancy data from the current experiment would support that these heifers may have needed to reach a greater targeted percent of mature BW for optimal reproductive success. Additionally, at approximately 360 d of age, a few heifers were observed attempting to suckle each other and began lactating. Likely driven by this behavior, 14 heifers (7 CON heifers and 7 MM heifers) were subsequently treated for mastitis. Of these heifers, 6 (1 CON and 5 MM heifers) were then removed at 383 ± 22 d of age from trial. The initiation of lactation involves numerous hormones including prolactin, glucocorticoids, and estrogen (Tucker, 2000) all of which could have substantial impacts on normal heifer growth and reproductive maturity.
Repeated supplementation of an injectable trace mineral to developing heifers resulted in an increase in Cu and Se status. However, minimal effects on heifer growth and symptoms of fescue toxicosis were noted. Interestingly, heifers administered the injectable trace mineral did have decreased attainment of cyclicity. However, this ultimately did not affect AI or overall pregnancy rates. These data suggest that additional mineral supplementation in the form of an injectable trace mineral may increase trace mineral status; however, no improvement in developing heifer performance or reproductive success was observed.
ACKNOWLEDGMENTS
The authors would like to thank Multimin USA for partial funding for this project and the staff at the University of Illinois Dixon Springs Agricultural Center, Simpson, IL, for care of the experimental animals and aiding in collection of data.
LITERATURE CITED
- Anderson K. J., LeFever D. G., Brinks J. S., and Odde K. G.. 1991. The use of reproductive tract scoring in beef heifers. Agri-Practice 12:19–26. [Google Scholar]
- AOAC. 1988. Official method 975.03: metals in plants and pet foods. Atomic absorption spectrophotometric method. In: Official methods of analysis. 13th ed. Assoc. Off. Anal. Chem., Gaithersburg, MD. [Google Scholar]
- Arthington J. D., Moriel P., Martins P. G., Lamb G. C., and Havenga L. J.. 2014. Effects of trace mineral injections on measures of performance and trace mineral status of pre- and postweaned beef calves. J. Anim. Sci. 92:2630–2640. doi: 10.2527/jas.2013-7164 [DOI] [PubMed] [Google Scholar]
- Arthington J. D., and Swenson C. K.. 2004. Effects of trace mineral source and feeding method on the productivity of grazing Braford cows. Prof. Anim. Sci. 20:155–161. doi: 10.15232/S1080-7446(15)31290-0 [DOI] [Google Scholar]
- Bernard J. K., Chestnut A. B., Erickson B. H., and Kelly F. M.. 1993. Effects of prepartum consumption of endopyte-infested tall fescue on serum prolactin and subsequent milk production of Holstein cows. J. Dairy Sci. 76:1928–1933. doi: 10.3168/jds.S0022-0302(93)77526-8 [DOI] [Google Scholar]
- Brasche C. J. 2015. Effect of a trace mineral injection on beef cattle performance. Master Thesis. Univ. of Nebraska–Lincoln, Lincoln, NE. [Google Scholar]
- Engle T. E., and Spears J. W.. 2000. Effects of dietary copper concentration and source on performance and copper status of growing and finishing steers. J. Anim. Sci. 78:2446–2451. doi: 10.2527/2000.7892446x [DOI] [PubMed] [Google Scholar]
- FASS 2010. Guide for the care and use of agricultural animals in agricultural research and teaching. Consortium for developing a guide for the care and use of agricultural research and teaching. Association Headquarters, Champaign, IL. [Google Scholar]
- Funston R. N., Martin J. L., Larson D. M., and Roberts A. J.. 2012. Physiology and endocrinology symposium: nutritional aspects of developing replacement heifers. J. Anim. Sci. 90:1166–1171. doi: 10.2527/jas.2011-4569 [DOI] [PubMed] [Google Scholar]
- Gadberry M. S., and Baldridge B.. 2013. Response of Angus cows and their suckling calves to an injectable trace mineral supplement. In: Arkansas Animal Science Department Report 2013. Rep. No. 612. Univ. of Arkansas, Fayetteville: p. 30–32. [Google Scholar]
- Gonzalez-Maldonado J., Rangel-Santos R., Rodríguez-de Lara R., and García-Peña O.. 2017. Effect of injectable trace mineral complex supplementation on development of ovarian structures and serum copper and zinc concentrations in over-conditioned Holstein cows. Anim. Reprod. Sci. 181:57–62. doi: 10.1016/j.anireprosci.2017.03.015 [DOI] [PubMed] [Google Scholar]
- Grings E. E., Staigmiller R. B., Short R. E., Bellows R. A., and MacNeil M. D.. 1999. Effects of stair-step nutrition and trace mineral supplementation on attainment of puberty in beef heifers of three sire breeds. J. Anim. Sci. 77:810–815. doi: 10.2527/1999.774810x [DOI] [PubMed] [Google Scholar]
- Gunn P. J., Schoonmaker J. P., Lemenager R. P., and Bridges G. A.. 2015. Feeding distiller’s grains as an energy source to gestating and lactating beef heifers: impact on female progeny growth, puberty attainment, and reproductive processes. J. Anim. Sci. 93:746–757. doi: 10.2527/jas.2014-8130 [DOI] [PubMed] [Google Scholar]
- Hackbart K. S., Ferreira R. M., Dietsche A. A., Socha M. T., Shaver R. D., Wiltbank M. C., and Fricke P. M.. 2010. Effect of dietary organic zinc, manganese, copper, and cobalt supplementation on milk production, follicular growth, embryo quality, and tissue mineral concentrations in dairy cows. J. Anim. Sci. 88:3856–3870. doi: 10.2527/jas.2010-3055 [DOI] [PubMed] [Google Scholar]
- Hansen S. L., Spears J. W., Lloyd K. E., and Whisnant C. S.. 2006. Growth, reproductive performance, and manganese status of heifers fed varying concentrations of manganese. J. Anim. Sci. 84:3375–3380. doi: 10.2527/jas.2005-667 [DOI] [PubMed] [Google Scholar]
- Hidiroglou M. 1979. Trace element deficiencies and fertility in ruminants: a review. J. Dairy Sci. 62:1195–1206. doi: 10.3168/jds.S0022-0302(79)83400-1 [DOI] [PubMed] [Google Scholar]
- Kincaid R. L. 2000. Assessment of trace mineral status of ruminants: a review. J. Anim. Sci. 77(E-Suppl):1–10. doi: 10.2527/jas2000.77E-Suppl1x [DOI] [Google Scholar]
- Lamb G. C., Brown D. R., Larson J. E., Dahlen C. R., Dilorenzo N., Arthington J. D., and Dicostanzo A.. 2008. Effect of organic or inorganic trace mineral supplementation on follicular response, ovulation, and embryo production in superovulated Angus heifers. Anim. Reprod. Sci. 106:221–231. doi: 10.1016/j.anireprosci.2007.04.007 [DOI] [PubMed] [Google Scholar]
- Miller W. J. 1975. New concepts and developments in metabolism and homeostasis of inorganic elements in dairy cattle. A review. J. Dairy Sci. 58:1549–1560. doi: 10.3168/jds.S0022-0302(75)84751-5 [DOI] [PubMed] [Google Scholar]
- Mundell L. R., Jaeger J. R., Waggoner J. W., Stevenson J. S., Grieger D. M., Pacheco L. A., Bolte J. W., Aubel N. A., Eckerle G. J., Macek M. J.,. et al. 2012. Effects of prepartum and postpartum bolus injections of trace minerals on performance of beef cows and calves grazing native range. Prof. Anim. Sci. 28:82–88. doi: 10.15232/S1080-7446(15)30318-1 [DOI] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine 2016. Nutrient requirements of beef cattle. 8th rev. ed. Natl. Acad. Press, Washington, DC. [Google Scholar]
- National Research Council 2001. Nutrient requirements of dairy cattle. 7th rev. ed. Natl. Acad. Sci, Washington, DC. [Google Scholar]
- Pogge D. J., Richter E. L., Drewnoski M. E., and Hansen S. L.. 2012. Mineral concentrations of plasma and liver after injection with a trace mineral complex differ among Angus and Simmental cattle. J. Anim. Sci. 90:2692–2698. doi: 10.2527/jas.2012-4482 [DOI] [PubMed] [Google Scholar]
- Reis M. M., Cooke R. F., Cappellozza B. I., Marques R. S., Guarnieri Filho T. A., Rodrigues M. C., Bradley J. S., Mueller C. J., Keisler D. H., Johnson S. E., et al. 2015. Creep-feeding to stimulate metabolic imprinting in nursing beef heifers: impacts on heifer growth, reproductive and physiological variables. Animal 9:1500–1508. doi: 10.1017/S1751731115000828 [DOI] [PubMed] [Google Scholar]
- Saker K. E., Allen V. G., Kalnitsky J., Thatcher C. D., Swecker W. S. Jr, and Fontenot J. P.. 1998. Monocyte immune cell response and copper status in beef steers that grazed endophyte-infected tall fescue. J. Anim. Sci. 76:2694–2700. doi: 10.2527/1998.76102694x [DOI] [PubMed] [Google Scholar]
- Stokes R. S., Ralph A. R., Mickna A. J., Chapple W. P., Schroeder A. R., Ireland F. A., and Shike D. W.. 2017. Effect of an injectable trace mineral at the initiation of a 14 day CIDR protocol on heifer performance and reproduction. Transl. Anim. Sci. 1. doi: 10.2527/tas2017.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoszek M. J., Oldfield J. E., Carter G. E., and Weswig P. H.. 1979. Effect of tall fescue and quackgrass on copper metabolism and weight gains of cattle. J. Anim. Sci. 48:893–899. doi: 10.2527/jas1979.484893x [DOI] [Google Scholar]
- Sunde R. A. 1997. Selenium. In: O’Dell B. L. and Sunde R. A., editors, Handbook of nutritionally essential mineral elements. Marcel Dekker Inc, New York: p. 335–356. [Google Scholar]
- Suttle N. F. 2010. The mineral nutrition of livestock. 4th ed. CABI Publishing, New York. [Google Scholar]
- Tucker H. A. 2000. Symposium: hormonal regulation of milk synthesis hormones, mammary growth, and lactation: a 41-year perspective. J. Dairy Sci. 83:874–884. doi: 10.3168/jds.S0022-0302(00)74951-4 [DOI] [PubMed] [Google Scholar]
- Vanegas J. A., Reynolds J., and Atwill E. R.. 2004. Effects of an injectable trace mineral supplement on first-service conception rate of dairy cows. J. Dairy Sci. 87:3665–3671. doi: 10.3168/jds.S0022-0302(04)73505-5 [DOI] [PubMed] [Google Scholar]
- Wagner J. J., Lusby K. S., Oltjen J. W., Rakestraw J., Wettemann R. P., and Walters L. E.. 1988. Carcass composition in mature Hereford cows: estimation and effect on daily metabolizable energy requirement during winter. J. Anim. Sci. 66:603–612. doi: 10.2527/jas1988.663603x [DOI] [PubMed] [Google Scholar]
- Wahlen R., Evans L., Turner J., and Hearn R.. 2005. The use of collision/reaction cell ICP-MS for the determination of elements in blood and serum samples. Spectroscopy. 20:84–89. [Google Scholar]