Abstract
Objective: We compared the clinical course of type 2 diabetic patients whose basal insulin preparations were replaced from insulin glargine (IGlar) 100 units/mL (U100) to IGlar biosimilar or IGlar 300 units/mL (U300).
Methods: After propensity score matching, 34 patients whose basal insulin preparation was switched from IGlar U100 to IGlar biosimilar and 102 switched to IGlar U300 were observed for 6 months.
Results: The HbA1c level and body weight did not change significantly after the replacement in the IGlar biosimilar or IGlar U300 groups. In the IGlar biosimilar group, the frequency of subjects who experienced hypoglycemia after the replacement (12%) was not different from before (12%). However, the frequency was significantly lower after the replacement (2%) than before (13%) in the IGlar U300 group. The change in the HbA1c level after the replacement showed a significant association with the HbA1c level at the baseline but not with the kind of IGlar. Hypoglycemia was frequently observed in subjects who had experienced hypoglycemia before the replacement.
Conclusions: IGlar biosimilar and IGlar U300 induced similar HbA1c and body weight changes among type 2 diabetic patients. IGlar biosimilar is a suitable option for patients with a low risk for hypoglycemia.
Keywords: Insulin glargine, biosimilar, hypoglycemia, type 2 diabetes mellitus
Introduction
Insulin glargine (IGlar), a long-acting basal insulin, is a human insulin analogue manufactured using recombinant DNA technology. It has been available since 2003 as Lantus (Sanofi K. K., Tokyo, Japan) in Japan and is widely prescribed for patients with types 1 and 2 diabetes. After the patent of this drug expired, the biosimilar insulin LY2963016 (Eli Lilly Japan K. K., Kobe, Japan), which has an identical amino acid sequence and the same pharmaceutical form and immunogenicity as IGlar [1,2], was admitted to the market of Japan in August 2015. A new IGlar comprising 300 U/mL (IGlar U300, Lantus XR; Sanofi K. K.) was then released in September of the same year. IGlar U300 is reported to reduce the risk of hypoglycemia in patients with types 1 and 2 diabetes compared with Lantus comprising 100 U/mL (IGlar U100) [3–13], as the pharmacokinetic and pharmacodynamic action profiles of IGlar U300 are more constant and longer than those of IGlar U100 [14].
IGlar biosimilar is also cheaper than IGlar U100 and IGlar U300 (Toujeo) in the United States [15]. Based on Japanese medical insurance system, the prices of IGlar BS Inj. [Lilly], which is IGlar biosimilar, Lantus and Lantus XR were 1481 yen/kit (300 U), 1936 yen/kit (300 U), and 2933 yen/kit (450 U), which is 1955 yen/300 U, respectively, as of April 2018. Although IGlar biosimilar and IGlar U300 are selected frequently instead of IGlar U100 when using IGlar as basal insulin, no study has compared the effects of IGlar biosimilar and IGlar U300 in patients with diabetes in actual clinical practice.
In this retrospective study, the clinical courses of patients with type 2 diabetes whose basal insulin preparation was switched from IGlar U100 to IGlar biosimilar or IGlar U300 were determined. We feel that our findings indicate which subjects are most suitable for using IGlar biosimilar after IGlar U100.
Methods
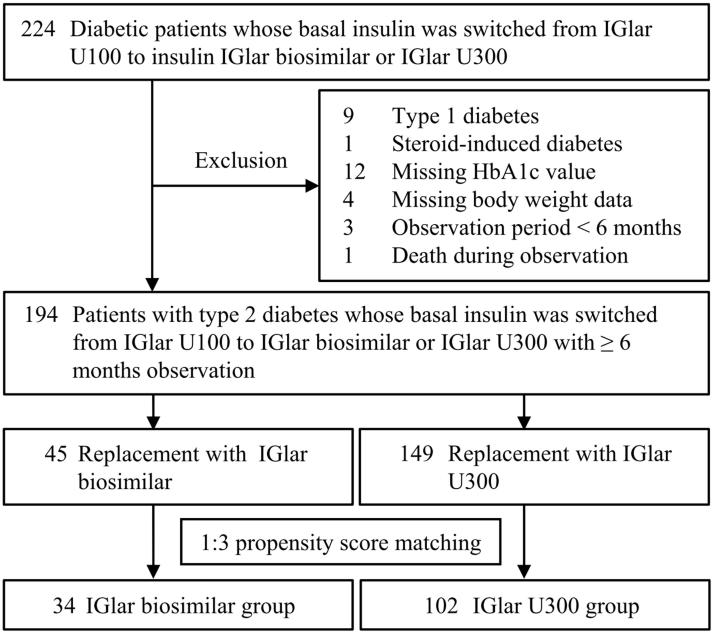
The flowchart of patients’ selection is shown in Figure 1. Initially, 224 patients with type 2 diabetes whose basal insulin was switched from IGlar U100 to IGlar biosimilar or IGlar U300 at the Department of Diabetes, Metabolism and Kidney Disease between April 2016 and April 2017 were eligible for this study. The patients with type 1 diabetes or steroid-induced diabetes, those in whom the HbA1c value or body weight had not been evaluated at the replacement, and those with observation periods of less than 6 months, including one dead case, were excluded from the analysis.
Figure 1.
Flowchart of patient selection. IGlar: insulin glargine, U100: 100 U/mL, and U300: 300 U/mL.
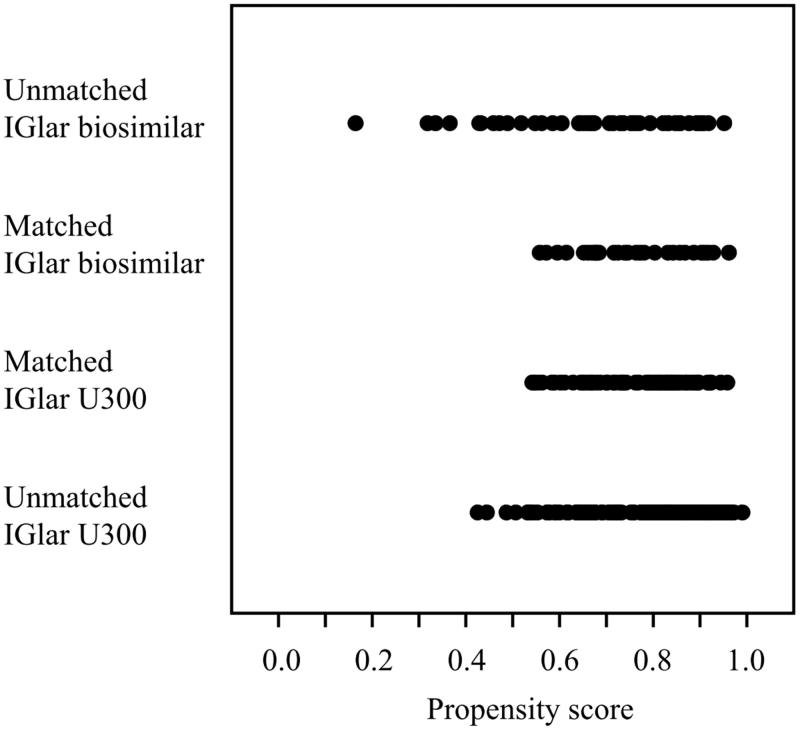
The subjects were divided into two groups (IGlar biosimilar group, n = 45 and IGlar U300 group, n = 149) based on the replacement IGlar administered. To guarantee the validity of the retrospective analysis, a propensity score was applied for 1:3 matching between the IGlar biosimilar and IGlar U300 groups. The propensity scores for the IGlar administered were calculated using a logistic analysis including the following covariates: sex, age, duration of diabetes, body mass index (BMI), dose of basal insulin and bolus insulin, HbA1c level, occurrence of hypoglycemia within the past 6 months, use of metformin, and use of dipeptidyl peptidase-4 (DPP-4) inhibitors. The distributions of propensity scores before and after the matching are illustrated in Figure 2. Based on the score of each subject, patients whose score fell within 0.03 of each other were selected at a 1:3 ratio to compare the clinical courses between the IGlar biosimilar and IGlar U300 groups. In total, 34 patients who received IGlar biosimilar and 102 who received IGlar U300 were investigated in this study.
Figure 2.
Distribution of propensity scores before and after propensity matching. IGlar: insulin glargine and U300: 300 U/mL.
The replacement IGlar administered and insulin dose in each subject were decided by the attending physician at each hospital visit. The clinical parameters and occurrence of hypoglycemia were determined retrospectively based on the medical records of the patients. Hypoglycemia was defined as blood glucose ≤3.9 mmol/L (≤70 mg/dL) or a sign or symptom associated with hypoglycemia [16].
In this study, we investigated the change in the HbA1c level for 6 months after switching from IGlar U100 to IGlar biosimilar or IGlar U300 as the primary outcome measure. The secondary outcome measure was the change in the frequency of hypoglycemia.
Ethical approval
The study was conducted in accordance with the principles expressed in the 2008 Declaration of Helsinki. The Ethics Committee of Edogawa Hospital approved the study protocol and waived the need for written informed consent because the data were analyzed anonymously for this retrospective analysis based on the information stored in the hospital (Approval number: 2018-4, approved date: April 5, 2018).
Statistical analysis
All of the data are presented as the mean ± standard deviations. The χ2 test was used for between-group comparisons of categorical variables. None of the continuous variables (age, duration of diabetes, body mass index, body weight, insulin dose, HbA1c level and number of concomitant agents) showed normal distribution in the Shapiro-Wilk tests. Thus, Wilcoxon’s signed rank test was assessed significance of differences in the continuous variables. Wilcoxon’s signed rank test was used to determine the presence of differences in the data for the BMI, body weight, dose of insulin, and HbA1c level during the observation period compared with the baseline values. A least squares model was used to evaluate the associations between the changes in the HbA1c levels and the clinical background factors of the patients. The odds ratios and respective 95% confidence intervals were determined to examine the strength of the relationship between the clinical characteristics of the patients and the occurrence of hypoglycemia after the replacement of IGlar U100 by a logistic regression analysis. A multivariate analysis was performed using only the factors that demonstrated a significant or marginal association (p < .10) with the changes in the HbA1c levels or the occurrence of hypoglycemia. Differences with a p < .05 (two-tailed) were considered to be statistically significant. The statistical software package JMP version 8.0.1 (SAS Institute, Cary, NC) was used to perform all analyses.
Results
The patients’ clinical and laboratory characteristics are shown in Table 1. After the propensity score matching, the clinical characteristics were not significantly different between the IGlar biosimilar and IGlar U300 groups. The other antidiabetic agents did not differ between the two groups. The subjects who had experienced hypoglycemia within the past 6 months before the replacement of IGlar U100 accounted for 12% and 13% of the IGlar biosimilar and IGlar U300 groups, respectively.
Table 1.
Clinical characteristics of the patients at the baseline.
| Before matching |
After matching |
|||||
|---|---|---|---|---|---|---|
| IGlar biosimilar | IGlar U300 | p | IGlar biosimilar | IGlar U300 | ||
| (n = 45) | (n = 149) | (n = 34) | (n = 102) | p | ||
| Male subjects (%) | 67 | 62 | .55 | 62 | 63 | .92 |
| Age (years) | 61 ± 12 | 67 ± 12 | <.01 | 65 ± 11 | 65 ± 12 | .87 |
| Duration of diabetes (years) | 12 ± 9 | 16 ± 11 | .03 | 14 ± 8 | 15 ± 10 | .99 |
| Body mass index (kg/m2) | 25.8 ± 5.6 | 25.4 ± 4.2 | .92 | 25.2 ± 5.3 | 25.9 ± 4.2 | .42 |
| Body weight (kg) | 69.1 ± 17.1 | 66.0 ± 13.7 | .44 | 65.9 ± 15.5 | 67.9 ± 13.7 | .29 |
| Dose of basal insulin (unit/day) | 10.6 ± 5.8 | 13.7 ± 7.6 | .01 | 11.6 ± 6.2 | 12.0 ± 6.3 | .67 |
| Dose of bolus insulin (unit/day) | 7.1 ± 8.6 | 10.0 ± 11.1 | .16 | 8.6 ± 9.2 | 8.7 ± 10.0 | .95 |
| Dose of total insulin (unit/day) | 17.7 ± 11.9 | 23.7 ± 14.6 | .01 | 20.2 ± 12.4 | 20.7 ± 13.4 | .96 |
| HbA1c (%) | 7.8 ± 1.1 | 8.1 ± 1.4 | .28 | 7.9 ± 1.1 | 7.9 ± 1.2 | .68 |
| Hypoglycemia within the past 6 months (%) | 9 | 16 | .21 | 12 | 13 | .88 |
| Concomitant anti-diabetic agents (%) | ||||||
| Sulfonylureas | 2 | 3 | .86 | 3 | 4 | .79 |
| Biguanides | 38 | 29 | .26 | 32 | 33 | .92 |
| Thiazolidinediones | 4 | 2 | .40 | 3 | 3 | .00 |
| α-glucosidase inhibitors | 11 | 9 | .55 | 12 | 8 | .50 |
| DPP-4 inhibitors | 56 | 41 | .08 | 47 | 46 | .92 |
| SGLT2 inhibitors | 16 | 10 | .16 | 12 | 10 | .75 |
| GLP-1 receptor agonists | 7 | 10 | .48 | 6 | 10 | .47 |
| Number of concomitant anti-diabetic agents | 1.4 ± 1.1 | 1.0 ± 0.8 | .05 | 1.2 ± 1.1 | 1.1 ± 0.8 | .98 |
Abbreviations. IGlar, insulin glargine; DPP-4, dipeptidyl peptidase-4; SGLT2, sodium glucose co-transporter 2; GLP-1, glucagon-like peptide-1.
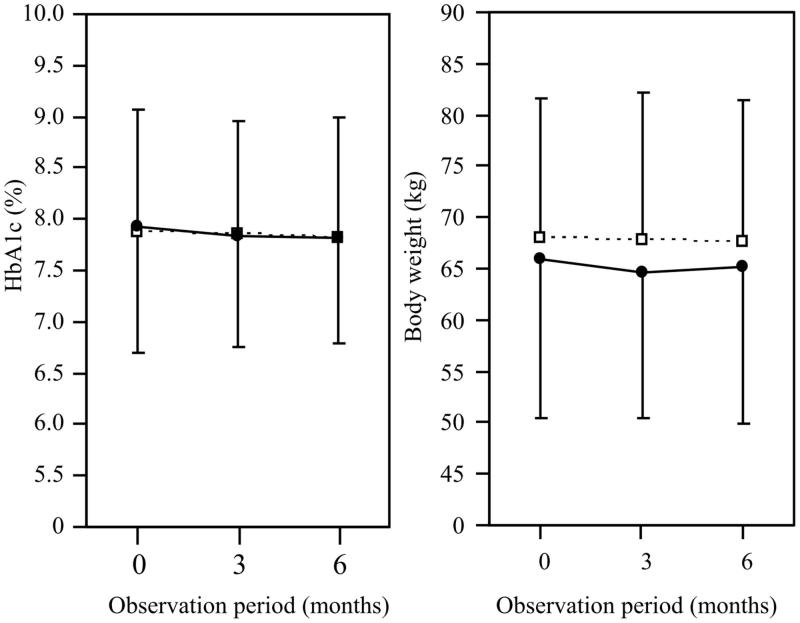
Figure 3 shows the changes in the HbA1c levels and body weight during the observation period. The HbA1c level and body weight did not change significantly during the observation period in either the IGlar biosimilar or IGlar U300 group. Table 2 shows the changes in the clinical parameters. In the IGlar biosimilar group, the doses of basal and bolus insulin and the frequency of subjects who experienced hypoglycemia after the replacement were not significantly different from those before the replacement. In the IGlar U300 group, the frequency of subjects who experienced hypoglycemia was significantly reduced after the replacement of IGlar U100 compared with that before, although the dose of basal insulin was significantly increased. In the patients before the propensity score matching, the change in the HbA1c level did not significantly differ between IGlar biosimilar (–0.0 ± 1.0%, n = 45) and IGlar U300 (–0.1 ± 1.0%, n = 149) groups. Hypoglycemia after the replacement of IGlar U100 was found in 9% in the IGlar biosimilar group and 3% in the IGlar U300 group (p = .15).
Figure 3.
Changes in the HbA1c levels and body weight during the observation period. Closed circles and open squares indicate glargine biosimilar and glargine U300, respectively.
Table 2.
Changes in the clinical parameters during the observation period.
| IGlar biosimilar (n = 34) |
IGlar U300 (n = 102) |
|||||
|---|---|---|---|---|---|---|
| Baseline | 6 months | p | Baseline | 6 months | p | |
| Body mass index (kg/m2) | 25.2 ± 5.3 | 24.9 ± 5.3 | .17 | 25.9 ± 4.2 | 25.7 ± 4.3 | .16 |
| Body weight (kg) | 65.9 ± 15.5 | 65.1 ± 15.2 | .18 | 67.9 ± 13.7 | 67.6 ± 13.8 | .16 |
| Dose of basal insulin (unit/day) | 11.6 ± 6.2 | 11.6 ± 6.9 | .91 | 12.0 ± 6.3 | 12.8 ± 7.3 | <.01 |
| Dose of bolus insulin (unit/day) | 8.6 ± 9.2 | 7.7 ± 9.1 | .12 | 8.7 ± 10.0 | 8.5 ± 9.6 | .50 |
| Dose of total insulin (unit/day) | 20.2 ± 12.4 | 19.3 ± 12.6 | .30 | 20. ±13.4 | 21.3 ± 14.1 | .18 |
| HbA1c (%) | 7.9 ± 1.1 | 7.8 ± 1.0 | .48 | 7.9 ± 1.2 | 7.8 ± 1.2 | .43 |
| Hypoglycemia within the past 6 months (%) | 12 | 12 | 1.00 | 13 | 2 | <.01 |
| Addition of anti-diabetic agents (%) | – | 6 | – | 6 | ||
| Decrease of anti-diabetic agents (%) | – | 12 | – | 9 | ||
Abbreviations. IGlar, insulin glargine; U300, 300 U/mL.
Table 3 shows the association between the change in HbA1c level and the clinical characteristic at the baseline. The change in the HbA1c level was not associated with the kind of IGlar. Based on a multivariate analysis of a least squares model, the change in the HbA1c level after the replacement showed a significantly weak negative association with the HbA1c level at the baseline. In the patients before the propensity score matching (n = 194), the change in the HbA1c level was significantly associated with only the HbA1c level at the baseline (correlation coefficient = –0.310, p < .01).
Table 3.
Association between the change in the HbA1c level and the clinical characteristics at the baseline.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| Correlation coefficient | p | Correlation coefficient | p | |
| Male sex | –0.143 | .40 | ||
| Age (year) | 0.013 | .05 | 0.003 | .62 |
| Duration of diabetes (year) | 0.009 | .26 | ||
| Body mass index (kg/m2) | –0.004 | .81 | ||
| Body weight (kg) | –0.005 | .35 | ||
| Dose of basal insulin (unit/day) | –0.008 | .53 | ||
| Dose of bolus insulin (unit/day) | –0.004 | .64 | ||
| Dose of total insulin (unit/day) | –0.004 | .52 | ||
| HbA1c at the baseline (%) | –0.347 | <.01 | –0.337 | <.01 |
| Hypoglycemia within the past 6 months before the replacement | –0.306 | .21 | ||
| Replacement to IGlar U300 | 0.051 | .79 | ||
Abbreviations. IGlar, insulin glargine; U300, 300 U/mL.
Table 4 shows the odds ratios of the clinical characteristics at the baseline with the occurrence of hypoglycemia after the replacement of IGlar U100. We first performed univariate analyses of the baseline characteristics and hypoglycemia and then included the factors that demonstrated a significant or marginal association according to a multivariate analysis. Hypoglycemia was frequently found in the subjects who had experienced hypoglycemia within the past 6 months before the replacement and was less frequently seen in the IGlar U300 group. In the patients before the propensity score matching (n = 194), hypoglycemia was also frequently found in those who had experienced hypoglycemia before the replacement (OR = 16.55, 95% CI = 3.34–109.9, p = .04)) and was less frequently found in the IGlar U300 group (OR = 0.19, 95% CI = 0.03–0.96, p = .04) based on a multivariate logistic regression analysis. Severe hypoglycemia, defined as an event requiring the assistance of another person to actively administer carbohydrates, glucagon, or take other corrective actions [16], was not noted during the observation period in either the IGlar biosimilar or IGlar U300 group.
Table 4.
Odds ratios of the clinical characteristics at the baseline with the occurrence of hypoglycemia after the replacement of insulin glargine 100 U/mL.
| Univariate |
Multivariate |
|||
|---|---|---|---|---|
| OR [95% CI] | p | OR [95% CI] | p | |
| Male sex | 0.28 [0.04–1.51] | .14 | ||
| Age (years) | 1.00 [0.93–1.07] | .92 | ||
| Duration of diabetes (years) | 0.97 [0.87–1.06] | .54 | ||
| Body mass index (kg/m2) | 0.86 [0.68–1.05] | .15 | ||
| Body weight (kg) | 0.94 [0.87–1.01] | .08 | 0.97 [0.90–1.04] | .39 |
| Dose of basal insulin (unit/day) | 0.96 [0.81–1.09] | .52 | ||
| Dose of bolus insulin (unit/day) | 1.03 [0.95–1.11] | .35 | ||
| Dose of total insulin (unit/day) | 1.01 [0.95–1.07] | .70 | ||
| HbA1c at the baseline (%) | 1.03 [0.47–1.98] | .94 | ||
| Hypoglycemia within the past 6 months before the replacement | 8.29 [1.42–48.73] | .02 | 8.04 [1.12–63.12] | .04 |
| Replacement to IGlar U300 | 0.15 [0.02–0.81] | .03 | 0.14 [0.02–0.84] | .03 |
Abbreviations. OR, odds ratio; CI, confidence interval; IGlar U300, insulin glargine 300 U/mL.
Discussion
The switching of IGlar U100 to IGlar biosimilar or IGlar U300 resulted in similar trends in the HbA1c level and body weight in patients with type 2 diabetes in this study. The frequency of subjects who experienced hypoglycemia was significantly lower in the IGlar U300 group than in the IGlar biosimilar group. It has been reported that IGlar U300 is as effective for glycemic control as IGlar U100 and superior in reducing hypoglycemia according to phase 3 clinical trials performed as multi-center, open-label, and parallel-group studies [3–13]. The daily blood glucose variability assessed using continuous glucose monitoring (CGM) system was also improved by IGlar 300 treatment compared with IGlar 100 in patients with types 1 [17] and 2 diabetes [18]. Because IGlar biosimilar has an identical amino acid sequence and the same pharmaceutical form as IGlar [1], both IGlar biosimilar and IGlar U100 demonstrated similar glucose control with similar safety profiles in patients with types 1 and 2 diabetes [19–21]. Yamada et al. reported that the meta-analysis of randomized controlled trials found no differences between long-term biosimilar (LY2963016 and MK-1293, Merck and Co., NJ) and originator insulin (IGlar U100) with regard to reduction in HbA1c and fasting blood glucose, hypoglycemia, mortality, injection site reactions, insulin antibodies, and allergic reactions [22]. Therefore, the findings in this study are not surprising. However, obtaining similar results in a real-world setting in patients with type 2 diabetes who develop variable adherence patterns in insulin treatment [23,24] is important. In their randomized crossover study using CGM system in 30 patients with type 2 diabetes, Takeishi et al. reported that the morning administration of insulin glulisine and IGlar U300 reduced the pre- and post-breakfast glucose levels and daily blood glucose variability without causing hypoglycemia compared with the morning administration of insulin lispro and IGlar biosimilar [25].
The body weight gain has been shown to be suppressed in diabetic patients treated with IGlar U300 compared with those treated with IGlar U100 [3–13]. In this study, the change in the body weight did not differ significantly between the IGlar biosimilar and IGlar U300 groups. This may have been caused by the lack of an increase in the dose of basal insulin in the IGlar biosimilar group, in contrast to the significant increase in the dose of IGlar U300 after the replacement.
One advantage associated with IGlar biosimilar is its relatively cheap price, as described above. The cost-effectiveness of antidiabetic agents is an important consideration in a patient-centered approach to diabetes care [15]. The median cost in the United States is US$253 and US$298 per 1000 units of IGlar biosimilar and IGlar U100/U300, respectively [15]. Therefore, IGlar biosimilar is a suitable option for patients with a low risk of hypoglycemia. We previously investigated the frequency of hypoglycemia using the CGM in type 2 diabetic patients undergoing basal-bolus insulin therapy. Although hypoglycemia was observed in more than 50% of the subjects, it was significantly less frequent in the patients using more than 40% of basal insulin in the total insulin dose [26]. Tamaki et al. [27] and Yokoyama et al. [28] also found that increasing the dose of basal insulin without changing the total insulin dose resulted in significantly better glycemic control without an increase in the frequency of hypoglycemia in type 2 diabetes on basal-bolus insulin therapy. Furthermore, prandial bolus insulin injected three times daily resulted in better HbA1c control in patients with type 2 diabetes than basal insulin therapy added to metformin and sulfonylurea but was associated with a greater risk of hypoglycemia [29]. Type 2 diabetic patients receiving bolus insulin therapy as the initial step of insulin therapy or those using basal insulin and a relatively small amount of bolus insulin are deemed most suited to receiving IGlar biosimilar treatment, considering the risk of hypoglycemia.
Several limitations associated with this study warrant mention. First, this study was a retrospective observational investigation of a relatively small number of patients. We must consider that changes in the clinical parameters and the association between hypoglycemia and IGlar may have occurred incidentally due to the low statistical power of the study. However, the patients’ background characteristics were matched using propensity scores, and we believe that the results obtained in this study will prove useful when considering switching basal insulin from IGlar U100. Further investigations in a larger number of patients should therefore be performed to confirm our results. Second, hypoglycemia was retrospectively evaluated based on the medical record. Given that the detection sensitivity of hypoglycemia might be inferior to reality, the occurrence of hypoglycemia is considered to have been underestimated in this study. Furthermore, nocturnal hypoglycemia was not determined although the previous phase 3 clinical trials comparing the IGlar U100 and IGlar U300 showed the reduction in hypoglycemia at any time of day and during the night in the subjects administered IGlar U300 [3–13]. A prospective study will be necessary for accurately comparing the frequency of hypoglycemia, especially during the night, between the IGlar biosimilar and IGlar U300 groups.
Conclusions
Upon switching basal insulin from IGlar U100 to IGlar biosimilar or IGlar U300, patients can expect similar HbA1c and body weight changes. Patients in whom hypoglycemia is already a problem before switching should be switched to IGlar U300. IGlar biosimilar is a suitable option for patients with a low risk of hypoglycemia.
Transparency
Declaration of funding
There is no funding to report for this study.
Declaration of financial/other relationships
Hiroyuki Ito has received lecture fees from Sanofi KK, Eli Lilly Japan KK, Novo Nordisk Pharma Ltd., MSD KK, Novartis Pharma KK, Takeda Pharmaceutical Company, Astellas Pharma, Daiichi Sankyo Company, Boehringer Ingelheim, Terumo Corporation, Mochida Pharmaceuticals, Teijin Pharma, Kissei Pharmaceuticals, Kowa Pharmaceuticals, Mitsubishi Tanabe Pharma Corporation, Sanwa Kagaku Kenkyusho, Dainippon Sumitomo Pharma, AstraZeneca KK, Kyowa Hakko Kirin, Shionogi and Co, Taisho Toyama Pharmaceutical Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Bayer Yakuhin, Ltd. Shigenori Ando has received lecture fees from Takeda Pharmaceutical Company. Suzuko Matsumoto has received lecture fees from Novo Nordisk Pharma Ltd., Astellas Pharma, and AstraZeneca KK. Shinya Nishio has received lecture fees from Sanofi KK, Taisho Toyama Pharmaceutical Co., Ltd., Kyowa Hakko Kirin, Bayer Yakuhin, Ltd., and Mitsubishi Tanabe Pharma Corporation. Shinichi Antoku has received lecture fees from Kyowa Hakko Kirin, Sanofi KK, Kyowa Hakko Kirin, Taisho Toyama Pharmaceutical Co., Ltd., Daiichi Sankyo Company, and Otsuka Pharmaceutical Co., Ltd.
Acknowledgments
The authors thank Tomoko Koyanagi in the secretarial section of Edogawa Hospital for her valuable help with data collection.
References
- 1.Linnebjerg H, Lam EC, Seger ME, et al. . Comparison of the pharmacokinetics and pharmacodynamics of LY2963016 insulin glargine and EU- and US-approved versions of Lantus insulin glargine in healthy subjects: three randomized euglycemic clamp studies. Diabetes Care. 2015;38:2226–2233. [DOI] [PubMed] [Google Scholar]
- 2.Ilag LL, Deeg MA, Costigan T, et al. . Evaluation of immunogenicity of LY2963016 insulin glargine compared with Lantus® insulin glargine in patients with type 1 or type 2 diabetes mellitus. Diabetes Obes Metab. 2016;18:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riddle MC, Bolli GB, Ziemen M, et al. . Edition 1. Study investigators. New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using basal and mealtime insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (edition 1). Diabetes Care. 2014;37:2755–2762. [DOI] [PubMed] [Google Scholar]
- 4.Riddle MC, Yki-Järvinen H, Bolli GB, et al. . One-year sustained glycaemic control and less hypoglycaemia with new insulin glargine 300 U/ml compared with 100 U/ml in people with type 2 diabetes using basal plus meal-time insulin: the edition 1 12-month randomized trial, including 6-month extension. Diabetes Obes Metab. 2015;17:835–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yki-Järvinen H, Bergenstal R, Ziemen M, et al. . New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 2 diabetes using oral agents and basal insulin: glucose control and hypoglycemia in a 6-month randomized controlled trial (edition 2). Diabetes Care. 2014;37:3235–3243. [DOI] [PubMed] [Google Scholar]
- 6.Yki-Järvinen H, Bergenstal RM, Bolli GB, et al. . Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the edition 2 randomized 12-month trial including 6-month extension. Diabetes Obes Metab. 2015;17:1142–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bolli GB, Riddle MC, Bergenstal RM, et al. . New insulin glargine 300 U/ml compared with glargine 100 U/ml in insulin-naïve people with type 2 diabetes on oral glucose-lowering drugs: a randomized controlled trial (edition 3). Diabetes Obes Metab. 2015;17:386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolli GB, Riddle MC, Bergenstal RM, et al. . Edition 3 study investigators. Glycaemic control and hypoglycaemia with insulin glargine 300U/mL versus insulin glargine 100U/mL in insulin-naïve people with type 2 diabetes: 12-month results from the EDITION 3 trial. Diabetes Metab. 2017;43:351–358. [DOI] [PubMed] [Google Scholar]
- 9.Ritzel R, Roussel R, Bolli GB, et al. . Patient-level meta-analysis of the edition 1, 2 and 3 studies: glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus glargine 100 U/ml in people with type 2 diabetes. Diabetes Obes Metab. 2015;17:859–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Home PD, Bergenstal RM, Bolli GB, et al. . New insulin glargine 300 units/mL versus glargine 100 units/mL in people with type 1 diabetes: a randomized, phase 3a, open-label clinical trial (edition 4). Diabetes Care. 2015;38:2217–2225. [DOI] [PubMed] [Google Scholar]
- 11.Home PD, Bergenstal RM, Bolli GB, et al. . Glycaemic control and hypoglycaemia during 12 months of randomized treatment with insulin glargine 300 U/mL versus glargine 100 U/mL in people with type 1 diabetes (edition 4). Diabetes Obes Metab. 2018;20:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuhisa M, Koyama M, Cheng X, et al. . New insulin glargine 300 U/ml versus glargine 100 U/mL in Japanese adults with type 1 diabetes using basal and mealtime insulin: glucose control and hypoglycaemia in a randomized controlled trial (edition JP 1). Diabetes Obes Metab. 2016;18:375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terauchi Y, Koyama M, Cheng X, et al. . New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (edition JP 2). Diabetes Obes Metab. 2016;18:366–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Becker RH, Dahmen R, Bergmann K, et al. . New insulin glargine 300 Units mL−1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units mL−1. Diabetes Care. 2015;38:637–643. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2018. Diabetes Care. 2018;41:S73–S85. [DOI] [PubMed] [Google Scholar]
- 16.Seaquist ER, Anderson J, Childs B, et al. . Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013;36:1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergenstal RM, Bailey TS, Rodbard D, et al. . Comparison of insulin glargine 300 units/mL and 100 units/mL in adults with type 1 diabetes: continuous glucose monitoring profiles and variability using morning or evening Injections. Diabetes Care. 2017;40:554–560. [DOI] [PubMed] [Google Scholar]
- 18.Iuchi H, Sakamoto M, Matsutani D, et al. . The Durability of Basal Insulin Affects Day-to-day glycemic variability assessed by continuous glucose monitoring in type 2 diabetes patients: a randomized crossover trial. Diabetes Technol Ther. 2017;19:457–462. [DOI] [PubMed] [Google Scholar]
- 19.Blevins TC, Dahl D, Rosenstock J, et al. . Efficacy and safety of LY2963016 insulin glargine compared with insulin glargine (Lantus®) in patients with type 1 diabetes in a randomized controlled trial: the element 1 study. Diabetes Obes Metab. 2015;17:726–733. [DOI] [PubMed] [Google Scholar]
- 20.Rosenstock J, Hollander P, Bhargava A, et al. . Similar efficacy and safety of LY2963016 insulin glargine and insulin glargine (Lantus®) in patients with type 2 diabetes who were insulin-naïve or previously treated with insulin glargine: a randomized, double-blind controlled trial (the element 2 study). Diabetes Obes Metab. 2015;17:734–741. [DOI] [PubMed] [Google Scholar]
- 21.Hadjiyianni I, Dahl D, Lacaya LB, et al. . Efficacy and safety of LY2963016 insulin glargine in patients with type 1 and type 2 diabetes previously treated with insulin glargine. Diabetes Obes Metab. 2016;18:425–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamada T, Kamata R, Ishinohachi K, et al. . Biosimilar vs originator insulins: systematic review and meta-analysis. Diabetes Obes Metab. 2018;20:1787–1792. [DOI] [PubMed] [Google Scholar]
- 23.Peyrot M, Barnett AH, Meneghini LF, et al. . Insulin adherence behaviours and barriers in the multinational Global Attitudes of Patients and Physicians in Insulin Therapy study. Diabet Med. 2012;29:682–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brod M, Rana A, Barnett AH. Adherence patterns in patients with type 2 diabetes on basal insulin analogues: missed, mistimed and reduced doses. Curr Med Res Opin. 2012;28:1933–1946. [DOI] [PubMed] [Google Scholar]
- 25.Takeishi S, Tsuboi H, Takekoshi S. Comparison of morning basal +1 bolus insulin therapy (insulin glulisine + insulin glargine 300 U/mL vs insulin lispro + insulin glargine biosimilar) using continuous glucose monitoring: a randomized crossover study. J Diabetes Investig. 2018;9:91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito H, Abe M, Shinozaki M, et al. . Hypoglycemia observed during continuous glucose monitoring in patients with type 2 diabetes mellitus treated by subcutaneous insulin injection. Diabetes Technol Ther. 2013;15:586–590. [DOI] [PubMed] [Google Scholar]
- 27.Tamaki M, Shimizu T, Kanazawa A, et al. . Effects of changes in basal/total daily insulin ratio in type 2 diabetes patients on intensive insulin therapy including insulin glargine (JUN-LAN Study 6). Diabetes Res Clin Pract. 2008;81:e1–e3. [DOI] [PubMed] [Google Scholar]
- 28.Yokoyama H, Tada J, Kamikawa F, et al. . Efficacy of conversion from bedtime NPH insulin to morning insulin glargine in type 2 diabetic patients on basal-prandial insulin therapy. Diabetes Res Clin Pract. 2006;73:35–40. [DOI] [PubMed] [Google Scholar]
- 29.Holman RR, Thorne KI, Farmer AJ, et al. . Addition of biphasic, prandial, or basal insulin to oral therapy in type 2 diabetes. N Engl J Med. 2007;357:1716–1730. [DOI] [PubMed] [Google Scholar]