Abstract
Lymphodepleting preconditioning with high-dose chemotherapy is commonly used to increase the clinical efficacy of adoptive T cell therapy (ACT) strategies, however, with severe toxicity for patients. Conversely, oncolytic adenoviruses are safe and, when engineered to express interleukin-2 (IL-2) and tumor necrosis factor alpha (TNF-α), they can achieve antitumor immunomodulatory effects similar to lymphodepletion. Therefore, we compare the safety and efficacy of such adenoviruses with a cyclophosphamide- and fludarabine-containing lymphodepleting regimen in the setting of ACT. Human adenovirus (Ad5/3-E2F-D24-hTNF-α-IRES-hIL-2; TILT-123) replication was studied using a Syrian hamster pancreatic tumor model (HapT1) infused with tumor-infiltrating lymphocytes (TILs). Using the oncolytic virus instead of lymphodepletion resulted in superior efficacy and survival. Immune cells responsive to TNF-α IL-2 were studied using an immunocompetent mouse melanoma model (B16.OVA) infused with ovalbumin-specific T (OT-I) cells. Here, the adenovirus approach improved tumor control together with increased intratumoral Th1 cytokine levels and infiltration of CD8+ T cells and CD86+ dendritic cells. Similar to humans, lymphodepleting preconditioning caused severe cytopenias, systemic inflammation, and damage to vital organs. Toxicity was minimal in adenovirus- and OT-I-treated mice. These findings demonstrate that ACT can be effectively facilitated by cytokine-coding adenovirus without requiring lymphodepletion, a rationale being clinically investigated.
Keywords: oncolytic adenovirus, adoptive T cell therapy, lymphodepleting preconditioning, interleukin-2, tumor necrosis factor alpha, chemotherapy, TIL therapy, TCR modified T cell therapy
Santos and colleagues show enhanced antitumor responses and major safety benefits in using cytokine-coding adenovirus therapy, rather than lymphodepleting preconditioning, to boost the efficacy of different adoptive T cell therapy (ACT) strategies. This study renders chemotherapy-based lymphodepleting regimens not required for ACT protocols utilizing cytokine-coding adenovirus.
Introduction
The use of adoptively transferred tumor-infiltrating lymphocytes (TILs), chimeric antigen receptor (CAR)-, or T cell receptor (TCR)-modified T cells to treat advanced malignancies has shown encouraging results in animal models and in humans.1, 2 In hematology, tisagenlecleucel (Kymriah) has been approved by the U.S. Food and Drug Administration (FDA) with outstanding results for the treatment of CD19+ acute lymphoblastic leukemia.3 In solid tumors, impressive results have been seen mostly in TIL treatment of melanoma, where efficacy requires both high-dose postconditioning interleukin-2 (IL-2) and high-dose preconditioning chemotherapy.4, 5 Without preconditioning, response rates to melanoma TIL therapy were 23%,6 while with lymphodepletion, a 51% response rate has been observed.5 Pre- and postconditioning are now universally used in TIL treatment of melanoma, and they are frequently used also in other forms of adoptive T cell therapy (ACT).1
It has been proposed that the depletion of endogenous host immune cells ensures the survival and proliferation of transferred cells that otherwise would be suppressed or deprived of key cytokines.7 Lymphodepleting preconditioning also acts as a modulator of the tumor microenvironment, enabling the infiltration and proliferation of effector cells and antigen-presenting cells (APCs). Thus, the many effects of lymphodepletion converge on decreasing tumor immunosuppression.7 The latter is thought to be the key obstacle to successful ACT of solid tumors, because the microenvironment of solid tumors can anergize, exclude, and inhibit the survival and proliferation of antitumor effector T cells.8, 9 The effects of a suppressive tumor microenvironment are not restricted to T cells but also act on other immune cell types, such as macrophages.10
Given the favorable clinical outcomes in various trials,4, 5, 11, 12, 13 lymphodepleting preconditioning has been integrated into ACT protocols, commonly consisting of a combination of chemotherapeutic agents.1 Yet, the increase in antitumor efficacy observed in preconditioned patients comes at a cost. Most patients experience severe and even life-threatening toxicities, such as opportunistic infections or acute sepsis, resulting from cytopenias caused by chemotherapeutics.5, 11, 12, 13 These can be particularly challenging for already-debilitated cancer patients, narrowing the spectrum of patients that can be treated with ACT.
Recently, oncolytic viruses have begun to transition from experimental therapeutics into routine clinical use. Talimogene laherparepvec (Imlygic) has been approved by the FDA and the European Medicines Agency following an impressive increase in durable response rates in non-resectable melanoma patients,14 and several other types of oncolytic viruses with different immunological effects are in development.15 Adenoviruses have been proposed attractive in this regard since they have strong effects on the adaptive and innate cellular immune systems.16
Oncolytic adenoviruses target and replicate only in tumor cells, and oncolytic cell death causes damage-associated molecular pattern (DAMP) and pathogen-associated molecular pattern (PAMP) signaling, rewiring the tumor microenvironment to become proinflammatory with the alteration of immune cell and cytokine content.16, 17, 18 Consequently, this triggers the immune system to develop a bispecific response against both the virus and tumor. Antiviral immunity helps the generation of antitumor responses against tumor epitopes (for instance, epitope spreading), which are generally weaker than evolutionarily conserved antiviral mechanisms.16, 17, 18 Tumor epitopes are presented after oncolysis, in the context of immunological danger signals. Accordingly, we previously reported that exploiting this circuit to deliver cytokines that favor T cell attraction and survival, such as IL-2 and tumor necrosis factor alpha (TNF-α), enables ACT in several preclinical models.19, 20, 21 Considering the aforementioned safety hazards of host lymphodepletion and the ability of armed adenoviruses to achieve similar biological effects in the tumor microenvironment, we hypothesize that we could use the latter to replace the former.
Here, we compared the antitumor efficacy and safety of IL-2- and TNF-α-coding adenoviruses with conventional lymphodepletion in animals that received ACT. We demonstrate that enabling ACT with adenovirus established effective antitumor responses, which were similar or superior to the ones observed in lymphodepleted animals that received ACT. Moreover, as seen in humans, host lymphodepletion caused severe toxicity that was not present in adenovirus-treated animals.
Results
Improved Tumor Control Is Associated with Intratumoral Infiltration of Immune Cells, which Mediate Potent Immunity against HapT1 Tumors
We studied whether intratumoral administration of TILs and oncolytic adenovirus promote effective tumor control and systemic immunity against HapT1 tumors, in comparison to hamsters that were lymphodepleted prior to TIL infusion (Figure 1A). The semi-permissive nature of the Syrian golden hamster allowed us to study the oncolytic capability of the human adenovirus coding for IL-2 and TNF-α. Overall, antitumor efficacy curves demonstrate that animals that received TIL therapy alone had statistically significant tumor regressions compared with the vehicle group (vehicle versus TILs, p < 0.0001; Figure 1B). Adding lymphodepleting preconditioning or oncolytic adenovirus to TIL therapy resulted in improvement in tumor control compared with the vehicle control (vehicle versus preconditioning + TILs, p < 0.00001; vehicle versus oncolytic adenovirus + TILs, p < 0.00001; Figure 1B). Adding lymphodepletion to oncolytic adenovirus treatment did not result in significant gains, although on the individual tumor level there were fewer escape variants (Figure S1). Also, response occurred faster with the triple combination, possibly because there was less inflammatory pseudoprogression when white blood cells had been eradicated with preconditioning (Figure 1B).
Figure 1.
Antitumor Efficacy and Systemic Effects of Local TIL and Oncolytic Adenovirus Therapy of Hamsters
(A) Hamsters with 5-day-old subcutaneous HapT1 tumors received a lymphodepleting regimen consisting of cyclophosphamide (550 mg/kg) and fludarabine (100 mg/kg) or saline intraperitoneally (i.p.). 1 × 108 virus particles (VPs) oncolytic adenovirus (OAd) coding for human IL-2 and TNF-α or PBS was injected intratumorally (i.t.) on day 1 and repeated 4 times. Tumor-infiltrating lymphocytes (TILs) or RPMI was injected i.t. a day after the first virus injection. (B) Tumor growth curves from different treatment groups (n = 5–6). Tumors were measured every 2–3 days after virus treatment initiation and followed until day 28 when animals were sacrificed and tumors and organs were collected for further analysis. (C) Ratio of CD4/CD8+ cells obtained by flow cytometric analysis on endpoint tumors. (D) Mitochondrial activity corresponding to cell viability obtained by performing MTS assay on co-cultures of endpoint-pooled splenocytes from each therapeutic group and tumor cell lines at a ratio of 20:1 splenocytes:tumor cells. Experiment was performed in triplicates. Data are presented as mean + SEM; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Interestingly, analysis of endpoint tumors demonstrated a progressive increase in the ratio of CD4/CD8 cells in groups with high tumor control (Figure 1C). In the spleens of hamsters that were preconditioned, we observed a significant decrease of CD8+ and CD4+ cells compared to the oncolytic adenovirus + TIL therapy group (Figure S2). The latter maintained levels of these cells similar to the control conditions (oncolytic adenovirus + TILs versus TILs, not significant [ns]; oncolytic adenovirus + TILs versus vehicle, ns). Regional lymph nodes revealed high infiltration of CD8+ and CD4+ cells in hamsters treated with TIL therapy only, while the other therapies (including the vehicle control) demonstrated mild alterations. Lymphodepletion also reduced the percentage of cells expressing major histocompatibility complex (MHC) class II in the lymph nodes, while the contrary was observed in any treatment combination containing adenovirus, including the triple therapy group. However, no significant changes in the percentage of MHC class II cells were observed between the groups. When we co-cultured endpoint splenocytes from different therapeutic groups with tumor cell lines, splenocytes from the hamsters that received the combination of oncolytic adenovirus and TILs were able to kill more HapT1 cells compared to the preconditioning groups (oncolytic adenovirus + TILs versus preconditioning + TILs, p < 0.05; oncolytic adenovirus + TILs versus preconditioning + oncolytic adenovirus + TILs, p < 0.05; Figure 1D). For other cell lines there were no differences between the groups, indicating the cell-specific nature of the immune response generated (Figure 1D).
Adenovirus Therapy Promotes Long-Term Survival of TIL-Treated Animals
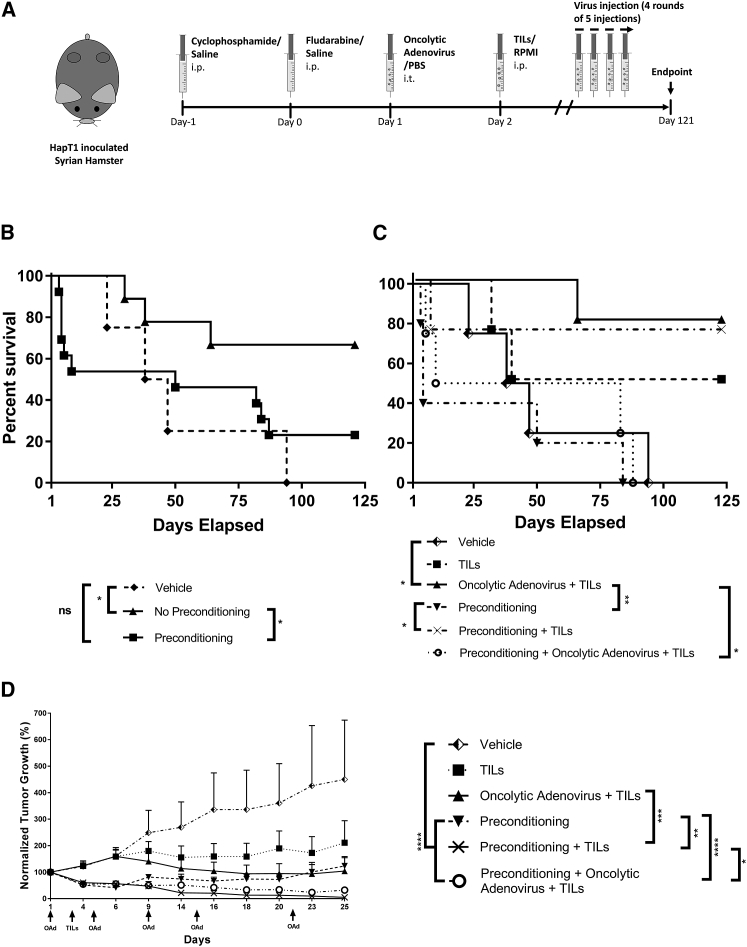
To understand if the oncolytic adenovirus therapy would translate into improved overall survival compared with lymphodepleted animals, we conducted a separate long-term hamster survival experiment (Figure 2A). Considering that TIL therapy can be administered with different approaches, such as intravenous, intratumoral, and intraperitoneal, here TIL therapy was administered systemically (intraperitoneally), resembling the majority of clinical trials that have used systemic administration.4, 5, 11, 12, 13, 22
Figure 2.
Overall Survival and Antitumor Efficacy in Hamsters Treated Systemically with TILs and Locally with Oncolytic Adenovirus
(A) Hamsters with 5-day-old subcutaneous HapT1 tumors received a lymphodepleting regimen consisting of cyclophosphamide (550 mg/kg) and fludarabine (100 mg/kg) or saline intraperitoneally (i.p.). 1 × 108 VPs oncolytic adenovirus (OAd) coding for human IL-2 and TNF-α or PBS was injected intratumorally (i.t.) on day 1 and repeated 4 times in intervals of 4–6 days. Three additional rounds of 5 injections were repeated until the end of the experiment. Tumor-infiltrating lymphocytes (TILs) or RPMI was injected i.p. after the first virus injection. Tumors were measured and animals (n = 4–5) were followed until day 121. (B) Overall survival of animals receiving vehicle, no preconditioning (including TIL and oncolytic adenovirus + TILs), or preconditioning (including preconditioning alone, preconditioning + TILs, and preconditioning + oncolytic adenovirus + TILs). (C) Overall survival of the different therapeutic groups. (D) Antitumor efficacy from the different therapeutic groups. Data are presented as mean + SEM; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
When we plotted the overall survival curve of hamsters that were preconditioned with cyclophosphamide and fludarabine, we observed that approximately 50% of the deaths occurred at early time points (days 4–9), indicating early complications after chemotherapy administration (Figure 2B), as seen in humans.5, 11, 12, 13 Survival of hamsters in the other therapeutic groups was determined by tumor control, and, thus, deaths occurred at later time points except in the vehicle control group, where there was poor tumor control. Overall survival curve analysis revealed that animals that were preconditioned had significantly decreased survival in comparison to animals that did not receive this regimen (hazard ratio [HR] = 3.059 [1.011; 9.258] preconditioning versus vehicle, p < 0.05) (Figure 2B).
Assessment of survival in different groups indicated that preconditioning was responsible for all deaths that occurred at early time points (before day 24). In comparison to oncolytic adenovirus + TILs or preconditioning + TILs (the two best groups), survival was poorer in animals that received all three treatments (preconditioning + oncolytic adenovirus + TILs) (Figure 2C). Preconditioning also increased the HR for death (HR = 9.134 [1.37; 60.88] preconditioning + oncolytic adenovirus + TILs versus oncolytic adenovirus + TILs; Figure 2C). In contrast, adenovirus therapy performed significantly better compared with the vehicle control (p < 0.05), preconditioning alone (p < 0.01), and the triple combination (p < 0.05) (Figure 2C). Moreover, oncolytic adenovirus was most consistent with regard to survival; the first casualty did not occur until day 64 (Figure 2C).
Repeated Administration of Adenovirus Enables Long-Term HapT1 Tumor Control in Hamsters
As a secondary endpoint, we recorded tumor growth in the survival experiment to assess if adenovirus therapy and/or preconditioning are able to provide long-term tumor control and efficacy. By day 6, we observed overall tumor growth separating between the two main therapeutic groups, hamsters that received vehicle and hamsters that received lymphodepleting preconditioning regimen (Figure 2D). This was likely caused by the antitumor effects of cyclophosphamide and fludarabine. Of note, dead animals were censored from the tumor size graph. Thus, observing only tumor size is misleading, since there were many treatment-related deaths in these groups as discussed in the previous paragraph. Much like our main efficacy experiment (Figure 1B), TIL therapy added little efficacy compared to the vehicle. In contrast, when oncolytic adenovirus injections were added, there was a prominent effect on therapeutic efficacy (Figure 2D).
If the animals survived preconditioning (and 53% did not), tumor sizes were smaller during the first 25 days (all preconditioning therapies versus vehicle, p < 0.05; Figure 2D). Nevertheless, preconditioning alone did not provide lasting tumor control (Figure S3). In parallel to what has been seen in humans, the combination of TILs with lymphodepletion resulted in good tumor control compared to hamsters that received only preconditioning (preconditioning versus preconditioning + TILs, p < 0.05). Repeated administration of oncolytic adenovirus in TIL-treated animals without lymphodepletion allowed long-term tumor control, as seen in individual tumor growth curves (Figure S3).
Adenoviruses Coding for IL-2 and TNF-α Achieve the Best Tumor Control in Mice Treated with Genetically Modified T Cells
Using another adoptive T cell therapy approach, we treated immunocompetent mice bearing B16.OVA tumors with TCR transgenic ovalbumin-specific T (OT-I) cells. Despite the fact that human serotype 5 adenovirus does not replicate in mice and, thus, oncolysis cannot be taken into consideration, this model enables the evaluation of the effects of the cytokines coded by adenovirus and testing of our hypothesis using a genetically modified T cell therapy approach (Figure 3A). Until day 4, all therapies resulted in similar tumor control (Figure 3B). Yet, after day 4, OT-I cell transfer alone was only able to delay tumor growth moderately, while the addition of adenovirus and/or lymphodepleting preconditioning therapies improved markedly the overall antitumor efficacy of OT-I cell transfer (Figure 3B).
Figure 3.
Antitumor Efficacy of Systemically Infused OT-I Cells and Adenovirus Therapy in Mice
(A) Mice with 7-day-old subcutaneous B16.OVA tumors received a lymphodepleting regimen consisting of cyclophosphamide (300 mg/kg) and fludarabine (100 mg/kg) or saline intraperitoneally (i.p.). 1 × 108 VPs adenovirus (Ad) coding for murine IL-2 and TNF-α in a ratio of 1:1 or PBS was injected intratumorally (i.t.) on day 1. OT-I cells or RPMI was injected i.p. following virus injection. (B) Tumors were measured and animals (n = 7–10) were followed until day 11. (C–G) Individual tumor growth curves from different treatment groups: vehicle (C), OT-I (D), adenovirus + OT-1 (E), preconditioning + OT-I (F), and preconditioning + adenovirus + OT-I (G). Data are presented as mean + SEM; **p < 0.01 and ****p < 0.0001.
Combining adenovirus therapy with OT-I cell transfer resulted in the best efficacy, with statistical significance when compared with the lymphodepleted group (OT-I + adenovirus versus preconditioning + OT-I, p < 0.01; Figure 3B). Moreover, the combination of adenovirus or preconditioning with OT-I cell transfer induced effective steady tumor control, contrasting with the vehicle and OT-I control groups (Figures 3C and 3D). As previously observed in hamsters (Figure 1B), triple treatment was not superior to double treatments (Figures 3B and 3G).
Adenovirus Therapy and Lymphodepleting Preconditioning Drive the Accumulation of Effector Cells and Convert APCs toward a Proinflammatory Status in the Tumor Microenvironment
To investigate the mechanism of action underlying the efficacy seen, we assessed immune cell populations within the tumor. Animals treated with OT-I cells showed a small increase in CD3+ T cells (Figure 4A), CD3+CD8+ T cells (Figure 4B), CD11c+ dendritic cells (Figure S4), and CD11c+CD86+ dendritic cells (Figure 4C) at the tumor site. Notably, in the groups with the best efficacy (adenovirus + OT-I and preconditioning + OT-I), all differences were significant versus controls (Figures 4A–4C). All of these cell populations, with the exception of CD11c+ dendritic cells (Figure S4), were equally significant in the triple group in comparison to controls. The expression levels of CD86 maturation marker in dendritic cells followed the same trend, with prominent increases in the three main therapeutic groups (OT-I + adenovirus, preconditioning + OT-I, and preconditioning + adenovirus + OT-I) compared to the vehicle group (Figure 4D). Interestingly, the biological effects of preconditioning were quite similar to those of the adenovirus coding for IL-2 and TNF-α. However, the triple combination did not have any additive effects over the double treatments.
Figure 4.
Immune Characterization and Cytokine Content of Endpoint B16.OVA Murine Tumors
Day 13 endpoint tumors (n = 4–8) were analyzed for the presence of (A) CD3+ T cells, (B) CD3+CD8+ T cells, (C) CD11c+CD86+ mature DCs, and (D) CD86 expression levels. Tumors (n = 2–8) were analyzed for (E) mIL-2, (F) mTNF-α, and (G) mIFNg. (H) Pooled Th1-type cytokines. All values from cytokine content are normalized to the mean vehicle value. Data are presented as mean + SEM; *p < 0.05, **p < 0.01, and ***p < 0.001.
Next, we evaluated the intratumoral levels of T helper 1 (Th1)-type proinflammatory cytokines (mIL-2, mTNF-α, and interferon gamma [mIFNg]) in endpoint B16.OVA tumors. Relative to the vehicle group, the levels of intratumoral mIL-2 were upregulated at least 5.1-fold in animals that received lymphodepleting preconditioning and/or adenovirus (Figure 4E). This was especially prominent in the latter group, where this upregulation was significant relative to animals in the vehicle (p < 0.05) and OT-I therapy (p < 0.05) groups (Figure 4E). mTNF-α and mIFNg were 5.5-fold and 3.5-fold increased, respectively, in animals that received the combination of OT-I and adenovirus versus vehicle (Figures 4F and 4G). In contrast, animals that received lymphodepleting preconditioning and OT-I therapy showed a moderate or low increase of intratumoral levels of these cytokines (Figures 4F and 4G).
The addition of adenovirus to animals that received preconditioning and OT-I treatment resulted in surprisingly low levels of mTNF-α and mIFNg at the tumor site (Figures 4F and 4G). Interestingly, except mTNF-α, the same trend was observed for all Th1-type cytokines in animals that received only OT-I therapy (Figures 4E–4G). Particularly, mIFNg was significantly upregulated in animals that received preconditioning + OT-I cells compared with the triple therapy and OT-I therapy (Figure 4G). Nevertheless, when we pooled all Th1-type response cytokines, we observed that animals that received adenovirus and OT-I cells had their Th1 cytokines increase 1.3-fold over animals that received the lymphodepleting regimen and OT-I cells (Figure 4H).
Lymphodepleting Chemotherapy Causes Toxicity to Vital Tissues
To investigate the effects of the lymphodepleting chemotherapeutics on different organs, we examined vital organs for pathological changes. In a setting where TIL therapy was not administered, we observed a drastic decrease in lymphocytes, neutrophils, and platelets in lymphodepleted animals compared to the vehicle and oncolytic adenovirus groups (Figure 5A). Heart tissue from preconditioned hamsters showed degenerative changes compatible with cardiac toxicity (Figure 5A). In contrast, adenovirus therapy resulted in only a mild decrease of platelets (vehicle versus oncolytic adenovirus, p < 0.05) but no major changes in lymphocytes or neutrophils (vehicle versus oncolytic adenovirus, ns; Figure 5A).
Figure 5.
Treatment-Related Systemic Adverse Events in Hamster and Mouse Tissues
(A) Hamsters that did not receive TIL therapy but were administered the vehicle, oncolytic adenovirus therapy, or lymphodepleting preconditioning (n = 5) were sacrificed at day 4, and blood was collected for lymphocyte, neutrophil, and platelet counting. The heart was collected for cardiac toxicity assessment through H&E staining. (B) Mice from different treatment groups (n = 7–10) were sacrificed on day 13 and the heart and lungs were collected for H&E staining and toxicity assessment. (C) 20×-magnified 4- to 5-mm thickness H&E-stained mouse lung and heart sections; Arrows indicate significant structural changes in the tissue. Data are presented as mean + SEM; *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.
Extensive toxic effects of lymphodepleting preconditioning were also seen in mice. The use of cyclophosphamide and fludarabine caused acute or severe degeneration in the cardiac muscle (Figures 5B and 5C). Furthermore, the lungs of the same animals had numerous alveolar and interstitial macrophages (Figure 5B). In addition, there was multifocal thickening and hyalinization of small pulmonary vessels, indicating degeneration (Figures 5B and 5C). These findings were observed to a minimal extent in mice treated with adenovirus. Nevertheless, the addition of adenovirus to lymphodepleted animals did seem to result in vascular degeneration in the lungs (Figures 5B and 5C). Thus, even though the triple combination did not add to efficacy or appealing immunological parameters, it did seem to add to toxicity.
When we assessed the blood serum for inflammatory cytokines, we found that mIL-2, mIL-6, mTNF-α, and mIFNg were highly increased in animals that received lymphodepleting preconditioning and OT-I therapy compared with the rest of the groups (Figure S5).
Discussion
This study reports that lymphodepleting preconditioning may not be required for effective ACT of solid tumors if replaced by an oncolytic adenovirus coding for IL-2 and TNF-α. We have focused our efforts in developing a viral platform that supports ACT in the absence of toxic high-dose chemotherapy lymphodepleting preconditioning regimens and high-dose IL-2 postconditioning. While our previous work demonstrated that vectorization of IL-2 by an oncolytic adenovirus can replace high-dose systemic IL-2 postconditioning while reducing toxicity,23 lymphodepleting preconditioning remained an open issue to be solved. Using the same animal tumor models and two different methods of ACT, the combination of adenovirus with TIL therapy resulted in prolonged survival and comparable (or superior) antitumor responses to those seen in animals receiving lymphodepleting preconditioning. Similar observations have been made in a clinical trial using a non-oncolytic adenovirus coding for IFNg and TIL therapy in patients with metastatic melanoma that did not receive preconditioning.22 Our experiments extend these findings to an oncolytic virus, which, unlike replication-incompetent adenovirus vectors, allows tumor-selective replication, enhanced infectivity in advanced malignancies, and tumor cell oncolysis. Moreover, IFNg as a transgene may be problematic with regard to the induction of rapid immunosuppression through Programmed cell death protein 1 (PD-1).22
Of note, our results show that lymphodepleting preconditioning may be counterproductive from a survival perspective. At a systemic level, the use of a lymphodepleting regimen could possibly harm the development of meaningful long-term systemic antitumor immunity and memory responses, because the relevant cells may be depleted. Accordingly, splenocytes from preconditioned hamsters had decreased ability to kill HapT1 tumor cells relative to spleens from hamsters that received oncolytic adenoviruses and TILs (Figure 1C), possibly caused by a reduction in the overall percentage of CD8+ and CD4+ cells (Figure S2). Moreover, the reconstitution of highly suppressive myeloid-derived suppressor cells (MDSCs) may also account for this result, since these cells have been shown to repopulate the spleens from mice that have undergone lymphodepletion.24 Interestingly, the triple combination (preconditioning + oncolytic adenovirus + TILs) did not result in favorable survival or efficacy. Even though oncolytic viruses have been proposed to have synergy with immunosuppressive drugs in some animal models, through increased virus replication,25, 26 the induction of tumor-specific immunity may be compromised by immunosuppression. This would explain why the triple combination seemed worse than the doublets. In addition, splenocytes from hamsters treated with oncoclytic adenovirus therapy were capable of achieving the highest HapT1 killing regardless of the comparable levels of splenic CD4+ and CD8+ cells in the different therapies (vehicle, TILs, and oncolytic adenovirus + TILs). A higher percentage of tumor-specific T cells could possibly explain this occurrence, since this has been demonstrated to be a splenic feature of animals treated with adenovirus therapy.27
One of the major challenges in ACT of solid tumors with TILs or gene-modified T cells is the poor infiltration of transferred T cells into the tumor microenvironment.8 In humans, melanoma and pancreatic cancer (the tumor types assessed in this study) are no exception, with the presence of TILs typically associated with longer survival.28, 29 We have shown that IL-2 and TNF-α can recruit natural killers and T cells to the tumor when produced from oncolytic adenoviruses.19, 20, 21, 23, 30, 31 This effect on trafficking could explain part of the good efficacy seen here. Additionally, preclinical work has proposed that host lymphodepletion associated the increased proliferation of CD8+ T cells with high antitumor efficacy in lymphodepleted hosts.32, 33 Yet, and concordantly with our previous work,20, 30 we found that hamsters that achieved high tumor control had a higher prevalence of CD4+ over CD8+ cells independently of the TIL combination treatment (Figures 1B and 1). Since Th1 CD4+ T cells are known to exert potent effector functions in vivo,34 it seems possible that this phenotype may comprise the majority of the CD4+ cells in tumors that responded better to therapy. Because the tumor microenvironment often dictates if CD4+ cells become effector or suppressor,34 one can hypothesize that the modulatory effects of the treatments may have favored the differentiation of CD4+ cells into a Th1 phenotype through adenovirus infection,35 the effects of IL-2,36 or lymphodepletion.37 In addition, MHC class II upregulation (a dendritic cell [DC] marker) in the draining lymph node may have contributed to the antitumor efficacy in hamsters treated with oncolytic adenovirus and TILs, given that this is required for effective CD4+ cell priming.20, 30 Nonetheless, this cannot be confirmed at present due to the lack of anti-hamster reagents.
In the B16.OVA tumor model, high antitumor efficacy was associated with the overall increase in CD3+ and CD8+ T cells, regardless of the therapeutic group (Figures 4A and 4B). This phenomenon may have arisen from the increased availability of IL-7 and IL-15 and depletion of Tregs and MDSCs, which are reported to contribute to the proliferation, activity, and lasting antitumor responses of effector T cells in lymphopenic mice.24, 38 Intratumoral APCs followed the same pattern as CD11c+ and their maturation marker, CD86, was increasingly expressed in lymphodepleted mice (Figure S4; Figures 4C and 4D). This result is consistent with Salem et al.,39 who proposed that cyclophosphamide-induced lymphopenia promotes the expansion of dendritic cells. Moreover, CD86 is a costimulatory molecule that has been linked to CD8+ T priming, activation, and survival,40 which are likely related to increased antitumor activity of CD8+ T cells. Interestingly, our experiments and others41 showed that the same events were observed in lymphoreplete mice that received adenovirus therapy. Infection of B16.OVA tumors with IL-2- and TNF-α-coding adenovirus in the context of OT-I cell transfer actively recruits CD8+ T cells to the tumor site,19, 21 and it triggers pathogen signaling that may have enhanced APC interaction with CD8+ T cells through CD86.17 Thus, the effects of adenovirus therapy and lymphodepletion may be similar at the tumor, even if the mechanisms are different.
Immunostimulatory cytokines also play a critical role in orchestrating an effective immune response against the tumor, either by interaction with immune cells or direct contact with cancer cells.31, 42 Cancer cells have the ability to exploit the absence of proinflammatory cytokines as one mechanism of evasion from the immune system.31 Sometimes the tumor microenvironment corrupts the physiological proinflammatory expression of cytokines, which is dynamic and often high level but short duration, into a chronic low-level expression, which is a recognized carcinogenic factor.31
Adenoviruses are advantageous carriers of cytokines, and we have previously shown in an ACT setting that the addition of IL-2 and TNF-α, at the tumor site, supports antitumor T cells and, thus, circumvents local tumor immunosuppression.15, 19, 21, 42 Accordingly, increased acute production of Th1 proinflammatory cytokines was seen, likely derived from virus transgenes, while increased IFNg may be attributed to the increase in CD8+ T cell activity in the tumor (Figures 4E and 4F). Notably, TNF-α and IFNg were highly upregulated in the highest efficacy group (adenovirus with OT-I therapy; Figures 4F and 4G), both of which are required for T cell-mediated B16 tumor rejection.43, 44 Of note, depletion of immune cells (by lymphodepletion) might have impaired the development of T cell immunity against adenovirus at the tumor site. As a result, this may have limited the production of IFNg and, consequently, resulted in lower levels of Th1-type proinflammatory cytokines in the triple combination group (Figure 4H).
While there is no question that lymphodepleting preconditioning enhances the effectiveness of ACT, its safety issues have been a concern, limiting its use in frail cancer patients.4, 5, 11, 12, 13 Considering that the large majority of ACT clinical trials use chemotherapy-based lymphodepleting regimens, especially in the context of solid tumors, we employed the same drugs here, high-dose cyclophosphamide (an alkylating agent) and fludarabine (a nucleoside analog).45 The combination of these two agents kills the majority of white blood cells in the patient or animal, preferentially those from lymphoid lineage cells, rendering the subject immunosuppressed. Consequently, and as observed in humans,4, 5, 11, 12, 13 blood from lymphodepleted hamsters that were not administered TIL therapy revealed an abrupt decrease of lymphocytes, neutrophils, and platelets (Figure 5A). While the decreased numbers of neutrophils and lymphocytes exposes the host to endogenous and exogenous pathogens, the low platelet count impedes blood clotting, increasing the risk of internal bleeding. We suspect that these conditions may have led to a high number of casualties present at an early time point in our hamster experiments. Preconditioning also causes mortality in humans,11, 12 but at a lower rate because humans are monitored more carefully; infections are treated with antibiotics, leukopenia can be treated with granulocyte colony-stimulating factor (GCSF), platelets or erythrocytes can be transferred, and intensive care is available.5, 11, 12, 13
At a cellular level, cyclophosphamide is metabolized into toxic metabolites that damage myocytes, leading to cardiac dysfunction and degeneration.45 As expected, this finding was particularly prominent in mice that received lymphodepleting preconditioning, which interestingly also demonstrated signs of hypertension with endothelial cell remodeling and hypertrophism (lungs; Figure 5C). These events may have resulted from multiple factors, including the direct effect of cyclophosphamide, cardiac dysfunction46, 47 and, as a by-product of increased levels of IL-6,48 TNF-α,49 and IFNg50 inflammatory cytokines (Figure S5). Moreover, because macrophages are directly regulated by activated T cells,51 their increase in combination with systemic inflammation possibly contributed to the numerical increase in interstitial macrophages and, thus, hypertension in lungs from lymphodepleted mice. In contrast, adenovirus therapy is well tolerated in humans, usually resulting in only mild to moderate adverse events,22, 26 including fatigue, fever, and flu-like symptoms. In line with previous observations,23 animals treated with adenovirus did not show significant tissue alterations in any of the two different species. Furthermore, it appears unlikely that this approach would potentiate a cytokine release syndrome, a toxicity arising from the use of gene-modified T cells for solid tumors.1 An important aspect of the oncolytic adenovirus design is that virus replication is tightly linked to transgene expression.20 Therefore, the adenovirus will replicate and transgenes will be expressed as long as tumor cells are present. This has been confirmed in animals20, 23 and humans,52 where cytokine shedding to the blood serum has remained minimal.
To summarize, our study suggests that oncolytic adenovirus coding for IL-2 and TNF-α is an attractive alternative to host lymphodepletion in solid tumor ACT protocols. Compared to lymphodepletion, the virus approach demonstrates a mild toxicity profile and high antitumor efficacy, which fundamentally determine the use of this modality in the clinic. Importantly, it was established that there is no rationale for using lymphodepletion together with the oncolytic adenovirus approach in ACT patients. A phase I clinical trial is underway, where melanoma patients receiving TIL therapy are treated with oncolytic adenovirus (Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2, also known as TILT-123). No lymphodepletion will be used in this trial.
Materials and Methods
Cell Lines and Viruses
The experiments performed in animals involved the use of HapT1, HAK, DDT1-MF2, and B16.OVA cell lines obtained and cultured as previously reported.17, 19, 20, 23, 30, 42, 53 Replication-deficient Ad5-CMV-mTNF-α, Ad5-CMV-mIL-2, and oncolytic Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 were constructed and obtained as previously described.19, 20
Animal Experiments
All animal protocols were carried out in agreement with the experimental animal committee of the University of Helsinki (Helsinki, Finland) and the Provincial Government of Southern Finland. 5- to 6-week-old immunocompetent male Golden Syrian Hamsters (HsdHan:AURA, Envigo, Huntington, UK) were implanted subcutaneously with 4 × 106 HapT1 cells in both flanks. For the short-term efficacy hamster experiment, tumors were grown for 5 days and hamsters were randomized into 5 therapeutic groups (n = 5–6). Following virus treatment, hamsters were followed for 28 days and their tumors were measured with a digital caliper. Alternatively, after virus treatment initiation, hamsters were followed until day 4 and humanely sacrificed for the collection of organs for histopathology and blood for white blood cell and platelet counting (n = 5). For the hamster survival experiment, tumors were grown for 5 days and hamsters were randomized into therapeutic groups (n = 4–5). Upon virus treatment, hamsters were followed for 121 days and their tumors were measured with a digital caliper. Endpoint criteria included tumor size limit (maximum diameter of 22,00 mm), skin ulcerations, visible decrease of well-being, or sudden death. For the testing of different doses of lymphodepleting chemotherapy (Figure S6), tumors were grown for 7 days, after which cyclophosphamide was administered. After 2 days, hamsters were sacrificed and the blood was collected for white blood cell counting (n = 3).
Similarly, 4- to 6-week-old immunocompetent C57BL/6 female mice (Harlan Laboratories, Indianapolis, IN, USA) were implanted subcutaneously with 0.25 × 106 B16.OVA cells in the right flank. Tumors were grown for 7 days and mice were randomized into 5 groups (n = 7–10). Following virus treatment, mice were followed for 13 days and their tumors were measured with a digital caliper.
All animals were humanely sacrificed and their blood and tumors were collected for blood count analysis and flow cytometry, respectively. Selected organs were also collected for histopathology. In all experiments, the tumor volume was obtained as 0.52 × length × width2 formula. Normalized tumor volume was obtained as percentage of daily tumor volume relative to day 1 tumor volume.
Lymphodepleting Preconditioning Regimen and Virus Treatments
Lymphodepleting preconditioning regimen was performed with cyclophosphamide (Sendoxan, Baxter, Deerfield, IL, USA) and fludarabine (Fludaribis, Actavis, Parsippany-Troy Hills, NJ, USA). After testing different doses of cyclophosphamide (550, 450, 350, and 250 mg/kg) with 100 mg/kg fludarabine for the depletion of white blood cells (Figure S6), hamsters received a single injection of 9 mg/mL saline (Braun, Aschaffenburg, Germany) or 550 mg/kg cyclophosphamide (maximum solubility 50 mg/mL) on day −1, followed by a single injection of 9 mg/mL saline or 100 mg/kg fludarabine on day 0. Moreover, hamsters were injected intratumorally with 50 μL PBS or 1 × 108 viral particles (VPs) per tumor of oncolytic Ad5/3-E2F-d24-hTNF-α-IRES-hIL-2 (also known as TILT-123) on day 1. Depending on the experiment, virus treatments were repeated on days 5, 9, 14, and 20 or continued 3 more rounds in blocks of 5 injections. Similarly, mice received a single injection of 9 mg/mL saline or 300 mg/kg cyclophosphamide on day −1 followed by a single injection of 9 mg/mL saline or 100 mg/kg fludarabine on day 0. The following day, mice were administered intratumorally only once with 50 μL PBS or 1 × 108 VPs/tumor Ad5-CMV-mIL-2 and Ad5-CMV-mTNF-α (1:1 ratio).
Adoptive Cell Transfer
Hamster TILs were obtained using a culturing method previously described by our group.20, 23, 30 Following the first virus treatment, hamsters in the short-term efficacy experiment received 4 × 106 TILs intratumorally, while hamsters in the survival experiment received 50 × 106 TILs intraperitoneally.
Mouse ovalbumin-specific T cell receptor transgenic T (OT-I) cells were obtained using a culturing method previously described by our group.17, 19, 23, 42 After 9 days culturing, 1 × 106 OT-I cells were injected intraperitoneally in mice following virus treatment.
Cytotoxicity Assay
HapT1, HAK, and DDT1-MF2 were seeded at density of 1 × 104 cells and co-cultured with 20 × 104 pooled splenocytes (from each therapeutic group) per well in 96-well plates, according to the protocols previously established.20, 30
Blood Collection, Counting, and Histopathology
Blood from hamsters was collected in heparinized 1-mL tubes (Greiner Bio-one, Kremsmünter, Austria). The neutrophil, lymphocyte, and platelet counts were obtained using ADVIA 2120i (Siemens, Erlangen, Germany). Alternatively, after the collection of blood from hamsters treated with different doses of chemotherapy, red blood cells were lysed with ammonium-chloride-potassium (ACK) lysing buffer (Thermo Fisher Scientific, Waltham, MA, USA), and white blood cells were analyzed through a BD Accuri C6 flow cytometer (BD Biosciences, San Jose, CA, USA) (Figure S6). Additionally, tissue samples from heart, lungs, and liver were prepared for H&E staining, as previously described.20, 23 Histopathological changes were assessed by a veterinary pathologist.
Cytokine Bead Array
Blood serum and homogenized snap-frozen tumor fragments were processed and stored at −80°C, as previously described.42 Levels of mIL-2, mTNF-α, mIFNg, and mIL-6 were measured using the CBA mouse Th1/Th2/Th17 kit (560485, BD Biosciences, San Jose, CA, USA) and BD Accuri C6 flow cytometer with FCAP Array software (BD Biosciences, San Jose, CA, USA). The levels obtained were normalized against the total protein content of the samples.
Flow Cytometry
As previously described,19, 20, 23, 30, 42 tumors, spleens, and lymph nodes collected from animals were prepared into a single-cell suspension and stored at −80°C. Hamster and murine organs were stained as previously reported.17, 19, 20, 23, 30, 42, 53 1,000 to 100,000 events were acquired during analysis on a BD Accuri C6 flow cytometer with CSampler software (BD Biosciences, San Jose, CA, USA).
Statistical Analysis
Statistical comparisons on log-transformed normalized tumor volumes from the different therapeutic groups were assessed using linear mixed models with SPSS version 24 (IBM, North Castle, NY, USA). Differences between tumor samples or cell viability and survival were obtained with unpaired Student’s t test with Welch’s correction and Kaplan-Meier survival analysis, respectively, using GraphPad Prism 7.04 software (GraphPad, La Jolla, CA, USA).
Author Contributions
J.M.S., V.C.-C., R.H., S.Z., M.S., and A.H. designed and conducted the experiments. J.M.S. and M.A. analyzed the results. S.S., A.K., and A.H. collected funding for the study. All the authors contributed to writing and reviewing the manuscript.
Conflicts of Interest
A.H. is an employee and shareholder of TILT Biotherapeutics Ltd. and a shareholder of Targovax ASA. J.M.S., M.S., R.H., S.S., and V.C.-C. are employees of TILT Biotherapeutics Ltd.
Acknowledgments
This study was supported by the European Commission Marie Curie Innovative Training Network (ITN) grant VIRION (H2020-MSCA-ITN-2014 project number 643130), the Jane and Aatos Erkko Foundation, HUCH Research Funds (EVO), the Sigrid Juselius Foundation, Finnish Cancer Organizations, the University of Helsinki, and TILT Biotherapeutics Ltd. The authors thank Professor Satu Sankari, Susanna Grönberg-Vähä-Koskela, Minna Oksanen, the Small Animal Hospital, the Pathology Unit from the Finnish Food Safety Authority (EVIRA), the Biomedicum Flow Cytometry Core, the Tissue Processing and Histochemistry unit, and the Laboratory Animal Center for their expertise and assistance during the study.
Footnotes
Supplemental Information includes six figures and can be found with this article online at https://doi.org/10.1016/j.ymthe.2018.06.001.
Supplemental Information
References
- 1.Aranda F., Buqué A., Bloy N., Castoldi F., Eggermont A., Cremer I., Fridman W.H., Fucikova J., Galon J., Spisek R. Trial Watch: Adoptive cell transfer for oncological indications. OncoImmunology. 2015;4:e1046673. doi: 10.1080/2162402X.2015.1046673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenberg S.A., Restifo N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maus M.V., June C.H. Making Better Chimeric Antigen Receptors for Adoptive T-cell Therapy. Clin. Cancer Res. 2016;22:1875–1884. doi: 10.1158/1078-0432.CCR-15-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenberg S.A., Yang J.C., Sherry R.M., Kammula U.S., Hughes M.S., Phan G.Q., Citrin D.E., Restifo N.P., Robbins P.F., Wunderlich J.R. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin. Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dudley M.E., Wunderlich J.R., Yang J.C., Sherry R.M., Topalian S.L., Restifo N.P., Royal R.E., Kammula U., White D.E., Mavroukakis S.A. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kradin R.L., Kurnick J.T., Lazarus D.S., Preffer F.I., Dubinett S.M., Pinto C.E., Gifford J., Davidson E., Grove B., Callahan R.J. Tumour-infiltrating lymphocytes and interleukin-2 in treatment of advanced cancer. Lancet. 1989;1:577–580. doi: 10.1016/s0140-6736(89)91609-7. [DOI] [PubMed] [Google Scholar]
- 7.Klebanoff C.A., Khong H.T., Antony P.A., Palmer D.C., Restifo N.P. Sinks, suppressors and antigen presenters: how lymphodepletion enhances T cell-mediated tumor immunotherapy. Trends Immunol. 2005;26:111–117. doi: 10.1016/j.it.2004.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiteside T.L., Demaria S., Rodriguez-Ruiz M.E., Zarour H.M., Melero I. Emerging Opportunities and Challenges in Cancer Immunotherapy. Clin. Cancer Res. 2016;22:1845–1855. doi: 10.1158/1078-0432.CCR-16-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen D.S., Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 10.Quatromoni J.G., Eruslanov E. Tumor-associated macrophages: function, phenotype, and link to prognosis in human lung cancer. Am. J. Transl. Res. 2012;4:376–389. [PMC free article] [PubMed] [Google Scholar]
- 11.Dudley M.E., Gross C.A., Langhan M.M., Garcia M.R., Sherry R.M., Yang J.C., Phan G.Q., Kammula U.S., Hughes M.S., Citrin D.E. CD8+ enriched “young” tumor infiltrating lymphocytes can mediate regression of metastatic melanoma. Clin. Cancer Res. 2010;16:6122–6131. doi: 10.1158/1078-0432.CCR-10-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Besser M.J., Shapira-Frommer R., Itzhaki O., Treves A.J., Zippel D.B., Levy D., Kubi A., Shoshani N., Zikich D., Ohayon Y. Adoptive transfer of tumor-infiltrating lymphocytes in patients with metastatic melanoma: intent-to-treat analysis and efficacy after failure to prior immunotherapies. Clin. Cancer Res. 2013;19:4792–4800. doi: 10.1158/1078-0432.CCR-13-0380. [DOI] [PubMed] [Google Scholar]
- 13.Ellebaek E., Iversen T.Z., Junker N., Donia M., Engell-Noerregaard L., Met Ö., Hölmich L.R., Andersen R.S., Hadrup S.R., Andersen M.H. Adoptive cell therapy with autologous tumor infiltrating lymphocytes and low-dose Interleukin-2 in metastatic melanoma patients. J. Transl. Med. 2012;10:169. doi: 10.1186/1479-5876-10-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andtbacka R.H., Ross M., Puzanov I., Milhem M., Collichio F., Delman K.A., Amatruda T., Zager J.S., Cranmer L., Hsueh E. Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Ann. Surg. Oncol. 2016;23:4169–4177. doi: 10.1245/s10434-016-5286-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerullo V., Koski A., Vähä-Koskela M., Hemminki A. Oncolytic adenoviruses for cancer immunotherapy: data from mice, hamsters, and humans. In: David T.C., Paul B.F., editors. Advances in Cancer Research. Volume 115. Academic Press; 2012. pp. 265–318. [DOI] [PubMed] [Google Scholar]
- 17.Tähtinen S., Grönberg-Vähä-Koskela S., Lumen D., Merisalo-Soikkeli M., Siurala M., Airaksinen A.J., Vähä-Koskela M., Hemminki A. Adenovirus Improves the Efficacy of Adoptive T-cell Therapy by Recruiting Immune Cells to and Promoting Their Activity at the Tumor. Cancer Immunol. Res. 2015;3:915–925. doi: 10.1158/2326-6066.CIR-14-0220-T. [DOI] [PubMed] [Google Scholar]
- 18.Kleijn A., Kloezeman J., Treffers-Westerlaken E., Fulci G., Leenstra S., Dirven C., Debets R., Lamfers M. The in vivo therapeutic efficacy of the oncolytic adenovirus Delta24-RGD is mediated by tumor-specific immunity. PLoS ONE. 2014;9:e97495. doi: 10.1371/journal.pone.0097495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siurala M., Havunen R., Saha D., Lumen D., Airaksinen A.J., Tähtinen S., Cervera-Carrascon V., Bramante S., Parviainen S., Vähä-Koskela M. Adenoviral Delivery of Tumor Necrosis Factor-α and Interleukin-2 Enables Successful Adoptive Cell Therapy of Immunosuppressive Melanoma. Mol. Ther. 2016;24:1435–1443. doi: 10.1038/mt.2016.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Havunen R., Siurala M., Sorsa S., Grönberg-Vähä-Koskela S., Behr M., Tähtinen S., Santos J.M., Karell P., Rusanen J., Nettelbeck D.M. Oncolytic Adenoviruses Armed with Tumor Necrosis Factor Alpha and Interleukin-2 Enable Successful Adoptive Cell Therapy. Mol. Ther. Oncolytics. 2016;4:77–86. doi: 10.1016/j.omto.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tähtinen S., Blattner C., Vähä-Koskela M., Saha D., Siurala M., Parviainen S., Utikal J., Kanerva A., Umansky V., Hemminki A. T-Cell Therapy Enabling Adenoviruses Coding for IL2 and TNFα Induce Systemic Immunomodulation in Mice With Spontaneous Melanoma. J. Immunother. 2016;39:343–354. doi: 10.1097/CJI.0000000000000144. [DOI] [PubMed] [Google Scholar]
- 22.Khammari A., Nguyen J.M., Saint-Jean M., Knol A.C., Pandolfino M.C., Quereux G., Brocard A., Peuvrel L., Saiagh S., Bataille V. Adoptive T cell therapy combined with intralesional administrations of TG1042 (adenovirus expressing interferon-γ) in metastatic melanoma patients. Cancer Immunol. Immunother. 2015;64:805–815. doi: 10.1007/s00262-015-1691-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos J.M., Havunen R., Siurala M., Cervera-Carrascon V., Tähtinen S., Sorsa S., Anttila M., Karell P., Kanerva A., Hemminki A. Adenoviral production of interleukin-2 at the tumor site removes the need for systemic postconditioning in adoptive cell therapy. Int. J. Cancer. 2017;141:1458–1468. doi: 10.1002/ijc.30839. [DOI] [PubMed] [Google Scholar]
- 24.Kodumudi K.N., Weber A., Sarnaik A.A., Pilon-Thomas S. Blockade of myeloid derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J. Immunol. 2012;189:5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koski A., Kangasniemi L., Escutenaire S., Pesonen S., Cerullo V., Diaconu I., Nokisalmi P., Raki M., Rajecki M., Guse K. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ranki T., Pesonen S., Hemminki A., Partanen K., Kairemo K., Alanko T., Lundin J., Linder N., Turkki R., Ristimäki A. Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer. 2016;4:17. doi: 10.1186/s40425-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cervera-Carrascon V., Siurala M., Santos J.M., Havunen R., Tähtinen S., Karell P., Sorsa S., Kanerva A., Hemminki A. TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. OncoImmunology. 2018;7:e1412902. doi: 10.1080/2162402X.2017.1412902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haanen J.B., Baars A., Gomez R., Weder P., Smits M., de Gruijl T.D., von Blomberg B.M., Bloemena E., Scheper R.J., van Ham S.M. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol. Immunother. 2006;55:451–458. doi: 10.1007/s00262-005-0018-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ino Y., Yamazaki-Itoh R., Shimada K., Iwasaki M., Kosuge T., Kanai Y., Hiraoka N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer. 2013;108:914–923. doi: 10.1038/bjc.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siurala M., Vähä-Koskela M., Havunen R., Tähtinen S., Bramante S., Parviainen S., Mathis J.M., Kanerva A., Hemminki A. Syngeneic syrian hamster tumors feature tumor-infiltrating lymphocytes allowing adoptive cell therapy enhanced by oncolytic adenovirus in a replication permissive setting. OncoImmunology. 2016;5:e1136046. doi: 10.1080/2162402X.2015.1136046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat. Rev. Cancer. 2004;4:11–22. doi: 10.1038/nrc1252. [DOI] [PubMed] [Google Scholar]
- 32.Wang L.-X., Shu S., Plautz G.E. Host lymphodepletion augments T cell adoptive immunotherapy through enhanced intratumoral proliferation of effector cells. Cancer Res. 2005;65:9547–9554. doi: 10.1158/0008-5472.CAN-05-1175. [DOI] [PubMed] [Google Scholar]
- 33.Cheever M.A., Greenberg P.D., Fefer A. Specificity of adoptive chemoimmunotherapy of established syngeneic tumors. J. Immunol. 1980;125:711–714. [PubMed] [Google Scholar]
- 34.Geginat J., Paroni M., Maglie S., Alfen J.S., Kastirr I., Gruarin P., De Simone M., Pagani M., Abrignani S. Plasticity of human CD4 T cell subsets. Front. Immunol. 2014;5:630. doi: 10.3389/fimmu.2014.00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heemskerk B., Veltrop-Duits L.A., van Vreeswijk T., ten Dam M.M., Heidt S., Toes R.E.M., van Tol M.J., Schilham M.W. Extensive cross-reactivity of CD4+ adenovirus-specific T cells: implications for immunotherapy and gene therapy. J. Virol. 2003;77:6562–6566. doi: 10.1128/JVI.77.11.6562-6566.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liao W., Lin J.-X., Wang L., Li P., Leonard W.J. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat. Immunol. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quezada S.A., Simpson T.R., Peggs K.S., Merghoub T., Vider J., Fan X., Blasberg R., Yagita H., Muranski P., Antony P.A. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gattinoni L., Finkelstein S.E., Klebanoff C.A., Antony P.A., Palmer D.C., Spiess P.J., Hwang L.N., Yu Z., Wrzesinski C., Heimann D.M. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salem M.L., Díaz-Montero C.M., Al-Khami A.A., El-Naggar S.A., Naga O., Montero A.J., Khafagy A., Cole D.J. Recovery from cyclophosphamide-induced lymphopenia results in expansion of immature dendritic cells which can mediate enhanced prime-boost vaccination antitumor responses in vivo when stimulated with the TLR3 agonist poly(I:C) J. Immunol. 2009;182:2030–2040. doi: 10.4049/jimmunol.0801829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas I.J., Petrich de Marquesini L.G., Ravanan R., Smith R.M., Guerder S., Flavell R.A., Wraith D.C., Wen L., Wong F.S. CD86 has sustained costimulatory effects on CD8 T cells. J. Immunol. 2007;179:5936–5946. doi: 10.4049/jimmunol.179.9.5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Watanabe K., Luo Y., Da T., Guedan S., Ruella M., Scholler J., Keith B., Young R.M., Engels B., Sorsa S. Pancreatic cancer therapy with combined mesothelin-redirected chimeric antigen receptor T cells and cytokine-armed oncolytic adenoviruses. JCI Insight. 2018;3:99573. doi: 10.1172/jci.insight.99573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tähtinen S., Kaikkonen S., Merisalo-Soikkeli M., Grönberg-Vähä-Koskela S., Kanerva A., Parviainen S., Vähä-Koskela M., Hemminki A. Favorable alteration of tumor microenvironment by immunomodulatory cytokines for efficient T-cell therapy in solid tumors. PLoS ONE. 2015;10:e0131242. doi: 10.1371/journal.pone.0131242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kline J., Zhang L., Battaglia L., Cohen K.S., Gajewski T.F. Cellular and molecular requirements for rejection of B16 melanoma in the setting of regulatory T cell depletion and homeostatic proliferation. J. Immunol. 2012;188:2630–2642. doi: 10.4049/jimmunol.1100845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang B., Karrison T., Rowley D.A., Schreiber H. IFN-γ- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers. J. Clin. Invest. 2008;118:1398–1404. doi: 10.1172/JCI33522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morandi P., Ruffini P.A., Benvenuto G.M., Raimondi R., Fosser V. Cardiac toxicity of high-dose chemotherapy. Bone Marrow Transplant. 2005;35:323–334. doi: 10.1038/sj.bmt.1704763. [DOI] [PubMed] [Google Scholar]
- 46.Guazzi M., Naeije R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J. Am. Coll. Cardiol. 2017;69:1718–1734. doi: 10.1016/j.jacc.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 47.Cameron A.C., Touyz R.M., Lang N.N. Vascular Complications of Cancer Chemotherapy. Can. J. Cardiol. 2016;32:852–862. doi: 10.1016/j.cjca.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahmud A., Feely J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension. 2005;46:1118–1122. doi: 10.1161/01.HYP.0000185463.27209.b0. [DOI] [PubMed] [Google Scholar]
- 49.Guzik T.J., Hoch N.E., Brown K.A., McCann L.A., Rahman A., Dikalov S., Goronzy J., Weyand C., Harrison D.G. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rabinovici R., Feuerstein G., Abdullah F., Whiteford M., Borboroglu P., Sheikh E., Phillip D.R., Ovadia P., Bobroski L., Bagasra O., Neville L.F. Locally produced tumor necrosis factor-alpha mediates interleukin-2-induced lung injury. Circ. Res. 1996;78:329–336. doi: 10.1161/01.res.78.2.329. [DOI] [PubMed] [Google Scholar]
- 51.Wang H., Kwak D., Fassett J., Hou L., Xu X., Burbach B.J., Thenappan T., Xu Y., Ge J.B., Shimizu Y. CD28/B7 deficiency attenuates systolic overload-induced congestive heart failure, myocardial and pulmonary inflammation, and activated T cell accumulation in the heart and lungs. Hypertension. 2016;68:688–696. doi: 10.1161/HYPERTENSIONAHA.116.07579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koski A., Bramante S., Kipar A., Oksanen M., Juhila J., Vassilev L., Joensuu T., Kanerva A., Hemminki A. Biodistribution Analysis of Oncolytic Adenoviruses in Patient Autopsy Samples Reveals Vascular Transduction of Noninjected Tumors and Tissues. Mol. Ther. 2015;23:1641–1652. doi: 10.1038/mt.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zafar S., Parviainen S., Siurala M., Hemminki O., Havunen R., Tähtinen S., Bramante S., Vassilev L., Wang H., Lieber A. Intravenously usable fully serotype 3 oncolytic adenovirus coding for CD40L as an enabler of dendritic cell therapy. OncoImmunology. 2016;6:e1265717. doi: 10.1080/2162402X.2016.1265717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.