Abstract
Recent studies have demonstrated sexual dimorphisms in the mechanisms contributing to the development of chronic pain. Here we tested the hypothesis that microglia might preferentially regulate hyperalgesic priming in male mice. We based this hypothesis on evidence that microglia preferentially contribute to neuropathic pain in male mice via ionotropic purinergic receptor (P2XR) or p38 mitogen activated protein kinase (p38) signaling. Mice given a single priming injection of the soluble human interleukin-6 receptor (IL-6r) and then a second injection of prostaglandin E2 (PGE2), which unmasks hyperalgesic priming, show a significant increase in levels of activated microglia at 3h following the PGE2 injection in both male and female mice. There was no change in microglia following PGE2. Intrathecal injection of the P2X3/4 inhibitor TNP-ATP blocked the initial response to IL-6r in both males and females, but only blocked hyperalgesic priming in male mice. Intrathecally applied p38 inhibitor, skepinone, had no effect on the initial response to IL-6r but attenuated hyperalgesic priming in males only. Neither TNP-ATP nor skepinone could reverse priming once it had already been established in male mice suggesting that these pathways must be inhibited early in the development of hyperalgesic priming to have an effect. Our work is consistent with previous findings that P2XR and p38 inhibition can lead to male-specific effects on pain behaviors in mice. However, given that we did not observe microglial activation at time points where these drugs were effective, our work also questions whether these effects can be completely attributed to microglia.
Introduction
It is becoming increasingly clear that the mechanisms underlying the way men and women develop chronic pain and respond to treatments are different. Several studies have demonstrated that the cell types responsible for mediating the development of chronic pain in male and female animals are different (Sorge et al., 2015),(Taves et al., 2016). Microglia are the resident immune cells of the central nervous system (CNS) and upon injury or insult they can become activated and begin releasing inflammatory and pro-nociceptive factors, such as cytokines and chemokines (Grace et al., 2014) (Block et al., 2007)(Ekdahl et al., 2003)(Scholz and Woolf, 2007)(Inoue and Tsuda, 2009) (Costigan et al., 2009). At least two studies have now shown that microglia contribute more prominently to chronic neuropathic pain in male than in female mice (Sorge et al., 2015),(Taves et al., 2016)(Sorge et al., 2011)(Mogil, 2012)(Coraggio et al., 2018)(Lopes et al., 2017). In the spared-nerve injury (SNI) model of neuropathic pain, when microglial activity was blocked, or these cells were depleted with selective toxins, the persistence of mechanical hypersensitivity, which is a key feature of neuropathic pain, was strongly reduced in male CD-1 mice but no effect was observed for these treatments in the same strain of female mice (Sorge et al., 2015). Additional studies have been conducted to demonstrate that these effects are found across laboratories and with different strains of mice creating a strong rationale to continue to explore mechanistic differences in the development of chronic pain in males and females (Mapplebeck et al., 2016).
Our study used the hyperalgesic priming model to examine the transition to a state of persistent pain plasticity where animals are susceptible to a normally sub-threshold stimulus promoting a long lasting pain state (Kandasamy and Price, 2015),(Price and Inyang, 2015),(Reichling and Levine, 2009). We hypothesized that in the mouse model of hyperalgesic priming, microglial activation would regulate this plasticity in male but not female mice. In this model we first inject a pro-nociceptive stimulus into the hindpaw to create a transient mechanical hypersensitivity around the site of injection (Dina et al., 2008). After this initial hypersensitivity had resolved, we then give a second stimulus, prostaglandin E2 (PGE2) into the same hindpaw at a dose that does not promote pain hypersensitivity in naïve mice. In animals that have been primed with an initial pro-nociceptive stimulus, there is a robust and extended response to the injection of PGE2 which reveals the presence of hyperalgesic priming (Reichling and Levine, 2009),(Dina et al., 2008). Because robust hyperalgesic priming can be induced in male and female mice with a broad variety of stimuli, this paradigm allowed us to test two primary hypotheses. First, we assessed whether microglial activation occurs in the spinal dorsal horn during the initiation of plasticity that causes hyperalgesic priming or at the time that priming is revealed by PGE2 injection. To our knowledge this has never been tested. Second, we sought to assess whether P2X3/4 antagonists or p38 inhibitors have sexually dimorphic effects in hyperalgesic priming in mice.
Our work demonstrates that modest microglial activation is noted in the spinal dorsal horn in both male and female mice in the hyperalgesic priming model around the time of PGE2 injection but not when priming is initiated. In contrast, pharmacological inhibition of P2X2/3 or p38 during the initial insult blocks the development of hyperalgesic priming in male mice, but has no impact in female mice. Once hyperalgesic priming has already been established, blocking P2X3/4 or p28 had no effect in either male or female mice. Our results are consistent with a male-specific role for P2X3/4 and p38 in the promotion of chronic pain plasticity but question whether these effects are dependent on microglia.
Methods and Materials
Animals
In all experiments naïve, 6 – 10 week old Swiss Webster mice were purchased from Taconic (Hudson, NY). Both male and female mice were used. Mice were housed in same-sex groups of 2 – 4 animals on a 12: 12 hr light/dark cycle. Food and water were available ad libitum. Animals were assigned to their experimental groups using a random number generator, with a minimum of one animal per drug treatment in each housing group. The Institutional Animal Care and Use Committee at the University of Texas at Dallas approved all animal procedures.
Drugs
The soluble human interleukin 6 receptor (IL-6r) was obtained from R&D Systems (Minneapolis, MN). Prostaglandin E2 (PGE2) from was from Cayman Chemical Company (Ann Arbor, MI). 2′,3′-O-(2,4,5-Trinitrophenyl) adenosine-5′-triphosphate tetra(triethylammonium) salt (TNP-ATP) and skepinone-L (skepinone) were obtained from Sigma-Aldrich (St. Louis, MO). IL-6r stock solution was made in sterile phosphate buffered saline (PBS). PGE2 stock solution was made in 100% ethanol. TNP-ATP and skepinone stocks were made in dimethyl sulfoxide. All drugs were diluted to final working concentration in sterile 0.9% saline prior to injection.
von Frey Testing and Hyperalgesic Priming
Animals were placed in acrylic boxes with mesh flooring and allowed to acclimate for at least 1 hr prior to testing. Calibrated von Frey Filaments were then used to determine the mechanical withdrawal threshold using the up-down method of Dixon with modification (Dixon, 1965),(Chapman et al., 1985). Baseline paw withdrawal threshold was determined before all injections.
In order to establish hyperalgesic priming, 0.1 ng of IL-6r was injected into the plantar surface of the left hindpaw. Paw withdrawal threshold was then measured at 3, 24, and 72 hr post IL-6r injection. Mice were then allowed to return to their baseline mechanical withdrawal threshold and were then injected with 100 ng PGE2 in a volume of 10 μL of 0.9% sterile saline at least 7 days after the initial injection of IL-6r. Mechanical withdrawal threshold was then determined at 3 and 24 hr after PGE2 injection.
Rotarod Testing
Rotarod testing was used to determine the impact of hindpaw IL-6r and PGE2 injections on locomotion in animals treated with I.T. Skepinone or vehicle. Prior to experimental testing animals were allowed to acclimate to the stationary rotarod device (rotarod treadmill, 1.25in diameter, IITC Life Science, Woodland Hills, CA) for 120 sec prior to baseline measurements. Animals were placed on rotarod rotating at a speed of 10 rotations per minute. Baseline measurements were taken 24 hr prior to intrathecal Skepinone injections over 3 trials with a maximal cutoff time of 90 seconds per trial. Rotarod testing was then performed 3 and 24 hr post IL-6r I.Pl. injections and 3 and 24 hr post PGE2 intraplantar injections. In this set of experiments locomotion was only assessed in female mice.
Intrathecal Injections
Immediately preceding hindpaw IL-6r injections male and female animals were anesthetized using isoflourane gas (4 % induction, 1.5 % maintenance). The animal was then given an intrathecal injection of either TNP-ATP (5 μg)(Sorge et al., 2015) or skepinone (30 μg)(Taves et al., 2016) in a total volume of 5 μL using a 50 μL Hamilton syringe with a 30 gauge needle (Hylden and Wilcox, 1980). In the second set of experiments males were given hindpaw injections of IL-6r and a minimum of 5 days later given an intrathecal injection of either TNP-ATP (5 μg) or skepinone (30 μg) in a volume of 5 μL immediately before receiving a hindpaw injection of PGE2.
Immunohistochemistry
In the first set of experiments, animals were injected with IL-6r and 3 hr later were deeply anesthetized with isoflourane and sacrificed. In the second set of experiments animals were injected with IL-6r and then 7 days later injected with 100 ng PGE2. Either 3 or 24 hr after PGE2 injections animals were sacrificed. We followed a previously reported immunohistochemistry protocol (Barragán-Iglesias et al., 2018). Immediately following sacrifice spinal cord tissue was dissected and the lumbar section was embedded into Optimal Cutting Temperature (O.C.T.) compound and flash frozen using dry ice. Tissues were stored at −80 degrees Celsius until they were cryosectioned at a 20 μm thickness and immediately mounted onto superfrost plus charged glass slides (VWR). The slides were stored at −80 degrees Celsius until immunohistochemistry (IHC) was done.
For IHC, slides were immediately fixed in chilled 4% paraformaldehyde for 1 hr after removal from −80 degree Celsius storage. The slides were then washed 3 times for 5 min each in PBS. Tissues were permeabilized for 30 min in 0.05 % Triton X-100 in PBS containing 5 % normal goat serum (NGS). These tissues were then blocked with PBS containing 5 % NGS for a minimum of 2 hr. Spinal cord tissue was stained with anti-ionized calcium-binding adaptor molecule (IBA-1) polyclonal antibody (1:1000)(Kim et al., 2012). The slides were then incubated with the appropriate secondary antibody for IBA1 for 1 hr. They were then stained with DAPI for 5 minutes (1:20,000). Microscopy images were acquired using an Olympus FluoView 1200 confocal microscope. Images were then analyzed in two separate ways using the Fiji extension package for ImageJ. The first method was to perform counts of microglia in the dorsal horn of the spinal cord by counting IBA1 positive cell bodies containing DAPI staining. In addition to microglia counts each image was analyzed using corrected total cell fluorescence where the background fluorescence was subtracted from the total fluorescence of each image to look at the fluorescent staining intensity between groups.
Statistics
Graphpad Prism Version 7 was use for all statistical analysis. Data are shown as mean ± standard error of the mean (SEM). The level of significance was set at α < 0.05. Behavioral differences between groups were measured using a two-Way ANOVA (Table 1) with Bonferroni’s post hoc test (Table 2). In experiments examining microglial response to IL-6r, differences between groups were measured using an unpaired t-test (Table 2). A p-value of < 0.05 was used to determine statistical significance.
Table 1. Two-way ANOVA.
Two-way ANOVA For each figure, the F-statistic and p-value information has been provided for each separate two-way ANOVA.
| Source of Variation Treatment | Time/Day, Location or Error type | Interaction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fig | Panel | dfn,dfd | F | p | dfn,dfd | F | p | dfn, dfd | F | p |
| 2 | C, Males | 1,10 | 29.01 | 0.0003 | 1,10 | 11.08 | 0.0076 | 1, 10 | 3.556 | 0.0887 |
| C, Females | 1,10 | 128.8 | <0.0001 | 1,10 | 15.55 | 0.0028 | 1, 10 | 4.872 | 0.0518 | |
| D, Males | 1,7 | 5.191 | 0.0568 | 1,7 | 4.781 | 0.0650 | 1, 7 | 9.823 | 0.0165 | |
| D, Females | 1,9 | 2.326 | 0.1616 | 1,9 | 0.7028 | 0.4235 | 1, 9 | 0.3276 | 0.5811 | |
| 3 | B, acute males | 1,40 | 60.65 | <0.0001 | 3,40 | 15.53 | <0.00001 | 3, 40 | 8.02 | 0.0003 |
| B, Priming males | 1,20 | 15.97 | 0.0007 | 1,20 | 48.57 | <0.0001 | 1, 20 | 30.33 | <0.0001 | |
| B, Acute females | 1,16 | 55.89 | <0.0001 | 3,16 | 26.34 | <0.0001 | 3, 16 | 6.808 | 0.0036 | |
| B, priming females | 1,8 | 0.8415 | 0.3858 | 1,8 | 366.7 | <0.0001 | 1, 8 | 0.5853 | 0.4662 | |
| C, acute males | 1.56 | 10.25 | 0.0023 | 3,6 | 58.18 | <0.0001 | 3, 56 | 1.032 | 0.3854 | |
| C, Priming males | 1,42 | 32.32 | <0.0001 | 2,42 | 50.46 | <0.0001 | 2, 42 | 12.89 | <0.0001 | |
| C, Acute females | 1,56 | 2.122 | 0.1508 | 3,56 | 93.38 | <0.0001 | 3, 56 | 0.3371 | 0.7986 | |
| C, priming females | 1,42 | 3.552 | 0.0064 | 2.42 | 87.81 | <0.0001 | 2, 42 | 0.139 | 0.8706 | |
| 4 | B, acute, TNP-ATP | 1,40 | 0.8198 | 0.3707 | 3,40 | 62.54 | <0.0001 | 3, 40 | 0.4399 | 0.7257 |
| B, priming, TNP-ATP | 1,20 | 4.695 | 0.0425 | 1,20 | 91.02 | <0.0001 | 1, 20 | 1.4104 | 0.2499 | |
| B, acute, skepinone | 1,40 | 1.874 | 0.1786 | 3,40 | 98.48 | <0.0001 | 3, 40 | 0.3043 | 0.8221 | |
| B, priming, skepinone | 1,20 | 1.513 | 0.2330 | 1,20 | 145.9 | <0.0001 | 1, 20 | 0.04539 | 0.8335 | |
| 5 | - | 1,30 | 0.7261 | 0.4009 | 4,30 | 1.822 | 0.1506 | 4, 30 | 0.5357 | 0.7105 |
Table 2.
Post-hoc Bonferroni for two-way ANOVA and unpaired t-test
P-values for Post-hoc Bonferroni for and upaired t-test P-values are provided for each comparison for unpaired t-tests in figure one and all post-hoc Bonferroni tests in the remaining experiments.
| Bonferroni | |||||
|---|---|---|---|---|---|
| Fig | Comparison | p | Fig | Comparison | p |
| 2C – M | IL-6r v. Saline (3h) | <0.00001 | 3C – F | Acute | |
| IL-6r v. Saline (24h) | 0.000017 | Skepinone v. Veh (BL) | 0.4979 | ||
| 2C – F | IL-6r v. Saline (3h) | <0.0001 | Skepinone v. Veh (3h) | >0.9999 | |
| IL-6r v. Saline (24h) | 0.0324 | Skepinone v. Veh (24h) | >0.9999 | ||
| 2D – M | IL-6r v. Saline (3h) | 0.0096 | Skepinone v. Veh (72h) | >0.9999 | |
| IL-6r v. Saline (24h) | >0.9999 | Priming | |||
| 2D – F | IL-6r v. Saline (3h) | >0.9996 | Skepinone v. Veh (7d) | 0.4104 | |
| IL-6r v. Saline (24h) | 0.3696 | Skepinone v. Veh (3h) | >0.9999 | ||
| 3B – M | Acute | Skepinone v. Veh (24h) | >0.9999 | ||
| TNP-ATP v. Veh (BL) | >0.9999 | 4B – M | Acute | ||
| TNP-ATP v. Veh (3h) | <0.0001 | TNP - ATP v. Veh (BL) | >0.9999 | ||
| TNP-ATP v. Veh (24h) | <0.0001 | TNP - ATP v. Veh (3h) | >0.9999 | ||
| TNP-ATP v. Veh (72h) | <0.0001 | TNP - ATP v. Veh (24h) | >0.9999 | ||
| Priming | TNP - ATP v. Veh (72h) | >0.9999 | |||
| TNP-ATP v. Veh (7d) | 0.5965 | Priming | |||
| TNP-ATP v. Veh (3h) | <0.0001 | TNP - ATP v. Veh (7d) | 0.9911 | ||
| 3B – F | Acute | TNP - ATP v. Veh (3h) | 0.05599 | ||
| TNP-ATP v. Veh (BL) | >0.9999 | Acute | |||
| TNP-ATP v. Veh (3h) | 0.0032 | Skepinone v. Veh (BL) | >0.9999 | ||
| TNP-ATP v. Veh (24h) | 0.0004 | Skepinone v. Veh (3h) | 0.7718 | ||
| TNP-ATP v. Veh (72h) | 0.0001 | Skepinone v. Veh (24h) | >0.9999 | ||
| Priming | Skepinone v. Veh (72h) | >0.9999 | |||
| TNP-ATP v. Veh (7d) | 0.5366 | Priming | |||
| TNP-ATP v. Veh (3h) | >0.9999 | Skepinone v. Veh (7d) | 0.9609 | ||
| 3C – M | Acute | Skepinone v. Veh (3h) | 0.6395 | ||
| Skepinone v. Veh (BL) | >0.999 | ||||
| Skepinone v. Veh (3h) | 0.4960 | 5 -Rotarod | Skepinone v. Veh (BL) | >0.9999 | |
| Skepinone v. Veh (24h) | 0.2364 | Skepinone v. Veh (3h) | >0.9999 | ||
| Skepinone v. Veh (72h) | 0.0396 | Skepinone v. Veh (24h) | >0.9999 | ||
| Priming | Skepinone v. Veh (3h+) | 0.5182 | |||
| Skepinone v. Veh (7d) | >0.999 | Skepinone v. Veh (24h+) | >0.9999 | ||
| Skepinone v. Veh (3h) | <0.0001 | ||||
| Skepinone v. Veh (24h) | <0.0001 | ||||
| Unpaired T - test | |||||
| 1C – M | IL-6 v. Saline (3h) | 0.9163 | 1D – M | IL - 6 v. Saline (3h) | 0.3938 |
| 1C – F | IL-6 v. Saline (3h) | 0.3969 | 1D – F | IL - 6 v. Saline(3h) | 0.05 |
Results
Initiation of priming using a single injection of IL-6r does not cause a significant increase in activated spinal microglia
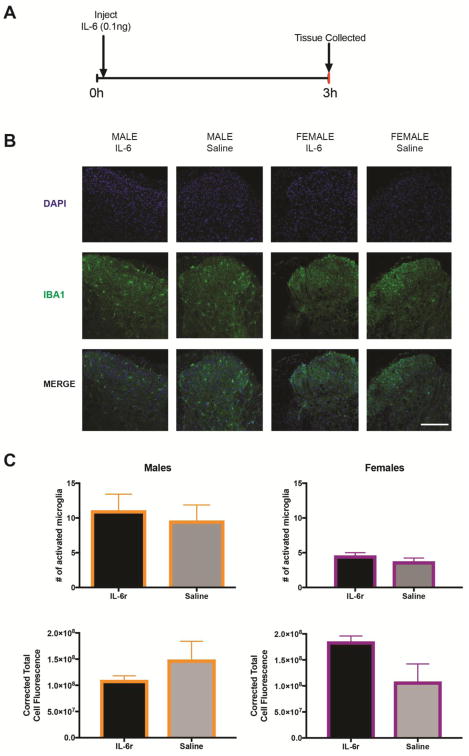
Our first objective was to determine if a single hindpaw injection of IL-6r creates an increase in levels of activated microglia in the spinal cord. We chose to use the soluble IL-6r for these experiments because previous studies have shown that IL-6 is constitutively expressed in peripheral tissues (König et al., 2016) and a rate-limiting step for IL-6-mediated activation of nociceptors is availability of soluble IL6r (Obreja et al., 2002). We reasoned that using this approach would reduce chances of sexual dimorphism in responses based simply on IL-6 or soluble IL-6r expression in mice. We injected IL-6r into the hindpaw of male and female mice and then removed the spinal cord at 3 hr after this single injection (FIG 1A). We did IHC using IBA-1, a marker for activated microglia, and co-stained for IB4, a marker of non-peptidergic neurons, and DAPI which labels all nuclei (FIG 1B). We found that a single injection of IL-6r did not increase numbers of activated microglia (FIG 1C) or intensity of total fluorescence (FIG 1D) in either male or female mice at this time point even though mechanical hypersensitivity is present (see below).
Figure 1. IL-6r injections do not cause an increase in levels of activated spinal microglia.
A. IL-6r was injected into plantar aspect of the hindpaw and spinal cord tissue was dissected 3 hr later. B. Spinal cord tissue was stained for IBA1 and DAPI in order to quantify microglia. Scale bar is 200 μm (n = 4–6 mice per group). C. There was no difference in either numbers of activated microglia or D. corrected total cell fluorescence Data are displayed as mean ± SEM.
Increased microglial activation when hyperalgesic priming is revealed by PGE2 injection in male and female mice
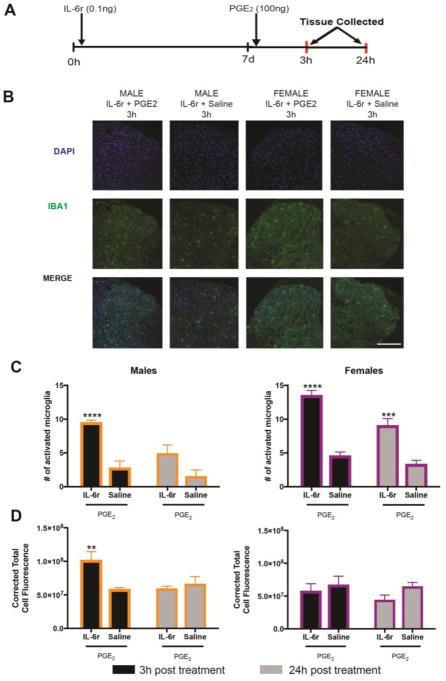
We next sought to determine if injection of PGE2 following priming with IL-6r would increase levels of activated microglia. In both male and female animals, IL-6r was injected into the hindpaw as described above. After 7 days, animals were injected with PGE2 into the same hindpaw (FIG 2A). Spinal cords were dissected at either 3 or 24 hr after this injection and again stained for IBA-1, and DAPI and levels of activated microglia were quantified (FIG 2B) as was corrected total cell fluorescence (FIG 2C). In both male and female mice that underwent hyperalgesic priming, there was a transient increase in numbers of activated microglia in the spinal cord at 3 hr post PGE2 injection, with this change returning to baseline levels at 24 hr in male mice. The increase in activated microglia was more persistent in female mice lasting for 24 hrs. The level of total fluorescence intensity was only elevated in male mice 3hr after the PGE2 injection, with no increase in total fluorescence seen in female mice at either 3hr or 24hr post the PGE2 injection (FIG 2C). We note that although significant changes were observed in these experiments, the magnitude of change observed in the priming model was modest compared to published reports in neuropathic pain models and to our own findings in the spared nerve injury paradigm in mice (data not shown)(Wodarski et al., 2009),(Sweitzer et al., 2002),(Romero-Sandoval et al., 2008).
Figure 2. Hyperalgesic priming revealed with PGE2 injection after IL-6r leads to increased spinal microglia at 3 hr after injection.
A. Animals received an intraplantar injection of IL-6 and then 7 d later received an intraplantar injection of PGE2. Spinal cords were dissected at 3 or 24h post (n = 4–6 mice per group) B. After PGE2 injection spinal cords were stained for IBA1 and DAPI. Scale bar is 200 μm. C. 3 hr following PGE2 injection in primed mice, levels of activated microglia were elevated in both males and females. D. Total cell fluorescence was only elevated in male mice at 3 hr post PGE2 injection. Data are displayed as mean ± SEM. Differences between groups were measured using a two-Way ANOVA with Bonferroni’s post hoc test, **p < 0.005, ***p < 0.0005, ****p < 0.0001.
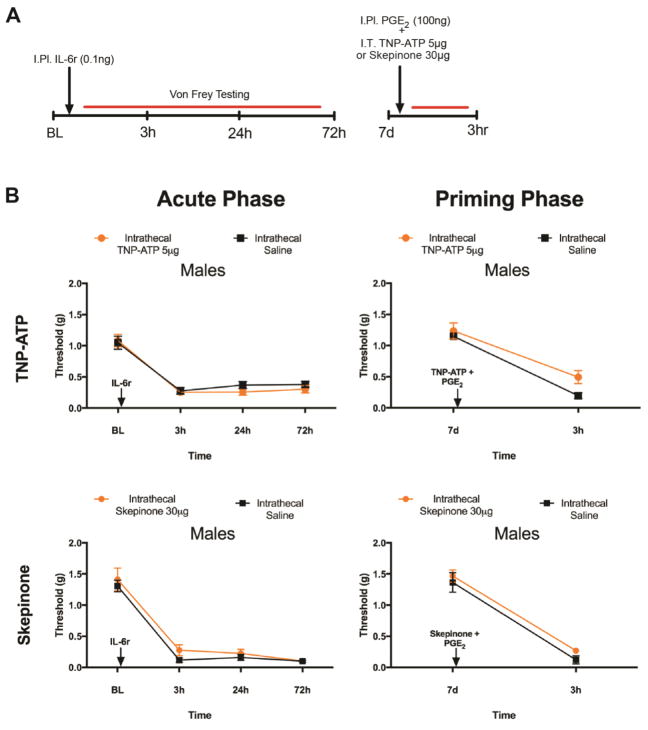
Intrathecal injection of a P2X3/4 inhibitor blocks the acute response to IL-6r in male and female mice and hyperalgesic priming exclusively in male mice
P2X4 expression on microglia is upregulated in states of neuropathic pain (Tsuda et al., 2003). This upregulation causes an increased activation of p38 MAPK and is linked to enhanced brain derived neurotrophic factor (BDNF) release from spinal microglia (Tsuda et al., 2003),(Ulmann et al., 2008). Pharmacological blockage of P2X4 receptors on spinal microglia causes a decrease in mechanical allodynia in neuropathic pain models (Tsuda et al., 2003). Many of these studies have relied on the use of TNP-ATP, which blocks both P2X3 and P2X4 (Lewis et al., 1998). P2X3 is prominently expressed by dorsal root ganglion (DRG) neurons (Guo et al., 1999) and single cell sequencing data from DRG neurons shows that P2X4 is also expressed by these neurons (Usoskin et al., 2015). We chose to use TNP-ATP because recent studies examining the microglial contributions to pain in males and females have used this drug with the interpretation that it is acting via P2X4 (Sorge et al., 2015).
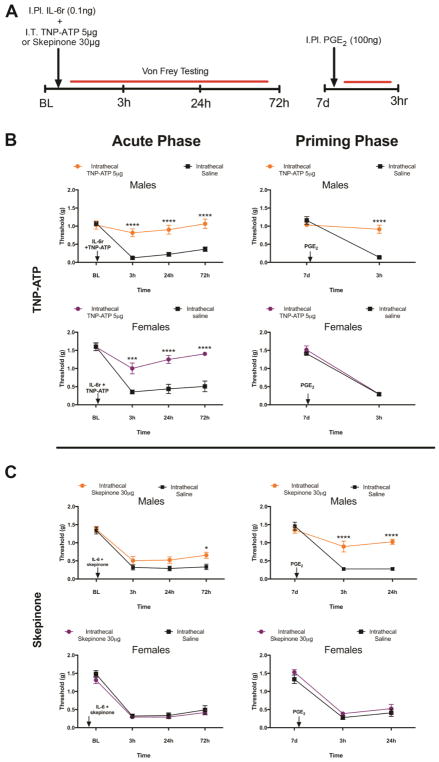
We assessed the action of intrathecal injection of TNP-ATP (5 μg) at the time of the initial hindpaw injection of IL-6r in males and females (FIG 3A). TNP-ATP injected intrathecally at this time point blocked the initial mechanical hypersensitivity response to IL-6r in both males and females (FIG 3B). The duration of the behavioral response to IL-6r was identical in male and female mice as was the magnitude of the inhibitory effect with TNP-ATP. When we then evaluated the presence of hyperalgesic priming, we noted that TNP-ATP completely blocked the response to the subsequent injection of PGE2 in males only, having no impact on mechanical hypersensitivity in response to PGE2 in females (FIG 3B). It is remarkable that there was no sign of initial mechanical hypersensitivity in female mice with TNP-ATP injection but hyperalgesic priming was still present. This shows that this form of priming can be present without any overt forms of pain in the priming phase. This is consistent with findings in type II priming which have recently been reported (Araldi et al., 2015),(Araldi et al., 2017),(Araldi et al., 2016). We attribute the acute effect of TNP-ATP on mechanical hypersensitivity to its affinity for P2X3 receptors, which are present on DRG nociceptors and are known to participate in the development of mechanical hypersensitivity in response to many pro-nociceptive factors (Souslova et al., 2000),(Barclay et al., 2002),(Mansoor et al., 2016).
Figure 3. Intrathecal injection of TNP-ATP or skepinone blocks hyperalgesic priming exclusively in male mice.
A. IL-6r was injected intraplantar into male mice at the same time as animals received an intrathecal injection of either TNP-ATP or skepinone. Mechanical hypersensitivity was then tested at indicated time points (n = 4–6 mice per group). B. Both male and female animals that received TNP-ATP at the time of IL-6r injection had the acute response to IL-6r blocked. In male mice that received TNP-ATP, priming was blocked. C. Skepinone had no impact on the acute response to IL-6r in males or females, but attenuated hyperalgesic priming in male mice. Data are displayed as mean ± SEM. Two-way anova with Bonferroni post hoc test, * p<0.05, *** p<0.001, **** p<0.0001.
Intrathecal injection of a p38 inhibitor blocks hyperalgesic priming exclusively in male mice
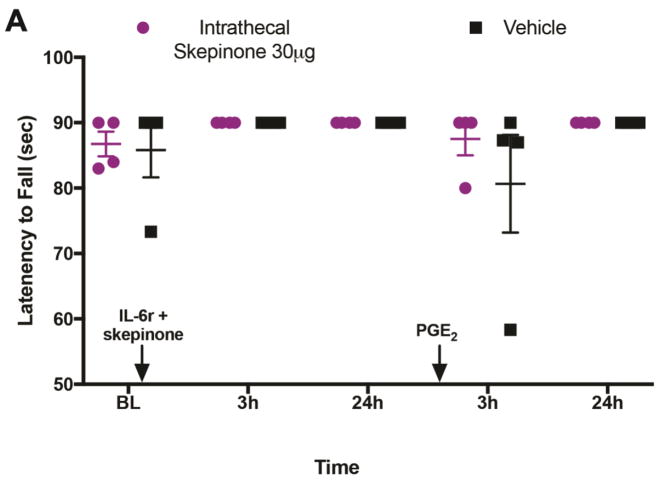
We next examined the impact of a distinct molecule that has also been described as a microglial inhibitor on hyperalgesic priming by using the p38 MAP kinase inhibitor skepinone (Koeberle et al., 2011). Previous studies have demonstrated that skepinone inhibits both inflammatory and neuropathic pain in male mice, but has no effect in female mice (Taves et al., 2016). We administered skepinone intrathecally to male and female mice at the time of the initial hindpaw IL-6r injection (FIG 3A). In male mice we found that this injection had no impact on the initial response to IL-6r, but significantly attenuated hyperalgesic priming which was revealed by injection of PGE2 (FIG 3C). Conversely, in female mice intrathecal injection of skepinone had no impact on mechanical hypersensitivity during the initial response to IL-6r or on the hyperalgesic priming revealed by injection of PGE2 (FIG 3C). In addition, intraplantar IL-6r and PGE2 did not impact locomotion in the rotorod test in female mice that received intrathecal injections of Skepinone or vehicle (FIG 4A).
Figure 4. Hyperalgesic priming does not impact locomotion in animals that receive skepinone or vehicle.
A. IL-6r was given intraplantarly and followed by a PGE2 intraplantar injection with skepinone intrathecal injections. Locomotion was assessed using rotarod and latency to fall was recorded in seconds at 3 and 24 hr following IL-6r and 3 and 24 hr following PGE2 (n = 4 mice for all groups, all mice are female). Data are displayed as individual values with mean ± SEM.
Hyperalgesic priming is not reversed by inhibiting P2X3/4 or p38 in male mice
Our previous experiments demonstrate that interfering with P2X3/4 or p38 signaling at the time of initial nociceptive insult blocks the development of hyperalgesic priming only in male mice. In our next set of experiments we sought to determine if hyperalgesic priming could be reversed by blocking these same pathways at the time of the hindpaw injection of PGE2 in male mice. We chose to examine male mice only because of the lack of efficacy of both TNP-ATP and skepinone in female mice during the priming phase of the paradigm in the previous experiments. Male mice were injected with IL-6r and after mechanical hypersensitivity returned to baseline levels, the mice were injected intrathecally with either TNP-ATP or skepinone at the same time as PGE2 injection into the hindpaw (FIG 5A). Neither TNP-ATP nor skepinone reversed priming as mechanical sensitivity was not affected by these drugs after PGE2 injection (FIG 5B).
Figure 5. Neither TNP-ATP nor skepinone reverse priming in male mice.
A. IL-6r was given intraplantar and followed by a PGE2 intraplantar injection with either TNP-ATP or skepinone intrathecal injections. B. Male mice respond acutely to IL-6r. Once hyperalgesic priming had been established with an IL-6r injection it could not be reversed with an injection at the time of PGE2 of either TNP-ATP (n=6 for all TNP-ATP groups) or skepinone (n = 8 mice for all skepinone groups). Data are displayed as mean ± SEM.
Discussion
Previous findings have repeatedly demonstrated that there is a difference in the cell types that mediate chronic pain, mostly in neuropathic pain models, with microglia having a male-specific role (Mapplebeck et al., 2016),(Sorge et al., 2015),(Taves et al., 2016). In our experiments in the hyperalgesic priming paradigm, some of our results are consistent with these findings but our results also suggest that the mechanisms engaged in a male specific fashion may not be specific for microglia. Interestingly, while we did observe an increase in activated microglia after PGE2 injection in the hyperalgesic priming in both males and females, inhibition of P2X3/4 or p38 had no behavioral effect at this time point in male mice. Conversely, we did not observe microglial activation at the initiation of priming in male or female mice, yet P2X3/4 antagonism blocked the acute response in both sexes and priming in males. Inhibition of p38 was effective in blocking development of priming in male but not female mice when given at time of initiation. Our interpretation of these findings is that it is likely that activation of P2XRs on neurons and microglia is responsible for the response to the acute response of IL-6r injections in males. Also, P2X3/4 or p38 inhibition can block development of an injury-induced chronic pain state selectively in male mice but these effects may not be due to direct effects on microglia.
We demonstrate that TNP-ATP strongly attenuates the initial response to IL-6r in male and female mice. We attribute this to TNP-ATPs antagonistic effects on P2X3 receptors, which are expressed presynaptically on nociceptive neurons that form synapses in the spinal dorsal horn (Mansoor et al., 2016). Plasticity in P2X3 receptor expression has been found in DRG neurons in chronic pain models, including in the chronic constriction injury (CCI) model of chronic neuropathic pain (Novakovic et al., 1999),(Souslova et al., 2000 p.3),(Barclay et al., 2002 p.3). Many previous reports have linked P2X3 to mechanical hypersensitivity following injury and a successful clinical trial has been reported in humans with P2X3 antagonists in the nociceptor-linked disease chronic cough (Abdulqawi et al., 2015),(Burnstock, 2017). While acute effects were not sexually dimorphic, the effects on hyperalgesic priming were only observed in males. We do not have a clear explanation for this finding, however, it could potentially be explained by sexual dimorphisms in expression or signaling for P2X3 or P2X4. Future experiments should explore this possibility.
In contrast to P2X3/4 antagonist experiments, we found that the specific p38 inhibitor, skepinone, had no effect on the initial behavioral response to IL-6r in either male or female mice even though this drug attenuated the development of priming exclusively in male mice. This finding of sexual dimorphism with p38 inhibition is consistent with the existing literature (Taves et al., 2016). Therefore, our results provide mixed evidence that microglia contribute to hyperalgesic priming but stronger evidence for a role of P2X3/4 and p38 signaling specifically in males. This contribution only appears to play an important role early in the development of hyperalgesic priming since treatments that block the development of priming are not capable of reversing it once it is established.
Despite enormous efforts, the emergence of new treatments for chronic pain has been disappointing. The majority of patients seen by physicians to treat chronic pain are female, and sex differences in the epidemiology of human pain conditions are obvious (Anon, 2011). The lack of new, effective treatments could be due to molecular differences in how males and females transition from an acute to a chronic pain state. Because the majority of pre-clinical and even clinical research has failed to analyze sex as a factor (Sorge and Totsch, 2016) this could be a strong contributing factor in the failure of translation that is decried across neuroscience. Until recently, very little work had been done to address sexual dimorphisms seen in chronic pain. While many groups have demonstrated male-specific mechanisms for chronic pain maintenance, there has been very little work to examine what female-specific mechanisms exist in chronic pain states. Our experiments contribute to the growing evidence for a sexual dimorphism in the transition to a chronic pain state, and highlight a need to further investigate molecular mechanisms where there is a particular gap in knowledge on what drives the development and maintenance of chronic pain states in females. An important priority should be the discovery of mechanisms that promote pain plasticity in females.
Highlights.
Both sexes of mice that undergo hyperalgesic priming have an increase in activated microglia.
A P2X3/4 antagonist blocks hyperalgesic priming in males only.
A p38 inhibitor attenuated hyperalgesic priming in males only.
Once priming was established it could not be reversed with a TNP-ATP or skepinone.
Acknowledgments
This work was supported by NIH grants R01NS065926 (TJP), R01NS098826 (TJP and GD) and The University of Texas STARS program (TJP and GD)
Footnotes
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- Abdulqawi R, Dockry R, Holt K, Layton G, McCarthy BG, Ford AP, Smith JA. P2X3 receptor antagonist (AF-219) in refractory chronic cough: a randomised, double-blind, placebo-controlled phase 2 study. The Lancet. 2015;385:1198–1205. doi: 10.1016/S0140-6736(14)61255-1. [DOI] [PubMed] [Google Scholar]
- Anonymous. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, D.C: National Academies Press; 2011. [Accessed January 14, 2016]. Available at: http://www.nap.edu/catalog/13172. [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD. Repeated Mu-Opioid Exposure Induces a Novel Form of the Hyperalgesic Priming Model for Transition to Chronic Pain. J Neurosci. 2015;35:12502–12517. doi: 10.1523/JNEUROSCI.1673-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD. Adenosine-A1 receptor agonist induced hyperalgesic priming type II: PAIN. 2016;157:698–709. doi: 10.1097/j.pain.0000000000000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araldi D, Ferrari LF, Levine JD. Hyperalgesic priming (type II) induced by repeated opioid exposure: maintenance mechanisms. PAIN. 2017;158:1204–1216. doi: 10.1097/j.pain.0000000000000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay J, Patel S, Dorn G, Wotherspoon G, Moffatt S, Eunson L, Abdel’al S, Natt F, Hall J, Winter J, Bevan S, Wishart W, Fox A, Ganju P. Functional Downregulation of P2X3 Receptor Subunit in Rat Sensory Neurons Reveals a Significant Role in Chronic Neuropathic and Inflammatory Pain. J Neurosci. 2002;22:8139. doi: 10.1523/JNEUROSCI.22-18-08139.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barragán-Iglesias P, Lou T-F, Bhat VD, Megat S, Burton MD, Price TJ, Campbell ZT. Inhibition of Poly(A)-binding protein with a synthetic RNA mimic reduces pain sensitization in mice. [Accessed May 30, 2018];Nat Commun. 2018 :9. doi: 10.1038/s41467-017-02449-5. Available at: http://www.nature.com/articles/s41467-017-02449-5. [DOI] [PMC free article] [PubMed]
- Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic Signalling: Therapeutic Developments. [Accessed February 21, 2018];Front Pharmacol. 2017 :8. doi: 10.3389/fphar.2017.00661. Available at: http://journal.frontiersin.org/article/10.3389/fphar.2017.00661/full. [DOI] [PMC free article] [PubMed]
- Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- Coraggio V, Guida F, Boccella S, Scafuro M, Paino S, Romano D, Maione S, Luongo L. Neuroimmune-Driven Neuropathic Pain Establishment: A Focus on Gender Differences. Int J Mol Sci. 2018;19:281. doi: 10.3390/ijms19010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD. Role of IL-6 in Chronic Muscle Hyperalgesic Priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon W. The up-and-down method for small samples. J Am Stat Assoc. 1965;60:967–978. [Google Scholar]
- Ekdahl CT, Claasen J-H, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace PM, Hutchinson MR, Maier SF, Watkins LR. Pathological pain and the neuroimmune interface. Nat Rev Immunol. 2014;14:217–231. doi: 10.1038/nri3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Intrathecal morphine in mice: a new technique. Eur J Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M. Microglia and neuropathic pain. Glia. 2009;57:1469–1479. doi: 10.1002/glia.20871. [DOI] [PubMed] [Google Scholar]
- Kandasamy R, Price TJ. The Pharmacology of Nociceptor Priming. In: Schaible H-G, editor. Pain Control. Berlin, Heidelberg: Springer Berlin Heidelberg; 2015. [Accessed February 21, 2018]. pp. 15–37. Available at: http://link.springer.com/10.1007/978-3-662-46450-2_2. [Google Scholar]
- Kim D-S, Li K-W, Boroujerdi A, Peter Yu Y, Zhou C-Y, Deng P, Park J, Zhang X, Lee J, Corpe M, Sharp K, Steward O, Eroglu C, Barres B, Zaucke F, Xu ZC, Luo ZD. Thrombospondin-4 Contributes to Spinal Sensitization and Neuropathic Pain States. J Neurosci. 2012;32:8977–8987. doi: 10.1523/JNEUROSCI.6494-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeberle SC, Romir J, Fischer S, Koeberle A, Schattel V, Albrecht W, Grütter C, Werz O, Rauh D, Stehle T, Laufer SA. Skepinone-L is a selective p38 mitogen-activated protein kinase inhibitor. Nat Chem Biol. 2011;8:141–143. doi: 10.1038/nchembio.761. [DOI] [PubMed] [Google Scholar]
- König C, Morch E, Eitner A, Möller C, Turnquist B, Schaible H-G, Ebersberger A. Involvement of Spinal IL-6 Trans-Signaling in the Induction of Hyperexcitability of Deep Dorsal Horn Neurons by Spinal Tumor Necrosis Factor-Alpha. J Neurosci Off J Soc Neurosci. 2016;36:9782–9791. doi: 10.1523/JNEUROSCI.4159-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis CJ, Surprenant A, Evans RJ. 2′,3′-O-(2,4,6-trinitrophenyl) adenosine 5′-triphosphate (TNP-ATP)--a nanomolar affinity antagonist at rat mesenteric artery P2X receptor ion channels. Br J Pharmacol. 1998;124:1463–1466. doi: 10.1038/sj.bjp.0702001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes DM, Malek N, Edye M, Jager SB, McMurray S, McMahon SB, Denk F. Sex differences in peripheral not central immune responses to pain-inducing injury. [Accessed May 31, 2018];Sci Rep. 2017 :7. doi: 10.1038/s41598-017-16664-z. Available at: http://www.nature.com/articles/s41598-017-16664-z. [DOI] [PMC free article] [PubMed]
- Mansoor SE, Lü W, Oosterheert W, Shekhar M, Tajkhorshid E, Gouaux E. X-ray structures define human P2X3 receptor gating cycle and antagonist action. Nature. 2016;538:66–71. doi: 10.1038/nature19367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapplebeck JCS, Beggs S, Salter MW. Sex differences in pain: a tale of two immune cells. PAIN. 2016;157:S2–S6. doi: 10.1097/j.pain.0000000000000389. [DOI] [PubMed] [Google Scholar]
- Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- Novakovic SD, Kassotakis LC, Oglesby IB, Smith JA, Eglen RM, Ford AP, Hunter JC. Immunocytochemical localization of P2X3 purinoceptors in sensory neurons in naive rats and following neuropathic injury. Pain. 1999;80:273–282. doi: 10.1016/s0304-3959(98)00225-5. [DOI] [PubMed] [Google Scholar]
- Obreja O, Schmelz M, Poole S, Kress M. Interleukin-6 in combination with its soluble IL-6 receptor sensitises rat skin nociceptors to heat, in vivo. Pain. 2002;96:57–62. doi: 10.1016/s0304-3959(01)00420-1. [DOI] [PubMed] [Google Scholar]
- Price TJ, Inyang KE. Commonalities between pain and memory mechanisms and their meaning for understanding chronic pain. Prog Mol Biol Transl Sci. 2015;131:409–434. doi: 10.1016/bs.pmbts.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichling DB, Levine JD. Critical role of nociceptor plasticity in chronic pain. Trends Neurosci. 2009;32:611–618. doi: 10.1016/j.tins.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Sandoval A, Chai N, Nutile-McMenemy N, DeLeo JA. A comparison of spinal Iba1 and GFAP expression in rodent models of acute and chronic pain. Brain Res. 2008;1219:116–126. doi: 10.1016/j.brainres.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- Sorge RE, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18:1081–1083. doi: 10.1038/nn.4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, LaCroix-Fralish ML, Tuttle AH, Sotocinal SG, Austin J-S, Ritchie J, Chanda ML, Graham AC, Topham L, Beggs S, Salter MW, Mogil JS. Spinal cord Toll-like receptor 4 mediates inflammatory and neuropathic hypersensitivity in male but not female mice. J Neurosci Off J Soc Neurosci. 2011;31:15450–15454. doi: 10.1523/JNEUROSCI.3859-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge RE, Totsch SK. Sex Differences in Pain: Sex Differences in Pain. [Accessed November 20, 2016];J Neurosci Res. 2016 Available at: http://doi.wiley.com/10.1002/jnr.23841.
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa L, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenius-Oosthuizen D, Smith AJH, Kidd EJ, Wood JN. Warm-coding deficits and aberrant inflammatory pain in mice lacking P2X 3 receptors. Nature. 2000;407:1015. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Sweitzer SM, White KA, Dutta C, DeLeo JA. The differential role of spinal MHC class II and cellular adhesion molecules in peripheral inflammatory versus neuropathic pain in rodents. J Neuroimmunol. 2002;125:82–93. doi: 10.1016/s0165-5728(02)00036-x. [DOI] [PubMed] [Google Scholar]
- Taves S, Berta T, Liu D-L, Gan S, Chen G, Kim YH, Van de Ven T, Laufer S, Ji R-R. Spinal inhibition of p38 MAP kinase reduces inflammatory and neuropathic pain in male but not female mice: Sex-dependent microglial signaling in the spinal cord. Brain Behav Immun. 2016;55:70–81. doi: 10.1016/j.bbi.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–783. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci Off J Soc Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usoskin D, Furlan A, Islam S, Abdo H, Lönnerberg P, Lou D, Hjerling-Leffler J, Haeggström J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P. Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci. 2015;18:145–153. doi: 10.1038/nn.3881. [DOI] [PubMed] [Google Scholar]
- Wodarski R, Clark AK, Grist J, Marchand F, Malcangio M. Gabapentin reverses microglial activation in the spinal cord of streptozotocin-induced diabetic rats. Eur J Pain. 2009;13:807–811. doi: 10.1016/j.ejpain.2008.09.010. [DOI] [PubMed] [Google Scholar]