Abstract
Background:
There is considerable evidence that pre- and post-natal factors are associated with a wide range of psychopathology in offspring during childhood and adolescence.
Objective:
The main aims of the present study were to examine the associations between pre- and perinatal factors and psychopathology in offspring during adulthood, and to explore whether family factors (i.e., family cohesion, mother’s social support, and father’s social support) mediate these relationships.
Method:
Information on pre- and perinatal events was collected from biological mothers of the participants (N=315) when they were between 14 and 18 years who were then followed up until they reached age 30.
Results:
Maternal obstetric history and illness during first year were significant predictors of offspring anxiety disorder. Maternal emotional health predicted offspring affective disorder. Difficult delivery and breast feeding predicted disruptive disorder. The relationship between maternal obstetric history/emotional health and anxiety/affective disorder were no longer significant after controlling for family cohesion.
Limitations:
The information was based on maternal recall when their offspring were between 14-18 years which may be subjected to recall bias.
conclusion:
The association between pre- and postnatal factors and psychopathology of offspring during adulthood is mediated by familial factors.
Keywords: Prenatal factors, postnatal factors, offspring, psychopathology, depression
1. Introduction
Research during the past three decades has provided considerable evidence that pre- and postnatal factors are associated with a wide range of psychopathology during childhood and adolescence (Allen et al., 1998; O’Connor et al., 2002, 2003; Stene-Larsen et al., 2009; Taylor et al., 2017). Pre- and postnatal factors can be grouped under (1) prenatal environment (e.g., maternal physical and mental health, experiences of stress during the pregnancy, use of drugs during pregnancy); (2) intrapartum events (e.g., surgical delivery, birth difficulties); (3) early neonatal environment (e.g., prematurity, anoxia); and (4) later neonatal environment (e.g., breast feeding, infant health during the first year of life) (Allen et al., 1998).
Amongst the prenatal environments, maternal anxiety or depression has consistently been linked with behavioral and/or emotional problems in the offspring (O’Connor et al., 2002). As reported in a series of studies conducted by O’Connor and colleagues (O’Connor et al., 2002, 2003), women with a high level of anxiety at 32 weeks’ gestation had double the risk of having children with behavioral problems at 4 and 7 years of age; children from this group of women also had high risk of having attention-deficit/hyperactivity disorder (ADHD), anxiety or depression, or conduct disorder symptoms; the attributable load in behavioral problems that are due to antenatal anxiety was estimated to be 15%. A recent study by Korhonen et al. (2012) showed maternal prenatal depression to be associated with adolescent boys’ (but not girls) poor psychosocial functioning and with externalizing problems.
Maternal smoking during pregnancy is another prenatal event that has consistently been linked with an increased risk of externalizing behaviors such as ADHD and conduct disorder among offspring (Indredavik et al., 2006; Nigg et al., 2007; Stene-Larsen et al., 2009; Schmitz et al., 2006; Wakschlag et al., 2006). Taylor et al. (2017) recently compared the associations of maternal smoking during pregnancy and mother’s partner’s smoking during pregnancy with offspring depression using four large data sets from the UK, Sweden, Brazil, and Norway. Maternal smoking during pregnancy was associated with an increased risk of offspring depression, but not with paternal smoking during pregnancy. Interestingly, individuals whose mothers smoked during pregnancy, compared to their siblings from another pregnancy in which the mother did not smoke, were no more likely to have depression; the authors suggested that the association between maternal smoking during pregnancy and offspring depression may have been confounded by unmeasured factors.
Intrapartum events such as obstetrical complications are significantly higher among individuals with a wide range of psychiatric disorders compared with those without any psychiatric disorders (Cantor-Graae et al., 1993; Kinney et al., 1994; see review by Serati et al., 2017). For example, in a study by Done et al. (1991) patients with mood disorder had significantly higher rates of obstetrical complications than participants from the general population. Xu et al. (2007) examined whether there is link between obstetrical complication and the presence of depression; their results showed obstetric and prenatal complications to be significantly more frequent among patients with depressive disorder compared to adult siblings without this disorder. In a recent study by Nguyen et al. (2012), women with severe mental illness (i.e., schizophrenia, bipolar, and non-psychotic disorders) were found to have a lower rate of spontaneous vaginal delivery and a higher rate of complications during pregnancy compared to women in the general population. A recent study by Buoli et al. (2016) found 17% of the patients with psychotic and mood disorders to have a history of obstetrical complications.
Early neonatal events such as low birth weight have frequently been reported to be linked with anxiety disorders (Nomura et al., 2007), as well as with anxiety and depressive symptoms (Alati et al., 2009). A recent review by Serati and colleagues (2017) have identified low bith weight to be a major risk factor for ADHD in children and adolescents. Betts and colleagues (Betts et al., 2011) recently examined the association between birth weight and anxiety disorder in young adults using data from the Mater University Study of Pregnancy. A linear and inverse association was found between birth weight and post-traumatic stress disorder. However, some other studies failed to find any associations between birth weight and mental health problems (Wiles et al., 2006), and others found this association only among females (Alati et al., 2007; Hack et al., 2009).
Late neonatal events, which include breastfeeding, have been associated with emotional and behavioral problems in offspring. Specifically, offspring who were not breastfed compared to those who were breastfed had a higher levels of emotional and behavioral problems, such as anxiety and depression (Allen etal.,1998; Hayatbakhsh et al., 2012; Heikkila et al., 2011; Liu et al., 2014; Oddy et al., 2010; Reynolds et al., 2014), and ADHD (Mimouni-Bloch et al., 2013; Sabuncuoglu et al., 2014_; Schmitt and Romanos, 2012; Stadler et al., 2016; Shamberger, 2012). In a more recent study, Loret de Mola and colleagues (Loret de Mola et al., 2016) examined the association between breastfeeding and mental health outcomes (i.e., depression, generalized anxiety disorder, social anxiety disorder, and common mental disorders) among young adults in Brazil. Information on breastfeeding was collected in early childhood and the participants were re-interviewed at young adulthood. Participants who were breastfed for more than six months were less likely to have more severe depressive symptoms. Furthermore, a longer duration of breastfeeding was associated with a lower risk of disease. However, findings of studies that examined the role of breastfeeding and children’s emotional problems have been inconsistent. For example, Allen et al. (1998) found no significant effect of breast feeding on anxiety and depression in adolescents.
While the above studies have examined specific types of pre- or postnatal factors, Allen and colleagues (1998) examined the association between a wider range of factors and non-schizophrenic psychopathology in offspring at the age of 18 years. Offspring depression at adolescence was found to be associated with not being breast fed and with maternal emotional problems during the pregnancy, whereas anxiety disorder was associated with fever and illness during late postnatal and with maternal history of miscarriage and stillbirth. Disruptive behavior disorder at adolescence was related to poor maternal emotional health during the pregnancy and with birth complications. Substance use disorders in the offspring were predicted by maternal substance (i.e., alcohol, cigarettes, caffeine, and marijuana) during the pregnancy.
While informative, little is known about the long-term impact (i.e., in adulthood) of pre- and postnatal factors in predicting the development of psychiatric disorders by using data from the same birth cohort. Studies that examined the factors that mediate the association between pre- and postnatal factors and psychopathology are also lacking. Previous studies suggest that familial environment is an important factor in the manifestation of specific pre- and perinatal behaviour (e.g., Cemadas et al., 2003), and thus the effects of family cohesion, mother’s social support, and father’s social support were examined in the present report as potential mediators.
Family cohesion and familial social support are influential factors that have been linked to prenatal maternal stress. For example, Kingston, Sword, Krueger, Hanna, & Markle-Reid (2012) showed that low family cohesion during childhood was indirectly associated with prenatal stress through current family cohesion and socioeconomic position. In this study, perceived social support also influenced prenatal stress indirectly through socioeconomic position and childhood stress.
Family functioning does not pose a risk only for the pregnant mother but for the offspring as well. Abell, Baker, Clover, & Ramsey (1991) found that women who perceived their families as dysfunctional were delivered of infants with lower birthweight. Pilowsky, Wickramaratne, Nomura, & Weissman (2006) showed that family discord factors were associated with both parental and offspring depression. Taken together, it can be hypothesized that prenatal and perinatal factors are influenced by family environment under which the pregnancy progress and these factors are predictive of future psychopathology. However, most studies that have been reported to date are cross-sectional in nature and the mediating effect of familial factors has not been adequately evaluated. Prenatal and perinatal factors occur either spontaneously or are determined by a complex interaction of biological factors; in contrast, the mediating effects of familial environment pose a target for intervention that can benefit the child as well as the mother.
Based on the above background, the present study reports the result of a 16-year longitudinal study on the association between pre- and perinatal factors and psychopathology in offspring at adulthood. The specific aims address the two following questions: (a) What is the association between pre- and perinatal factors and psychopathology in offspring at age 30 The prenatal and perinatal factors examined cover a range of effects during the pregnancy, intrapartum (at birth), and neonatal periods. The types of offspring psychopathology examined were anxiety, affective, disruptive, and substance use disorders, (b) Do specific familial factors (i.e., father and mother social support and family cohesion) mediate the relationship between pre- and perinatal factors and psychopathology in offspring at age 30?
The hypotheses to be tested in this study were as follows: First, based on previous studies in children and adolescents (O’Hara, 1995; Heikkila et al., 2011; Liu et al., 2014; Oddy et al., 2010; Reynolds et al., 2014), pre- and perinatal factors experienced during the pregnancy, intrapartum (birth), and neonatal periods are expected to be associated with psychopathology at age 30. Second, the association between pre- and perinatal factors and adult psychopathology is predicted to be mediated by low levels of family cohesion, as well as by low social support from mother and father (Allen et al., 1998).
2. Methods
2.1. Participants
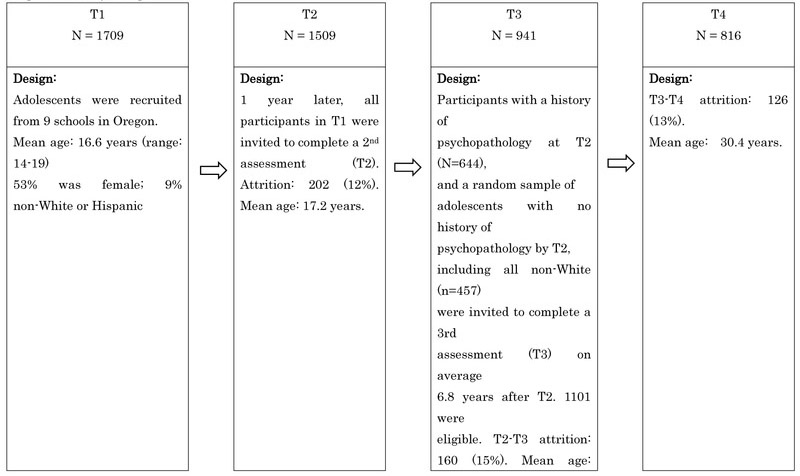
The present study used data from the Oregon Adolescent Depression Project (OADP; Lewinsohn et al., 1993), a 16-year longitudinal study of a large cohort of high school students who were randomly selected from nine high schools in western Oregon. The participants were assessed twice during adolescence, a third time when the average age was 24, and a fourth time when the average age was 30. Atotal of 1,709 adolescents (ages 14–18; mean age 16.6, SD=1.2) completed the initial (T1) assessments between 1987 and 1989, with a response rate at T1 was 61%. Approximately one year later, 1,507 of the adolescents (88%) returned for a second evaluation (T2). Between 1994 and 1999, as participants reached their 24th birthday, a third wave of questionnaires and interviews (T3) was conducted. For the third assessment, all adolescents with a history of a depressive disorder by T2 (n=360) or a history of non-mood disorders (n=284), and a random sample of adolescents with no history of psychopathology by T2 (n=457) were invited to participate in a third (T3) evaluation. At the same time, all non-white T2 participants were retained in the T3 sample to maximize ethnic diversity. This strategy reduces the number of participants necessary to follow-up and reduces study costs and does so by maximizing representativeness of the study population. Of the 1,101 T2 participants who took part in the T3 interview, 941 (85%) completed the assessment at age 24. At age 30, all T3 participants were asked to complete another interview assessment (mean age=30.45, SD=0.70, range=28–34 years). Of the 941 who participated in the T3 assessment, 816 (87%) completed the T4 assessment.
Information on pre- and perinatal events was collected from a subset of the T1–T2 sample. Specifically, 1,165 adolescents who completed the T1 assessment, and their parents were contacted and asked to take part in a study that required participation of both the adolescent and their parents (Hops et al., 1992), of which 697 (60%) agreed to participate. However, the final sample that was included in the analyses consisted of 315 participants, whose pre- and perinatal events questionnaire was completed by their biological mother when the participants were between 14 and 18 years of age, and who had complete data up through the follow-up period when the participants were 30 years old. The participants were 61% (n=191) female and 89% White (n=279). These percentage did not significantly differ from the earlier report by Allen and colleagues (1998). Mean mother age at child birth was 24.9 (SD=4.6), and mean father age at child birth was 27.3 (SD=5.8).
2.2. Measures
2.2.1. Diagnostic measures
All participants were interviewed with the Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS; Orvaschel et al., 1982) at T1 and T2. At T2 and T3, the participants were additionally administered with the Longitudinal Interval Follow-Up Evaluation (LIFE; Keller et al., 1987). The T4 interview consisted of a joint administration of the LIFE and the Structured Clinical Interview for DSM-IV (SCID; First et al., 1996) to collect information for new or continuing episodes since T3. Diagnoses were based on DSM-III-R criteria for T1 and T2 and Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association, 1994) criteria for T3 and T4. The interviews were conducted by interviewers who had degrees in a mental health discipline and completed a 70-hour course in diagnostic interviewing, and were closely supervised throughout the study.
Interviewers were required to show a minimum kappa of 0.80 across all symptoms for at least two consecutive training interviews and on one videotaped interview of a participant who showed symptoms of psychopathology before conducting interviews. Interviewer performance was carefully monitored to maintain reliability. The interrater reliabilities (n = 263 at T1, n = 162 at T2, n = 190 at T3, and n = 124 at T4) good to excellent agreement for both MDD (k = .81-.86) and nonmood disorders (k = .76- .89; Rohde et al., 2005; Seeley et al., 2011).
The psychiatric disorders included in the present study included anxiety disorders (generalized anxiety disorder, overanxious disorder, post-traumatic stress disorder, panic disorder, agoraphobia, social phobia, simple phobia, obsessive–compulsive disorder, and separation anxiety disorder), substance use disorders ([SUD]; alcohol abuse or dependence [AUD] and sedative/hypnotic/anxiolytic, cannabis, stimulant, opioid, cocaine, and hallucinogen/PCP abuse or dependence, and polydrug dependence), disruptive disorders (ADHD, oppositional defiant disorder and conduct disorder), and depressive disorders (major depressive disorder and dysthymia). The outcomes were defined as lifetime occurrence of each disorder category by age 30. The prevalence rates of each disorder were: anxiety disorders 24%, affective disorders 58%, disruptive disorders 9%, and substance use disorders 45%.
2.2.2. Pre- and peri-natal events
Information about pre- and perinatal event were obtained from mothers by means of a questionnaire. As was done in the Allen et al. report (1998), the pre- and perinatal variables were assigned to one of the 12 dimensions (see Table 1) according to the multidimensional normal ogive models of latent trait theory (McDonald, 1985) using the NOHARM program (Fraser, 1988). These dimensions were classified as prenatal factors, intrapartum factors, early neonatal factors, and late neonatal factors. Test-retest reliability based on a subscale of mothers who completed both T1 and T2 assessment were excellent (Kappa>.70) for almost all dimensions except for maternal emotional health (Kappa=.58) and maternal substance use during pregnancy (Kappa=.62), surgical (Kappa=.57), and difficult delivery (Kappa=.61).
Table 1.
Assignment of prenatal and perinatal items into 12 scales and four time frames with the prevalence of each variable
| Time Frame/ Scale/ Items | Percent |
|---|---|
| Prenatal | |
| Maternal physical health | |
| Bleeding from vagina | 12.1 |
| Premature contractions | 13.8 |
| Swelling of face and hands | 39.6 |
| High blood pressure | 8.4 |
| Seizures and convulsions | 1.3 |
| Rubella | 1.0 |
| Any other infectious diseases | 1.6 |
| Diabetes mellitus | 1.3 |
| Anemia | 16.0 |
| Serious injury | 2.6 |
| X rays | 13.2 |
| Maternal emotional health | |
| Depression during pregnancy | 11.8 |
| Anxiety during pregnancy | 18.0 |
| Use of prescribed drugs | |
| Morning sickness | 24.9 |
| Pain | 4.8 |
| High blood pressure | 1.3 |
| Hormones | 1.3 |
| Valium | 3.5 |
| Thyroid medication | 5.1 |
| Maternal substance use | |
| Cigarettes | 23.7 |
| Alcohol | 19.0 |
| Coffee/ tea | 57.9 |
| Marijuana | 6.8 |
| Maternal obstetric history | |
| Previous miscarriage | 18.0 |
| Previous stillbirth | 1.6 |
| Medications to prevent miscarriage | 2.3 |
| Intrapartum | |
| Surgical delivery | |
| Caesarean delivery | 10.6 |
| General anesthesia | 19.6 |
| Difficult delivery | |
| Local anesthesia | 58.9 |
| Breech birth | 7.6 |
| Forceps used | 20.2 |
| Early neonatal | |
| Prematurity | |
| Low birth weight | 5.6 |
| Premature delivery | 14.7 |
| Baby required incubator | 9.1 |
| Acute anoxia/ hypoxia | |
| Cord around neck | 5.4 |
| Blue baby | 4.5 |
| Slow heart beat | 1.6 |
| Baby did not breathe | 3.2 |
| Baby had convulsions | 0.3 |
| Baby required oxygen | 4.2 |
| Hematological problems | |
| Rhesus incompatibility | 13.1 |
| Baby had jaundice | 15.4 |
| Baby required blood transfusion | 1.0 |
| Late neonatal | |
| Illness in first year | |
| Fever | 23.5 |
| Infection | 25.0 |
| Breast feeding | 65.0 |
2.2.3. Family factors
Family relation and parental support was measured using the Cohesion subscale of the Family Environment Scale (Moos, 1974). It consist of five items, with a Cronbach Alpha of .80. The degree of conflict between mother and the child was measured using Mother’s Appraisal of Dyad subscale of the Conflict Behavior Questionnaire (Prinz et al., 1979). It consists of 7 items, with a Cronbach Alpha of .62.
3. Results
3.1. Pre- and perinatal factors
Table 1 shows the prevalence of prenatal, intrapartum, early neonatal, and late neonatal factors. During pregnancy, 12% had depression and 18% had anxiety. In terms of substance consumption, 24% smoked cigarettes, and 19% and 7% consumed alcohol and marijuana, respectively. Approximately, 15% of the participants were delivered prematurely; in 20% of the cases, forceps were used. At late neonatal, 65% of the participants were breastfed.
3.2. Associations among predictor variables, mediating variables, and later psychopathology
To compare the relative effect sizes of the odds ratios that are reported in the result section, each of the 12 dimensions was reduced to a binary variable. The cutoff score for each variable was designated such that the factor was considered present for those participants in the upper quartile of the distribution of that variable, with the exception of those variables where the number of participants endorsing the presence of the problem was less than 25% (i.e. maternal emotional health, maternal obstetric history, surgical delivery, prematurity, and acute anoxia/hypoxia). The correlations among the dichotomized prenatal and perinatal events (Table 2), family factors (Table 3), and offspring psychopathology (Table 4) were calculated.
Table 2.
Correlations among the pre- and post-natal variables
| Maternal physical health |
Maternal emotional health |
Use of prescribed drugs |
Maternal substance use |
Surgical delivery |
Maternal obstetric history |
Difficult delivery |
Pre-mat urity |
Acute anoxia/ hypoxia |
Hematologi cal problems |
Illness first year |
Breast feeding Maternal |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal physical health |
- | |||||||||||
| Maternal emotional health |
.49 | |||||||||||
| Use of prescribed drugs |
.55 | .53 | ||||||||||
| Maternal substance use |
.49 | .32 | .36 | |||||||||
| Maternal obstetric history |
.54 | .43 | .37 | .19 | ||||||||
| Surgical delivery |
.48 | .41 | .37 | .26 | .19 | |||||||
| Difficult delivery |
.50 | .36 | .43 | .49 | .47 | .39 | ||||||
| Pre-maturity | .46 | .44 | .48 | .42 | .44 | .25 | .51 | |||||
| Acute anoxia/ hypoxia |
.44 | .27 | .31 | .37 | .47 | .48 | .42 | .54 | ||||
| Hematological problems |
.47 | .25 | .33 | .22 | .35 | .39 | .51 | .49 | .46 | |||
| Illness first year | .54 | .50 | .61 | .45 | .28 | .39 | .52 | .49 | .30 | .41 | ||
| Breast feeding | .56 | .51 | .58 | .61 | .45 | .33 | .68 | .47 | .44 | .57 | .54 |
Note: All correlations reached significance
Table 3.
Correlations between family factors, pre- and perinatal factors, and offspring mental disorders
| Family cohesion |
Mother support |
Father support |
|
|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | |
| 3.36 (1.80) | 0.10 (4.25) | −0.08 (4.21) | |
| Family factors | |||
| Mother support | −.53 | - | |
| Father support | −.37 | .20 | - |
| Pre- and postnatal factors | |||
| Maternal physical health | .01 | −.05 | .05 |
| Maternal emotional health | −.05 | .06 | .14 |
| Use of prescribed drugs | .08 | ||
| Maternal substance use | .00 | −.02 | −.01 |
| Maternal obstetric history | .07 | −.02 | .00 |
| Surgical delivery | −.06 | .07 | .07 |
| Difficult delivery | −.01 | −.08 | .09 |
| Pre-maturity | .01 | −.08 | .09 |
| Acute anoxia/ hypoxia | .06 | −.11 | .05 |
| Hematological problems | .04 | −.09 | −.05 |
| Illness first year | .07 | −.05 | .00 |
| Breast feeding | .05 | −.06 | −.07 |
|
Offspring mental disorders |
|||
| Anxiety disorders | −.16 | .11 | .07 |
| Affective disorders | −.19 | .15 | .12 |
| Disruptive disorders | −.26 | .20 | .17 |
| Substance use disorders | −.17 | .15 | .05 |
Note: Values in bold represent significant correlations
Table 4.
Correlations between pre- and perinatal factors and offspring mental disorders in adulthood
| Pre- and postnatal factors |
Offspring mental disorders | |||
|---|---|---|---|---|
| Anxiety disorders |
Affective disorders |
Disruptive disorders |
Substance use disorders |
|
| Maternal physical health |
−.05 | −.04 | .07 | −.04 |
| Maternal emotional health |
.04 | .16 | .13 | .09 |
| Use of prescribed drugs |
.00 | −.02 | −.10 | −.12 |
| Maternal substance use |
.04 | .00 | −.07 | .16 |
| Maternal obstetric history |
.14 | −.06 | −.03 | .01 |
| Surgical delivery |
.12 | −.05 | .06 | −.06 |
| Difficult delivery |
.01 | .00 | .15 | −.01 |
| Pre-maturity | .14 | .13 | .08 | −.06 |
| Acute anoxia/ hypoxia |
.11 | −.09 | .04 | −.06 |
| Hematological problems |
.01 | −.02 | .14 | −.09 |
| Illness first year |
.20 | .04 | −.11 | −.11 |
| Breast feeding | −.04 | −.10 | −.07 | .05 |
Note: Values in bold represent significant correlations
Based on the bivariate associations calculated between the prenatal/perinatal scales and the diagnostic outcome measures (i.e., anxiety disorder, affective disorder, disruptive disorder, and substance use disorder), variables to be included in the logistic regression equations for the prediction of each diagnosis was chosen. Table 5 shows the unadjusted odds ratios with 95% confidence intervals for each of the variables that showed p < .05 associations. Maternal obstetric history and illness during first year were significant predictors of offspring anxiety disorder. Maternal emotional health predicted offspring affective disorder, and maternal substance use predicted offspring substance use disorder.
Table 5.
Summary of the logistic regression analyses predicting psychopathology
| Disorder/ Prenatal Variable | Unadjusted OR (95% CI) |
Adjusted OR (95% CI)a |
Adjusted OR (95% cI)a,b |
|---|---|---|---|
| Anxiety disorder | |||
| Maternal obstetric history | 1.96 (1.08-3.56)* | 2.20 (1.02-4.74)* | 1.98 (1.00-3.92) |
| Illness during first year | 2.40 (1.41-4.08)** | 2.22 (1.10-4.44)* | 2.67 (1.46-4.87)** |
| Affective disorder | |||
| Maternal emotional health | 2.10(1.10-4.00)* | 1.74 (0.88-3.40) | |
| Disruptive disorder | |||
| Difficult delivery | 4.30(1.25-14.84)* | 9.87(1.27-77.06)* | 9.70 |
| Breast feeding | 0.81 (0.38-1.75) | 0.27 (0.09-0.78)* | (2.05-46.02)** |
| 0.77 (0.30-1.97) | |||
| Substance use disorder | |||
| Maternal substance use | 2.66(1.39-5.08)** | 2.05 (1.24-3.38)** |
Note: p < .05
p < .01
Adjusted for the pre/perinatal variables that were significantly associated with the offspring psychiatric disorder. Not calculated if there was only one pre/perinatal variable that was significantly associated with the dependent variable.
Adjusted for the set of covariates (family cohesion, mother’s social support, and father’s social support)
Note: 3rd column adjusted for both the mediator and the other prenatal variables
3.3. Multivariate analyses
To examine whether family cohesion, mother’s social support, and father’s social support functioned as mediating variables, hierarchical logistic regression was utilized. The odds ratios (ORs) and their 95% confidence intervals (CIs) are shown in Table 5. The second column stands for ORs and CIs for the predictors when controlling for other significant prenatal/perinatal predictors. The third column shows the ORs and CIs when controlling for the three mediating variables.
For anxiety disorders, maternal obstetric history was only marginally significant (p=.05) after the inclusion of the mediating variable block. Post hoc analyses showed that the key variable in reducing the association between obstetric history and anxiety disorders was family cohesion (unadjusted OR=.86, p < .01). Illness during first year remained a significant predictor controlling for the three mediating variables (i.e., family cohesion, mother’s social support, and father’s social support).
Maternal emotional health did not predict affective disorders after the inclusion of the familial variables (p=.11). As was with the anxiety disorders, family cohesion (unadjusted OR=.84, p<001) acted as a mediating variable.
For disruptive disorder, father and mother support acted as significant mediators of one of the two prenatal/perinatal factors. Unadjusted OR for mother support was 1.10 (p<001) and father support was 1.09 (p<01). The effect of breast feeding was no longer significant (p=.58) after controlling for these variables; however, the effects of difficult delivery remained significant (p<01).
No mediating effects of familial variables were found for substance use disorder. The only variable that predicted offspring substance use disorder was maternal substance abuse.
4. Discussion
To our knowledge, the present study is the first to have systematically (a) examined the association of pre- and perinatal factors on offspring’s psychopathology at adulthood, and (b) to investigate factors in adolescence that potentially mediate these associations. The results contribute to our understanding of the long-term impact of early life conditions in predicting offspring’s psychopathology at adulthood in several ways. First, the participants comprised large community sample, and as such it does not have the selection bias inherent in the clinical sample. Second, the study contained a wide range of medical complications, maternal psychological symptoms and substance use during pregnancy, at birth and postpartum periods of the participants, which enabled the examination of their specific impact for specific psychopathology at adulthood. Third, participant’s psychopathology was examined using a reliable, valid, and widely used interview schedule which ensured examining the presence of psychopathology in confidence (Allen et al., 1998). Finally, the longitudinal nature of this study allowed the participants to be followed-up from childhood and adolescence (14-18 years) to adulthood (30 years). Furthermore, by having four assessments over the 16-year period, potential cofounders could be controlled and potential recall bias on the part of the participants could be eliminated.
The findings can be summarized as follows: First, in line with previous studies (Korhonen et al., 2012; O’Connor et al., 2002, 2003) maternal obstetric history and emotional health predicted anxiety and depressive disorders in offsprings, respectively. However, after controlling for the effect of family factors (i.e., family cohesion, maternal and paternal support), both of these neonatal variables failed to predict subsequent internalizing disorders in the offspring. The present results suggests that family factors acted as a mediating factor between offspring anxiety/affective disorder and prenatal factors. This is an interesting finding because to our knowledge, previous studies (Korhonen et al., 2012; O’Connor et al., 2002, 2003) which have shown maternal anxiety and depression to be linked with offspring’s behavioral and/or emotional problems did not examine factors that mediate these associations. Furthermore, in almost all studies, the age of the offspring being examined was only up to adolescence (e.g., Allen et al., 1998), whereas in the present study, the participants were in their adulthood, i.e., 30 years old. Regardless of these gaps, our result seemed to suggest that family cohesion acted as a buffer against the negative consequences of maternal emotional health. As argued by several resilience researchers (e.g., Garmezy, 1993; Luthar, 2006; Rutter, 2000), family support and family cohesion are important factors which help to reduce the negative impact and reduce the likelihood of negative chain reactions of having mothers with mental health problems and to promote resilience. Indeed in the present study, moderate intercorrelations were found between family cohesion, maternal support, and paternal support.
In contrast, illness during first year remained a significant predictor of anxiety after controlling for the family factors. Many infections or serious illnesses before 12 months occur simply by chance or as a result of biological predisposition; these incidents, difficult to alter solely by positive family environment, may influence the child’s view of the world as a dangerous place and heighten the risk of developing future anxiety disorder. It can be inferred that some pre- and perinatal variables are less influenced by familial environment than others. Mothers’ health condition was strengthened by familial support, but the same factors had little effect in altering children’s health condition. This topic warrants continued research.
Second, difficult delivery and breastfeeding was a significant predictor of disruptive behavior. Between these two factors, the former was not influenced by familial factors, but for the latter, father and mother support acted as significant mediators. Difficult delivery poses a risk of oxygen deprivation and neurological complications, thereby providing a physiological background in the development of disruptive behavior regardless of familial environment. On the other hand, breastfeeding is a behavioral routine that can influence mother-child bonding and the process of attachment. Father and mother support are suggestive of a positive family environment, fostering healthy attachment that can function as a protective factor from oppositional behavior.
This finding could be interpreted as being consistent with Rutter’s (2000) notion that a protective process can help to mitigate a negative consequences which are linked to the original risks. For example, parental support in the form of parental monitoring has been reported to be strongly associated with children’s resilience even for individuals with mental illness (Brennan et al., 2003; Garber, 2005; Knoche et al., 2007; Tiet et al., 2001). Parents’ knowledge of what their children do outside of the home (e.g., knowledge of their peers and activities) could help to prevent children from pursuing unfavorable developmental trajectories and fosters age-appropriate competence (Tiet et al., 2001).
Third, the only variable that predicted offspring substance use disorder during adulthood was maternal substance abuse and this association was not mediated by family cohesion or parental support. While it is beyond the scope of the present report to explore the mechanism responsible for the association between maternal substance abuse and offspring substance abuse, the attitudes and behaviors of substance-abusing mother’s parenting style have been used to explain this association (Parolin and Simonelli, 2016).
The study is not without methodological problems which must be considered when interpreting the findings. First, the information on pre- and postnatal factors were obtained based on maternal recall when their offspring (i.e., participants) were between 14-18 years; as such this information may be subjected to recall bias. However, previous studies have shown adequate agreement between maternal recall and objective measures of obstetric complications (O’Callaghan et al., 1990) and substance use (Jacobson et al., 1991). Furthermore, previous studies have reported maternal recall of breastfeeding initiation and duration as quite accurate (Li et al., 2005). Second, due to the small number of participants with specific disorder subgroups (e.g., panic disorder), aggregate disorders (e.g., anxiety disorders) were used to ensure that the statistical power was retained (Allen et al., 1998). Thus, it is unclear whether pre- and perinatal factors may be relevant to all or to some subtypes of anxiety disorders. In addition, the large number of statistical tests used in the study may have inflated the chance for type I error; some of our findings could have been due to chance. Third, information on family cohesion and support was provided only by the mothers and not by the fathers. These limitations notwithstanding, our findings suggest that the association between pre- and postnatal factors and psychopathology of offspring during adulthood is mediated by family cohesion.
The implication of the present research is that family cohesion and providing support to females with anxiety and/or depression during pregnancy is important and has the potential of reducing the incidence of emotional and behavioral problems in their offsprings later in adulthood. Some specific programs for supporting females during pregnancy may include prevention actions in the pregnant women routine visits to health institutions, home visits from nurses or social workers, informational meetings, and involvement in local social groups (Bullock et al., 2002; Lumley et al., 2006; Matthey et al., 2004).
Specifically, it is likely that maternal physical and mental health can be sustained or boosted by familial support, although other factors such as child illness in first year are less influenced. Future research is needed to explore the way in which family support and cohesion could have an impact on the hormonal and other mechanisms that underlay the frequently reported association between maternal anxiety/depression and offspring’s mental health problems. Such information will help to design the timing for an effective antenatal prevention.
Figure 1:
Study Design
Highlights.
Maternal obstetric history and emotional health predicted anxiety and depressive disorders in offsprings, respectively. However, after controlling for the effect of family factors, these neonatal variables failed to predict these disorders in the offspring.
Child’s illness during first year remained a significant predictor of anxiety after controlling for the family factors
For disruptive disorders, father and mother support acted as significant mediators.
No mediating effects of familial variables were found for substance use disorder, except for maternal substance abuse.
Acknowledgement
The authors are grateful to all those who participated in the study.
Role of Funding Source
This research was supported in part by National Institute of Mental Health awards MH40501 and MH50522 (Dr Lewinsohn). The NIMH had no further role in study design; in collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Conflict of Interest
The authors have no conflict of interest to report in relation to the research presented in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alati R, Najman JM, O’Callaghan M, Bor W, Williams GM, & Clavarino A, 2009. Fetal growth and behavioural problems in early adolescence: Findings from the Mater University Study of Pregnancy. Int J Epidemiol 38, 1390–1400. [DOI] [PubMed] [Google Scholar]
- Allen NB, Lewinsohn PM, Seeley JR, 1998. Prenatal and perinatal influences on risk for psychopathology in childhood and adolescence. Dev Psychopathol 10, 513–529. [DOI] [PubMed] [Google Scholar]
- Ashford J, van Lier PA, Timmermans M, Cuijpers P & Koot HM, 2008. Prenatal smoking and internalizing and externalizing problems in children studied from childhood to late adolescence. J Am Acad Child Adolesc Psychiatry, 47, 779–787. [DOI] [PubMed] [Google Scholar]
- Betts KS, Williams GM, Najman JM, & Alati R, 2011. The association between birth weight and anxiety disorders in young adults. J. Anxiety Disord. 25, 1060–1067. [DOI] [PubMed] [Google Scholar]
- Bullock LF, Browning C, Geden E, 2002. Telephone social support for low-income pregnant women, J Obstet Gynecol Neonatal Nurs, 31, 658–664. [PubMed] [Google Scholar]
- Buoli M, Bertino V, Caldiroli A, Dobrea C, Serati M, Ciappolino V, Altamura AC, 2016. Are obstetrical complications really involved in the etiology and course of schizophrenia and mood disorders? Psychiatry Research, 241, 297–301. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Brocque R, Hammen C, 2003. Maternal depression, parent–child relationships, and resilient outcomes in adolescence. J Am Acad Child Adolesc Psychiatry, 42 (12), 1469–1477. [DOI] [PubMed] [Google Scholar]
- Brook JS, Brook DW, Whiteman M, 2000. The influence of maternal smoking during pregnancy on toddler’s negativity. Arch Pediatr Adolesc Med, 154:381–385. [DOI] [PubMed] [Google Scholar]
- Cantor-Graae E, McNeil TF, Sjostrom K, 1993. Obsteric complications and their relationship to other etiological risk factors in schizophrenia. J Nerv Ment Dis 182(11), 645–650. [DOI] [PubMed] [Google Scholar]
- Cernadas JMC, Noceda G, Barrera L, Martinez AM, & Garsd A, 2003. Maternal and Perinatal Factors Influencing the Duration of Exclusive Breastfeeding During the First 6 Months of Life. J Hum Lact, 19, 136–144. [DOI] [PubMed] [Google Scholar]
- Done DJ, Johnstone EC, Frith CD, 1991. Complication of pregnancy and delivery in relation to psychosis in adult life: Data from the British perinatal mortality survey sample. Br Me d J, 302, 1576–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblad M, Gissler M, Lehtonen L, Korkeila J, 2010. Prenatal smoking exposure and the risk of psychiatric morbidity into young adulthood. Arch. Gen. Psychiatry 67, 841–849. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, 1996. Structured Clinical Interview for DSMIV Axis I disorders: Nonpatient edition (SCID-I, Version 2.0). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Fraser C, 1988. NOHARM: A computer program for fitting both unidimensional and multidimensional normal ogive models of latent trait theory [Computer software]. Armidale, Australia: Author. [Google Scholar]
- Garber J, 2005. Depression and the family In: Hudson JL, Rapee RM, editors. Psychopathology and the family. Oxford, UK: Elsevier; pp. 227–283. [Google Scholar]
- Garmezy N, 1993. Children in poverty: Resilience despite risk. Psychiatry, 56, 127–136. [DOI] [PubMed] [Google Scholar]
- Hack M, Hudson G Taylor HG, Schluchter M, Andreias L, Drotar D, Klein N, 2009. Behavioral Outcomes of Extremely Low Birth Weight Children at Age 8 Years. J Dev Behav Pediatr 30(2): 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayatbakhsh MR, O’Callaghan MJ, Bor W, Williams GM, Najman JM, 2012. Association of breastfeeding and adolescents’ psychopathology: a large prospective study. Breastfeed. Med,7:480–486. [DOI] [PubMed] [Google Scholar]
- Heikkila K, Sacker A, Kelly Y, Renfrew MJ, Quigley MA, 2011. Breast feeding and child behaviour in the millennium cohort study. Arch. Dis. Child 96, 635–642 [DOI] [PubMed] [Google Scholar]
- Hook B, Cederblad M, Berg R, 2006. Prenatal and postnatal maternal smoking as risk factors for preschool children’s mental health. Acta Paediatr, 95, 671–677. [DOI] [PubMed] [Google Scholar]
- Hops H, & Seeley JR, 1992. Parent participation in studies of family interaction: Methodological and substantive considerations. Behav Assess 14, 229–243. [Google Scholar]
- Indredavik MS, Brubakk AM, Romundstad P, Vik, T., 2006. Prenatal smoking exposure and psychiatric symptoms in adolescence. Acta Paediatr, 96: 377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indredavik MS, Brubakk AM, Romundstad P, Vik T, 2007. Prenatal smoking posure and psychiatric symptoms in adolescence. Acta Paediatrica 96, 377–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, & Martier SS, 1991. Maternal recall of alcohol, cocaine, and marijuana use during pregnancy. Neurotoxicol Teratol 13, 535–540. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PW, Friedman B, Nielsen E, Endicott J, McDonald-Scott PA, 1987. Longitudinal Interval FollowUp Evaluation (LIFE): A comprehensive method for assessing outcome in prospective longitudinal studies. Arch. Gen. Psychiatry 44, 540–548. [DOI] [PubMed] [Google Scholar]
- Kinney DK, Levy DL, Yurgelun-Todd DA, 1994. Season of birth and obstetrical complications in schizophrenics. J Psychiat Res, 28(6): 499–509. [DOI] [PubMed] [Google Scholar]
- Knoche LL, Givens JE, Sheridan SM, 2007. Risk and protective factors for children of adolescents: maternal depression and parental sense of competence. Journal of Child and Family Studies, 16, 684–695. [Google Scholar]
- Korhonen M, Luoma I, Salmelin R, & Tamminen T, 2012. A longitudinal study of maternal prenatal, postnatal and concurrent depressive symptoms and adolescent well-being. Journal of Affective Disorders, 136, 680–692. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Hops H, Roberts RE, Seeley JR, & Andrews JA, 1993. Adolescent psychopathology: I. Prevalence and incidence of depression and other DSM-III-R disorders in high school students. J. Abnorm. Psychol, 102, 133–144. [DOI] [PubMed] [Google Scholar]
- Li R, Scanlon KS, Serdula MK, 2005. The validity and reliability of maternal recall of breastfeeding practice. Nutrition Reviews. 63(4), 103–110. doi: 10.1111/j.1753-4887.2005.tb00128.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Leung P, Yang A, 2014. Breastfeeding and active bonding protects against children’s internalizing behavior problems. Nutrients, 6, 76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loret de Mola C, Horta BL, Goncalves H, Quevedo LDL, Pinheiro R, Gigante DP, Motta JVDS, & Barros FC, 2016. Breastfeeding and mental health in adulthood: Abirth cohort study in Brazil. J. Affect. Disord 202, 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley J, Watson L, Small R, Brown S, Mitchell C, Gunn J, 2006. PRISM (Program of Resources Information and Support for Mothers): A community randomised trial to reduce depression and improve women’s physical health six months after birth [ISRCTNO3464021], BMC Public Health, 6, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthar SS, 2006. Resilience in development: A synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Risk, Disorder, and Adaptation. New York: Wiley, pp. 740–795. [Google Scholar]
- Matthey S, Kavanagh DJ, Howie P, Barnett B, Charles M, 2004. Prevention of postnatal distress or depression: An evaluation of an intervention at preparation for parenthood classes, J Affect Disord, 79, 113–126. [DOI] [PubMed] [Google Scholar]
- McDonald RP, 1985. Unidimensional and multidimensional models for item response theory. In Weiss DJ, (Ed.), Proceedings of the 1982 item response theory and computer adaptive testing conference Minneapolis: University of Minnesota. [Google Scholar]
- Mimouni-Bloch A, Kachevanskaya A, Mimouni FB, Shuper A, Raveh E, & Linder N, 2013. Breastfeeding may protect from developing attention-deficit/hyperactivity disorder. Breastfeed Med Journal, 8, 363–367. [DOI] [PubMed] [Google Scholar]
- Moos RH, 1974. Family Environment Scale and preliminary manual. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Nguyen RHN, Stewart EG, Harlow BL, 2012. A population-based study of pregnancy and delivery characteristics among women with vulvodynia. Pain Ther, 1(1): 2. doi: 10.1007/s40122-012-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg JT, Breslau N, 2007. Prenatal smoking exposure, low birth weight, and disruptive behavior disorders. J Am Acad Child Adolesc Psychiatry, 46:362–369. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Wickramaratne PJ, Pilowsky DJ, Newcorn JH, Bruder-Costello B, Davey C et al. 2007. Low birth weight and risk of affective disorders and selected medical illness in offspring at high and low risk for depression. Compr Psychiatry, 48, 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan E, Larkin C, Waddington JL, 1990. Obstetric complications in schizophrenia and the validity of maternal recall. Psychol Med, 20, 89–94. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Glover V, 2003. Maternal antenatal anxiety and behavioural/emotional problems in children: a test of a programming hypothesis. J Child Psychol Psychiatry 44:1025–1036. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Glover V, the ALSPAC Study Team, 2002. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adoles Psychiatry 41:1470–1477. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Heron J, Golding J, Beveridge M, Glover V, 2002. Maternal antenatal anxiety and children’s behavioural/ emotional problems at 4 years: report from the Avon Longitudinal Study of Parents and Children. Br J Psychiatry 180:502–508 [DOI] [PubMed] [Google Scholar]
- O’Hara MW, 1995. Postpartum depression: Causes and consequences. New York: Springer-Verlag. [Google Scholar]
- Oddy WH, Kendall GE, Li J, Jacoby P, Robinson M, de Klerk NH, Silbum SR, Zubrick SR, Landau LI, Stanley FJ., 2010. The long-term effects of breast-feeding on child and adolescent mental health: A pregnancy cohort study followed for 14 years. J Pediatr, 156, 568–574. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich J, Chambers W, Tabrizi MA, Johnson R, 1982. Retrospective assessment of prepubertal major depression with the Kiddie-SADS-E. J Am Acad Child Adolesc Psychiatry, 21, 392–397. [DOI] [PubMed] [Google Scholar]
- Parolin M, Simonelli A, Mapelli D, Sacco M, & Cristofalo P, 2016. Parental substance abuse as an early traumatic event. Preliminary findings on neuropsychological and personality functioning in young drug addicts exposed to drugs early. Front Psychol, 7, 887. doi: 10.3389/fpsyg.2016.00887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds LC, Inder TE, Neil JJ, Pineda RG, and Rogers CE, 2014. Maternal obesity and increased risk for autism and developmental delay among very preterm infants. J. Perinatol 34, 688–692. doi: 10.1038/jp.2014.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rückinger S, Rzehak P, Chen CM., Sausenthaler S, Koletzko S, Bauer CP, Hoffmann U, Kramer U, Berdel D, von Berg A, Bayer O, Wichmann HE, von Kries R, & Heinrich J, for the GINI-plus Study Group, 2010. Prenatal and Postnatal Tobacco Exposure and Behavioral Problems in 10-Year-Old Children: Results from the GINI-plus Prospective Birth Cohort Study. Environ. Health Perspect, 118, 150–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M, 2000. Resilience reconsidered: Conceptual considerations, empirical findings, and policy implication In: Shonkoff JP.; Meisels SJ, editors. Handbook of early childhood intervention. New York: Cambridge University Press; p. 651–682. [Google Scholar]
- Sabuncuoglu O, Alin Basgul A, 2014. Pregnancy health problems and low birth weight associated with maternal insecure attachment style. J Health Psychol 21 (6), 934–943. 10.1177/1359105314542819. [DOI] [PubMed] [Google Scholar]
- Schmitt J, Romanos M, 2012. Prenatal and perinatal risk factors for attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med, 166(11), 1074–5. doi: 10.1001/archpediatrics.2012.1078. [DOI] [PubMed] [Google Scholar]
- Schmitz M, Denardin D, Silva TL et al. 2006. Smoking during pregnancy and attention-deficit/hyperactivity disorder, predominantly inattentive type: a case-control study. J Am Acad Child Adolesc Psychiatry, 45, 1338–1345. [DOI] [PubMed] [Google Scholar]
- Serati M, Barkin JL, Orsenigo G, Altamura AC, & Buoli M (2017). Research Review: The role of obstetric and neonatal complications in childhood attention deficit and hyperactivity disorder – a systematic review. J Child Psychol and Psychiatry, 58, 1290–1300 [DOI] [PubMed] [Google Scholar]
- Shamberger R, 2012. Attention-deficit disorder associated with breastfeeding: A brief report. J Am Coll Nutr 31, 239–242. [DOI] [PubMed] [Google Scholar]
- Stadler DD, Musser ED, Holton KF, Shannon J, Nigg JT, 2016. Recalled initiation and duration of maternal breastfeeding among children with and without ADHD in a well characterized case-control sample. J Abnorm Child Psychol, 44(2), 347–355. doi: 10.1007/sl0802-015-9987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stene-Larsen K, Borge AI, Vollrath ME, 2009. Maternal smoking in pregnancy and externalizing behavior in 18-month-old children: results from a population-based prospective study. J Am Acad Child Adolesc Psychiatry, 48, 283–289. [DOI] [PubMed] [Google Scholar]
- Taylor AE, Carslake D, de Mola CL, Rydell M, Nilsen TIL, Bjørngaard JH, Horta BL, Pearson R, Rai D, Galanti MR, Barros EC., Romundstad PR, Smith GD, & R. Marcus Munafò MR, 2017. Maternal Smoking in Pregnancy and Offspring Depression: a cross cohort and negative control study. Scientific Reports 7, Article number: 12579. doi: 10.1038/s41598017-11836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiet QQ, Bird HR, Hoven CW, Wu P, 2001. Resilience in the face of maternal psychopathology and adverse life events. J. Child Fam. Stud 10, 347–365. [Google Scholar]
- Wakschlag LS, Pickett KE, Cook E, Benowitz NL, Leventhal BL, 2002. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 92, 966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag LS, Pickett KE, Kasza KE, Loeber R., 2006. Is prenatal smoking associated with a developmental pattern of conduct problems in young boys? J Am Acad Child Adolesc Psychiatry, 45:461–467. [DOI] [PubMed] [Google Scholar]
- Wiles NJ, Peters TJ, Heron J, Gunnell D, Emond A, Lewis G, 2006. Fetal growth and childhood behavioral problems: Results from the ALSPAC cohort. Am J Epidemiol 163 (9), 829–837. [DOI] [PubMed] [Google Scholar]
- Xu J, Duan X, Yang Y, 2007. Association study of obstetrical complication and depressive disorder. Journal of Nanjing Medical University, 21(6), 394–397. [Google Scholar]