Abstract
Background
Many randomized controlled trials have investigated the effects of exercise on the rehabilitation of patients with breast cancer. However, the exercise forms used in most previous studies were monotonous. Therefore, we designed a protocol to estimate the effects of combined exercise intervention using Internet and social media software on the rehabilitation of postoperative patients with BC.
Methods/Design
This study protocol is a randomized control trial with an intervention time of 12 weeks. After completing baseline questionnaire and physical fitness tests, the participants are randomized to the study group or the control group. Procedure contents of exercise intervention in the study group include: via phone step-recording app, ask the individuals to complete the target number of steps within a specified period of exercise, four times per week; face-to-face remote video guidance of individuals on muscle training, three times per week; common knowledge of physical exercise BC rehabilitation will be pushed regularly by social media apps every day. The control group will receive normal treatment and rehabilitation according to daily specifications of the hospital. The primary outcome will be the quality of life. The secondary outcomes are physical fitness and social cognitive indicators.
Discussion
This study is a clinical trial to estimate the effects of combined exercise intervention based on the Internet and social media software for postoperative patients with breast cancer (BC). If expected results are achieved in this study, measures and methods of BC rehabilitation will be enriched.
Trial registration
Chinese Clinical Trial Register, ChiCTR-IPR-17012368. Registered on 14 August 2017.
Electronic supplementary material
The online version of this article (10.1186/s13063-018-2857-3) contains supplementary material, which is available to authorized users.
Keywords: Internet and social media software, Combined exercise, Breast cancer , Quality of life, SF-36, Physical fitness
Background
Breast cancer (BC) is a type of malignant cancer that severely harms women’s health worldwide and is the most common cancer in women in both developed and developing countries. In developing countries, the incidence of breast cancer is rising due to increased life expectancy, the expansion of urbanization, and the adoption of a western lifestyle [1]. The most common manifestations in postoperative BC patients are mental fatigue, anxiety, and even depression [2]. The functions of the upper extremities may be affected, which also leads to limited physical activity. Long-term reduced physical activity will cause decreased adult bone mineral density and decreased muscle and cardiopneumatic endurance, which will severely affect emotions and quality of life (QOL) [3].
It has been demonstrated in modern medical studies, both domestic and abroad, as well as clinical practice that sport activities may improve muscle strength and cardiopneumatic endurance in BC patients during BC rehabilitation and further improve their emotions and QOL [3–9]. In addition, some studies have shown that resistance training has a certain effect on the improvement of postoperative complications of lymphedema [10–12]. Therefore, it has been recommended that both resistance training and aerobic exercise be included in the postoperative BC rehabilitation plan [3]. Qigong is one type of traditional Chinese exercise which is a combination of physical activity, breathing exercises, and psychological adjustment that has long been applied in health promotion and the treatment of various chronic diseases [13–16]. Theoretically, fitness Qigong may improve postoperative QOL, physical fitness, and psychological status of BC patients. With the universalization and extensive applications of the Internet, exercise intervention and guidance via phone social media apps and remote video to BC patients becomes possible. Currently, some related studies have been carried out [17–19], but most of the previous studies express the same problem that the means of exercise intervention is overly simple, which may impact the effect of the intervention.
Therefore, we have designed a combined exercise intervention protocol applying multiple Internet social media and plan to evaluate the effectiveness of the protocol by a clinical trial using a high-quality methodology: to conduct a randomized controlled trial (RCT) to estimate the effects of combined exercise intervention based on the Internet and social media software for postoperative patients with BC.
Methods and design
Study design
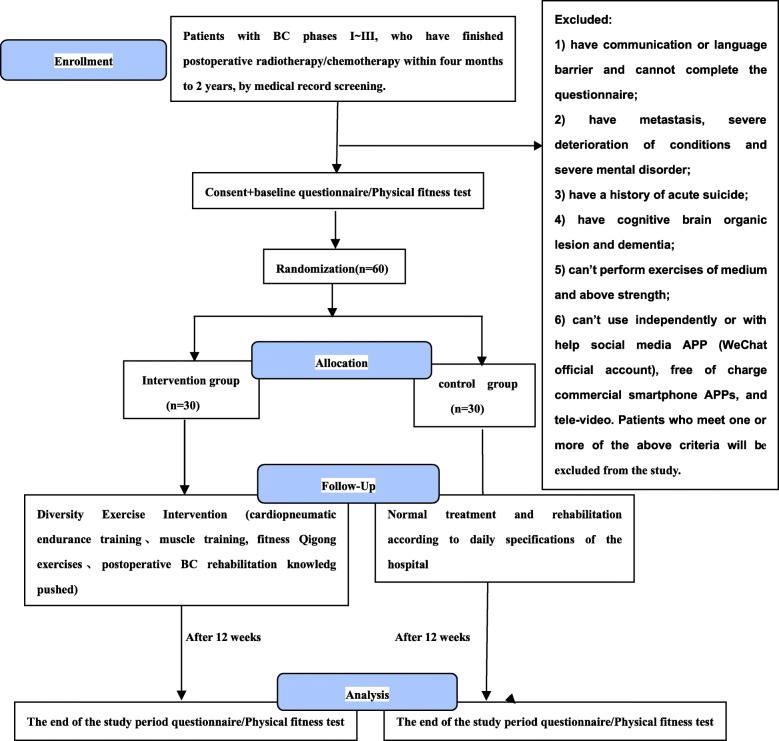
This study is a RCT with an intervention time of 12 weeks. Trial participants were recruited starting in August 2017 and the data will be analyzed after the sample size is achieved according to the study design. The flow chart of the study design is shown in Fig. 1. Apart from the time and contents of the designed exercise plan being different between the study group and the control group, relative regulations of the hospital at other times are followed to ensure regular work performance in the hospital and ensure that the treatment of BC patients is not affected in the hospital. All participants will provide informed consent forms before entering the study.
Fig. 1.
The flow chart of the trial design
Participants and recruitment
Individuals who meet the inclusion criteria will be briefly introduced to this study and informed about the benefits as well as possible risks by the clinician. The participants will then be required to fill in consent forms and are free to withdraw from the study at any time without any specific reason, penalty, or loss of benefits.
Inclusion criteria
Patients with BC phases I–III, who have finished postoperative radiotherapy/chemotherapy within four months to two years will be included.
Exclusion criteria
Individuals who meet any of the following criteria will be excluded: (1) those with communication or language barriers and inability to complete the questionnaire; (2) those with metastasis, severe deterioration of conditions, or severe mental disorders; (3) those with a history of acute attempted suicide; (4) those with cognitive organic brain lesions and dementia; (5) those who cannot perform exercises of medium exertion and above; and (6) those who cannot use independently or with help social media app (WeChat official account), free-of-charge commercial smartphone apps, and remote video. Patients who meet one or more of the above criteria will be excluded from the study.
Recruitment strategies
Participants will be recruited from the Department of Breast Surgery, the Second Hospital of Shandong University, Jinan, Shandong. Recruitment is mainly through the doctors’ recommendations, patients publicizing the trial, and the distribution of leaflets. Patients interested in this trial will first be evaluated by physicians. If they meet the criteria and decide to participate, they will be asked to sign the informed consent. They will then be included in the trial for randomization.
Blinding
After completing the baseline questionnaire and physical fitness tests, the participants will be enrolled and a computer-generated random list generated by SPSS 16.0 will be used for randomization. The random number list is kept strictly confidential by the Data Coordination Committee (DCC) staff. Eligible patients will be randomized to the experimental group or the control group at a 1:1 ratio. The group numbers will be provided in envelopes made from carbonless paper. The envelopes will be kept by a study administrator who will not directly participate in the recruitment or follow-up of any participant and the group numbers will be subsequently disclosed. Different people will enroll participants and assign participants to interventions.
The division results are kept by the study designer until the end of the study to ensure allocation concealment, which means the allocation is bound to both the result evaluator and the data analyzer. The participants and the treatment provider will be instructed not to disclose the allocation to the result evaluator.
Control group
Participants who have been randomized into the control group will receive normal treatment and examination according to the daily specifications of the hospital. The rehabilitation plan will also be performed according to the regular requirements and postoperative BC rehabilitation-related health education will be provided.
Intervention group
Basic contents of intervention in the study group include cardiopneumatic endurance training, muscle training, conventional fitness exercises, and postoperative rehabilitation knowledge which pushed combined interventions: (1) cardiopneumatic endurance training: to be performed four times per week, determine the strength of the exercise via rate of perceived exertion (RPE), ask the individuals to complete the target number of steps within the specified time and record the number of steps via a phone step-recording app; (2) muscle training, fitness Qigong exercises: muscle training includes muscle strength, muscle endurance, and muscle function training while fitness Qigong exercise is scheduled after completion of muscle training, practicing certain Qigong Baduanjin fitness routines. Face-to-face remote video guidance on physical exercise rehabilitation is performed three times per week for 30 min each time, including a 5-min warm-up, 20 min of muscle training, and 5 min of relaxation. The primary content of muscle training is endurance training in the first month, strength training in the second month, and muscle function training in the third month. Traditional fitness exercise is scheduled after warming up and relaxing exercises and may include one or more routines each time; (3) postoperative BC rehabilitation knowledge: common knowledge of physical exercise BC rehabilitation pushed regularly by social media apps every day as well as knowledge of simple physical exercise rehabilitation and psychological adjustment relative to BC survivors.
Physical exercise rehabilitation training is performed under the guidance of the participants’ professional physiotherapists. The measures of exercise intervention will be gradually advanced and the strength of exercise will be gradually increased. The side effects and severe adverse events will be recorded and reported to the study designer by physiotherapists; the study designer will then determine further measures to take. In this case, other sports-related exercise interventions will stop, while treatment by medication will continue. If necessary, training will be discontinued according to the reports from the therapists and the consultation results from the BC specialists. We call the roll through every video guide, endurance training, and weekly group meeting to improve adherence to intervention protocols. There are no concomitant or prohibited interventions during this trial.
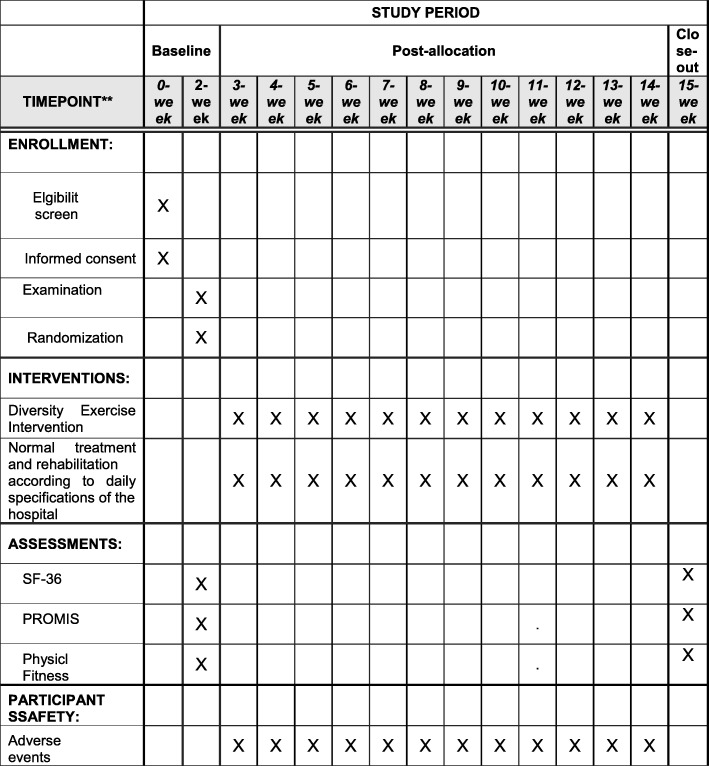
Participant timeline
The Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) table in Fig. 2 shows details on the schedule of enrollment, interventions, and assessments.
Fig. 2.
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) diagram of enrolment, treatment, and assessments over time
Measurements
The primary outcome of the trial will be the MOS 36-item Short Form Health Survey mental component summary scale (SF-36 MCS). The secondary outcomes are other sub-items of the MOS 36-item Short Form Health Survey physical component summary scale (other sub items of SF36) [20, 21], PROMIS, physical fitness, and social cognitive theory. Measurements will be conducted in the same place and by the same tester for both the study group and control group strictly adhering to the precautions and operational rules, to ensure that the measurements are scientific and precise.
Quality of life (QOL)
-
The MOS 36-item Short Form Health Survey (SF-36)
SF-36 is a reliable and manageable questionnaire of QOL, which has been extensively applied in the assessment of the health conditions of patients with chronic diseases. SF-36 includes eight dimensions and two summary tables; the score of each dimension is in the range of 1–100 (100 represents the best health condition).
The Patient Reported Outcome Measurement Information System [22] (PROMIS) will be used in the evaluation of physical function, anxiety, and depression of the individuals mainly, including physical functions (such as: can you run or do shopping?), anxieties during the past seven days (such as: I feel fearful), frustrating (such as: I feel useless), sleeping disorders (such as: I have trouble falling asleep), and pain disruptions (does pain limit your housekeeping activities?). The options are categorized into five levels from not at all/no/very weak as the first option to always/very much/very good as the fifth option. It has been demonstrated that PROMIS can be applied in the evaluation of the population of clinic patients [23].
Physical fitness
Maximal oxygen uptake: this is determined by an improved Bruce plank test [24] which will be strictly adhered to; the heart rate will be monitored by a Polar watch during the test.
Stand-up and sit-down chair test (number of times standing up from the chair within 30 s): these outcomes will be used to evaluate leg strength and endurance of the individual. Test procedure: (i) put an upright chair (or a folding chair) against the wall (for the sake of safety); (ii) the individual sits in the middle of the chair with the right left feet apart, equal to the shoulders (one foot may be placed slightly in front of the other) and both arms are crossed around the waist and close to the chest; (iii) during the test, the individual should stand up completely and then sit down completely; (iv) the number of times that the individual stands up from and sits down in the chair within 30 s is recorded; (v) for safety purposes, or when necessary, the individual may use her arms for assistance.
Number of arm raises (30 s with a dumbbell of 5 lbs or 2.3 kg): this outcome is used to evaluate the strength and endurance of the upper extremities. Test procedure: (i) a 2.3 kg (5 lbs) dumbbell is used; (ii) put an upright chair (or a folding chair) against the wall (for the sake of safety); (iii) the individual sits close to the side of chair if she has a stronger arm on that side; (iv) the stronger arm holds the dumbbell with the arm naturally hanging straight down over one side of the chair while the body takes a push-up position; (v) the non-moving arm is fixed close to the body so that only the moving arm is moving; (vi) bend and lift the arm to its maximum extent when gradually rotating to the outward position with the palm facing up; (vii) the arm returns to the push-up position and is placed on one side of the chair; (viii) the number of times that the arm bends and lifts is recorded; (ix) precautions include making sure the arm is completely bent and the elbow is completely straightened—it is very important that the upper extremities are stable without swaying.
Percentage of body fat [24]: measuring the (i) upper arm (triceps)—vertical direction of skin folds; both arms fall naturally on both sides of the body, measure at the midpoint of the straight line connecting the acromion and the olecranon of the posterior upper arm; (ii) anterior superior iliac spine—oblique direction of the skin folds; measure at the axillary line above the anterior superior iliac spine and follow the natural wing of the ilium; (iii) abdominal skin folds—vertical direction of the skin folds, at 2 cm from the right side of umbilicus. Measure the thickness of the skin folds at these three points, calculate the body density with the three-point method according to the female skin fold measurement and calculation formula, and then calculate the individual’s percentage of body fat.
Height, body weight, and flexibility tests.
Social cognitive indicator
The participant’s social cognitive belief will be evaluated by standard self-report questionnaire [25] including: sport exercises: social support questionnaire; sport exercises: obstruction questionnaire; sport exercises: expected outcome questionnaire; sport exercises: self-efficiency questionnaire. For example: sport exercises: social support questionnaire is to survey whether your family and friends have completed the following items during the past 30 days, including five social support questions (encourage you to exercise, discuss the disadvantages of not exercising, remind you to exercise, share some methods of exercise with you, do exercises together with you). The options include “almost none,” “once,” “sometimes,” “often,” and “always” in sequence. These questionnaires have been subject to normal distribution validation and content validity analysis in previous literature.
Sample size
A total of 52 participants (26 in each group) completing follow-up will provide 90% power (α = 0.05) to detect 9.5 points [26] in SF-36 MCS assuming a standard deviation of 11.6 points [27]. Assuming a 15% drop-out rate between baseline and follow-up, 60 participants will be enrolled in the study.
Data collection and management
To ensure the accuracy of outcome assessments and data collection, all of the physicians, nurses, and research assistants will attend a training workshop before the start of the trial. All attendees will be provided with a protocol and standard operation procedures and will discuss the topics they may feel confused about until everyone is totally clear about the procedures. We do not have plans to promote participant retention and complete follow-up.
After screening and signing of informed consent and end-of-study measurements, the SF-36, physical fitness, and social cognitive theory will be carefully recorded. Monitoring will be performed by personnel independent of the investigators and the sponsor and will consist of checking all informed consent forms and completeness of all data and source data verification of patients. Data are collected and recorded on a standard report form; when the visit is completed, all the recorded data will be entered into the web-based data system by the double-entry method. All errors will need to be corrected by crossing out, with the researcher’s signature and date. Any participant may quit the study at any time for any reason. If any patients want to quit, clinicians will ask whether they agree to finish the follow-up according to the study schedule. All patients who quit and are lost to follow-up will be recorded. If our study fails to recruit 50% of patients within six months, we will stop the study. The process will be independent from investigators and the sponsor.
Statistical analyses
The study results will undergo statistical analysis with SPSS software version 19.0. Comparisons between groups will be an analysis of covariance (ANCOVA) with treatment and baseline entered in the model. Estimate, 95% confidence intervals (CIs), and p values will be reported. The analysis population will adopt Intent to treat (ITT) and ignoring missing data.
Ethical approval and dissemination
This study follows the principles of the Declaration of Helsinki (Version Edinburgh 2000). Before participating in this research, every potential participant should be informed of the detailed study information, while participants can ask questions by phone, e-mail, and face to face. Only patients who sign the informed consent form will be included. This study has been approved by the Ethics Committee of the Second Hospital of Shandong University (KYLL-2017(KJ)P-0003). Personal information about potential and enrolled participants will be collected and stored separately from medical records and will be only accessible for the assessors who contact the participants for data assessment. In case of an unexpected serious adverse event (e.g. life-threatening event or permanent damage) over the course of the study, we will report the serious adverse event to the local Ethics Committee. The Ethics Committee and the study team will then decide in accordance, in the best interests of the patient, if the study procedures will be continued or terminated. The results of this study will be disseminated by presenting at international conferences and publication in peer-reviewed journals. Participants will learn about the results of the study.
The principal investigator is responsible for publication of the results of this study in whole or in part. Neither the complete nor any part of the results of the study carried out under this protocol, nor any of the information provided by the sponsor for the purposes of performing the study, will be published or passed on to any third party without the consent of the study sponsor. The study designer will have access to the final trial dataset. Any investigator involved with this study cannot access the trial data. Data sharing statement is as follows: no later than three years after the collection of the one-year post-randomization interviews, we will deliver a completely deidentified dataset to an appropriate data archive for sharing purposes.
Discussion
There have been studies, both domestic and abroad, on physical exercise interventions with social media apps or smartphone apps in postoperative BC patients. However, as far as we know, the ways of intervention and measures of practice in these studies are relatively simple and few combined intervention studies have been conducted. Therefore, we propose this protocol of combined physical exercise intervention based on Internet and social media apps and plan to validate the feasibility and effectiveness of this protocol in BC rehabilitation via empirical research. If the expected results are achieved in this study, measures and methods of BC rehabilitation will be enriched. Meanwhile, it will also provide support for physical exercise interventions for other diseases, promoting the development of prevention and rehabilitation through physical exercise for patients with chronic diseases, who may also experience social and economic benefits as a result.
In this study, our objective is to perform a clinical trial with a relatively high standard of methods. The protocol of this trial, implementation, and reports will comply with the recommendations in Consolidate Standards of Reporting Trial (CONSORT). A Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) [28] diagram is provided in Additional file 1.
Trial status
At the time of manuscript submission, recruitment for the study is underway but has not been completed.
Additional file
SPIRIT 2013 checklist: Recommended items to address in a clinical trial protocol and related documents. (DOC 142 kb)
Acknowledgements
We thank the Specialized Key Subjects of China National Science & Technology Fundamental Work for funding this trial.
Funding
The trial is funded by the Specialized Key Subjects of China National Science & Technology Fundamental Work (2015FY111600). The funder has not taken part in the study design, data collection and analysis, the decision to publish, or the preparation of the manuscript.
Abbreviations
- BC
Breast cancer
- CONSORT
Consolidated Standards of Reporting Trials
- DCC
Data Coordination Committee
- QOL
Quality of life
- RPE
Rate of perceived exertion
- SF-36
36-item Short Form Health Survey
Authors’ contributions
DX was responsible for drafting the manuscript. YX obtained grant funding to support the study. DM, DX, and CM made several revisions. GD and HS wrote the ethical review confirmation. DM conceived and designed the study protocol. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study has been approved by the Ethics Committee of the Second Hospital of Shandong University (KYLL-2017(KJ)P-0003). Informed consent will be obtained from all participants before participation in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Yi Xiangren and Dong Xiaosheng regarded as co-first authors.
Yi Xiangren and Dong Xiaosheng contributed equally to this work.
Contributor Information
Dong Xiaosheng, Email: dxiaosheng@hotmail.com.
Yi Xiangren, Email: 459274626@qq.com.
Huang Shuyuan, Email: huangshuyuan@163.com.
Gao Dezong, Email: gaodezong1@163.com.
Chao Mengyao, Email: chaomengyo@163.com.
Ding Meng, Email: dingmengpy@aliyun.com.
References
- 1.Breast cancer: prevention and control. http://www.who.int/topics/cancer/breastcancer/zh/. Accessed 16 Feb 2018.
- 2.Gokal K, Wallis D, Ahmed S, Boiangiu I, Kancheria K, Munir F. Effects of a self-managed home-based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: a randomised controlled trial. Support Care Cancer. 2016;24(3):1139–1166. doi: 10.1007/s00520-015-2884-5. [DOI] [PubMed] [Google Scholar]
- 3.Leach HJ, Danyluk JM, Nishimura KC, Culos-Reed SN. Evaluation of a community-based exercise program for breast Cancer patients undergoing treatment. Cancer Nurs. 2015;38(6):417–425. doi: 10.1097/NCC.0000000000000217. [DOI] [PubMed] [Google Scholar]
- 4.Ng AV, Cybulski AN, Engel AA, Papanek PE, Sheffer MA, Waltke LJ, et al. Triathlon training for women breast cancer survivors: feasibility and initial efficacy. Support Care Cancer. 2017;25(5):1465–1473. doi: 10.1007/s00520-016-3531-5. [DOI] [PubMed] [Google Scholar]
- 5.Stan DL, Croghan KA, Croghan IT, Jenkins SM, Sutherland SJ, Cheville AL, et al. Randomized pilot trial of yoga versus strengthening exercises in breast cancer survivors with cancer-related fatigue. Support Care Cancer. 2016;24(9):4005–4015. doi: 10.1007/s00520-016-3233-z. [DOI] [PubMed] [Google Scholar]
- 6.Baruth M, Wilcox S, Der Ananian C, Heiney S. Effects of home-based walking on quality of life and fatigue outcomes in early stage breast Cancer survivors: a 12-week pilot study. J Phys Act Health. 2015;12(Suppl 1):S110–S118. doi: 10.1123/jpah.2012-0339. [DOI] [PubMed] [Google Scholar]
- 7.Kim TH, Chang JS, Kong ID Effects of exercise training on physical fitness and biomarker levels in breast cancer survivors. J Lifestyle Med. 2017;7(2):55–62. doi: 10.15280/jlm.2017.7.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dos Santos WDN, Gentil P, de Moraes RF, Ferreira Junior JB, Campos MH, CAB d L, et al. Chronic effects of resistance training in breast cancer survivors. Biomed Res Int. 2017;2017:8367803. doi: 10.1155/2017/8367803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knobf MT, Jeon S, Smith B, Harris L, Thompson S, Stacy MR, et al. The Yale fitness intervention trial in female cancer survivors: cardiovascular and physiological outcomes. Heart Lung. 2017;46(5):375–381. doi: 10.1016/j.hrtlng.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ammitzboll G, Lanng C, Kroman N, Zerahn B, Hyldegaard O, Kaae Anderson K, et al. Progressive strength training to prevent LYmphoedema in the first year after breast CAncer - the LYCA feasibility study. Acta Oncol. 2017;56(2):360–366. doi: 10.1080/0284186X.2016.1268266. [DOI] [PubMed] [Google Scholar]
- 11.Nelson NL. Breast cancer-related lymphedema and resistance exercise: a systematic review. J Strength Cond Res. 2016;30(9):2656–2665. doi: 10.1519/JSC.0000000000001355. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed RL, Thomas W, Yee D, Schmitz KH. Randomized controlled trial of weight training and lymphedema in breast cancer survivors. J Clin Oncol. 2006;24(18):2765–2772. doi: 10.1200/JCO.2005.03.6749. [DOI] [PubMed] [Google Scholar]
- 13.Jahnke RA, Larkey LK, Rogers R Dissemination and benefits of a replicable Tai Chi and Qigong program for older adults. Geriatr Nurs. 2010;31(4):272–280. doi: 10.1016/j.gerinurse.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Lee MS, Chen KW, Choi TY, Ernst E. Qigong for type 2 diabetes care: a systematic review. Complement Ther Med. 2009;17(4):236–242. doi: 10.1016/j.ctim.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 15.Oh B, Butow PN, Mullan BA, Clarke SJ, Beale PJ, Pavlakis N, et al. Effect of medical qigong on cognitive function, quality of life, and a biomarker of inflammation in cancer patients: a randomized controlled trial. Support Care Cancer. 2012;20(6):1235–1242. doi: 10.1007/s00520-011-1209-6. [DOI] [PubMed] [Google Scholar]
- 16.Chan CL, Wang CW, Ho RT, Ho AH, Ziea ET, Taam Wong VC, et al. A systematic review of the effectiveness of qigong exercise in cardiac rehabilitation. Am J Chin Med. 2012;40(2):255–267. doi: 10.1142/S0192415X12500206. [DOI] [PubMed] [Google Scholar]
- 17.Uhm KE, Yoo JS, Chung SH, Lee JD, Lee I, Kim JI, et al. Effects of exercise intervention in breast cancer patients: is mobile health (mHealth) with pedometer more effective than conventional program using brochure? Breast Cancer Res Treat. 2017;161(3):443–452. doi: 10.1007/s10549-016-4065-8. [DOI] [PubMed] [Google Scholar]
- 18.McCarroll ML, Armbruster S, Pohle-Krauza RJ, Lyzen AM, Min S, Nash DW, et al. Feasibility of a lifestyle intervention for overweight/obese endometrial and breast cancer survivors using an interactive mobile application. Gynecol Oncol. 2015;137(3):508–515. doi: 10.1016/j.ygyno.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 19.Coughlin SS, Whitehead M, Sheats JQ, Mastromonico J, Smith S A Review of smartphone applications for promoting physical activity. Jacobs J Community Med. 2016;2(1):021. [PMC free article] [PubMed] [Google Scholar]
- 20.Serra MC, Ryan AS, Ortmeyer HK, Addison O, Goldberg AP. Resistance training reduces inflammation and fatigue and improves physical function in older breast cancer survivors. Menopause. 2018;25(2):211–216. doi: 10.1097/GME.0000000000000969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dolan LB, Barry D, Petrella T, Davey L, Minnes A, Yantzi A, et al. The cardiac rehabilitation model improves fitness, quality of life, and depression in breast cancer survivors. J Cardiopulm Rehabil Prev. 2018;38:246–252. doi: 10.1097/HCR.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 22.Pineda MD, White E, Kristal AR, Taylor V. Asian breast cancer survival in the US: a comparison between Asian immigrants, US-born Asian Americans and Caucasians. Int J Epidemiol. 2001;30(5):976–982. doi: 10.1093/ije/30.5.976. [DOI] [PubMed] [Google Scholar]
- 23.Unger JB, Reynolds K, Shakib S, Spruijt-Metz D, Sun P, Johnson CA Acculturation, physical activity, and fast-food consumption among Asian-American and Hispanic adolescents. J Community Health. 2004;29:467–481. doi: 10.1007/s10900-004-3395-3. [DOI] [PubMed] [Google Scholar]
- 24.Pescatello LS, Arena R, Riebe D, Thompson PD. SNEAK PEEK preview of ACSM’s guidelines for exercise testing and prescription, ninth edition. ACSMs Health Fit J. 2013;17(2):16–20. doi: 10.1249/FIT.0b013e318282a46d. [DOI] [PubMed] [Google Scholar]
- 25.Carlson JA, Sallis JF, Wagner N, Calfas KJ, Patrick K, Groesz LM, et al. Brief physical activity-related psychosocial measures: reliability and construct validity. J Phys Act Health. 2012;9(8):1178–1186. doi: 10.1123/jpah.9.8.1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klein D, Mercier M, Abeilard E, Puyraveau M, Danzon A, Dalstein V, et al. Long-term quality of life after breast cancer: a French registry-based controlled study. Breast Cancer Res Treat. 2011;129(1):125–134. doi: 10.1007/s10549-011-1408-3. [DOI] [PubMed] [Google Scholar]
- 27.Kwiatkowski F, Mouret-Reynier MA, Duclos M, Leger-Enreille A, Bridon F, Hahn T, et al. Long term improved quality of life by a 2-week group physical and educational intervention shortly after breast cancer chemotherapy completion. Results of the ‘Programme of accompanying women after breast Cancer treatment completion in thermal resorts’ (PACThe) randomised clinical trial of 251 patients. Eur J Cancer. 2013;49(7):1530–1538. doi: 10.1016/j.ejca.2012.12.021. [DOI] [PubMed] [Google Scholar]
- 28.Agha RA, Altman DG, Rosin D The SPIRIT 2013 statement - defining standard protocol items for trials. Int J Surg. 2015;13:288–291. doi: 10.1016/j.ijsu.2014.12.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPIRIT 2013 checklist: Recommended items to address in a clinical trial protocol and related documents. (DOC 142 kb)