Abstract
Purpose
Palliative radiation therapy (RT) can improve quality of life but also incurs time and financial costs. The aim of this study was to evaluate factors associated with use and intensity of palliative RT for incurable non-small cell lung cancer (NSCLC).
Methods and materials
This was a retrospective analysis of Medicare's Surveillance, Epidemiology and End Results data. We identified patients who were diagnosed with incurable (American Joint Committee on Cancer 6th edition stage IIIB with malignant effusion or stage IV) NSCLC between 2004 and 2011. Univariable and multivariable logistic regressions were used to identify factors associated with the receipt of palliative RT and the use of >10 fractions during the first course of radiation. Among patients who were treated with radiation, freestanding versus hospital-based center information was collected on the basis of the location of the RT delivery claim.
Results
Among 55,258 patients with incurable NSCLC, 38% (21,053 patients) received palliative RT during the first year after diagnosis. Among patients who received RT, 56% (11,717 patients) received >10 fractions. On multivariable analysis, factors associated with greater RT use included younger age group (overall P < .01), lower modified Charlson comorbidity score (overall P < .01), female sex (odds ratio [OR]: 1.1; P < .01), marital status (OR: 1.1; P < .01), and chemotherapy use (OR: 3.6; P < .01). Predictors for >10 fractions were chemotherapy use (OR: 1.7; P < .01) and treatment at a freestanding versus hospital-based facility (58% vs 43%; OR: 1.7; P < .01).
Conclusions
More than a third of patients diagnosed with incurable lung cancer receive palliative RT and 56% received >10 fractions. The use of RT varied by region and patient characteristics, and patients treated at freestanding RT centers were more likely to receive >10 fractions. Further research into factors that influence treatment decisions including potential financial incentives may contribute to the high value and strategic utilization of palliative RT.
Summary.
In patients with incurable non-small cell lung cancer, palliative radiation therapy (RT) can improve quality of life but prior studies suggest that patients may receive more fractions than necessary. Using data from Medicare's Surveillance, Epidemiology and End Results, we identify temporal trends in the use of palliative RT among Medicare patients with incurable non-small cell lung cancer and identify predicting factors for extended fractionation. Although patient factors were not strong predictors of fractionation, patients who were treated at freestanding RT centers were more likely to receive >10 fractions.
Alt-text: Unlabelled box
Introduction
Nearly half of patients who present with non-small cell lung cancer (NSCLC) have incurable disease at the time of diagnosis and harbor a 5-year survival of <5%.1 The goals of care focus primarily on the prolongation of survival and palliation of symptoms rather than curing.2, 3 Palliative radiation therapy (RT) is frequently employed in patients with incurable NSCLC, and the most common radiation treatment sites are the brain, thorax, and bone.4 Despite its ability to stabilize or improve symptoms,3 prior studies have shown significant variations in the use of palliative RT that ranged from 31% to 66% in patients with incurable disease.4, 5, 6, 7, 8, 9, 10 Even in instances when palliative RT is employed, RT fractionation schemes vary and are not always well-supported by randomized data.4, 11, 12
Over the past decade, there have been several significant clinical developments with the potential to influence the use of palliative RT including new radiation technologies such as stereotactic radiosurgery and stereotactic body radiation therapy. New biologically targeted therapies also can alter the timing and need for conventional palliative RT. Prior studies4, 8, 13 that investigated RT receipt among patients with incurable NSCLC largely included only patients diagnosed prior to adoption of these new treatment advances. Even less is known about the factors that influence the number of radiation fractions used in patients with incurable NSCLC. On the basis of the results of multiple trials that showed no difference in pain control between single fraction and longer radiation courses for patients with bone metastases,14, 15, 16, 17, 18 the American Society for Radiation Oncology Choosing Wisely Campaign recommended against the routine use of extended fractionation schemes (>10 fractions) for palliation of bone metastases.19 Additionally, although 1 meta-analysis found that higher dose schedules (30 Gy per ≥10 fraction-equivalent) were associated with small improvements in symptom control and survival at the cost of increased short-term side effects,20, 21 the general consensus is that shorter courses should be considered for patients with a poor prognosis or performance status.18, 21 Because the use of extended RT courses is associated with increased cost of care and inconvenient for patients with a significant disease burden and limited life expectancy,22, 23 understanding the factors that influence their use is critical.
The goal of our study was to elucidate palliative RT practice patterns and identify patient and health care service factors associated with the use of palliative RT and extended fractionation (>10 fractions) among patients with incurable NSCLC.
Methods and materials
Sources of data
We used data from the National Cancer Institute's Surveillance, Epidemiology and End Results (SEER) cancer registries linked to Medicare claims data. The SEER database consists of tumor registries across the United States and provides a near representative sample of approximately 28% of the U.S. population.24 SEER registries include data on patient demographics, cancer site, stage, histology, and dates of diagnosis and death. SEER data for patients diagnosed between January 1, 2004 and December 31, 2011 were linked to inpatient and outpatient Medicare claims data through December 31, 2012.24
Study cohort
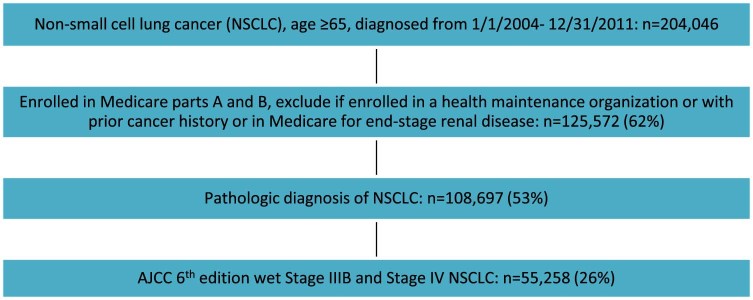
Our cohort included Medicare-enrolled patients age >65 years diagnosed with incurable lung cancer (defined as stage IIIB with malignant effusion [wet IIIB] or stage IV NSCLC [American Joint Committee on Cancer 6th Edition]) between 2004 and 2011 in a SEER surveillance area. NSCLC had to be pathologically confirmed and diagnosed prior to death as the first cancer diagnosis. Subjects were continuously enrolled in Medicare Parts A and B and not in a health maintenance organization from the time of diagnosis to death. Patients with a prior history of cancer who were enrolled in Medicare due to end stage renal disease or cancer that was diagnosed at the time of an autopsy were excluded (Fig 1).
Figure 1.
Flowchart of cohort selection. NSCLC, non-small cell lung cancer.
Definition of outcomes variables
We identified patients who had at least 1 Medicare claim for radiation delivery for conventional RT (2-dimensional, 3-dimensional, or intensity modulated RT [IMRT]) or stereotactic radiation surgery and stereotactic body RT within 1 year of diagnosis. Conventional palliative RT was defined as external beam 2-dimensional, 3-dimensional, or IMRT on the basis of the common procedural terminology (CPT) or Healthcare Common Procedure Coding System (HCPCS) codes 77401-77416, 77418, 0073T, and G0174 (Table A1). Stereotactic RT was defined separately from conventional palliative RT and based on CPT/HCPCS codes 77371-77373, G0173, G0243, G0251, G0339, and G0340.
Among the patients who received conventional palliative RT, we determined whether the RT delivery code had an associated International Classification of Diseases 9th Revision (ICD-9) diagnosis code of brain metastases (198.3), bone metastases (198.5), or other (nonbrain, nonbone). Patients with multiple ICD-9 codes were first assigned to brain metastases, then bone, then lung or other sites (nonbrain, nonbone). Fractionation by associated ICD-9 code was calculated. Furthermore, among patients who received conventional palliative RT, we also calculated the number of RT fractions delivered during the first course of treatment on the basis of the number of RT delivery codes for conventional RT. RT courses were considered separate if there was more than a 14-day break between treatment deliveries. Extended fractionation for this study was defined as >10 fractions, which corresponds to about 2 weeks of daily treatment.
Definition of explanatory variables
Demographic variables including age, sex, race, and marital status were obtained from the SEER data and categorized as shown in Table 1. Patients were categorized in income quartiles on the basis of the median household income in the ZIP code of residence. A modified Charlson comorbidity index was determined using Deyo's implementation of the Charlson score applied to both inpatient and outpatient claims over the 12 months prior to the diagnosis month as suggested by Klabunde.25, 26 Treatment factors included use of any hospice service or chemotherapy during the first year after diagnosis. Patients who had any claims in the SEER-Medicare Hospice file24 were categorized as having received hospice services. Receipt of chemotherapy was defined by the CPT/HCPCS codes listed in Table A1.
Table 1.
Baseline characteristics among patients with incurable non-small cell lung cancer (2004-2011)
| N | % | |
|---|---|---|
| Demographic | ||
| Age at time of diagnosis (years) | ||
| 65-69 | 13,904 | 25% |
| 70-74 | 13,904 | 25% |
| 75-79 | 12,773 | 23% |
| >80 | 14,677 | 27% |
| Sex | ||
| Female | 25,966 | 47% |
| Male | 29,292 | 53% |
| Race | ||
| White | 47,359 | 86% |
| Black | 4993 | 9% |
| Asian/Pacific Islander | 2654 | 5% |
| Other | 252 | 1% |
| Marital status | ||
| Not married | 27,840 | 50% |
| Married | 27,418 | 50% |
| Diagnosis year | ||
| 2004-2005 | 15,165 | 27% |
| 2006-2007 | 14,413 | 26% |
| 2008-2009 | 13,234 | 24% |
| 2010-2011 | 12,446 | 23% |
| Median income quartile (percentile)* | ||
| ≤25th | 13,549 | 25% |
| 25th-50th | 13,547 | 25% |
| 50th-75th | 13,589 | 25% |
| >75th | 13,598 | 25% |
| Clinical and treatment | ||
| Modified Charlson comorbidity score† | ||
| 0 | 28,449 | 51% |
| 1 | 14,211 | 26% |
| 2 | 6129 | 11% |
| >3 | 6469 | 12% |
| Radiation treatment‡ | ||
| No | 33,506 | 62% |
| Yes | 20,435 | 38% |
| Chemotherapy‡ | ||
| No | 31,812 | 58% |
| Yes | 23,446 | 42% |
| Hospice services‡ | ||
| No | 28,926 | 52% |
| Yes | 26,332 | 48% |
| Regional and health care services | ||
| SEER region | ||
| Northeast | 10,300 | 19% |
| South | 15,798 | 28% |
| Midwest | 7544 | 14% |
| West | 21,616 | 39% |
| Living in urban area | ||
| No | 6537 | 12% |
| Yes | 48,721 | 88% |
| Radiation oncologist density§ | ||
| Lowest tertile | 18,230 | 33% |
| Middle tertile | 20,054 | 36% |
| Highest tertile | 16,968 | 31% |
SEER, Surveillance, Epidemiology and End Results.
By ZIP code.
Measured using the Klabunde modification.
Within 1 year of diagnosis.
Number of radiation oncologists (per 100,000 population) practicing within each patient's county of residence, divided into top (>1.5), middle (1-1.5), and bottom (<1) tertiles.
The health service characteristics included urban residence, SEER region, and density of radiation oncology providers. Urban residence was obtained from the SEER data. SEER registries were grouped geographically into the following regions: West (San Francisco, San Jose, Los Angeles, Greater California, Hawaii, New Mexico, Seattle, and Utah), Midwest (Detroit and Iowa), South (Atlanta, rural Georgia, Kentucky, Louisiana, and Oklahoma), and Northeast (Connecticut and New Jersey). On the basis of the 2011 state and county data, the Center for Medicare and Medicate Services (CMS) Area Resource File27 was utilized to ascertain the density of radiation oncologists, which was defined as the number of radiation oncologists (per 100,000 population) who practiced within each patient's county of residence. The density of radiation oncologists was reported by top, middle, and bottom tertiles, which corresponded to ≥1.5, 1 to 1.5, or <1 radiation oncologist per 100,000 population.
Among patients who were treated with palliative radiation, freestanding versus hospital-based center information was collected. Patients were assigned as receiving RT in a hospital-associated outpatient clinic if the RT delivery claims were only present in the SEER-Medicare outpatient data file.24 Patients who received RT in a freestanding RT center were assigned if the RT delivery claim was only in the SEER-Medicare carrier claims file.24 Patients who had RT delivery claims in both the outpatient and carrier files were categorized as having received RT at a freestanding center.
Statistical methods
χ2 tests were used to compare categorical baseline demographic and clinical characteristics between patients treated with conventional palliative RT and those who were not. Univariable and multivariable fixed-effects logistic regressions were used to identify patient, treatment and health care system factors associated with receipt of RT and number of treatment fractions received with a correction for clustering at the county level. All variables that were included in the univariable analysis were included in the multivariable analysis. SAS software version 9.4 (SAS Institute, Cary, NC) was used for the analyses.
Results
Among the 55,258 patients diagnosed with incurable NSCLC, the median age was 76 years, 53% were male, and 84% were white. The remaining characteristics of the patient cohort are described in Table 1.
Use of radiation therapy in patients with incurable non-small cell lung cancer
Overall, 38% (21,053 patients) received conventional palliative RT within 1 year of diagnosis. Among these patients, 3% received a single fraction and 56% received >10 fractions for their first course. Use of conventional RT trended downward from 40% in 2004 to 35% in 2011 (χ2 trend test P < .01) but the use of stereotactic RT increased from 0.8% in 2004 to 5% in 2011 (χ2 trend test P < .01; Fig 2).
Figure 2.
Percent of patients with incurable non-small cell lung cancer who received conventional and stereotactic radiation therapy by year of diagnosis.
Among patients who received conventional palliative RT, 26% of courses were associated with a brain metastasis, 18% with bone metastases, and 57% with lung or other diagnosis codes. Among patients who received radiation associated with brain or bone metastasis diagnosis codes, >50% received ≤10 fractions (Table 2). Among those who received radiation associated with lung or other metastases, close to two-thirds received >10 fractions and 35% received >20 fractions.
Table 2.
Number of palliative radiation therapy fractions by site of metastases
| 1 fraction | 2-5 fractions | 6-10 fractions | 11-15 fractions | 16-20 fractions | >20 fractions | |
|---|---|---|---|---|---|---|
| Brain | 132 (2%) | 623 (12%) | 2042 (38%) | 1767 (33%) | 458 (8%) | 393 (7%) |
| Bone | 152 (4%) | 595 (16%) | 1400 (38%) | 968 (26%) | 340 (9%) | 262 (7%) |
| Lung and other | 298 (3%) | 1364 (11%) | 2730 (23%) | 2234 (19%) | 1104 (9%) | 4191 (35%) |
Predictors of conventional palliative radiation therapy receipt
On multivariable analysis, demographic factors associated with use of conventional palliative RT included marital status (married vs unmarried; 42% vs 35%; P < .01), younger age group (48% vs 43% vs 37% vs 27% for age groups 65-69, 70-75, 76-80, and 80 + years; overall P < .01), and female sex (38% vs 39%; P < .01). Clinical and treatment factors that were associated with the receipt of RT were a lower modified Charlson comorbidity score (42% vs 38% vs 34 % vs 29% for modified Charlson 0, 1, 2, and 3 + , respectively; overall P < .01) and receipt of chemotherapy (57% vs 25%; P < .01).
In terms of regional and health service factors associated with the use of RT, patients in the West were less likely to receive RT relative to patients in the Northeast, Midwest, or South of the United States (36% vs 39% vs 40% vs 41%; overall P < .01). There appeared no clear trend in the percentage of patients who received RT between patients living in counties with low, middle, and high densities of radiation oncologists (39% vs 37% vs 40% for lowest, middle, and top tertiles). Race, urban residence, median census income quartile, and use of hospice services were not significant predictors of RT receipt. Table 3 lists the univariable and multivariable predictors of conventional RT use.
Table 3.
Predictors of receipt of RT among patients with incurable non-small cell lung cancer
| Univariable |
Multivariable |
|||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | RT Yes | RT No | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Demographic | ||||||||
| Age at time of diagnosis (years) | ||||||||
| 65-69 | 48% | 52% | ref | < .01 | ref | < .01 | ||
| 70-74 | 43% | 57% | 0.82 | 0.79-0.86 | 0.88 | 0.84-0.93 | ||
| 75-79 | 37% | 63% | 0.64 | 0.61-0.67 | 0.74 | 0.70-0.79 | ||
| >80 | 27% | 73% | 0.41 | 0.39-0.43 | 0.60 | 0.56-0.64 | ||
| Sex | ||||||||
| Female | 38% | 62% | ref | .54 | ref | < .01 | ||
| Male | 39% | 61% | 1.01 | 0.98-1.05 | 0.93 | 0.89-0.97 | ||
| Race | ||||||||
| White | 39% | 61% | ref | < .01 | ref | .12 | ||
| Black | 36% | 64% | 0.89 | 0.84-0.94 | 0.93 | 0.87-1.00 | ||
| Asian/Pacific Islander | 35% | 65% | 0.84 | 0.78-0.92 | 1.02 | 0.90-1.14 | ||
| Other | 32% | 68% | 0.74 | 0.57-0.97 | 0.78 | 0.58-1.07 | ||
| Marital status | ||||||||
| Not married | 35% | 65% | ref | < .01 | ref | < .01 | ||
| Married | 42% | 58% | 1.38 | 1.34-1.43 | 1.15 | 1.10-1.20 | ||
| Diagnosis year | ||||||||
| 2004-2005 | 40% | 60% | ref | < .01 | ref | .03 | ||
| 2006-2007 | 39% | 61% | 0.98 | 0.93-1.02 | 1.02 | 0.97-1.07 | ||
| 2008-2009 | 38% | 62% | 0.94 | 0.89-0.98 | 0.97 | 0.91-1.04 | ||
| 2010-2011 | 37% | 63% | 0.89 | 0.85-0.93 | 0.92 | 0.87-0.98 | ||
| Median income quartile (percentile)* | ||||||||
| ≤25th | 38% | 62% | ref | .06 | ref | .90 | ||
| 25th to 50th | 38% | 62% | 1.04 | 0.99-1.09 | 1.00 | 0.93-1.07 | ||
| 50th-75th | 39% | 61% | 1.08 | 1.00-1.10 | 1.00 | 0.93-1.07 | ||
| >75th | 39% | 61% | 1.07 | 1.02-1.12 | 1.02 | 0.95-1.10 | ||
| Clinical and treatment | ||||||||
| Modified Charlson comorbidity score† | ||||||||
| 0 | 42% | 58% | ref | < .01 | ref | < .01 | ||
| 1 | 38% | 62% | 0.88 | 0.85-0.92 | 0.91 | 0.87-0.95 | ||
| 2 | 34% | 66% | 0.73 | 0.69-0.77 | 0.81 | 0.75-0.88 | ||
| 3 + | 29% | 71% | 0.58 | 0.55-0.61 | 0.69 | 0.64-0.74 | ||
| Chemotherapy‡ | ||||||||
| No | 25% | 75% | ref | < .01 | ref | < .01 | ||
| Yes | 57% | 43% | 3.98 | 3.84-4.13 | 3.57 | 3.42-3.72 | ||
| Hospice services‡ | ||||||||
| No | 41% | 59% | ref | < .01 | ref | .23 | ||
| Yes | 36% | 64% | 0.83 | 0.80-0.86 | 1.02 | 0.98-1.07 | ||
| Regional and health care services | ||||||||
| SEER region | ||||||||
| Northeast | 39% | 61% | ref | < .01 | ref | < .01 | ||
| South | 41% | 59% | 1.12 | 1.07-1.18 | 1.14 | 1.04-1.24 | ||
| Midwest | 40% | 60% | 1.06 | 1.00-1.13 | 1.08 | 1.00-1.18 | ||
| West | 36% | 64% | 0.89 | 0.85-0.94 | 0.91 | 0.83-0.99 | ||
| Living in urban area | ||||||||
| No | 40% | 60% | ref | < .01 | ref | .41 | ||
| Yes | 38% | 62% | 0.92 | 0.88-0.97 | 0.97 | 0.89-1.05 | ||
| Radiation oncology county density§ | ||||||||
| Lowest tertile | 39% | 61% | ref | < .01 | ref | < .01 | ||
| Middle tertile | 37% | 63% | 0.95 | 0.91-0.99 | 1.14 | 1.05-1.23 | ||
| Highest tertile | 40% | 60% | 1.04 | 0.99-1.08 | 1.14 | 1.05-1.22 | ||
CI, confidence interval; OR, odds ratio; ref, reference group; RT, radiation therapy; SEER, Surveillance, Epidemiology and End Results.
By ZIP code.
Measured using the Klabunde modification.
Within 1 year of diagnosis.
Number of radiation oncologists (per 100,000 population) practicing within each patient's county of residence, divided into top (>1.5), middle (1-1.5), and bottom (<1) tertiles.
Predictors of receipt of extended fractionation (>10 fractions)
None of the demographic factors (sex, marital status, race, year of diagnosis, and median ZIP code income) in our model was associated with the use of extended fractionation RT on multivariable analysis and there was no consistent age-related trend. The receipt of chemotherapy in the year after diagnosis (54% vs 39%; P < .01) was associated with use of extended fractionation but the modified Charlson comorbidity score was not a significant predictor of extended fractionation.
With regard to health system factors, radiation oncologist density, urban residence, and SEER region were not significant predictors of receiving extended fractionation. However, we found that patients treated at freestanding RT facilities were more likely to receive extended fractionation compared with those treated at hospital-based facilities (58% vs 44%; P < 0.01). Table 4 lists the univariable and multivariable predictors of extended fractionation.
Table 4.
Predictors of receipt of >10 fractions of RT among patients who received conventional RT
| Univariable |
Multivariable |
|||||||
|---|---|---|---|---|---|---|---|---|
| Predictors | >10 fractions | ≤10 fractions | OR | 95% CI | P-value | OR | 95% CI | P-value |
| Demographic | ||||||||
| Age at time of diagnosis (years) | ||||||||
| 65-69 | 50% | 50% | ref | < .01 | .02 | |||
| 70-74 | 49% | 51% | 0.97 | 0.90-1.04 | 1.02 | 0.95-1.10 | ||
| 75-79 | 47% | 53% | 0.89 | 0.83-0.96 | 0.98 | 0.91-1.06 | ||
| >80 | 47% | 53% | 0.89 | 0.82-0.96 | 1.13 | 1.04-1.23 | ||
| Sex | ||||||||
| Female | 48% | 52% | ref | .70 | .63 | |||
| Male | 49% | 51% | 1.01 | 0.96-1.07 | 0.99 | 0.93-1.05 | ||
| Race | ||||||||
| White | 49% | 51% | ref | .04 | .16 | |||
| Black | 46% | 54% | 0.88 | 0.80-0.97 | 0.92 | 0.81-1.04 | ||
| Asian/Pacific Islander | 48% | 52% | 0.93 | 0.82-1.06 | 0.97 | 0.66-1.44 | ||
| Other | 48% | 52% | 1.25 | 0.81-1.93 | 1.51 | 1.00-2.30 | ||
| Marital status | ||||||||
| Not married | 47% | 53% | ref | < .01 | .46 | |||
| Married | 49% | 51% | 1.10 | 1.04-1.16 | 1.02 | 0.97-1.08 | ||
| Diagnosis year | ||||||||
| 2004-2005 | 48% | 52% | ref | .65 | .68 | |||
| 2006-2007 | 48% | 52% | 1.00 | 0.93-1.08 | 1.03 | 0.97-1.10 | ||
| 2008-2009 | 48% | 52% | 1.02 | 0.94-1.10 | 1.02 | 0.94-1.10 | ||
| 2010-2011 | 49% | 51% | 1.05 | 0.97-1.13 | 1.05 | 0.96-1.14 | ||
| Median income quartile (percentile)* | ||||||||
| ≤25th | 49% | 51% | ref | .11 | .23 | |||
| 25th-50th | 49% | 51% | 1.02 | 0.94-1.10 | 1.07 | 0.94-1.21 | ||
| 50th-75th | 47% | 53% | 0.93 | 0.87-1.01 | 0.96 | 0.85-1.09 | ||
| >75th | 48% | 52% | 0.96 | 0.89-1.03 | 1.03 | 0.91-1.17 | ||
| Clinical and treatment | ||||||||
| Modified Charlson comorbidity score† | ||||||||
| 0 | 49% | 51% | ref | .30 | .57 | |||
| 1 | 49% | 51% | 1.00 | 0.94-1.07 | 1.00 | 0.93-1.08 | ||
| 2 | 47% | 53% | 0.94 | 0.86-1.04 | 0.95 | 0.85-1.07 | ||
| 3 + | 47% | 53% | 0.93 | 0.84-1.02 | 0.95 | 0.87-1.04 | ||
| Chemotherapy‡ | ||||||||
| No | 39% | 61% | ref | < .01 | < .01 | |||
| Yes | 54% | 46% | 1.79 | 1.69-1.89 | 1.74 | 1.61-1.88 | ||
| Hospice services‡ | ||||||||
| No | 52% | 48% | ref | < .01 | < .01 | |||
| Yes | 44% | 56% | 0.73 | 0.69-0.77 | 0.77 | 0.73-0.82 | ||
| Regional and health care services | ||||||||
| SEER region | ||||||||
| Northeast | 43% | 57% | ref | .09 | .07 | |||
| South | 50% | 50% | 1.34 | 1.24-1.45 | 1.19 | 1.02-1.39 | ||
| Midwest | 49% | 51% | 1.27 | 1.15-1.39 | 1.23 | 1.04-1.46 | ||
| West | 49% | 51% | 1.29 | 1.19-1.39 | 1.13 | 0.97-1.33 | ||
| Living in urban area | ||||||||
| No | 50% | 50% | ref | .09 | .40 | |||
| Yes | 48% | 52% | 0.93 | 0.86-1.01 | 1.06 | 0.93-1.20 | ||
| Radiation oncologist density§ | ||||||||
| Lowest tertile | 50% | 50% | ref | < .01 | .63 | |||
| Middle tertile | 47% | 53% | 0.90 | 0.84-0.96 | 0.96 | 0.85-1.09 | ||
| Highest tertile | 48% | 52% | 0.92 | 0.86-0.99 | 1.01 | 0.90-1.13 | ||
| RT facility type | ||||||||
| Hospital-based | 44% | 56% | ref | < .01 | < .01 | |||
| Freestanding | 58% | 43% | 1.74 | 1.65-1.84 | 1.74 | 1.60-1.89 | ||
CI, confidence interval; OR, odds ratio; ref, reference group; RT, radiation therapy; SEER, Surveillance, Epidemiology and End Results.
By ZIP code.
Measured using the Klabunde modification.
Within 1 year of diagnosis.
Number of radiation oncologists (per 100,000 population) practicing within each patient's county of residence, divided into top (>1.5), middle (1-1.5), and bottom (<1) tertiles.
Discussion
This study characterizes the delivery of RT among patients diagnosed with incurable NSCLC including factors that influenced the use of conventional palliative RT and choice of extended RT fractionation in the year after diagnosis. Although we observed decreases in the use of conventional palliative RT between 2004 and 2011, this was offset by a synchronous increase in the utilization of stereotactic RT.
A combination of patient and treatment factors were associated with the use of conventional RT. Younger age, female sex, lower modified Charlson comorbidity score, marital status, and chemotherapy use were associated with greater conventional RT use, which is consistent with prior studies8, 9 Chemotherapy receipt may be a surrogate for a preference for higher intensity of care and patients fit enough to receive chemotherapy may also have a higher chance of receiving RT.
More than 50% of patients with incurable NSCLC received >10 fractions of palliative RT including 42% who received RT associated with a bone metastasis diagnosis code despite national guidelines that suggest shorter courses.19 This is consistent with findings from other studies28, 29, 30 and suggests that there may be opportunities to streamline or consolidate care by implementing programs to evaluate whether and when shorter courses of radiation are appropriate.
Furthermore, a proportion of patients in our study likely received palliative radiation directed to the chest. Prior studies suggest that approximately 20% of patients with metastatic lung cancer receive palliative chest RT.4, 31 Although several RCTs failed to find a statistically significant benefit to prolonged courses of RT to the chest,20, 32, 33, 34 an exploratory meta-analysis suggested that more aggressive radiation schedules (greater than 30 Gy/10 fractions) was associated with modest improvements in survival,21 though with greater acute side effects. In our cohort, 22% of patients received radiation associated with a nonmetastatic diagnosis code, many of whom likely received RT directed to the chest. In this group, approximately 28% of patients received >10 fractions and 9% received >15 fractions. Many patients who received >10 fractions may also have good overall performance or were treated more aggressively for oligometastatic disease.
We found that many of the patient factors such as sex, comorbidity, and marital status, which predicted a patient receiving conventional RT, did not influence the choice of fractionation scheme. Once the decision to deliver palliative RT is made, other factors appear to influence the fractionation schedule. Patients who received any chemotherapy and those treated at a freestanding RT facility were respectively 1.79 and 1.74 times more likely to receive >10 fractions. Although unobserved differences in patient characteristics or preferences could account for some of this variation, differences in reimbursement of radiation or ownership structure by facility type may also be contributing factors. Prior studies have shown that radiation oncologists are overwhelmingly likely to recommend treatment once a patient is referred to them,35 which suggests that practice type may be more likely to influence decisions about radiation intensity than decisions about whether to treat.
Previous work has shown a nearly two-fold differential in the reimbursement for IMRT between freestanding centers versus hospital-based outpatient clinics.36 Although the reimbursement gap has decreased over time, another study has shown more a rapid adoption of IMRT at freestanding versus hospital-based centers.37 Additionally, Mitchell et al. reported a 29% increase in the use of IMRT for patients with prostate cancer among physician-owned practices compared with nonphysician-owned practices38 but data on practice ownership by freestanding versus hospital-based facility type are not readily available.
Further research is needed to determine the relative impact of financial incentives on the choice of treatment schema and develop effective strategies to properly align incentives. One possible solution may be the implementation of bundled payments that are piloted currently by the CMS and other insurers in close collaboration with providers and hospitals.39 Even though the American Society for Radiation Oncology Choosing Wisely campaign's initial recommendations were released in 2013, data in support of these recommendations existed well before then. Furthermore, our data can serve as a baseline from which to evaluate the impact of the campaign.
Our study has several limitations. First, SEER-Medicare data are observational and lack the clinical granularity needed to identify radiation specifics including radiation treatment fields and doses. In addition, RT delivered in the inpatient setting is not captured in claims data.40 SEER-Medicare data also do not directly provide information on treatment site but we expect that radiation that is administered to patients with incurable disease would be palliative in intent.
In our patient cohort, patients possibly received RT to the primary or a metastatic site. Although we attempted to glean potential treatment site by determining the associated ICD-9 code with the radiation delivery claim, diagnosis codes do not necessarily indicate the site of treatment and physicians may bill according to the primary rather than the secondary diagnosis code even when treating a metastatic site. Furthermore, we do not have information on symptom burden or performance status, which may influence decisions about palliative RT and the delivery of long or short courses of treatment.
Second, our analysis is limited to fee-for-service Medicare-insured patients who lived in SEER regions; therefore, the study results may not be generalizable to all patients with incurable NSCLC. However, since a majority of the lung cancer diagnoses occurred in patients age >65 years and more than 95% of Americans age >65 years are covered by Medicare, our cohort's experiences are likely to represent typical patterns of care for NSCLC. In addition, the SEER-Medicare population has been shown to be reasonably representative of the general U.S. adult population.41, 42 Finally, although we controlled for available demographic and clinical characteristics in our models, the possibility of additional confounding from other patient characteristics exists.
Conclusions
Our study characterizes the use of RT for patients with incurable NSCLC and identifies factors associated with the use of conventional palliative RT and extended fractionation. Although there are potential clinical scenarios when extended fractionation may be warranted, in most palliative settings, radiation treatments can safely and effectively be given in ≤10 fractions. Further research into the underlying factors, including potential financial incentive, that shape treatment decisions will allow for development of effective policies aimed at improving access and efficient administration of RT in an era when high-value care for patients is the focus.
Acknowledgments
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services, Inc.; and the SEER Program tumor registries in the creation of the SEER-Medicare database. Jennifer Wind provided exceptional project management.
Footnotes
Sources of support: This study was supported by a grant from the Gloria Spivak Fund. No funding was obtained from the National Institutes of Health.
Conflicts of interest: None.
Supplementary material for this article (https://doi.org/10.1016/j.adro.2018.04.005) can be found at www.practicalradonc.org.
Supplementary data
The following is the supplementary data to this article:
Supplementary materials.
References
- 1.Howlader N., Noone A., Krapcho M. SEER cancer statistics review, 1975-2012. http://seer.cancer.gov/csr/1975_2012/ National Cancer Institute; Available at: based on November 2014 SEER data submission.
- 2.Lutz S., Korytko T., Nguyen J., Khan L., Chow E., Corn B. Palliative radiotherapy: When is it worth it and when is it not? Cancer J. 2010;16:473–482. doi: 10.1097/PPO.0b013e3181f28b4d. [DOI] [PubMed] [Google Scholar]
- 3.Lutz S.T., Jones J., Chow E. Role of radiation therapy in palliative care of the patient with cancer. J Clin Oncol. 2014;32:2913–2919. doi: 10.1200/JCO.2014.55.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen A.B., Cronin A., Weeks J.C. Palliative radiation therapy practice in patients with metastatic non-small-cell lung cancer: A Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) Study. J Clin Oncol. 2013;31:558–564. doi: 10.1200/JCO.2012.43.7954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bekelman J.E., Epstein A.J., Emanuel E.J. Single- vs multiple-fraction radiotherapy for bone metastases from prostate cancer. JAMA. 2013;310:1501–1502. doi: 10.1001/jama.2013.277081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guadagnolo B.A., Liao K.P., Elting L., Giordano S., Buchholz T.A., Shih Y.C. Use of radiation therapy in the last 30 days of life among a large population-based cohort of elderly patients in the United States. J Clin Oncol. 2013;31:80–87. doi: 10.1200/JCO.2012.45.0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guadagnolo B.A., Huo J., Liao K.P., Buchholz T.A., Das P. Changing trends in radiation therapy technologies in the last year of life for patients diagnosed with metastatic cancer in the United States. Cancer. 2013;119:1089–1097. doi: 10.1002/cncr.27835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayman J.A., Abrahamse P.H., Lakhani I., Earle C.C., Katz S.J. Use of palliative radiotherapy among patients with metastatic non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2007;69:1001–1007. doi: 10.1016/j.ijrobp.2007.04.059. [DOI] [PubMed] [Google Scholar]
- 9.Murphy J.D., Nelson L.M., Chang D.T., Mell L.K., Le Q.T. Patterns of care in palliative radiotherapy: a population-based study. J Oncol Pract. 2013;9:e220–e227. doi: 10.1200/JOP.2012.000835. [DOI] [PubMed] [Google Scholar]
- 10.Wong J., Xu B., Yeung H.N. Age disparity in palliative radiation therapy among patients with advanced cancer. Int J Radiat Oncol Biol Phys. 2014;90:224–230. doi: 10.1016/j.ijrobp.2014.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haddad P., Wong R.K.S., Pond G.R. Factors influencing the use of single vs multiple fractions of palliative radiotherapy for bone metastases: a 5-year review. Clin Oncol (R Coll Radiol) 2005;17:430–434. doi: 10.1016/j.clon.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 12.Bradley N.M.E., Husted J., Sey M.S.L. Review of patterns of practice and patients' preferences in the treatment of bone metastases with palliative radiotherapy. Support Care Cancer. 2007;15:373–385. doi: 10.1007/s00520-006-0161-3. [DOI] [PubMed] [Google Scholar]
- 13.Kapadia N.S., Mamet R., Zornosa C., Niland J.C., D'Amico T.A., Hayman J.A. Radiation therapy at the end of life in patients with incurable nonsmall cell lung cancer. Cancer. 2012;118:4339–4345. doi: 10.1002/cncr.27401. [DOI] [PubMed] [Google Scholar]
- 14.Steenland E., Leer J.W., van Houwelingen H. The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol. 1999;52:101–109. doi: 10.1016/s0167-8140(99)00110-3. [DOI] [PubMed] [Google Scholar]
- 15.Chow E., Zeng L., Salvo N., Dennis K., Tsao M., Lutz S. Update on the systematic review of palliative radiotherapy trials for bone metastases. Clin Oncol (R Coll Radiol) 2012;24:112–124. doi: 10.1016/j.clon.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Fairchild A., Barnes E., Ghosh S. International patterns of practice in palliative radiotherapy for painful bone metastases: evidence-based practice? Int J Radiat Oncol Biol Phys. 2009;75:1501–1510. doi: 10.1016/j.ijrobp.2008.12.084. [DOI] [PubMed] [Google Scholar]
- 17.Hartsell W.F., Scott C.B., Bruner D.W. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97:798–804. doi: 10.1093/jnci/dji139. [DOI] [PubMed] [Google Scholar]
- 18.Lutz S., Berk L., Chang E. Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–976. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Hahn C., Kavanagh B., Bhatnagar A. Choosing wisely: the American Society for Radiation Oncology's top 5 list. Pract Radiat Oncol. 2014;4:349–355. doi: 10.1016/j.prro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Rodrigues G., Videtic G.M.M., Sur R. Palliative thoracic radiotherapy in lung cancer: an American Society for Radiation Oncology evidence-based clinical practice guideline. Pract Radiat Oncol. 2011;1:60–71. doi: 10.1016/j.prro.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fairchild A., Harris K., Barnes E. Palliative thoracic radiotherapy for lung cancer: a systematic review. J Clin Oncol. 2008;26:4001–4011. doi: 10.1200/JCO.2007.15.3312. [DOI] [PubMed] [Google Scholar]
- 22.Hanly P., Céilleachair A.Ó., Skally M., O'Neill C., Sharp L. Direct costs of radiotherapy for rectal cancer: a microcosting study. BMC Health Serv Res. 2015;15:184. doi: 10.1186/s12913-015-0845-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayman J.A., Lash K.A., Tao M.L., Halman M.A. A comparison of two methods for estimating the technical costs of external beam radiation therapy. Int J Radiat Oncol Biol Phys. 2000;47:461–467. doi: 10.1016/s0360-3016(00)00427-2. [DOI] [PubMed] [Google Scholar]
- 24.National Cancer Institute Surveillance, epidemiology, and end results program: SEER data. http://healthcaredelivery.cancer.gov/seermedicare/ Available at:
- 25.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 26.Klabunde C.N., Potosky A.L., Legler J.M., Warren J.L. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 27.U.S. Department of Health and Human Services Health resources and services administration health professions: Area resource file (ARF) national county-level health resource information database. http://arg.hrsa.gov Available at:
- 28.Rutter C.E., Yu J.B., Wilson L.D., Park H.S. Assessment of national practice for palliative radiation therapy for bone metastases suggests marked underutilization of single-fraction regimens in the United States. Int J Radiat Oncol Biol Phys. 2015;91:548–555. doi: 10.1016/j.ijrobp.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 29.Conway J.L., Yurkowski E., Glazier J. Comparison of patient-reported outcomes with single versus multiple fraction palliative radiotherapy for bone metastasis in a population-based cohort. Radiother Oncol. 2016;119:202–207. doi: 10.1016/j.radonc.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 30.McDonald R., Chow E., Lam H., Rowbottom L., Soliman H. International patterns of practice in radiotherapy for bone metastases: a review of the literature. J Bone Oncol. 2014;3:96–102. doi: 10.1016/j.jbo.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koshy M., Malik R., Mahmood U., Husain Z., Weichselbaum R.R., Sher D.J. Prevalence and predictors of inappropriate delivery of palliative thoracic radiotherapy for metastatic lung cancer. J Natl Cancer Inst. 2015;107:djv278. doi: 10.1093/jnci/djv278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stevens R., Macbeth F., Toy E., Coles B., Lester J.F. Palliative radiotherapy regimens for patients with thoracic symptoms from non-small cell lung cancer. In: Stevens R., editor. Vol. 1. John Wiley & Sons; Chichester, UK: 2015. (Cochrane Database of Systematic Reviews). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lester J.F., Macbeth F., Toy E., Coles B. Palliative radiotherapy regimens for non-small cell lung cancer. In: Lester J.F., editor. Cochrane Database of Systematic Reviews. John Wiley & Sons; Chichester, UK: 2006. [DOI] [PubMed] [Google Scholar]
- 34.Macbeth F., Toy E., Coles B., Melville A., Eastwood A. Palliative radiotherapy regimens for non-small cell lung cancer. In: MacBeth F., editor. The Cochrane Database of Systematic Reviews. John Wiley & Sons; Chichester, UK: 2001. [Google Scholar]
- 35.Coia L.R., Owen J.B., Maher E.J., Hanks G.E. Factors affecting treatment patterns of radiation oncologists in the United States in the palliative treatment of cancer. Clin Oncol. 1992;4:6–10. doi: 10.1016/s0936-6555(05)80762-9. [DOI] [PubMed] [Google Scholar]
- 36.Smith B.D., Pan I.W., Shih Y.C.T. Adoption of intensity-modulated radiation therapy for breast cancer in the United States. J Natl Cancer Inst. 2011;103:798–809. doi: 10.1093/jnci/djr100. [DOI] [PubMed] [Google Scholar]
- 37.Shen X., Showalter T.N., Mishra M.V. Radiation oncology services in the modern era: evolving patterns of usage and payments in the office setting for Medicare patients from 2000 to 2010. J Oncol Pract. 2014;10:e201–e207. doi: 10.1200/JOP.2013.001270. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell J.M. Urologists' use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med. 2013;369:1629–1637. doi: 10.1056/NEJMsa1201141. [DOI] [PubMed] [Google Scholar]
- 39.Falit B.P., Chernew M.E., Mantz C.A. Design and implementation of bundled payment systems for cancer care and radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:950–953. doi: 10.1016/j.ijrobp.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 40.Mundt A.J., Roeske J.C. Vol. 1. PMPH-USA; Raleigh, NC: 2005. (Intensity Modulated Radiation Therapy: A Clinical Perspective). [Google Scholar]
- 41.American Cancer Society Detailed guide: Lung cancer (non-small cell) http://www.cancer.org/Cancer/LungCancer-Non-SmallCell/DetailedGuide/non-small-cell-lung-cancer-key-statistics Available at:
- 42.Nattinger A.B., McAuliffe T.L., Schapira M.M. Generalizability of the surveillance, epidemiology, and end results registry population: Factors relevant to epidemiologic and health care research. J Clin Epidemiol. 1997;50:939–945. doi: 10.1016/s0895-4356(97)00099-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials.