Abstract
Objectives
Stereotactic radiation surgery (SRS) and hypofractionated stereotactic radiation surgery (HF-SRS) have become an alternative to external beam radiation therapy (EBRT) in the adjuvant treatment of meningiomas. The purpose of this study was to identify national treatment patterns and survival outcomes for meningiomas on the basis of radiation treatment modality in the adjuvant setting.
Methods and materials
The National Cancer Database was queried for patients with meningioma diagnosed between 2010 and 2012. World Health Organization grade I disease with subtotal resection and all cases of grade II disease regardless of the extent of the resection were included. Logistic regression was used to determine factors that were associated with receipt of SRS/HF-SRS compared with EBRT. Cox regression was used to determine covariables associated with differences in overall survival (OS).
Results
A total of 802 patients met the inclusion criteria of which 173 patients received SRS/HF-SRS (22%) and 629 patients (78%) received EBRT. The 3-year OS rate was 97.3% for the SRS/HF-SRS group and 93.4% for the EBRT group (P = .018). On subgroup analysis by grade, for grade I the 3-year OS rate was 98.3% for the SRS/HF-SRS group versus 96.7% for the EBRT group (P = .117). For grade II disease, the 3-year OS rate was 94.4% in the SRS/HF-SRS group versus 92.4% in the EBRT group (P = .199). On multivariable analysis, World Health Organization grade II histology (odds ratio [OR]: 0.34; 95% confidence interval [CI], 0.21-0.56; P < .001) and gross total resection (OR: 0.29; 95% CI, 0.15-0.57; P < .001) were associated with a decreased likelihood of receiving SRS/HF-SRS but private insurance (OR: 8.89; 95% CI, 1.15-68.47; P = .036) and Medicare (OR: 10.03; 95% CI, 1.28-78.69; P = .028) were associated with an increased likelihood of receiving SRS/HF-SRS. Year of diagnosis was not associated with receipt of SRS/HF-SRS. The multivariable Cox regression demonstrated a trend toward improved OS for treatment with SRS/HF-SRS (hazard ratio: 0.24; 95% CI, 0.06-1.03; P = .055).
Conclusions
SRS and HF-SRS are associated with similar survival as EBRT; however, SRS/HF-SRS is used infrequently and usage has not increased over time.
Summary.
We analyzed patterns of care and outcomes of postoperative stereotactic radiation surgery (SRS) and hypofractionated-SRS (HF-SRS) among patients who underwent resection for meningioma. Patients with a higher World Health Organization (WHO) grade and gross total resection were less likely to receive SRS/HF-SRS over standard external beam radiation therapy. On multivariable Cox regression, there was a trend toward improved survival for the SRS/HF-SRS group (hazard ratio: 0.24: P = .055). When stratified by grade, the Kaplan-Meier curves were not significant between the 2 treatment groups (WHO grade I: P = .117; WHO grade II: P = .199).
Alt-text: Unlabelled box
Introduction
Meningioma is one of the most common intracranial tumors and comprises approximately 36% of all diagnosed intracranial neoplasms.1 Treatment is typically based on whether the tumor is benign, atypical, or anaplastic (grades I, II, and III, respectively) as defined by the World Health Organization (WHO) classification scheme as well as the presence of symptoms.2, 3 The decision to use adjuvant radiation in the treatment of meningiomas is somewhat more nuanced but again depends on the grade of the tumor as well as the extent of the surgical resection.4 Adjuvant radiation has been suggested to improve survival in anaplastic meningiomas5, 6, 7 and recent data support its use for select benign or atypical meningiomas.8, 9, 10, 11
Radiation Therapy Oncology Group study 0539 explored the role of adjuvant treatment for meningiomas divided into three different risk groups on the basis of grade, extent of surgical resection, and recurrence. However, this study used external beam radiation therapy (EBRT) only. Several retrospective reports have suggested that stereotactic radiation surgery (SRS) or hypofractionated SRS (HF-SRS) may be a viable alternative to standard EBRT.12, 13, 14, 15 The purpose of this study was to identify the national trends in the utilization of SRS for the adjuvant treatment of meningiomas as well as evaluate the impact of treatment modality on survival using patients from the National Cancer Database (NCDB).
Methods and materials
The NCDB is a nationwide, hospital-based registry that consists of patients who received care at cancer centers accredited by the American College of Surgeons Commission on Cancer and currently captures approximately 70% of all patients newly diagnosed with cancer.16, 17 The NCDB and the accredited facilities that participate are the source of the de-identified data that were used in this study. However, they have not verified and are not responsible for the statistical validity or conclusions derived by the authors of this study. An exemption was obtained from the New York Harbor Veterans Affairs Committee for Research and Development prior to the initiation of this study.
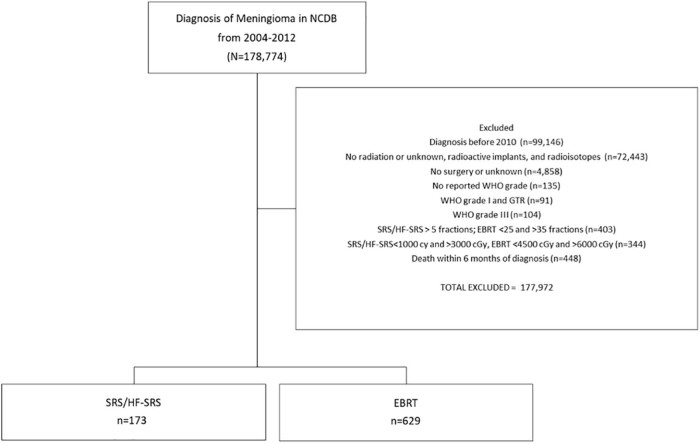
The NCDB was queried for patients with meningioma diagnosed between 2010 and 2012 and underwent resection followed by adjuvant radiation. Patients who had a biopsy only were excluded. Grade I disease with subtotal resection (STR) and all cases of grade II disease regardless of the extent of the resection were included in the study but grade III disease was excluded. Patients were divided into 2 groups on the basis of the number of fractions and dose of radiation received.
SRS was defined as 1 fraction and 12 Gy to 20 Gy. HF-SRS was defined as 2 to 5 fractions and 20 Gy to 30 Gy. For the purposes of this analysis, SRS and HF-SRS were included in 1 group. EBRT was defined as 25 to 35 fractions and 45 Gy to 60 Gy. Patients with nonstandard dose/fractionation schedules were excluded (Fig 1). We also excluded patients with unknown values related to radiation and surgery as well as those with an unknown WHO grade. Patients diagnosed prior to 2010 were excluded due to previous inconsistencies with the coding of the extent of surgical resection. Additionally, to account for immortal time bias, patients who were alive for <6 months from the time of diagnosis were excluded.18
Figure 1.
Cohort selection diagram.
The primary goal of this analysis was to study the patterns of care with regard to SRS/HF-SRS use and the secondary goal to analyze survival. Vital status was available in the data but not cause of death. Demographic, clinical, and treatment details were obtained and compared via the χ2 test between those patients treated with SRS/HF-SRS and those with EBRT. Patient demographic details included age, sex, and race. Clinical and treatment details included tumor size, Charlson-Deyo comorbidity score, extent of surgical resection, radiation dose, categorization of academic or nonacademic cancer center, U.S. region, insurance type, and year of diagnosis. The Charlson-Deyo comorbidity score is based on a sum of scores of comorbid conditions that are listed in the Charlson Comorbidity Mapping Table and coded 0, 1, 2, 3, or higher in the NCDB.19
Univariable logistic regression was performed to assess for predictors of SRS/HF-SRS. The included variables were age, sex (male/female), tumor size (≤3 cm, 3-5 cm, and >5 cm), modified Charlson-Deyo comorbidity score (0, 1, and ≥2), race (white and nonwhite), WHO grade (grades I and II), surgical resection (gross total resection [GTR] and STR), facility type (academic and nonacademic), U.S. region (Northeast, Midwest, South, and West), insurance status (none, private insurance, Medicare, Medicaid, other government, and unknown), and year of diagnosis (2010, 2011, and 2012). Variables with a P-value of < .10 on univariable analysis were planned for inclusion in the multivariable analysis.
Overall survival (OS) curves to compare patients who received SRS/HF-SRS with those who received EBRT were generated using the Kaplan-Meier method and compared via the log-rank test. Univariable and multivariable Cox regressions were used to determine covariables associated with differences in OS. An analysis was done separately for WHO grade I tumor status post-STR and then repeated for patients with WHO grade II tumors. Factors that are associated with P < .10 on univariable analysis were included in the multivariable analysis. The variables included in these analyses were age, sex (male/female), tumor size (≤3 cm, 3-5 cm, and >5 cm), modified Charlson-Deyo comorbidity score (0, 1, and ≥2), race (white and nonwhite), WHO grade (grades I and II), surgical resection (GTR and STR), facility type (academic and nonacademic), and radiation therapy modality (EBRT, SRS/HF-SRS). All analyses were performed using SPSS version 23 (IBM Inc, Armonk, NY).
Results
A total of 802 patients met the inclusion criteria of which 173 patients (22%) received SRS/HF-SRS and 629 patients (78%) received EBRT. Of the patients in the SRS/HF-SRS group, 78 patients (45%) received single-fraction SRS and 95 patients (55%) received HF-SRS delivered in 2 to 5 fractions. The median dose was 54 Gy in the EBRT group and 21 Gy in the SRS/HF-SRS group. The median follow-up time was 30.7 months. Table 1 provides a summary of the patient demographic and clinical characteristics. White patients made up a larger percentage of the SRS/HF-SRS group: 87.3% versus 78.1% in the EBRT group (P = .011). Additionally, female patients made up a larger percentage of the SRS/HF-SRS group compared with the EBRT group (65.9% vs 59.6%; P = .026). Eighty-seven percent of patients who received SRS/HF-SRS had an STR but 56.6% of patients in the EBRT group had an STR (P < .001). Grade I disease comprised of 67.6% in the SRS/HF-SRS group compared with 31.6% in the EBRT group (P < .001).
Table 1.
Demographic and clinical characteristics of patients
| EBRT (n = 629) | SRS/HF-SRS (n = 173) | P-value | |
|---|---|---|---|
| Age (median) | 56 years | 56 years | .562 |
| Sex | .136 | ||
| Male | 254 (40.4%) | 59 (34.1%) | |
| Female | 375 (59.6%) | 114 (65.9%) | |
| Tumor size | < .001 | ||
| ≤3 cm | 93 (14.8%) | 44 (25.4%) | |
| 3-5 cm | 218 (34.7%) | 71 (41.0%) | |
| >5 cm | 318 (50.6%) | 58 (33.5%) | |
| Charlson Deyo comorbidity score | .880 | ||
| 0 | 487 (77.4%) | 137 (79.2%) | |
| 1 | 96 (15.3%) | 24 (13.9%) | |
| ≥2 | 46 (7.3%) | 12 (6.9%) | |
| Race | .007 | ||
| White | 491 (78.1%) | 151 (87.3%) | |
| Nonwhite | 138 (21.9%) | 22 (12.7%) | |
| WHO grade | < .001 | ||
| I | 199 (31.6%) | 117 (67.6%) | |
| II | 430 (68.4%) | 56 (32.4%) | |
| Surgery | < .001 | ||
| STR | 356 (56.6%) | 150 (86.7%) | |
| GTR | 248 (39.4%) | 17 (9.8%) | |
| Resection, NOS | 25 (4.0%) | 6 (3.5%) | |
| Facility type | .450 | ||
| Nonacademic | 237 (43.0%) | 57 (39.3%) | |
| Academic | 314 (57.0%) | 88 (60.7%) | |
| Region | .002 | ||
| Northeast | 121 (22.0%) | 33 (22.8%) | |
| Midwest | 146 (26.5%) | 22 (15.2%) | |
| South | 169 (30.7%) | 41 (28.3%) | |
| West | 115 (20.9%) | 49 (33.8%) | |
| Insurance | .087 | ||
| Not insured | 47 (7.5%) | 5 (2.9%) | |
| Private insurance | 345 (54.8%) | 109 (63.0%) | |
| Medicaid | 59 (9.4%) | 10 (5.8%) | |
| Medicare | 153 (24.3%) | 43 (24.9%) | |
| Other government/unknown | 25 (4.0%) | 6 (3.5%) | |
| Year of diagnosis | .263 | ||
| 2010 | 222 (35.3%) | 54 (31.2%) | |
| 2011 | 191 (30.4%) | 48 (27.7%) | |
| 2012 | 216 (34.3%) | 71 (41.0%) |
EBRT, external beam radiation therapy; GTR, gross total resection; HF-SRS, hypofractionated stereotactic radiation surgery; NOS, not otherwise specified; SRS, stereotactic radiation surgery; STR, subtotal resection; WHO, World Health Organization.
On multivariable analysis, WHO grade II histology (odds ratio [OR]: 0.34; 95% confidence interval [CI], 0.21-0.56; P < .001) and GTR (OR: 0.29; 95% CI, 0.15-0.57; P = .001) were associated with a decreased likelihood of receiving SRS/HF-SRS. Patients who lived in the Midwest (OR: 0.47; 95% CI, 0.25-0.90; P = .022) were less likely to be treated with SRS/HF-SRS. Additionally, private insurance (OR: 8.89; 95% CI, 1.15-68.47; P = .036) and Medicare (OR: 10.03; 95% CI, 1.28-78.69; P = .028) were associated with an increased likelihood of receiving SRS/HF-SRS. Tumor size at the time of diagnosis was not predictive for treatment modality nor were facility type (academic or nonacademic). There was no difference in receipt of SRS/HF-SRS on the basis of year of diagnosis. The results of the analysis of patterns of care for SRS/HF-SRS receipt are summarized in Table 2.
Table 2.
Univariable and multivariable logistic regressions for receipt of adjuvant SRS/HF-SRS over adjuvant EBRT
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | 1.00 (0.98-1.01) | .586 | — | — |
| Sex | ||||
| Male | 1 | — | — | |
| Female | 1.31 (0.92-1.86) | .135 | — | — |
| Tumor size | ||||
| ≤3 cm | 1 | 1 | ||
| 3-5 cm | 0.69 (0.44-1.08) | .102 | 1.17 (0.68-2.03) | .575 |
| >5 cm | 0.39 (0.25-0.61) | < .001 | 0.64 (0.37-1.12) | .118 |
| Charlson Deyo comorbidity score | ||||
| 0 | 1 | — | — | |
| 1 | 0.89 (0.55-1.45) | .634 | — | — |
| ≥2 | 0.93 (0.48-1.80) | .823 | — | — |
| Race | ||||
| White | 1 | 1 | ||
| Nonwhite | 0.52 (0.32-0.84) | .008 | 0.70 (0.39-1.27) | .238 |
| WHO grade | ||||
| I | 1 | 1 | ||
| II | 0.22 (0.16-0.32) | < .001 | 0.34 (0.21-0.56) | < .001 |
| Surgery | ||||
| STR | 1 | 1 | ||
| GTR | 0.16 (0.10-0.28) | < .001 | 0.29 (0.15-0.57) | < .001 |
| Resection, NOS | 0.57 (0.23-1.42) | .226 | 0.80 (0.28-2.27) | .674 |
| Facility type | ||||
| Nonacademic | 1 | — | — | |
| Academic | 1.17 (0.80-1.69) | .422 | — | — |
| Region | ||||
| Northeast | 1 | 1 | ||
| Midwest | 0.55 (0.31-1.00) | .553 | 0.47 (0.25-0.90) | .022 |
| South | 0.89 (0.53-1.49) | .656 | 0.67 (0.38-1.18) | .166 |
| West | 1.56 (0.94-2.60) | .086 | 1.27 (0.72-2.23) | .416 |
| Insurance | ||||
| Not insured | 1 | 1 | ||
| Private Insurance | 2.97 (1.15-7.66) | .024 | 8.89 (1.15-68.47) | .036 |
| Medicaid | 1.59 (0.51-4.98) | .423 | 3.72 (0.39-35.30) | .253 |
| Medicare | 2.64 (0.99-7.05) | .053 | 10.03 (1.28-78.69) | .028 |
| Other government/unknown | 2.26 (0.63-8.13) | .214 | 8.41 (0.85-83.54) | .069 |
| Year of diagnosis | ||||
| 2010 | 1 | — | — | |
| 2011 | 1.03 (0.67-1.60) | .883 | — | — |
| 2012 | 1.35 (0.91-2.02) | .141 | — | — |
CI, confidence interval; EBRT, external beam radiation therapy; GTR, gross total resection; HF-SRS, hypofractionated stereotactic radiation surgery; NOS, not otherwise specified; OR, odds ratio; SRS, stereotactic radiation surgery; STR, subtotal resection; WHO, World Health Organization.
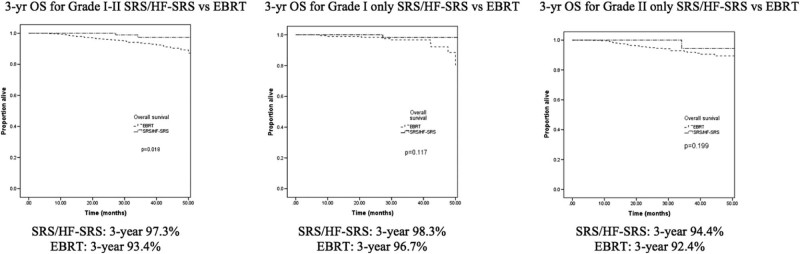
Kaplan-Meier curves that depict the survival in patients grouped by receipt of SRS versus EBRT are shown in Figure 2. The 3-year OS rate was 97.3% for the SRS/HF-SRS group and 93.4% for the EBRT group (P = .018). When stratified by grade, there were no differences in 3-year OS rates on the basis of treatment modality. For grade I disease, the 3-year OS rate was 98.3% for the SRS/HF-SRS group versus 96.7% for the EBRT group (P = .117). For grade II disease, the 3-year OS rate was 94.4% in the SRS/HF-SRS group versus 92.4% in the EBRT group (P = .199).
Figure 2.
Kaplan-Meier for entire cohort and subgroup analyses.
The multivariable Cox regression demonstrated a trend toward improved OS for treatment with SRS/HF-SRS (hazard ratio [HR]: 0.24; 95% CI, 0.06-1.03; P = .055). Increasing age was associated with decreased OS (HR: 1.07; 95% CI, 1.04-1.11; P < .001). Female sex was also associated with improved survival (HR: 0.44; 95% CI, 0.22-0.88; P = .019). Tumor size at the time of diagnosis, tumor grade, race, extent of resection, and facility type were not found to be associated with decreased survival. A summary of the findings of the univariable and multivariable Cox regressions for OS are shown in Table 3.
Table 3.
Univariable and multivariable Cox regressions for overall survival
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.08 (1.05-1.11) | < .001 | 1.07 (1.04-1.11) | < .001 |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 0.29 (0.15-0.56) | < .001 | 0.44 (0.22-0.88) | .019 |
| Tumor size | ||||
| ≤3 cm | 1 | 1 | ||
| 3-5 cm | 3.28 (0.75-14.35) | .115 | 3.14 (0.71-13.85) | .132 |
| >5 cm | 4.30 (1.02-18.18) | .048 | 2.85 (0.66-12.24) | .160 |
| Charlson Deyo cormobidity score | ||||
| 0 | 1 | 1 | ||
| 1 | 2.13 (1.06-4.29) | .035 | 1.42 (0.69-2.91) | .345 |
| ≥2 | 1.26 (0.38-4.15) | .705 | 0.75 (0.22-2.50) | .638 |
| Race | ||||
| White | 1 | — | — | |
| Nonwhite | 1.00 (0.46-2.17) | 1.000 | — | — |
| WHO grade | ||||
| I | 1 | 1 | ||
| II | 1.98 (0.97-4.03) | .061 | 0.75 (0.30-1.87) | .538 |
| Surgery | ||||
| STR | 1 | 1 | ||
| GTR | 2.08 (1.11-3.91) | .022 | 1.62 (0.73-3.60) | .237 |
| Resection, NOS | 1.81 (0.42-7.83) | .425 | 2.12 (0.47-9.50) | .328 |
| Facility type | ||||
| Nonacademic | 1 | — | — | |
| Academic | 0.66 (0.35-1.23) | .190 | — | — |
| RT Modality | ||||
| EBRT | 1 | 1 | ||
| SRS/HF-SRS | 0.21 (0.05-0.88) | .032 | 0.24 (0.06-1.03) | .055 |
CI, confidence interval; EBRT, external beam radiation therapy; GTR, gross total resection; HF- HR, hazard ratio; NOS, not otherwise specified; RT, radiation therapy; SRS, hypofractionated stereotactic radiation surgery; SRS, stereotactic radiation surgery; STR, subtotal resection; WHO, World Health Organization.
Discussion
In this large, hospital-based, database analysis, we found that SRS/HF-SRS was utilized in the adjuvant setting 22% of the time and usage has not increased over time. Although there was a small survival benefit noted for the whole cohort, this was no longer significant on multivariable analysis with a 3-year OS for SRS/HF-SRS at 97.3% compared with 93.4% for EBRT (P = .018).
Even though the need for adjuvant radiation for anaplastic meningiomas is more or less established, the role of adjuvant radiation in the treatment of atypical and certain benign meningiomas is less clear6, 20 and often based on clinical and pathologic factors such as the extent of the resection, the presence of symptoms, and whether or not the tumor is recurrent.6, 8, 10, 11, 21 A recent analysis of the NCDB demonstrated that GTR in combination with adjuvant radiation was found to be the most important factor for improved survival even though GTR was associated with lower rates of adjuvant radiation usage.22 The Radiation Therapy Oncology Group investigated the role of postoperative radiation in the treatment of meningiomas on the basis of risk stratification (https://clinicaltrials.gov/ct2/show/NCT00895622). Patients with recurrent grade I disease or grade II after GTR received 54 Gy in 30 fractions adjuvantly. The first clinical outcomes report from this trial showed that the 3-year actuarial local failure rate was 4.1% and the 3-year OS rate was 96% for the intermediate-risk group.9
Several other clinical trials are also underway but also include standard fractionated radiation. The Radiation versus Observation following surgical resection of Atypical Meningioma trial 1308 by the European Organisation for Research and Treatment of Cancer is an ongoing randomized study that compares the role of standard fractionated radiation over 6 weeks versus observation in atypical meningiomas that have been completely resected (http://roam-trial.org.uk). Additionally, NRG BN-003 is an ongoing phase III trial that compares observation versus irradiation for atypical meningioma after GTR (http://clinicaltrials.gov/ct2/show/NCT03180268). Mature data from these trials may provide further insight into optimal adjuvant treatment strategies for meningiomas on the basis of risk stratification defined by grade and extent of resection. By identifying risks groups that benefit from adjuvant radiation, the results of these trials may be used to select patients for whom SRS or HF-SRS may be a suitable alternative to EBRT in the adjuvant setting.
SRS and HF-SRS may be an appealing adjuvant treatment strategy for meningiomas given the shortened treatment time as well as the decreased cumulative dose to the surrounding bran tissue. However, the role of SRS and HF-SRS as an adjuvant treatment of meningioma has not been clearly defined because fewer studies with long-term follow-up after adjuvant SRS and HF-SRS treatment are available. Detailed guidelines for patient selection, target volumes, and dose and fractionation schemes have yet to be developed due to the paucity of prospective trials that involve these treatment modalities.21
However, there are data to suggest that SRS and HF-SRS may be used in certain settings. In a series of patients with grades II and III meningiomas treated with surgery and SRS, the 10-year OS rate was 86%.23 Another retrospective study using SRS adjuvantly for grade II meningiomas showed 3- and 5-year OS rates of 88.6% and 81.1%, respectively.24 Ferraro et al. found a 3-year OS rate of 78% for patients with grades II and III treated adjuvantly with SRS.25 In the present analysis, the grade II 3-year OS rate was 94.4% in the SRS/HF-SRS group versus 92.4% in the EBRT group (P = .199).
Despite these data, there is concern for treatment failure with the use of SRS. A single institution retrospective analysis of atypical meningiomas using SRS as the primary or salvage treatment found treatment failure in 14 of 24 patients.26 Patients were treated with a median marginal prescription dose of 14 Gy with a range of 10.5 Gy to 18 Gy. A total of 8 recurrences were infield, 4 were marginal failures, and 2 were distant failures. However, these patients differed from those in the current analysis because either no resection was performed or the tumor was recurrent. In another study that investigated postoperative radiation including salvage treatment, loco-marginal control was improved with a minimum dose of >12 Gy and an extended target volume along the dural insertion.27 Many of the analyses that investigated the use of SRS for the treatment of meningiomas included a heterogenous group of patients that consisted of patients with recurrent tumors, patients treated primarily with SRS, and patients treated with SRS in the adjuvant setting. Therefore, more prospective data specific to SRS and HF-SRS in the adjuvant setting are needed.
In theory, the high doses of radiation per fraction that are delivered with SRS and HF-SRS may provide better tumor control and overall results compared with standard fractionated radiation.28 However, to date there are no prospective data to show that SRS is superior to EBRT in the adjuvant setting. Even though we noted a small survival benefit that favored HF-SRS for the whole cohort, this benefit lost significance on multivariable analysis. As such, the small survival benefit found may be in part due to differences between the patients selected for SRS/HF-SRS versus those selected for EBRT. For example, the majority of patients in the SRS/HF-SRS group had grade I disease whereas the majority of patients in the EBRT has grade II disease. Additionally, other factors such as proximity of residual tumor or tumor bed to critical structures may preclude the use of SRS/HF-SRS. However, this information is not captured in the NCDB. Thus, patients selected for EBRT may have been those with less favorable clinical and pathologic characteristics compared with patients in the SRS/HF-SRS group.
Tumor size is often an important factor when selecting patients for treatment with SRS or HF-SRS given the volume limitations with these treatment modalities. In this study, tumor size at the time of diagnosis was not predictive for the receipt of SRS/HF-SRS. However, selection for this treatment modality may be based on the size of gross residual disease or the size of the postoperative cavity and these data are not currently available from the NCDB.
The use of adjuvant SRS/HF-SRS for meningiomas has not increased over time. Despite multiple single-institutional retrospective series that show favorable results for the use of SRS/HF-SRS in the adjuvant setting, there may be hesitancy to adopt this treatment strategy due to a dearth of prospective data, less long-term data, lack of detailed treatment guidelines, and concerns for treatment failure. This may also be related to the relatively short timeframe analyzed in this study. Similarly, an analysis of Surveillance, Epidemiology, and End Results Medicare data by Amsbaugh et al. also found that the use of adjuvant SRS had remained stable over time in patients age >65 years between 2000 and 2010.29 Additionally, the current analysis showed that private insurance and Medicare were associated with an increased likelihood of receiving SRS/HF-SRS, which may be a reflection of the referral patterns for insured patients. However, there was no association with academic versus nonacademic facility type.
There are challenges and limitations associated with hospital-based registries. Data that are reported to the NCDB are highly standardized but there may still be variances with data abstraction and particularly with community-based oncology practices. In addition, there is no central pathologic review and some WHO grade I tumors may have been really grade II or III tumors that were misidentified. Another important limitation is the lack of coding with regard to recurrence and lack of detail with the extent of resection per the Simpson grading score. Given the high survival rates for patients with WHO grades 1 and II meningiomas, differences in progression-free survival on the basis of treatment modality may be a more clinically relevant outcome but cannot be determined from the NCDB. Additionally, there is a lack of data with regard to salvage therapy and cause of death. Most notably, we were unable to account for all possible reasons why a patient may have been more likely to receive adjuvant SRS/HF-SRS compared with EBRT.
Conclusions
Given the shortened treatment time for SRS/HF-SRS compared with EBRT, SRS/HF-SRS may be favorable options for select patients. However, the current analysis demonstrates that SRS/HF-SRS is infrequently used in the adjuvant setting compared with EBRT and usage has not increased over time. Additional prospective data with regard to the use of adjuvant SRS/HF-SRS in conjunction with mature data from trials related to adjuvant radiation for meningiomas may help define groups of patients for whom SRS/HF-SRS may be a suitable treatment strategy.
Footnotes
Sources of support: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest: The authors do not have any actual or potential conflicts of interest.
References
- 1.Ostrom Q.T., Gittleman H., Xu J. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. 2016;18:v1–v75. doi: 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louis D.N., Ohgaki H., Wiestler O.D. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabors L.B., Portnow J., Ammirati M. Central nervous system cancers, Version 1.2017. J Natl Compr Canc Netw. 2017;15:1331–1345. doi: 10.6004/jnccn.2017.0166. [DOI] [PubMed] [Google Scholar]
- 4.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hug E.B., Devries A., Thornton A.F. Management of atypical and malignant meningiomas: Role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48:151–160. doi: 10.1023/a:1006434124794. [DOI] [PubMed] [Google Scholar]
- 6.Orton A., Frandsen J., Jensen R., Shrieve D.C., Suneja G. Anaplastic meningioma: An analysis of the National Cancer Database from 2004 to 2012. J Neurosurg. 2017:1–6. doi: 10.3171/2017.2.JNS162282. [DOI] [PubMed] [Google Scholar]
- 7.Yang S.Y., Park C.K., Park S.H., Kim D.G., Chung Y.S., Jung H.W. Atypical and anaplastic meningiomas: Prognostic implications of clinicopathological features. J Neurol Neurosurg Psychiatry. 2008;79:574–580. doi: 10.1136/jnnp.2007.121582. [DOI] [PubMed] [Google Scholar]
- 8.Condra K.S., Buatti J.M., Mendenhall W.M., Friedman W.A., Marcus R.B., Jr, Rhoton A.L. Benign meningiomas: Primary treatment selection affects survival. Int J Radiat Oncol Biol Phys. 1997;39:427–436. doi: 10.1016/s0360-3016(97)00317-9. [DOI] [PubMed] [Google Scholar]
- 9.Rogers L., Zhang P., Vogelbaum M.A. Intermediate-risk meningioma: Initial outcomes from NRG Oncology RTOG 0539. J Neurosurg. 2017:1–13. doi: 10.3171/2016.11.JNS161170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soyuer S., Chang E.L., Selek U., Shi W., Maor M.H., DeMonte F. Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol. 2004;71:85–90. doi: 10.1016/j.radonc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Wang C., Kaprealian T.B., Suh J.H. Overall survival benefit associated with adjuvant radiotherapy in WHO grade II meningioma. Neuro Oncol. 2017;19:1263–1270. doi: 10.1093/neuonc/nox007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J.Y., Niranjan A., McInerney J., Kondziolka D., Flickinger J.C., Lunsford L.D. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97:65–72. doi: 10.3171/jns.2002.97.1.0065. [DOI] [PubMed] [Google Scholar]
- 13.Malik I., Rowe J.G., Walton L., Radatz M.W., Kemeny A.A. The use of stereotactic radiosurgery in the management of meningiomas. Br J Neurosurg. 2005;19:13–20. doi: 10.1080/02688690500080885. [DOI] [PubMed] [Google Scholar]
- 14.Nicolato A., Foroni R., Alessandrini F., Maluta S., Bricolo A., Gerosa M. The role of Gamma Knife radiosurgery in the management of cavernous sinus meningiomas. Int J Radiat Oncol Biol Phys. 2002;53:992–1000. doi: 10.1016/s0360-3016(02)02802-x. [DOI] [PubMed] [Google Scholar]
- 15.Pollock B.E., Stafford S.L., Utter A., Giannini C., Schreiner S.A. Stereotactic radiosurgery provides equivalent tumor control to Simpson Grade 1 resection for patients with small- to medium-size meningiomas. Int J Radiat Oncol Biol Phys. 2003;55:1000–1005. doi: 10.1016/s0360-3016(02)04356-0. [DOI] [PubMed] [Google Scholar]
- 16.Boffa D.J., Rosen J.E., Mallin K. Using the National Cancer Database for outcomes research: A review. JAMA Oncol. 2017;3:1722–1728. doi: 10.1001/jamaoncol.2016.6905. [DOI] [PubMed] [Google Scholar]
- 17.Mohanty S., Bilimoria K.Y. Comparing national cancer registries: The National Cancer Data Base (NCDB) and the Surveillance, Epidemiology, and End Results (SEER) program. J Surg Oncol. 2014;109:629–630. doi: 10.1002/jso.23568. [DOI] [PubMed] [Google Scholar]
- 18.Park H.S., Gross C.P., Makarov D.V., Yu J.B. Immortal time bias: A frequently unrecognized threat to validity in the evaluation of postoperative radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1365–1373. doi: 10.1016/j.ijrobp.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 19.Deyo R.A., Cherkin D.C., Ciol M.A. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 20.Sun S.Q., Hawasli A.H., Huang J., Chicoine M.R., Kim A.H. An evidence-based treatment algorithm for the management of WHO Grade II and III meningiomas. Neurosurg Focus. 2015;38:E3. doi: 10.3171/2015.1.FOCUS14757. [DOI] [PubMed] [Google Scholar]
- 21.Rogers L., Barani I., Chamberlain M. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J Neurosurg. 2015;122:4–23. doi: 10.3171/2014.7.JNS131644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rydzewski N.R., Lesniak M.S., Chandler J.P. Gross total resection and adjuvant radiotherapy most significant predictors of improved survival in patients with atypical meningioma. Cancer. 2018;124:734–742. doi: 10.1002/cncr.31088. [DOI] [PubMed] [Google Scholar]
- 23.Lubgan D., Rutzner S., Lambrecht U. Stereotactic radiotherapy as primary definitive or postoperative treatment of intracranial meningioma of WHO grade II and III leads to better disease control than stereotactic radiotherapy of recurrent meningioma. J Neurooncol. 2017;134:407–416. doi: 10.1007/s11060-017-2540-7. [DOI] [PubMed] [Google Scholar]
- 24.Refaat T., Gentile M., Sachdev S. Gamma Knife stereotactic radiosurgery for grade 2 meningiomas. J Neurol Surg B Skull Base. 2017;78:288–294. doi: 10.1055/s-0036-1597834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferraro D.J., Funk R.K., Blackett J.W. A retrospective analysis of survival and prognostic factors after stereotactic radiosurgery for aggressive meningiomas. Radiat Oncol. 2014;9:38. doi: 10.1186/1748-717X-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Attia A., Chan M.D., Mott R.T. Patterns of failure after treatment of atypical meningioma with gamma knife radiosurgery. J Neurooncol. 2012;108:179–185. doi: 10.1007/s11060-012-0828-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valery C.A., Faillot M., Lamproglou I. Grade II meningiomas and Gamma Knife radiosurgery: Analysis of success and failure to improve treatment paradigm. J Neurosurg. 2016;125:89–96. doi: 10.3171/2016.7.GKS161521. [DOI] [PubMed] [Google Scholar]
- 28.Kirkpatrick J.P., Soltys S.G., Lo S.S., Beal K., Shrieve D.C., Brown P.D. The radiosurgery fractionation quandary: Single fraction or hypofractionation? Neuro Oncol. 2017;19:ii38–ii49. doi: 10.1093/neuonc/now301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amsbaugh M., Ugiliweneza B., Burton E., Skirboll S., Woo S., Boakye M. Patterns of care and outcomes of adjuvant radiotherapy for meningiomas: A Surveillance, Epidemiology, and End Results and Medicare linked analysis. Cureus. 2016;8:e567. doi: 10.7759/cureus.567. [DOI] [PMC free article] [PubMed] [Google Scholar]