Abstract
Purpose
Head and neck surgery and radiation cause tissue fibrosis that leads to functional limitations and lymphedema. The objective of this study was to determine whether lymphedema therapy after surgery and radiation for head and neck cancer decreases neck circumference, increases cervical range of motion, and improves pain scores.
Methods and materials
A retrospective review of all patients with squamous cell carcinoma of the oral cavity, oropharynx, or larynx who were treated with high-dose radiation therapy at a single center between 2011 and 2012 was performed. Patients received definitive or postoperative radiation for squamous cell carcinoma of the oral cavity, oropharynx, or larynx. Patients were referred to a single, certified, lymphedema therapist with specialty training in head and neck cancer after completion of radiation treatment and healing of acute toxicity (typically 1-3 months). Patients underwent at least 3 months of manual lymphatic decongestion and skilled fibrotic techniques. Circumferential neck measurements and cervical range of motion were measured clinically at 1, 3, 6, 9, and 12 months after completion of radiation therapy. Pain scores were also recorded.
Results
Thirty-four consecutive patients were eligible and underwent a median of 6 months of lymphedema therapy (Range, 3-12 months). Clinically measured total neck circumference decreased in all patients with 1 month of treatment. Cervical rotation increased by 30.2% on the left and 27.9% on the right at 1 month and continued to improve up to 44.6% and 55.3%, respectively, at 12 months. Patients undergoing therapy had improved pain scores from 4.3 at baseline to 2.0 after 1 month.
Conclusions
Lymphedema therapy is associated with objective improvements in range of motion, neck circumference, and pain scores in the majority of patients.
Summary.
Lymphedema therapy after surgery and radiation for head and neck cancer improves neck circumference, cervical range of motion, and pain scores. This retrospective review showed that clinically measured total neck circumference decreased in a majority of patients with treatment and cervical rotation increased. Patients undergoing therapy had improvement in their pain scores. Lymphedema therapy is associated with objective improvements in range of motion, neck circumference, and pain scores in the majority of patients.
Alt-text: Unlabelled box
Introduction
Aggressive multimodality treatment has improved survival in patients with head and neck cancer but at the cost of increased treatment-related complications.1, 2 The number of long-term survivors after treatment for head and neck cancer is expected to increase with improved cure rates in the era of human papilloma virus-related oropharynx cancers.3, 4 More investigations are necessary to find ways to reduce the impact of acute and long-term complications in these cancer survivors. A recent study showed that 75% of patients with head and neck cancer had some form of lymphedema ≥3 months after completion of treatment.5 Llymphedema presence that persists in the early post-treatment period can lead to impaired swallowing, neck fibrosis, pain, decreased range of motion, negative body image, and social isolation.6, 7, 8, 9 Improved management of lymphedema during the early months after treatment provides an opportunity to enhance the quality of life in head and neck cancer survivors.
The mechanism of lymphedema after treatment is believed to be the disruption of lymphatic drainage by surgery and/or radiation therapy (RT).10 When lymphatic structures in the head and neck are damaged, lymphatic fluid accumulates in the interstitial space and activates an inflammatory response, which results in fibrosis and worsening of the lymphatic function.11, 12, 13 Both internal and external structures are affected, which compromises function and quality of life.14, 15 Lymphedema therapy performed post-treatment by a trained, lymphedema, occupational therapist may reduce complications that arise from chronic lymphedema.16 Early studies of lymphedema therapy report a reduced symptom burden.16, 17, 18 In the present study, we evaluate the quantitative effect of lymphedema therapy in patients with head and neck cancer using clinical data.
Methods and materials
Patient population
A retrospective review of all patients with squamous cell carcinoma of the oral cavity, oropharynx, or larynx who were treated with high dose RT at a single medical center between 2011 and 2012 was performed. Eligible patients received either definitive or postoperative radiation that targeted the primary site and drained lymphatics with or without chemotherapy. Patients with recurrent or persistent disease were excluded as well as patients with active radiation dermatitis, recurrent stroke or transient ischemic attack, or renal insufficiency. Patient characteristics including weight, smoking status, subjective pain score using the Wong-Baker FACES pain rating scale, duration of percutaneous endoscopic gastrostomy (PEG) tube use, and use of lymphedema therapy were collected.
Clinical assessment of lymphedema
Thirty-four patients were referred to a single, certified, lymphedema therapist with specialty training in head and neck cancer by the radiation oncologist after completion of radiation treatment and healing of acute toxicity (typically 1-3 months). Patients were referred early in their course of recovery because they were deemed high risk. Although formal baseline MD Anderson or Foldi lymphedema assessments were not recorded, these patients typically have MD Anderson 1a (soft nonpitting edema) or 1b (reversible pitting edema) lymphedema.19 Patients underwent at least 3 months of manual lymphatic decongestion and skilled fibrotic techniques (Range, 3-12 months; median: 6 months).
Complete decongestive therapy was done by a single, hospital-based, occupational therapist/Lymphology Association of North America-certified lymphatic therapist with specialty training in treatment for head and neck lymphedema. The complete decongestive therapy program includes manual lymphatic decongestion per anterior and posterior pathways (dependent on the presentation of the head and neck congested areas), head and neck compression, skin care education, neck range of motion, and skilled techniques to decrease fibrosis, decrease pain, and increase range of motion. Manual lymphatic decongestion for the patient was conducted by using anterior and posterior sequences. The completion of therapy was determined either by durable long-term improvement confirmed by the lymphedema therapist or by patient choice and compliance.
Patients who underwent lymphedema therapy were assessed at the beginning and end of treatment as well as at various monthly intervals. Superior, middle, and inferior circumferential neck measurements were taken using the MD Anderson Cancer Center Head and Neck Tape Measurement Protocol. Cervical range of motion was measured using a goniometer as the number of degrees the patient was able to rotate to the left and right from midline. Changes were calculated from the beginning to the last date of treatment. Pain scores using a visual analogue scale of 0 to 10 were assessed as part of routine clinical management and obtained from patients' charts. The institutional review board approved the retrospective review of the results.
Statistical analysis
Patient characteristics and outcome measures were summarized by mean and standard deviation (SD) or quartiles for continuous variables and by frequency count and percent for categorical variables. For outcome measures of total circumference and cervical motion (left and right), percent changes were computed for each follow-up time point using the baseline (approximately 1 month post-RT) measures as denominators. Outcome measures of total circumference, cervical motion (left and right), and pain scores (Range, 0-10) were analyzed by mixed-effects models with weight and cumulative smoking exposure (pack/year) before RT as covariates.
Least square means and Tukey's procedure were used to make post hoc comparisons. The changes in total neck circumference were also categorized into decrease/clinical improvement (>2% reduction), stable (changes between −2% to 2%), and increase (>2% change). The time to clinical improvement was assumed to follow the Weibull distribution and analyzed by parametric regression. All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
Patient characteristics
This study evaluated 34 patients with squamous cell carcinoma of the oropharynx, oral cavity, or larynx of whom 16 patients received definitive RT and 18 patients had surgery including resection of the primary. Three patients received ipsilateral radical neck dissections, 2 patients received bilateral modified radical neck dissections, and 13 patients had ipsilateral-modified radical neck dissections (Table 1). All patients received radiation to the primary and bilateral neck using intensity modulated RT with doses that ranged from 54 Gy to 70 Gy. Patients were referred for lymphedema therapy beginning at ≥1 month after completion of RT. Patients participated in therapy for a median of 6 months.
Table 1.
Patient characteristics
| Characteristic | Patients with lymphedema therapy (n = 34) |
|---|---|
| Age, years* | 57.4 ± 8.9 |
| Female, n (%) | 4 (11.8%) |
| Ethnicity, n (%) | |
| Non-Hispanic, Latino, or Spanish | 34 (100%) |
| Race, n (%) | |
| White/Caucasian | 33 (97.1%) |
| Black/African-American | 1 (2.9%) |
| Primary tumor location, n (%) | |
| Oral cavity | 7 (20.6%) |
| Oropharynx | 24 (70.6%) |
| Larynx | 3 (8.8%) |
| Radiation indication, n (%) | |
| Definitive | 16 (47.1%) |
| Postoperative | 18 (52.9%) |
| Neck dissection, n (%) | (n = 18) |
| Ipsilateral radical | 3 (16.7%) |
| Ipsilateral modified radical | 13 (72.2%) |
| Bilateral modified radical | 2 (11.1%) |
| Weight (lb) 1-month post RT, n (95% CI)† | 174.6 (157.6, 201.4) |
| Weight (lb) 3-month post RT, n (95% CI)† | 178.9 (154.2, 204.0) |
| Smoking (pack-year) before RT, n (95% CI)† | 9.5 (0, 30) |
| Total neck circumference (mm) post-RT* | 138.1 ± 10.2 |
| Baseline pain scores† | 4.3 ± 2.6 |
RT, radiation therapy.
mean ± standard deviation.
median (lower and upper quartiles).
Clinical measures
Using the methods described, the mean total neck circumference was 144.6 ± 11.1 mm (SD) at baseline. After 1, 3, 6, 9, and 12 months of lymphedema therapy, total neck circumference decreased by 2.2%, 3.7%, 3.2%, 3.1%, and 38.4%, respectively (95% confidence interval [CI], −1.25% to −3.47%; −2.16% to −5.58%; 0.39% to −5.49%; −0.26% to −4.02%; and −37.13% to −39.67%; respectively). Figures 1A and B show the trend of individual patients toward improvement and percent change in neck circumference. Figure 2 shows the percent of patients undergoing lymphedema therapy who had clinically improved or worsened neck circumferences at each time point. A change in total neck circumference of ≥2% was considered clinically significant on the basis of previously reported studies.16
Figure 1.
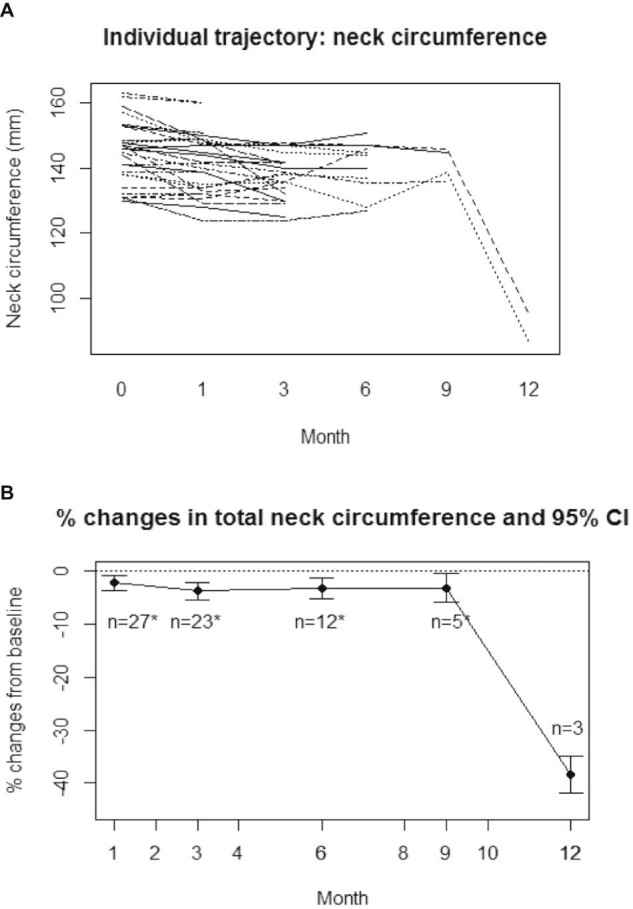
(A) Individual trajectory of clinically measured neck circumference over time for patients who received lymphedema therapy. (B) Mean percent changes in clinically measured neck circumference over time for patients who received lymphedema therapy. The 95% confidence interval is shown.
Figure 2.
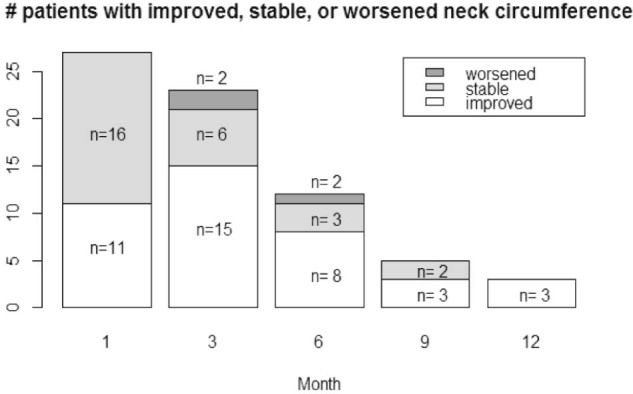
Number of patients with improved, stable, or worsened clinically measured neck circumference over time in patients who received lymphedema therapy.
All patients (100%) showed improvement or stability in total neck circumference at 1 month (n = 27), 9 months (n = 5), and 12 months (n = 3). At months 3 and 6, 91% and 92%, respectively, showed improvement or stability. The 3 patients who were found to have increases in neck circumference at these time points had more extensive surgery with a bilateral modified radical neck dissection, a unilateral radical neck dissection, and a revision unilateral-modified radical neck dissection. Neck circumference was not measured in 7 of 34 patients (21%).
When evaluating clinical improvement alone, 11 of 27 patients (41%) showed a clinical decrease in neck total circumference (>2% reduction) at 1 month and 16 patients' measurements (59%) were stable (changes between −2% to 2% change from baseline). At 3 months, 15 of 23 patients (65%) showed a decrease in circumference, 6 patients (26%) showed stability, and 2 patients showed an increase (>2% change). The percentages remained similar at 6 month when 8 of 12 patients with measurements (67%) showed a decrease, 3 patients (25%) were stable, and 1 patient (8%) increased in size. At 9 months, 3 of 5 patients with measurements (60%) showed a decrease and 2 patients (40%) were stable. At 12 months, all 3 patients with available measurements showed a decrease in clinically measured neck circumference.
Cervical range of motion also improved with lymphedema therapy. The average cervical motion was 49.2 ± 14.8 degrees on the left and 49.0 ± 13.8 degrees on the right at baseline, which was decreased from the expected rotation of approximately 80 degrees in a healthy population.20 After controlling for weight and smoking exposure, at 1 month cervical motion increased by 30.2% on the left and 27.9% on the right. This trend of improvement continued and at 12 months, a 44.6% increase was observed on the left and 55.3% increase on the right (Figs 3A and B).
Figure 3.
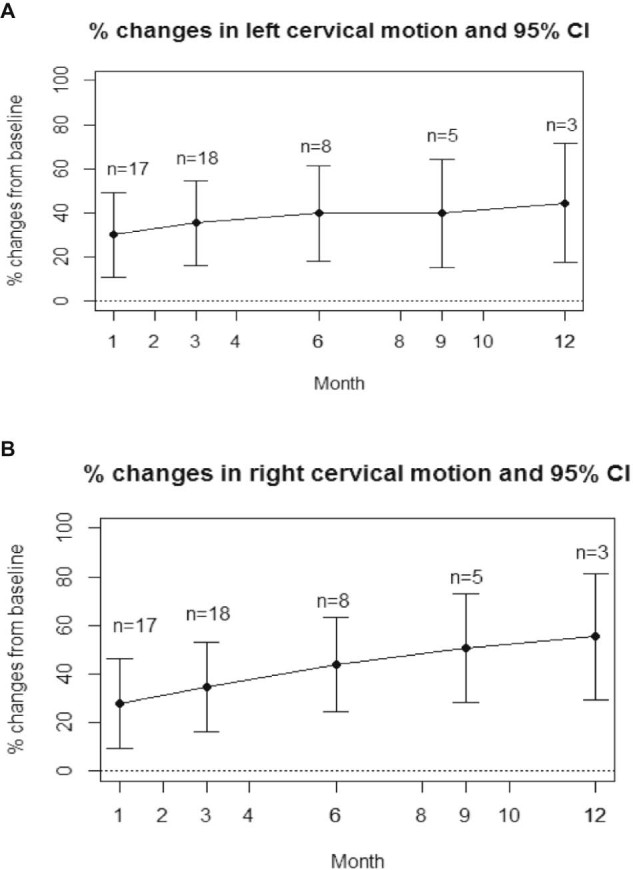
(A). Mean percent change in left cervical range of motion over time in patients who received lymphedema therapy. The 95% confidence interval is shown. (B) Mean percent change in right cervical range of motion over time in patients who received lymphedema therapy. The 95% confidence interval is shown.
Pain scores reduced in patients when receiving lymphedema therapy
The mean pain score was 4.3 for patients at baseline. Mean pain scores were 4.3, 2.0, 1.4, and 1.2 at baseline and 1, 3, and 6 months of lymphedema therapy. The reduction in pain scores was significant at all follow-up time points (P < .0001 at 1, 3, and 6 months vs baseline). Figure 4 shows the change in mean pain score at baseline and 1, 3, and 6 months.
Figure 4.
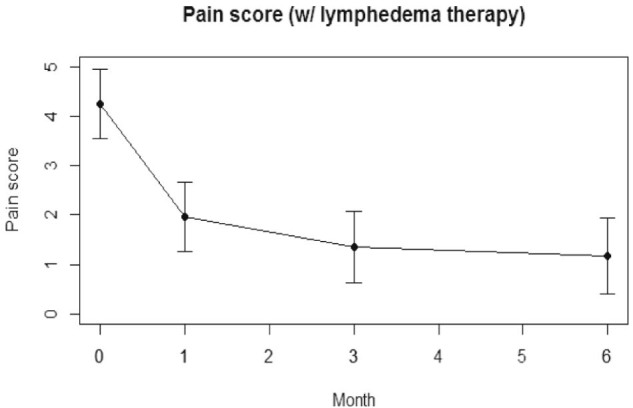
Patient-reported pain scores over time of patients with lymphedema therapy.
Percutaneous endoscopic gastrostomy tube use duration
Of the 34 patients who received lymphedema therapy, 27 patients had a PEG tube placed during radiation treatment to allow for supplemental nutrition. Three patients remained at least partially dependent on the PEG tube indefinitely and the tube remained in place until the patients' deaths. The mean duration of PEG tube use from the final day of RT to the date of PEG tube removal was 274.3 days. When censoring the 3 patients who remained dependent on the PEG tube indefinitely, the mean duration of PEG tube use for the remaining 24 patients was 128.3 days (Range, 40-446 days).
Discussion
In 2010, Smith et al. published a review article in which they noted the dearth of recent publications on head and neck cancer and lymphedema.19 Since then, there has been an outburst of activity looking at the prevalence of head and neck lymphedema, evaluating therapy, and investigating imaging modalities for study. Extensive work from Vanderbilt has characterized internal versus external lymphedema, associations with swallowing difficulties, and other patient symptoms. The literature continues to evolve as Deng et al. reported on the development of a clinician-administered instrument to assess lymphedema.21 Our work adds to the literature on the efficacy of lymphedema therapy and the natural history of this complication of treatment for head and neck cancer.
Our study shows that patients with head and neck cancer with lymphedema had improvement in neck circumference after receiving manual lymphatic decongestion from a certified lymphedema therapist. More than 90% of patients showed either stability or improvement at all time points and lymphedema therapy prevented the worsening progression of edema. With more therapy from 1 to 3 months, more patients saw improvements of >2%. Furthermore, patients tended to see larger changes in neck circumference over time.
Prior studies have not characterized cervical rotation. Our study demonstrates that patients undergoing therapy also saw steady clinical improvements in cervical range of motion after the first treatment each month until the completion of therapy. We did not see an influence of weight or smoking status when we explored these as possible confounders. This population of patients has a longer follow up period than those in other reported studies. Existing published data do not capture changes in lymphedema over time as this study does.
This study is limited in that there is no control group of patients who received head and neck RT and clinical measurements to compare with the group of patients who received lymphedema therapy. This is an area that warrants prospective investigation to delineate the natural course of head and neck lymphedema after surgery and radiation. Optimal timing and duration of lymphedema therapy have yet to be characterized. Furthermore, at extended time points in this study, there were few patients remaining in therapy and thus conclusions should not be drawn from the 12-month data. However, this does suggest that due to the natural course of lymphedema after cancer treatment, prolonged therapy may be warranted to improve symptoms. Patient-reported quality-of-life outcomes would help qualify the degree of clinical significance of this therapeutic intervention.
The duration of response after completion of lymphedema therapy is an area for further investigation. Many series do not report on the prospective study of patients over time. The large MD Anderson series suggests that many patients may have an initial assessment and exercise at home. We observed that some patients have symptom flare-ups after initial treatment and benefit from a reassessment and additional treatment. Patients who undergo more extensive surgery are at a higher risk for lymphedema and early referral to a lymphedema therapist should be considered.
Conclusions
This study has important implications for the clinical care and recovery of patients with head and neck cancer. Clinician awareness of lymphedema is likely on the rise with the recent increase in publications. In addition to lymphedema therapy, pharmacologic interventions such as selenium or pentoxifylline/vitamin E may be of interest in patients with significant morbidity.22, 23
Acknowledgments
The authors acknowledge the contributions of Lexie Brown, MS.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
References
- 1.Murphy B.A., Gilbert J., Ridner S.H. Systemic and global toxicities of head and neck treatment. Expert Rev Anticancer Ther. 2007;7:1043–1053. doi: 10.1586/14737140.7.7.1043. [DOI] [PubMed] [Google Scholar]
- 2.Machtay M., Moughan J., Trotti A. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: An RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang K.K., Harris J., Wheeler R. Human papillomavirus and survival of patients with oropharyngeal cancer. New Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaturvedi A.K., Engels E.A., Pfeiffer R.M. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deng J., Ridner S.H., Dietrich M.S. Prevalence of secondary lymphedema in patients with head and neck cancer. J Pain Symptom Manage. 2012;43:244–252. doi: 10.1016/j.jpainsymman.2011.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Deng J., Murphy B.A., Dietrich M.S. Impact of secondary lymphedema after head and neck cancer treatment on symptoms, functional status, and quality of life. Head Neck. 2013;35:1026–1035. doi: 10.1002/hed.23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenthal D.I., Lewin J.S., Eisbruch A. Prevention and treatment of dysphagia and aspiration after chemoradiation for head and neck cancer. J Clin Oncol. 2006;24:2636–2643. doi: 10.1200/JCO.2006.06.0079. [DOI] [PubMed] [Google Scholar]
- 8.Jackson L.K., Ridner S.H., Deng J. Internal lymphedema correlates with subjective and objective measures of dysphagia in head and neck cancer patients. J Palliat Med. 2016;19:949–956. doi: 10.1089/jpm.2016.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patterson J.M., Hildreth A., Wilson J.A. Measuring edema in irradiated head and neck cancer patients. Ann Otol Rhinol Laryngol. 2007;116:559–564. doi: 10.1177/000348940711600801. [DOI] [PubMed] [Google Scholar]
- 10.Deng J., Ridner S.H., Aulino J.M., Murphy B.A. Assessment and measurement of head and neck lymphedema: State-of-the-science and future directions. Oral Oncol. 2015;51:431–437. doi: 10.1016/j.oraloncology.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Avraham T., Zampell J.C., Yan A. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. 2013;27:1114–1126. doi: 10.1096/fj.12-222695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X., Yoshida E., Cassidy R.J. Quantitative ultrasonic Nakagami imaging of neck fibrosis after head and neck radiation therapy. Int J Radiat Oncol Biol Phys. 2015;92:407–414. doi: 10.1016/j.ijrobp.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Straub J.M., New J., Hamilton C.D., Lominska C., Shnayder Y., Thomas S.M. Radiation-induced fibrosis: Mechanisms and implications for therapy. J Cancer Res Clin Oncol. 2015;141:1985–1994. doi: 10.1007/s00432-015-1974-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langendijk J.A., Doornaert P., Verdonck-de Leeuw I.M., Leemans C.R., Aaronson N.K., Slotman B.J. Impact of late treatment-related toxicity on quality of life among patients with head and neck cancer treated with radiotherapy. J Clin Oncol. 2008;26:3770–3776. doi: 10.1200/JCO.2007.14.6647. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill M., Heron D.E., Flickinger J.C., Smith R., Ferris R.L., Gibson M. Posttreatment quality-of-life assessment in patients with head and neck cancer treated with intensity-modulated radiation therapy. Am J Clin Oncol. 2011;34:478–482. doi: 10.1097/COC.0b013e3181f4759c. [DOI] [PubMed] [Google Scholar]
- 16.Smith B.G., Hutcheson K.A., Little L.G. Lymphedema outcomes in patients with head and neck cancer. Otolaryngol Head Neck Surg. 2015;152:284–291. doi: 10.1177/0194599814558402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tacani P.M., Franceschini J.P., Tacani R.E. Retrospective study of the physical therapy modalities applied in head and neck lymphedema treatment. Head Neck. 2016;38:301–308. doi: 10.1002/hed.23899. [DOI] [PubMed] [Google Scholar]
- 18.Purcell A., Nixon J., Fleming J., McCann A., Porceddu S. Measuring head and neck lymphedema: The “ALOHA” trial. Head Neck. 2016;38:79–84. doi: 10.1002/hed.23853. [DOI] [PubMed] [Google Scholar]
- 19.Smith B.G., Lewin J.S. Lymphedema management in head and neck cancer. Curr Opin Otolaryngol Head Neck Surg. 2010;18:153–158. doi: 10.1097/MOO.0b013e32833aac21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Youdas J.W., Garrett T.R., Suman V.J., Bogard C.L., Hallman H.O., Carey J.R. Normal range of motion of the cervical spine: an initial goniometric study. Phys Ther. 1992;72:770–780. doi: 10.1093/ptj/72.11.770. [DOI] [PubMed] [Google Scholar]
- 21.Deng J., Dietrich M.S., Ridner S.H., Fleischer A.C., Wells N., Murphy B.A. Preliminary evaluation of reliability and validity of head and neck external lymphedema and fibrosis assessment criteria. Eur J Oncol Nurs. 2016;22:63–70. doi: 10.1016/j.ejon.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Chua D.T., Lo C., Yuen J., Foo Y.C. A pilot study of pentoxifylline in the treatment of radiation-induced trismus. Am J Clin Oncol. 2001;24:366–369. doi: 10.1097/00000421-200108000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Micke O., Bruns F., Mücke R. Selenium in the treatment of radiation-associated secondary lymphedema. Int J Radiat Oncol Biol Phys. 2003;56:40–49. doi: 10.1016/s0360-3016(02)04390-0. [DOI] [PubMed] [Google Scholar]


