Abstract
Purpose
Hypofractionation (HF) of whole breast irradiation has become a standard treatment regimen because randomized trials continue to demonstrate equivalence in survival and local control compared with conventional fractionation. In 2011, the American Society for Radiation Oncology (ASTRO) adopted clinical guidelines on the proper selection of HF. Nevertheless, utilization remains lower than predicted. We evaluate the effects of clinical directives that serve as default treatment decisions and prospective contouring rounds on the implementation of HF in a large, multicenter radiation oncology department.
Methods and materials
In 2010, we implemented consensus-driven and evidence-based clinical directives to guide treatment decisions. Five directives were available for adjuvant breast cancer treatment, including conventional fractionation and HF approaches, and were selected on the basis of disease specifics and clinical judgment. In 2012, we instituted prospective contouring rounds wherein the treating physicians presented their directive selection and patient contours for peer-review and consensus opinion. For this study, charts for patients with early stage breast cancer were reviewed. A total of 1043 cases of breast cancer were identified. Patients receiving HF were analyzed on the basis of the ASTRO 2011 guidelines and adherence to our more inclusive clinical directives.
Results
For the ASTRO-endorsed group (n = 685), 49% of patients received HF in 2011, and 80% received HF in 2015. For the directives-endorsed group (n = 1042), 47% of patients received HF in 2011, and 73% received HF in 2015.
Conclusions
HF is underutilized despite equivalent local control, superior toxicity profile, and noninferior late effects. Our study demonstrates the possibility of achieving high levels of utilization in a large, multisite, outpatient setting. Factors responsible may include default rules established through the development of consensus-based treatment directives, peer review by faculty, and strong financial leadership to implement HF when indicated. To our knowledge, this is the first example of combining both consensus-based treatment directives and prospective contouring rounds in an attempt to change practice patterns.
Summary.
Hypofractionation of radiation schedules for early stage breast cancer in the United States has been demonstrated to be below the utilization found elsewhere in the world. This institution developed a default-rule process in the form of clinical directives as well as daily contouring rounds to help standardize care within our large, multisite department. A review of breast cancer cases was performed to examine hypofractionation utilization over time, which has increased since these changes.
Alt-text: Unlabelled box
Introduction
Until recently, hypofractionated radiation therapy in women with breast cancer was considered a harmful practice, potentially resulting in significant injury from late toxicity, such as soft-tissue necrosis and fibrosis.1, 2 In hindsight, this concern was perhaps due to a limited understanding of the radiobiology of breast cancer. This limitation resulted in an inadequate decrease in total radiation dose in conjunction with the associated increase in dose per fraction.3 However, prospective randomized trials have demonstrated that dose-adjusted hypofractionated regimens not only result in equivalent survival and local control4, 5, 6, 7, 8 but also have comparable, if not better, acute toxicity profiles.9, 10 With the release of 10-year data supporting hypofractionation (HF) and with endorsement by the American Society for Radiation Oncology (ASTRO) clinical guidelines since 2011, HF has become the new standard of care for women with early stage breast cancer.11
Despite the strength of available data, recent studies suggest that hypofractionated whole breast irradiation remains significantly underutilized in the United States.12, 13 A paper by Bekelman et al. showed a utilization of HF in the United States of 34% in 2013 for those matching the characteristics delineated by the ASTRO guidelines.12 In contrast, approximately 70% of patients in Ontario, Canada, receiving whole breast irradiation (without regional lymph node irradiation) received hypofractionated treatment in 2008. In the United Kingdom, most patients with early stage breast cancer have received hypofractionated treatment since 2009.
Factors influencing this discrepancy have previously been examined, suggesting that although patient factors play a role, the greater influence likely comes from the individual practitioners' preferences and the institutional bias of their training.14 Our department has instituted the use of clinical directives for all radiation therapy treatments to standardize the practice of multiple physicians from diverse backgrounds. In addition, we have added the requirement of consensus opinion on the choice of clinical directives during our daily peer review contouring rounds. Herein, we review how these changes increased our utilization rates of hypofractionated radiation therapy for early stage breast cancer to levels that are comparable to those in Canada and the United Kingdom.
Methods and materials
Within our academic and community department of radiation medicine, we implemented a default process for care in 2010 that included a series of consensus-driven and evidence-based clinical directives to guide decisions on patient care.15 A working committee that consisted of physicians, dosimetrists, nurses, and physics and therapy staff was given the task of reviewing available data for the support of each directive, specifying patient selection criteria, and providing prescription, dosimetry, simulation instructions, and imaging choices. These directives were incorporated into the electronic treatment-information system where they could be applied to any patient receiving treatment.
For the adjuvant treatment of breast cancer, 5 treatment directives were developed and available for our physicians to select, based upon inclusion criteria and in combination with their clinical judgment (Table 1). Each directive contained a default prescription with accompanying simulation instructions, dosimetry constraints, and supporting data from the literature. For HF, the main support came from the Canadian and UK Standardisation of Breast Radiotherapy (START) trials, with the ASTRO guidelines serving to provide precautions in choosing HF for specific subgroups that were not studied in sufficient numbers to garner full support (eg, patients <50 years old).4, 6, 7, 8, 11
Table 1.
Available directive choices with defaulted doses
| Clinical directive | Prescription dose |
|---|---|
| Hypofractionation whole breast | 4240 cGy/16 fx + 1000 cGy/5 fx (optional) |
| Standard fractionation whole breast | 5000/25 fx + 1000 cGy/5 fx (optional) |
| Standard fractionation, whole breast and regional nodal irradiation | 5000/25 fx + 1000 cGy/ 5fx (optional) |
| Standard fractionation, postmastectomy with regional nodal irradiation | 5000/25 fx + 1000 cGy/5 fx (optional) |
| Accelerated partial breast irradiation | 3400 cGy/10 fx (brachytherapy) |
fx, fractions.
The choice of fraction size for HF and boost was taken from the Canadian and START trials. Although the Canadian trial did not use a boost, the START trials did at the physician's discretion.4, 6, 7 Our physicians generally extrapolated from the boost criteria in Radiation Therapy Oncology Group Study 1005 when deciding whether to boost the tumor bed.16 Along with this, our department instituted peer-reviewed prospective contouring rounds, during which the chosen directive was reviewed for a consensus opinion. When there was a disagreement, the treating physician was expected to defend his or her choice of a specific directive to guide management or change that choice to match the consensus opinion.17
The patient cohort was defined as patients undergoing adjuvant radiation therapy for breast cancer with curative intent between 2011 and 2015. Patients undergoing partial breast irradiation or treatment involving regional nodal irradiation were excluded. The hypofractionated patient cohort was defined as patients receiving >200 cGy per fraction. A total of 1043 cases of breast cancer were identified in a total of 1021 patients. There were 22 patients with cancer of the contralateral breast (either synchronously or metachronously) during this period.
The patient cohort receiving HF was divided into 2 groups based on those whose treatment was reflective of the selection criteria that followed the ASTRO 2011 guidelines11 versus our clinical directives, which were more inclusive (Table 2). Our clinical directives were more liberal in those patients with ductal carcinoma in situ and those who had received prior chemotherapy. Both could be treated with HF on the basis of available retrospective data showing equivalence and no detriment compared with conventional fractionation.
Table 2.
Subgroup characteristics of patients who received hypofractionation
| ASTRO-endorsed cohort | Directive-endorsed cohort | |
|---|---|---|
| T stage | T1-T2 | Tis-T2 |
| N stage | N0 | N0-Nmi |
| Age | ≥50 | No limit |
| Prior chemotherapy | No | No |
ASTRO, American Society for Radiation Oncology.
We also treated patients with left-sided disease, provided that the heart was sufficiently avoided, and patients with large separations of >25 cm as long as the hot spots were <107% on the basis of planning criteria outlined in Radiation Therapy Oncology Group Study 1005, which was open at that time.16, 18
Results
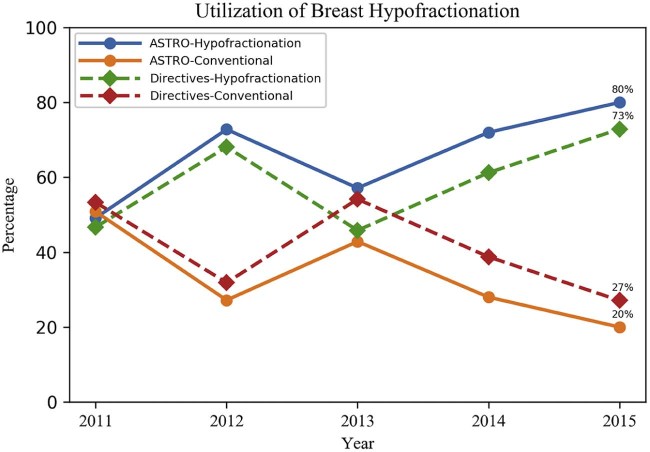
For the ASTRO-endorsed group (n = 685), 49% of patients received hypofractionated therapy in 2011, and an upward trend was noted with 80% receiving hypofractionated therapy in 2015. For the directives-endorsed group (n = 1042), 47% of patients received hypofractionated therapy in 2011; again, an upward trend was noted with 73% of patients receiving hypofractionated therapy in 2015 (Fig 1).
Figure 1.
Utilization of breast hypofractionation for both American Society for Radiation Oncology- and directive-endorsed cohorts (2011-2015).
Discussion
The use of breast HF and its relative underutilization in the United States has come into focus within the past several years.12, 13, 14, 19, 20 As clinical data on long-term local control accrue, so do data with regard to favorable acute toxicities and long-term cosmetic outcomes supporting HF.6, 9, 10 HF has become the clear choice over conventional fractionation on the basis of clinical outcomes and patient preference. The lag in adopting HF is likely multifactorial but based mostly on physician preference. Our utilization of hypofractionated therapy for breast cancer matches that already seen in Canada and the United Kingdom.21 We believe that the implementation of clinical directives and peer-reviewed rounds lead to this high level of utilization.
The combination of evidence-based medicine coupled with faculty consensus to develop department-wide treatment directives served as the foundation for how patients were treated. A limited set of breast treatment directives was designed to apply to the majority of clinical scenarios. Having a set of directives to choose from eliminated the need for the physician to develop a treatment plan for each patient and resort to ad hoc therapy. Certain aspects of the radiation prescription were defaulted to streamline this choice toward best practice, such as conventional fractionation to 5000 cGy in 25 fractions and HF to 4240 cGy in 16 fractions.
However, given the relative complexity of breast cancer presentations, no attempt was made to create a one-size-fits-all choice of therapy. Additionally, there were no barriers to making changes to the directives for special clinical scenarios deemed warranted by the physician. The choice of directive and any alterations were subject to peer-review rounds for a consensus opinion. We observed that the dose heterogeneity for breast cancer essentially disappeared from our practice. Our data show that the vast majority of breast treatments during the study period (97%) were based on a standard unmodified directive.15
This combination of directive selection and peer review can be likened to that of default rules. Default rules have previously been shown to increase consumers' use of green energy,22 organ-donor status,23 and enrollment in pension plans.24, 25 For example, Johnson and Goldstein performed an analysis examining countries' consent to organ donation by whether they had an opt-in (default nondonor) versus an opt-out (default donor) system of consent. They demonstrated a large disparity between the organ donor status of countries with opt-in versus opt-out systems (eg, Germany's opt-in policy nets approximately 12% donors vs Austria's 99% with opt-out).23 Default pathways in medicine work similarly and may steer physicians in a particular direction (ie, preferred practice) while maintaining the autonomy of choice.25
Although there was a department-wide expectation that the directives would be followed, no punitive measures were taken against a physician for changing a directive to suit an individual patient's needs. Also, even though there was no direct incentive to use a particular directive, physicians were encouraged to choose the most appropriate directive for each patient. When a nonhypofractionated directive was selected for a patient who appeared to meet the criteria for HF, the treating physician was expected to defend that decision in a peer-review environment. As such, an important component of directive compliance is daily, peer-reviewed, prospective contouring rounds by all available faculty and residents.
This interaction commonly invites discussions on areas of radiation medicine that have experienced states of fluctuation, such as fractionation schedules for breast cancer, and allows for cultural changes to happen more effectively in our multisite institution.17 In this way, recommendations are also routinely forwarded to a working committee to update a given directive. Ultimately, changes occurred in a minority of cases, and most changes were based on anatomy and setup details, not dose or fractionation.15 Plans were later finalized by the individual physician.17
In 2013, we observed a decrease in the utilization of hypofractionated therapy. The reasons are unclear, but this may have been due to a small cluster of patients treated with hypofractionated therapy who developed symptomatic subacute morphea-type changes within 6 to 12 months after treatment, leading to investigations by the breast cancer treatment teams. These complications generally resolved with supportive care. Nevertheless, in advance of the 10-year START trial publication later in 2013, these anecdotal cases likely led to more caution with hypofractionated therapy.4, 7 This example helps illustrate the impact that anecdotal experience can exert on physician decision making in advance of evidence.
Although there was encouragement from leadership to apply the most appropriate directive to each patient, there was simultaneously a lack of discouragement toward the use of any particular directive. An expectation that utilization of HF would increase with time was taken into account when estimating a future budget. Given the current reimbursement models in the United States, a forward-looking financial infrastructure must also be in place for these changes to occur. Konski et al.26 demonstrated that, for a predicted HF utilization of 40% within a hospital-based radiation oncology practice, there would be approximately a $500,000 decrease in yearly global revenue on the basis of current Medicare and Medicaid service reimbursement with increased HF to patients with breast cancer, lung cancer, prostate cancer, and palliative care.
The current reimbursement trends in the United States are likely designed to reward long episodes of care and would not support utilization of hypofractionated therapy as a reason for the underutilization of hypofractionated therapy in the United States compared with other countries. For example, given the prevalence of breast cancer treatments at most centers, adopting a call for greater HF and changing from a 5-week to a 3-week regimen results in significant financial losses. This gap is likely to widen as investigations into even more abbreviated regimens (eg, UK FAST trial, comparing 5 versus 25 fractions) continue.27, 28 Minding this gap requires forward-thinking financial planning and leadership.
Moreover, the trend for shorter treatment regimens can be contrasted with the relatively rapid adoption of intensity modulated radiation therapy (IMRT) for breast cancer. When Jagsi et al. performed a Surveillance, Epidemiology, and End Results analysis to determine the use of hypofractionated whole breast irradiation in the United States, they found that its use for invasive disease had increased from 3.9% to 9.5% between 2004 and 2008. However, in that same period, IMRT use for invasive disease increased from 9.8% to 20%. Although no formal analysis was performed to compare the reasons for the uptake in either modality, the higher absolute increase in IMRT (despite weaker evidence) was postulated to suggest that providers are more likely to utilize treatment modifications that increase, rather than decrease, reimbursement.13 Hypofractionated therapy fits well with the changes in reimbursement considered by the Centers for Medicare and Medicaid Services Innovation Center, which is currently testing bundled payment models for oncologic care.29 Given that hypofractionated breast regimens appear to not only be clinically equivalent and match patient preferences21 but also to lower costs,19, 30 one would expect a significant increase in hypofractionated therapy as financial incentives align with best clinical practice.
Although we focus on just one aspect of implementing both a clinical directive system with defaulted choices and prospective peer-review rounds, namely the impact on breast HF, making the changes required ultimately reshapes an entire department. The committee that created and manages the directives involved elements from all clinical staff to ensure the continued buy-in and usefulness of these treatment approaches. Early on, there was acculturation of clinicians to the system as they exposed their contours and prescriptions not only to peer scrutiny but possibly the scrutiny of nonphysician staff who were present. As jarring as this may be at first, we believe it was essential to developing the culture necessary to make these changes quickly. As a bonus, it created an atmosphere in which the staff was encouraged to address issues and conflicts in the open, which we believe will ultimately decrease rates of medical errors.
This study's limitations are that it is a retrospective review with incomplete data available for full clinicopathologic analysis. As such, the study makes it difficult to define causal relationships between the increase in HF and use of clinical directives with default rules and peer-reviewed prospective contouring rounds. For example, a small but significant proportion of patients continued to receive conventional fractionation. At this time, the reasons for this are not clear. Previously, Jagsi et al. examined potential drivers within this patient population, and their analysis suggested that nonpathologic factors (eg, age, treatment year, education, and geography) were more likely to be implicated in the decision to give HF.13 Boero et al. expanded on this analysis and confirmed that the significant predictors of receiving HF were much higher if a woman was treated during a later treatment year (after 2005), was of an older age, or was treated by a female physician. Interestingly, pathologic factors such as grade, size, and laterality did not appear to contribute significantly to the choice of therapy.
Again, geographic considerations appeared to have a moderate impact, but individual radiation oncologists appeared to contribute a considerable amount of heterogeneity to treatment choices. For example, if a patient in this population study were to see 2 different radiation oncologists at random within the same geographic area, there could be up to a 3 × difference in her odds of receiving HF on the basis of provider heterogeneity alone.31 These provider-level differences present opportunities for interventions such as ours that attempt to decrease provider heterogeneity through treatment standardization.
Additionally, the impact of outside variables, such as the ASTRO guidelines, long-term results of trials, and entities such as the Choosing Wisely campaign,32 is not well defined and may only be inferred. Nevertheless, we believe that our institutional factors played a role in changing the culture in our department and adopting hypofractionated therapy. With this and the support of future payment models favoring shorter courses of therapy, we predict continued increases in hypofractionated therapy utilization.
Conclusions
HF is underutilized despite equivalent local control, superior toxicity profile, and noninferior late effects compared with conventional fractionation. Our study demonstrates the possibility of achieving high levels of utilization in a large, multisite, outpatient setting. Factors likely to be responsible include the development of consensus-based treatment directives with default choices on the basis of physician consensus, peer review by faculty of all cases, a forward-looking financial structure, and a supportive environment for feedback to implement HF when indicated. To our knowledge, this is the first example of combining both consensus-based treatment directives and prospective contouring rounds in an attempt to change practice patterns.
Footnotes
Conflicts of interest: None.
Sources of support: No authors received a grant or financial support for this research.
Meeting information: Presented in part at the 58th Annual Meeting of the American Society for Radiation Oncology, September 25-28, 2016 in Boston, Massachusetts.
References
- 1.Fletcher G.H. Hypofractionation: Lessons from complications. Radiother Oncol. 1991;20:10–15. doi: 10.1016/0167-8140(91)90106-q. [DOI] [PubMed] [Google Scholar]
- 2.Friberg S., Rudén B.I. Hypofractionation in radiotherapy. An investigation of injured Swedish women, treated for cancer of the breast. Acta Oncol. 2009;48:822–831. doi: 10.1080/02841860902824917. [DOI] [PubMed] [Google Scholar]
- 3.Yarnold J., Bentzen S.M., Coles C., Haviland J. Hypofractionated whole-breast radiotherapy for women with early breast cancer: Myths and realities. Int J Radiat Oncol Biol Phys. 2011;79:1–9. doi: 10.1016/j.ijrobp.2010.08.035. [DOI] [PubMed] [Google Scholar]
- 4.Bentzen S.M., Agrawal R.K., Aird E.G. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet. 2008;371:1098–1107. doi: 10.1016/S0140-6736(08)60348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owen J.R., Ashton A., Bliss J.M. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: Long-term results of a randomised trial. Lancet Oncol. 2006;7:467–471. doi: 10.1016/S1470-2045(06)70699-4. [DOI] [PubMed] [Google Scholar]
- 6.Whelan T.J., Pignol J.P., Levine M.N. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 7.Bentzen S.M., Agrawal R.K., Aird E.G. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: A randomised trial. Lancet Oncol. 2008;9:331–341. doi: 10.1016/S1470-2045(08)70077-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haviland J.S., Owen J.R., Dewar J.A. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 9.Jagsi R., Griffith K.A., Boike T.P. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule: Comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA Oncol. 2015;1:918–930. doi: 10.1001/jamaoncol.2015.2590. [DOI] [PubMed] [Google Scholar]
- 10.Shaitelman S.F., Schlembach P.J., Arzu I. Acute and short-term toxic effects of conventionally fractionated vs hypofractionated whole-breast irradiation: A randomized clinical trial. JAMA Oncol. 2015;1:931–941. doi: 10.1001/jamaoncol.2015.2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith B.D., Bentzen S.M., Correa C.R. Fractionation for whole breast irradiation: An American Society for Radiation Oncology (ASTRO) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 12.Bekelman J.E., Sylwestrzak G., Barron J. Uptake and costs of hypofractionated vs conventional whole breast irradiation after breast conserving surgery in the United States, 2008-2013. JAMA. 2014;312:2542–2550. doi: 10.1001/jama.2014.16616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jagsi R., Falchook A.D., Hendrix L.H., Curry H., Chen R.C. Adoption of hypofractionated radiation therapy for breast cancer after publication of randomized trials. Int J Radiat Oncol Biol Phys. 2014;90:1001–1009. doi: 10.1016/j.ijrobp.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Jagsi R., Griffith K.A., Heimburger D. Choosing wisely? Patterns and correlates of the use of hypofractionated whole-breast radiation therapy in the state of Michigan. Int J Radiat Oncol Biol Phys. 2014;90:1010–1016. doi: 10.1016/j.ijrobp.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 15.Potters L., Raince J., Chou H. Development, implementation, and compliance of treatment pathways in radiation medicine. Front Oncol. 2013;3:105. doi: 10.3389/fonc.2013.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman G.M., White J.R., Arthur D.W., Allen Li X., Vicini F.A. Accelerated fractionation with a concurrent boost for early stage breast cancer. Radiother Oncol. 2013;106:15–20. doi: 10.1016/j.radonc.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Cox B.W., Kapur A., Sharma A. Prospective contouring rounds: A novel, high-impact tool for optimizing quality assurance. Pract Radiat Oncol. 2015;5:e431–e436. doi: 10.1016/j.prro.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Williamson D., Dinniwell R., Fung S., Pintilie M., Done S.J., Fyles A.W. Local control with conventional and hypofractionated adjuvant radiotherapy after breast-conserving surgery for ductal carcinoma in-situ. Radiother Oncol. 2010;95:317–320. doi: 10.1016/j.radonc.2010.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan M.S., Flickinger J.C., Heron D.E., Beriwal S. Changing practice patterns for breast cancer radiation therapy with clinical pathways: An analysis of hypofractionation in a large, integrated cancer center network. Pract Radiat Oncol. 2015;5:63–69. doi: 10.1016/j.prro.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Dwyer P., Hickey B., Burmeister E., Burmeister B. Hypofractionated whole-breast radiotherapy: Impact on departmental waiting times and cost. J Med Imaging Radiat Oncol. 2010;54:229–234. doi: 10.1111/j.1754-9485.2010.02163.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoopes D.J., Kaziska D., Chapin P. Patient preferences and physician practice patterns regarding breast radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:674–681. doi: 10.1016/j.ijrobp.2010.11.077. [DOI] [PubMed] [Google Scholar]
- 22.Sunstein C.R., Reisch L.A. Automatically green: Behavioral economics and environmental protection. Harvard Environ Law Rev. 2014;38:127–158. [Google Scholar]
- 23.Johnson E.J., Goldstein D.G. Defaults and donation decisions. Transplantation. 2004;78:1713–1716. doi: 10.1097/01.tp.0000149788.10382.b2. [DOI] [PubMed] [Google Scholar]
- 24.Chetty R., Friedman J.N., Leth-Petersen S., Nielsen T., Olsen T. 2013. Active vs. passive decisions and crowdout in retirement savings accounts: Evidence from Denmark. In: Working Paper 18565: National Bureau of Economic Research. [Google Scholar]
- 25.Sunstein C.R. Deciding by default. Univ PA Law Rev. 2013;162:1–57. [Google Scholar]
- 26.Konski A., Yu J.B., Freedman G., Harrison L.B., Johnstone P.A. Radiation oncology practice: Adjusting to a new reimbursement model. J Oncol Pract. 2016;12:e576–e583. doi: 10.1200/JOP.2015.007385. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal R.K., Alhasso A., Barrett-Lee P.J. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015) Radiother Oncol. 2011;100:93–100. doi: 10.1016/j.radonc.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 28.Brunt A.M., Wheatley D., Yarnold J. Acute skin toxicity associated with a 1-week schedule of whole breast radiotherapy compared with a standard 3-week regimen delivered in the UK FAST-Forward Trial. Radiother Oncol. 2016;120:114–118. doi: 10.1016/j.radonc.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Press M.J., Rajkumar R., Conway P.H. Medicare's new bundled payments: Design, strategy, and evolution. JAMA. 2016;315:131–132. doi: 10.1001/jama.2015.18161. [DOI] [PubMed] [Google Scholar]
- 30.Lievens Y. Hypofractionated breast radiotherapy: Financial and economic consequences. Breast. 2010;19:192–197. doi: 10.1016/j.breast.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Boero I.J., Gillespie E.F., Hou J. The impact of radiation oncologists on the early adoption of hypofractionated radiation therapy for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2017;97:571–580. doi: 10.1016/j.ijrobp.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Hahn C., Kavanagh B., Bhatnagar A. Choosing wisely: The American Society for Radiation Oncology's top 5 list. Pract Radiat Oncol. 2014;4:349–355. doi: 10.1016/j.prro.2014.06.003. [DOI] [PubMed] [Google Scholar]