Abstract
Aims
Ca2+/calmodulin-dependent protein kinase II (CaMKII) plays a critical role in the development of heart failure and in the induction of myocardial mitochondrial injury. Recent evidence has shown that hydrogen sulfide (H2S), produced by the enzyme cystathionine γ-lyase (CSE), improves the cardiac function in heart failure. However, the cellular mechanisms for this remain largely unknown. The present study was conducted to determine the functional role of H2S in protecting against mitochondrial dysfunction in heart failure through the inhibition of CaMKII using wild type and CSE knockout mouse models.
Results
Treatment with S-propyl-L-cysteine (SPRC) or sodium hydrosulfide (NaHS), modulators of blood H2S levels, attenuated the development of heart failure in animals, reduced lipid peroxidation, and preserved mitochondrial function. The inhibition CaMKII phosphorylation by SPRC and NaHS as demonstrated using both in vivo and in vitro models corresponded with the cardioprotective effects of these compounds. Interestingly, CaMKII activity was found to be elevated in CSE knockout (CSE-/-) mice as compared to wild type animals and the phosphorylation status of CaMKII appeared to relate to the severity of heart failure. Importantly, in wild type mice SPRC was found to promote S-sulfhydration of CaMKII leading to reduced activity of this protein, however, in CSE-/- mice S-sulfhydration was abolished following SPRC treatment.
Innovation and conclusions
A novel mechanism depicting a role of S-sulfhydration in the regulation of CaMKII is presented. SPRC mediated S-sulfhydration of CaMKII was found to inhibit CAMKII activity and to preserve cardiovascular homeostasis.
Abbreviations: CaMKII, Ca2+/calmodulin-dependent protein kinase II; CSE, cystathionine γ-lyase; H2O2, hydrogen peroxide; H2S, hydrogen sulfide; ISO, isoprenaline; mPTP, mitochondrial permeability transition pore; NaHS, sodium hydrogen sulfide; NO, nitric oxide; PAG, D,L-propargylglycine; PLN, phospholamban; SERCA2a, sarco/endoplasmic reticulum Ca2+-ATPase 2a; SPRC, S-propyl-L-cysteine
Keywords: Hydrogen Sulfide, Mitochondria, Heart Failure, Ca2+/calmodulin-dependent protein kinase II, S-sulfhydration
1. Introduction
Heart failure is one of the leading causes of morbidity and mortality globally [38]. In Western countries like, the United States, some 5 million people are affected by this condition and it is posing a significant economic burden on associated health care systems [21]. Similarly, concerns regarding the prevalence of heart failure in South East Asia and China have also come to light [2], [3]. The complex pathophysiological effects of heart failure are diverse and are in part a result of damage to the myocardium. This damage being influenced due to changes in myocardial Ca2+ homeostasis, increased activity of Ca2+ and calmodulin dependent protein kinase II (CaMKII), and mitochondrial stress coupled with increased production of reactive oxygen species (ROS). In the main, changes to the homeostatic systems in the heart during stress induction activate signaling cascades linked to apoptosis and inflammation. Interestingly, aside from the pronounced roles that Ca2+ plays in various physiological processes dysregulation also leads to the activation of CaMKII and the opening of the mitochondrial permeability transition pore (mPTP) [8], [11]. CaMKII plays multiple roles associated with the regulation of cardiac excitation-contraction coupling, apoptotic signaling, Ca2+ homeostasis, and ROS signaling [26], [33]. Indeed, in the cardiovascular system elevated CaMKII activity is observed in ischemia reperfusion, myocardial infarction and heart failure, which is a result of dysregulation in Ca2+ homeostasis, mitochondrial Ca2+ handling and increased ROS [10], [33]. Importantly, the overexpression of CaMKII in mice results in heart failure and recent evidence has shown a clear link between CaMKII activity, the expression of the mitochondrial Ca2+ uni-porter and mPTP opening [13]. Indeed, inhibition of CaMKII prevents Ca2+ uptake into mitochondria and reduces damage to these organelles. Thus, growing evidence supports the targeting of the CaMKII and the mPTP as a means to prevent tissue damage within the cardiovascular system [12]. Therefore, the rational design and development of pharmacological agents that can target CaMKII signaling and the mPTP could provide new strategies in the prevention of tissue damage within the cardiovascular system.
Hydrogen sulfide (H2S) is a known biologically active gasotransmitters that mediates its effects via membrane receptor-independent cellular signaling transduction cascades [44]. Endogenous H2S is produced via the catabolism of cysteine by the enzymes cystathionine β-synthase, and cystathionine γ-lyase (CSE). Current research indicates that H2S acts as an endothelium relaxing factor similar to that of nitric oxide (NO) [20], in addition to having pro-angiogenic effects [5], vascular remodeling effects [31], anti-atherosclerotic activity [19], and platelet anti-aggregatory properties [9]. Emerging evidence also indicates that dysregulation in the endogenous production of H2S either using pharmacological inhibition of CBS and CSE or utilizing CSE knockout (CSE-/-) animals can influence cardiovascular function [25]. Therefore it is now recognized that H2S plays an important role in cardiovascular homeostasis and dysregulation in endogenous levels contributes to a range of pathophysiological conditions like myocardial ischemia, spontaneous hypertension, and hypoxic pulmonary hypertension. Intervention using donor molecules like sodium hydrosulfide (NaHS) or GYY4137, to restore tissue H2S, preserves organ function and elicits cytoprotective effects [24], [32]. An interesting aspect of H2S biology is its ability to modify target proteins via S-sulfhydration due to its ability to react with cysteine residues [41]. This post-translational modification controls a range of physiological processes, for example, in the regulation of NF-κB [27], protein-tyrosine phosphatase 1B [17], Parkin [34], and MEK1 [47]. Thus, an understanding of the specific modification of key cysteine residues present within proteins not only plays an important role in regulating protein function, but also provides a novel target for the design of new drugs focused on treating cardiovascular diseases. Specifically, a better understanding of the molecular mechanisms and cellular targets that contribute to the cytoprotective effects of H2S could provide a rational route for the exploitation and development of potential therapeutic agents.
S-propyl-L-cysteine (SPRC) is a H2S donor. Unlike inorganic salts, such as NaHS, SPRC releases H2S relaying on endogenous H2S producing enzymes CSE or CBS (Fig. S1), and for this reason we refer to SPRC as an endogenous H2S donor [36]. Researches indicated that SPRC could improve endothelium cells proliferation and had anti-inflammatory effect in cardiomyocytes [16], [23]. In the present study, SPRC was found to have cardioprotective effects in vivo by inhibiting mitochondria dysfunction and by promoting S-sulfhydration of CaMKII.
2. Results
2.1. H2S had protective effect against isoprenaline-induced heart failure
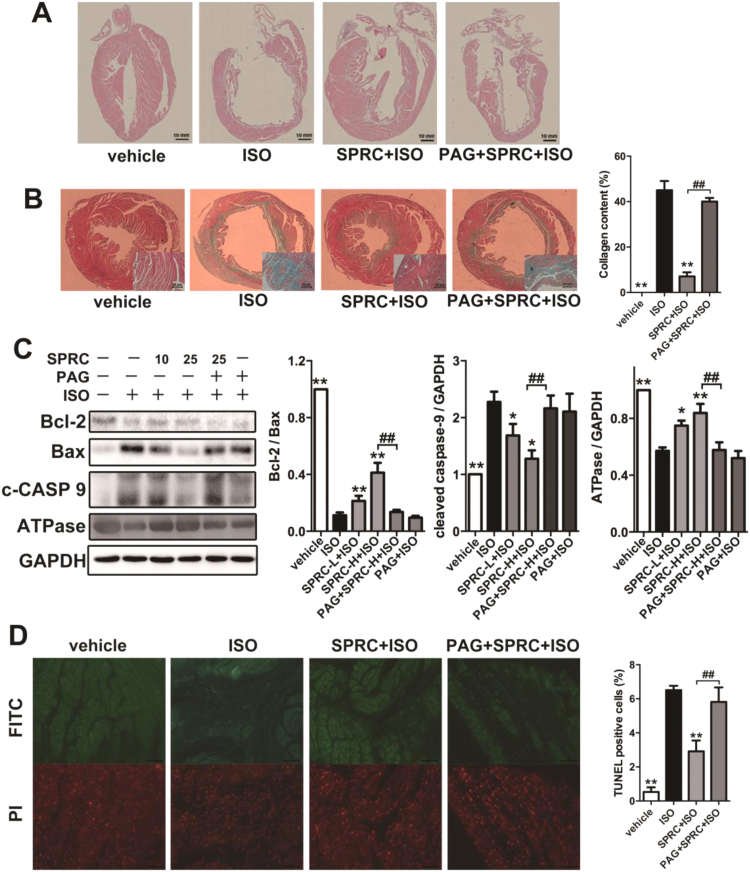
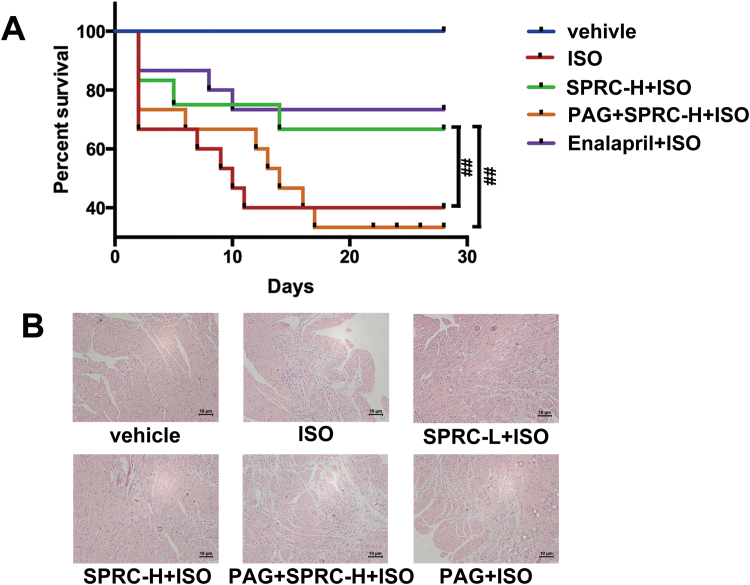
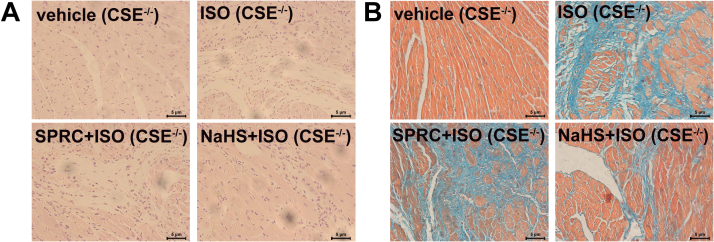
To detect the cardioprotective effect of SPRC, we used isoprenaline (ISO) to induce heart failure in C57 mice. Enalapril is an angiotensin-converting enzyme inhibitor, and it is used to treat heart failure patient in clinics. As shown in the Kaplan-Meier survival plot (Fig. S2A), SPRC at 25 mg/kg/d and enalapril at 20 mg/kg/d had a significant effect on survival rates in ISO (7.5 mg/kg/d) treated animals. Echocardiography revealed that ejection fraction, fraction shortening, left ventricular anterior wall systole and left ventricular posterior wall systole were significantly decreased, whereas left ventricular internal diameter-diastole, left ventricular internal diameter-systole, left ventricular end-diastolic volume and left ventricular end-systolic volume were increased in ISO group (Table 1). ISO treatment resulted in ventricular dilation, thinning of ventricular wall and increased collagen deposition within tissues (Fig. 1A). Moreover, a significant loss of myocardium and an increase in fibrosis, as determined using H&E staining and Masson trichome staining, was particularly evident in the left ventricular wall (Fig. 1B and Fig. S2B).
Table 1.
H2S donor improved cardiac function in ISO-induced heart failure mice.
| Measurement | Vehicle (n= 6) | ISO (n= 6) | SPRC-L+ISO (n= 6) | SPRC-H+ISO (n= 6) | PAG+SPRC-H+ISO (n= 6) | PAG+ISO (n= 6) | Enaprili+ISO (n= 6) |
|---|---|---|---|---|---|---|---|
| EF (%) | 78.48 ± 2.52** | 19.27 ± 3.04 | 45.72 ± 4.99** | 65.62 ± 4.16** | 30.71 ± 3.99## | 20.23 ± 10.05 | 56.24 ± 13.28** |
| FS (%) | 46.48 ± 2.41** | 8.81 ± 1.49 | 22.66 ± 2.92** | 35.83 ± 3.02** | 14.5 ± 2.11## | 9.31 ± 4.75 | 29.47 ± 8.78** |
| LVAWs (mm) | 1.18 ± 0.03** | 0.73 ± 0.10 | 0.99 ± 0.07* | 1.12 ± 0.1** | 0.84 ± 0.15## | 0.66 ± 0.11 | 0.92 ± 0.06 |
| LVPWs (mm) | 1.1 ± 0.13** | 0.48 ± 0.03 | 0.8 ± 0.1* | 1 ± 0.03** | 0.58 ± 0.12## | 0.57 ± 0.02 | 0.98 ± 0.27** |
| LVIDd (mm) | 3.83 ± 0.24** | 5.21 ± 0.23 | 4.4 ± 0.47 | 4.29 ± 0.42 | 4.91 ± 0.31 | 4.98 ± 0.34 | 4.08 ± 0.74* |
| LVIDs (mm) | 2.05 ± 0.19** | 4.75 ± 0.16 | 3.41 ± 0.38** | 2.76 ± 0.37** | 4.19 ± 0.25## | 4.53 ± 0.55 | 2.92 ± 0.84** |
| LV Vold (μl) | 63.30 ± 9.57** | 130.38 ± 13.42 | 88.88 ± 21.65 | 83.47 ± 19.25* | 113.74 ± 16.82## | 117.63 ± 18.89 | 75.87 ± 29.66* |
| LV Vols (μl) | 13.71 ± 3.22** | 105.16 ± 8.25 | 48.25 ± 12.18** | 29.1 ± 9.48** | 78.68 ± 11.3## | 95.09 ± 27.92 | 35.82 ± 21.24** |
p < 0.05 vs ISO group.
p < 0.01 vs ISO group.
p < 0.01 vs SPRC-H+ISO group.
Fig. 1.
H2S donor had protective effect against heart failure. (A) Treatment with ISO resulted in ventricular dilation and thinning of ventricular wall compared to the vehicle group, which could be improved by SPRC. (B) Masson trichrome stain showed fibrosis of the left ventricular endocardium in each group. The collagen area in each group was quantified by IPP software. (C) Expression levels of Bcl-2, Bax, cleaved caspase 9 (c-CASP 9) and ATPase in ISO-induced heart failure mice treated with SPRC-L (10 mg/kg/d) or SPRC-H (25 mg/kg/d) with or without PAG for 4 weeks. (D) TUNEL-stain showed cardiomyocytes apoptosis in each group. Green: TUNEL staining representing apoptotic cells. Red: Cell stained by PI. Data represent means ± SEM, n = 8–12 for each group. **p < 0.01 compared with isoprenaline group, ##p < 0.01.
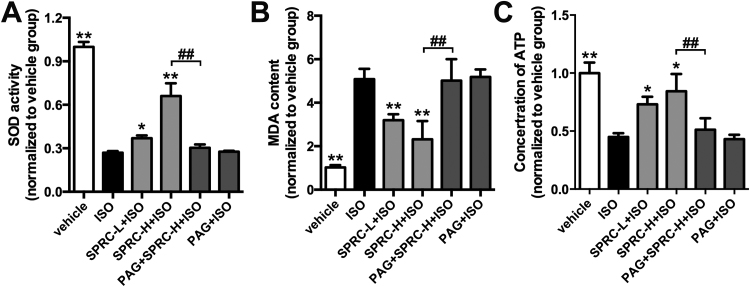
ISO appeared to promote oxidative stress and the induction of apoptosis in cardiac tissues. Indeed, changes in the levels of lipid peroxidation products, a decrease activity of the antioxidant enzyme superoxide dismutase (SOD), an increase of malondialdehyde (MDA) content, a reduction in cellular ATP concentrations and reduced expression of ATPase were noted in this study (Fig. S3A–C). Similarly, a decrease in the expression levels of the anti-apoptotic protein Bcl-2 and an increase in the pro-apoptotic protein Bax and activated caspase-9 were observed (Fig. 1C). TUNEL staining clearly showed an increase in apoptotic cells in the cardiac tissues of ISO treated animals (Fig. 1D). In contrast, in all of the parameters assessed SPRC+ISO were found to have a significant opposing effect on the tissue damage evoked by ISO treatment.
2.2. SPRC mediated H2S production is CSE dependent
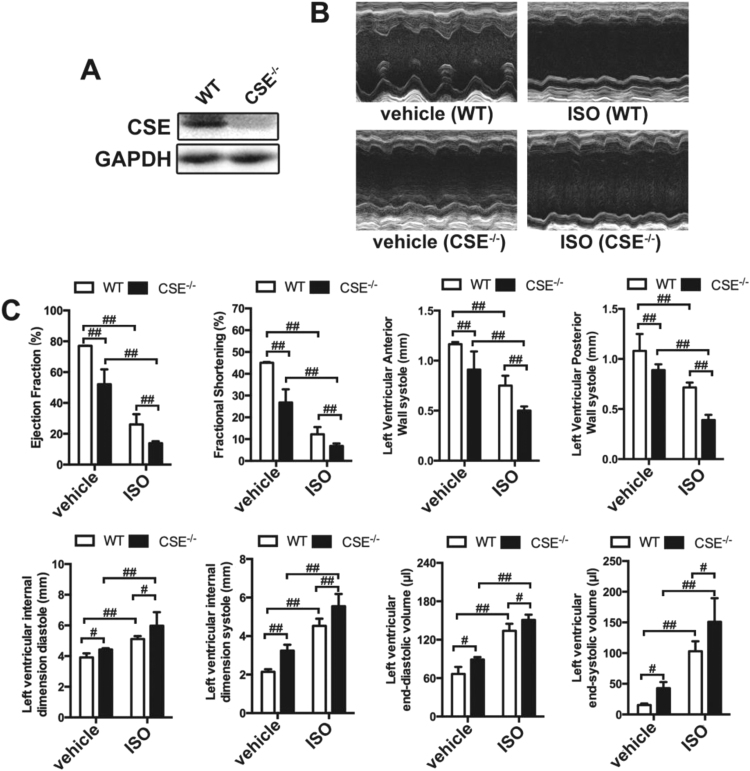
We hypothesized, based on previous work, that SPRC would induce CSE and that this may alter endogenous levels of H2S. To confirm the effects of SPRC are dependent on a functional CSE protein, CSE-/- mice were utilized. CSE expression was confirmed by western blot and noted to be absent in the CSE-/- animals (Fig. 2A). Interestingly, basal ejection fraction, fraction shortening, left ventricular anterior wall systole and left ventricular posterior wall systole in CSE-/- mice were lower than that in their wild type counterparts and left ventricular end-diastolic volume, left ventricular end-systolic volume, left ventricular internal diameter-diastole and left ventricular internal diameter-systole were higher. Furthermore, cardiac function damage induced by ISO was more pronounced in CSE-/- mice (Figs. 2B and 2C).
Fig. 2.
ISO caused more severe HF in CSE-/-mice. (A) Expression levels of CSE in the heart of wild type (WT) and CSE-/- mice. (B-C) The echocardiography data showed the changes in ejection fraction, fractional shortening, left ventricular anterior wall systole, left ventricular posterior wall systole, left ventricular internal dimension diastole, left ventricular internal dimension systole, left ventricular end-diastolic volume and left ventricular end-systolic volume in CSE-/- mice and WT mice treated with or without ISO for 4 weeks. Data represent means ± SEM, n = 8–12 for each group. #p < 0.05, ##p < 0.01, NS = no significance.
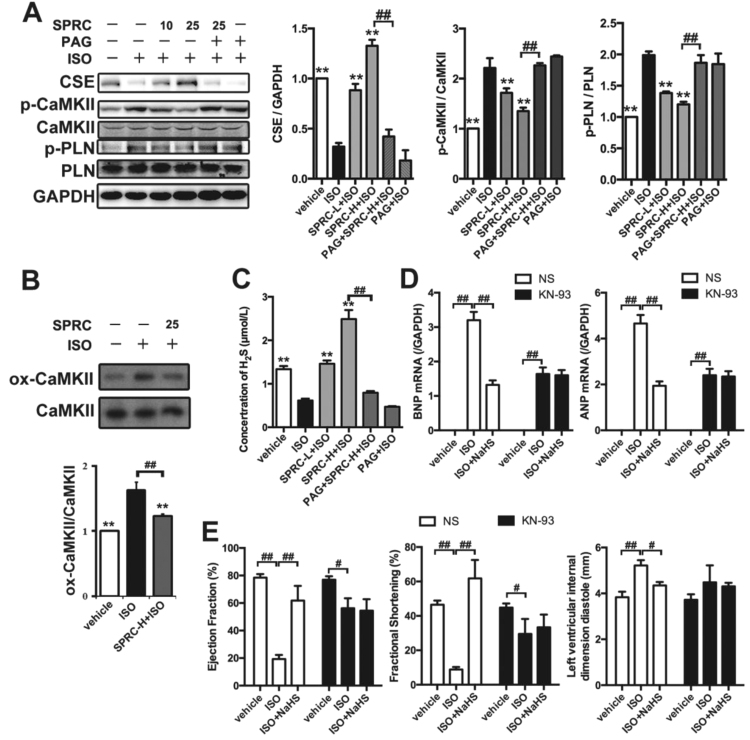
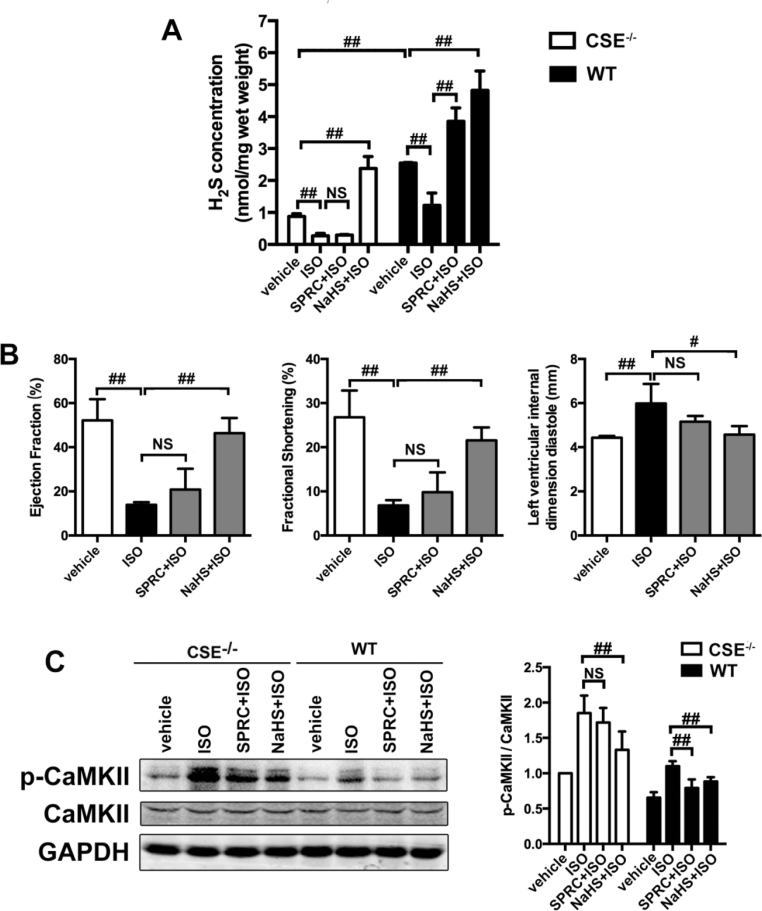
We next assessed whether there were any changes in the expression level of the H2S producing enzyme, CSE, in cardiac tissue. As shown in Fig. 3A, SPRC increased CSE protein expression levels in a dose dependent manner. This led us to ascertain whether the degradation of CSE prevented by SPRC influenced plasma H2S levels. In ISO treated animals, plasma H2S concentrations were obviously lower compared to that of the vehicle group. However, in SPRC+ISO treated animal's plasma H2S levels were significant increased from the ISO group (p < 0.01) (Fig. 3C). Importantly, using the CSE inhibitor, D, L- Propargylglycine (PAG), plasma H2S levels were reduced and the cytoprotective effects ascribed to SPRC were abolished. Moreover, in wild type mice, both 25 mg/kg/d SPRC and 60 μmol/kg/d NaHS could enhance plasma H2S level. However, in CSE-/- mice, only NaHS could increase H2S level in cardiac tissue (Fig. 4A). SPRC could not protect against tissue injury (Fig. S4A and S4B) and cardiac function damage (Fig. 4B and Table 2) induced by ISO in CSE-/- mice. These data above suggest that SPRC mediated H2S production are dependent on a functional CSE protein.
Fig. 3.
H2S donor improved cardiac function through CaMKII pathway. (A) Expression levels of CSE, CaMKII, p-CaMKII PLN and p-PLN in ISO-induced HF mice treated with indicated treatments for 4 weeks. (B) Expression levels of CaMKII and ox-CaMKII in ISO-induced HF mice treated with indicated treatments for 4 weeks. (C) The concentration of plasma H2S in ISO-induced HF mice with indicated treatments for 4 weeks. (D) Quantitative real-time PCR for BNP and ANP. (E) The echocardiography data showed the changes in ejection fraction, fractional shortening, left ventricular internal dimension diastole. SPRC-L = 10 mg/kg/d and SPRC-H = 25 mg/kg/d. PAG: D, L-propargylglycine. KN-93 = 10 μM/kg/d. Data represent means ± SEM, n = 8–12 for each group. **p < 0.01 compared with ISO group, #p < 0.05, ##p < 0.01.
Fig. 4.
The cardioprotective effect of H2S donor SPRC through CaMKII pathway relayed on CSE expression. (A) H2S concentrations of hearts in ISO-induced heart failure with indicated treatments for 4 weeks in WT and CSE-/- mice. (B) The echocardiographic data in CSE-/- mice with indicated treatments for 4 weeks. n = 7–9. (C) Expression levels of CaMKII and p-CaMKII in WT and CSE-/- mice treated with SPRC or NaHS for 4 weeks. Data represent means ± SEM, n = 8–12 for each group. *p < 0.05 and **p < 0.01 compared with ISO group, #p < 0.05, ##p < 0.01, NS = no significance.
Table 2.
H2S donor NaHS rather than SPRC could improve cardiac function in ISO-induced heart failure CSE-/- mice.
| Measurement | Vehicle (n= 6) | ISO (n= 6) | SPRC+ISO (n= 6) | NaHS+ISO (n= 6) |
|---|---|---|---|---|
| EF (%) | 52.16 ± 9.61** | 13.9 ± 1.2 | 20.84 ± 9.42NS | 46.36 ± 6.90** |
| FS (%) | 26.79 ± 6.06** | 6.83 ± 1.19 | 9.86 ± 4.47NS | 21.54 ± 2.94** |
| LVAWs (mm) | 0.91 ± 0.18** | 0.50 ± 0.04 | 0.53 ± 0.18NS | 0.72 ± 0.06* |
| LVPWs (mm) | 1.1 ± 0.13** | 0.48 ± 0.03 | 0.8 ± 0.1NS | 1 ± 0.03* |
| LVIDd (mm) | 4.43 ± 0.08** | 5.98 ± 0.89 | 5.16 ± 0.26NS | 4.57 ± 0.39** |
| LVIDs (mm) | 3.24 ± 0.31** | 5.55 ± 0.64 | 5.70 ± 0.82NS | 4.04 ± 0.48* |
| LV Vold (μl) | 89.12 ± 3.88** | 150.93 ± 8.24 | 145.10 ± 15.94NS | 105.67 ± 19.60* |
| LV Vols (μl) | 42.77 ± 9.94** | 150.94 ± 38.52 | 134.40 ± 26.15NS | 68.89 ± 15.52* |
p < 0.05 vs ISO group.
p < 0.01 vs ISO group.
No significant vs ISO group.
2.3. SPRC altered the phosphorylation status of CaMKII in vivo
Changes in the expression levels and phosphorylation status of CaMKII were also investigated in the current study. Recent evidence has shown that this kinase plays a significant role in the promotion of myocardial hypertrophy and heart failure. Phospholamban (PLN) was used as a biomarker for the indication of the CaMKII activity. Interestingly, no significant changes were found in the expression levels of total CaMKII and PLN in any of the treatment groups. However, ISO was found to induce CaMKII phosphorylation at T286, which was also the indicative of its kinase activity. Importantly, SPRC was found to inhibit phosphorylation of CaMKII (T286) and PLN (T17) in treated animals (Fig. 3A), and in CSE-/- mice SPRC failed to inhibit the phosphorylation status of CaMKII (Fig. 4C). Furthermore, ISO led to oxidative stress and caused CaMKII oxidation, which could be suppressed by SPRC supplementing (Fig. 3B). Moreover, treatment of CaMKII inhibitor KN93 together with ISO prevented the cardioprotective effects of H2S, such as hypertrophic markers (BNP and ANP, Fig. 3D), EF, FS and LVIDd (Fig. 3E). Thus, the H2S could regulate CaMKII activity in ISO-induced mice heart failure.
2.4. H2O2 mediated CaMKII activation in H9c2 cardiomyocytes
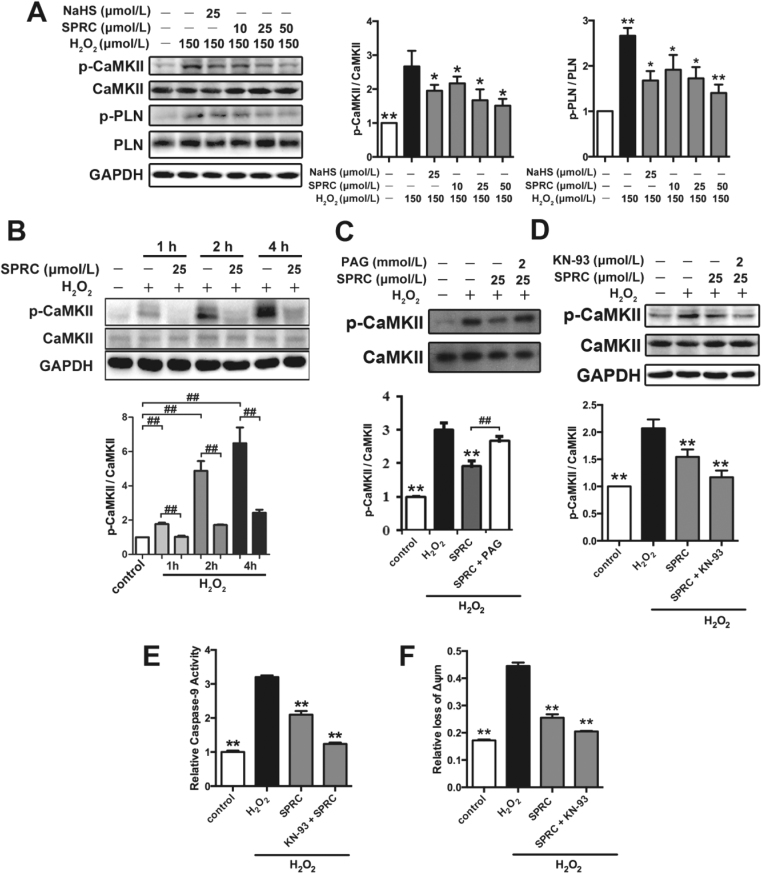
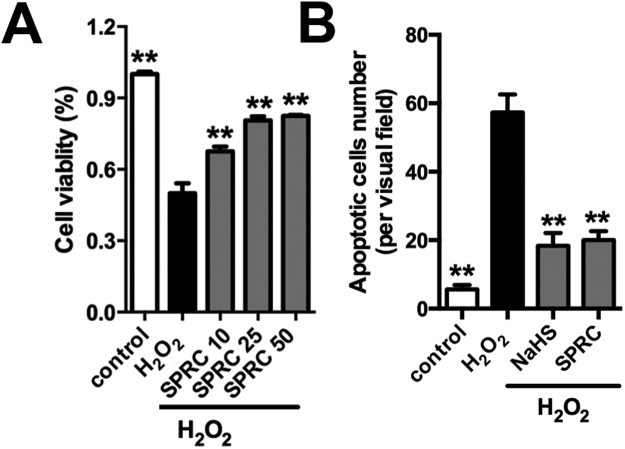
Based on the in vivo data we further explored the mechanism(s) linked to the cardioprotective effects of SPRC studies utilizing the immortalized H9c2 cardiomyocytes model coupled to oxidative stress induction. In this instance we also assessed the expression and phosphorylation status of CaMKII in hydrogen peroxide (H2O2) exposed cells (Fig. 5A). H2O2 significantly increased the phosphorylation status of CaMKII (T286) and its target protein PLN (T17) in H9c2 cardiomyocytes. CaMKII activity is known to influence myocardial Ca2+ homeostasis, intracellular calcium flux and mitochondrial Ca2+ handling. This result is indicative of a link between H2O2 mediated stress mediated calcium mobilization, CaMKII and cell viability. Indeed, using western blot analysis SPRC was found to inhibit CaMKII (T286) phosphorylation status in a time and concentration dependent manner (Figs. 5A and 5B). The effect of SPRC on CaMKII activity could be suppressed by PAG (Fig. 5C). Moreover, co-treatment of SPRC with the KN-93 had an additive effect on the inhibition of CaMKII (T286) phosphorylation status in H9c2 cardiomyocytes (Fig. 5D). Importantly, SPRC co-treatment with KN-93 inhibited caspase-9 activity, the loss of mitochondrial membrane potential and preserved cell viability in H2O2 exposed cells (Figs. 5E and 5F). These data confirms that CaMKII is a cellular target of SPRC in this model.
Fig. 5.
H2S donor had protective effect against oxidative through CaMKII pathway. (A) The levels of p-CaMKII and p-PLN in H9c2 cardiomyocytes with indicated treatments. (B) The levels of CaMKII activity were decreased by SPRC in a time-dependent manner. (C) SPRC inhibited CaMKII activity depended on CSE. (D) SPRC and CaMKII inhibitor KN-93 decreased CaMKII activity. (E) The activity of caspase 9 was decreased after treatment with SPRC or KN-93. (F) SPRC and KN-93 increased the mitochondrial membrane potential. Data represent means ± SEM, n = 6. *p < 0.05, **p < 0.01 compared with H2O2 group, ##p < 0.01.
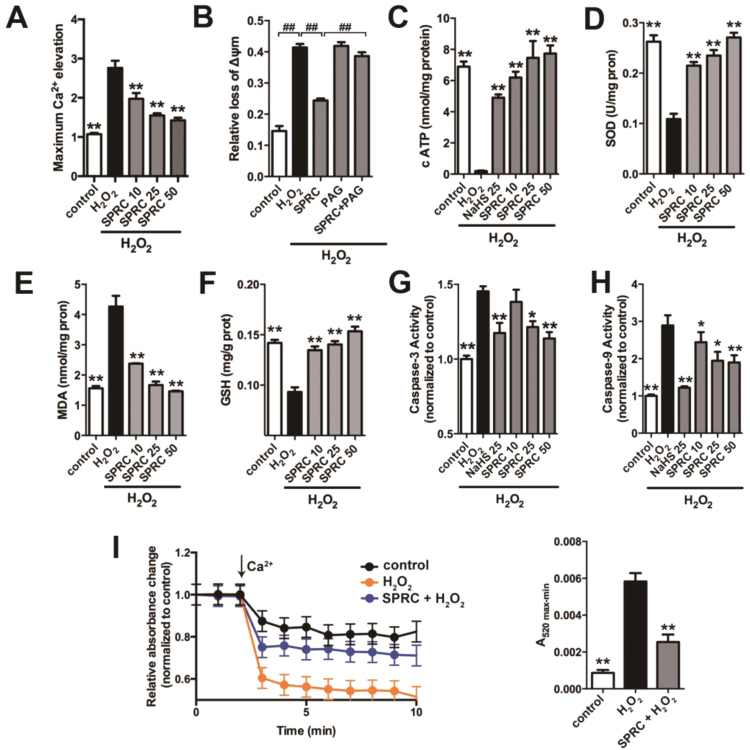
In addition, the maximum intracellular Ca2+ elevation was also determined in H9c2 cells using the fluorescent probe Fluo3-AM. H2O2 exposure promoted the maximum intracellular Ca2+ elevation compared to all treatment groups. Interestingly, SPRC could inhibit the elevation of intracellular Ca2+ in a concentration dependent manner (Fig. 6A). Furthermore, elevated cytoplasmic Ca2+ correlated with CaMKII phosphorylation status and the loss of mitochondrial membrane potential (ΔΨm), as determined using the ratiometric fluorescent probe, JC-1 (Fig. 6B). Similarly, it was also found that SPRC prevented H2O2 mediated loss of ATP (Fig. 6C). Increased SOD activity, inhibited lipid peroxidation and the preservation of cellular glutathione status in H9c2 cells were also noted. The expression and activities of caspase-3 and caspase-9 were reduced in SPRC treated cells correlating with the available in vivo data described above (Fig. 6D–H). Collectively, these findings indicated that SPRC had a marked effect on stress signaling in H2O2 treated cells with this preserving cell viability as determined using the MTT assay and reducing the number of apoptotic cells in H2O2 exposed cells (Fig. S5A and S5B). Lastly, since H2O2 induced the mobilization of Ca2+ stores and loss of ΔΨm in H9c2 cells we next explored the potential role of the mPTP using isolated mitochondria. The combined exposure of isolated mitochondria to 200 µmol/L Ca2+ and 150 µmol/L H2O2 led to mitochondrial swelling and mPTP opening, as noted by a decrease in absorbance at 540 nm. As shown in Fig. 6I, SPRC significantly reduced the degree and rate of mitochondrial swelling and mPTP opening, suggesting that SPRC preserved mitochondrial integrity under stress conditions.
Fig. 6.
H2S donor regulated CaMKII activity to protect against oxidative stress-induced mitochondrial dysfunctionin vitro. (A) The maximum intracellular Ca2+ elevation was decreased by SPRC treatment in H9c2 cardiomyocytes. (B) The change of mitochondria membrane potential in H9c2 cardiomyocytes treated with SPRC. (C) The concentration of ATP in H9c2 cardiomyocytes. (D-F) The activity of SOD and the content of MDA and GSH were measured in the mitochondria isolated from H9c2 cells. (G and H) The activity of caspase 9 and caspase 3 in H9c2 cardiomyocytes. Data represent means ± SEM, n = 6. *p < 0.05 and **p < 0.01, ##p < 0.01.
2.5. SPRC mediated inhibition of CaMKII signaling
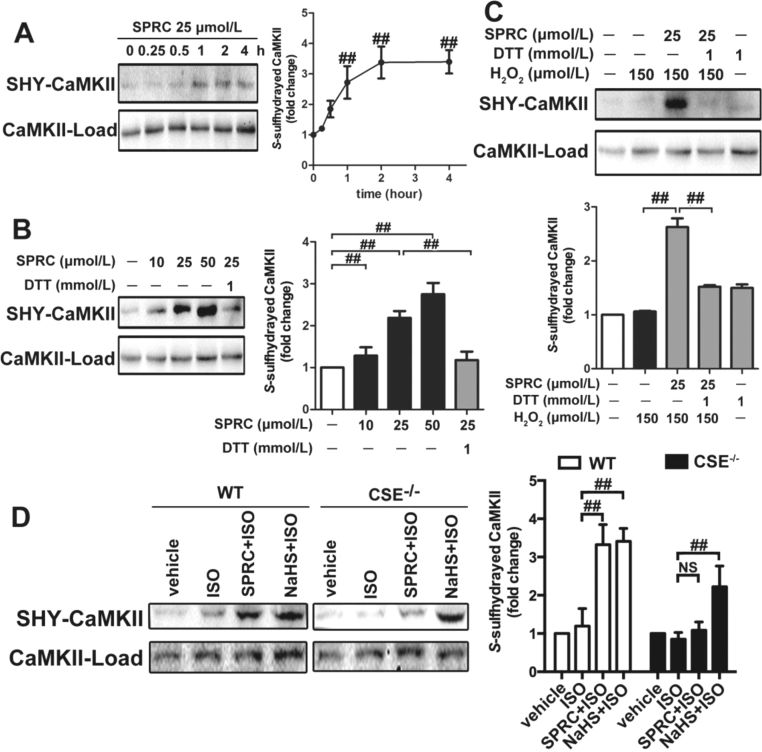
Since SPRC inhibited the phosphorylation status of CaMKII, we questioned which mechanisms were responsible for this process. Emerging evidence has highlighted that S-sulfhydration is a key post-translation modification step responsible for some of the physiological effects ascribed to H2S and allied donor molecules [27]. Indeed, in the current model it was found that SPRC mediated inhibition of CaMKII activity corresponded to S-sulfhydration. Under the conditions used S-sulfhydration of CaMKII was concentration (10–50 µmol/L) and time dependent (0–4 h) in SPRC exposed cells, with maximal levels occurring at 1 h and at a concentration of 50 µmol/L (Fig. 7A–C). Furthermore, treatment with dithiothreitol (DTT) reversed SPRC-induced S-sulfhydration, likely due to the liberation of the SH- group from key cysteine residues. This observation indicated that SPRC mediated S-sulfhydration of CaMKII and that this post-translational step played an important function role in its cardioprotective effects. Interestingly, in CSE-/- animals SPRC mediated S-sulfhydration of CaMKII could not detected (Fig. 7D). Again, this data is in agreement with the notion that a functional CSE protein is required for the biological effects of SPRC in this model.
Fig. 7.
H2S donor decreased the CaMKII activity throughS-sulfhydration of CaMKII. (A) H2S stimulated CaMKII S-sulfhydration in H9c2 cardiomyocytes in a time-dependent manner. (B) SPRC stimulated CaMKII S-sulfhydration in H9c2 cardiomyocytes in a dose-dependent manner. (C) SPRC stimulated CaMKII S-sulfhydration under oxidative stress. (D) Levels of CaMKII S-sulfhydration of hearts in ISO-induced heart failure mice with indicated treatments. DTT: dithiothreitol, SHY-CaMKII: S-sulfhydrated CaMKII. Data represent means ± SEM, n = 6 in vitro, and n = 8–12 for each group in vivo.##p < 0.01, NS: no significance.
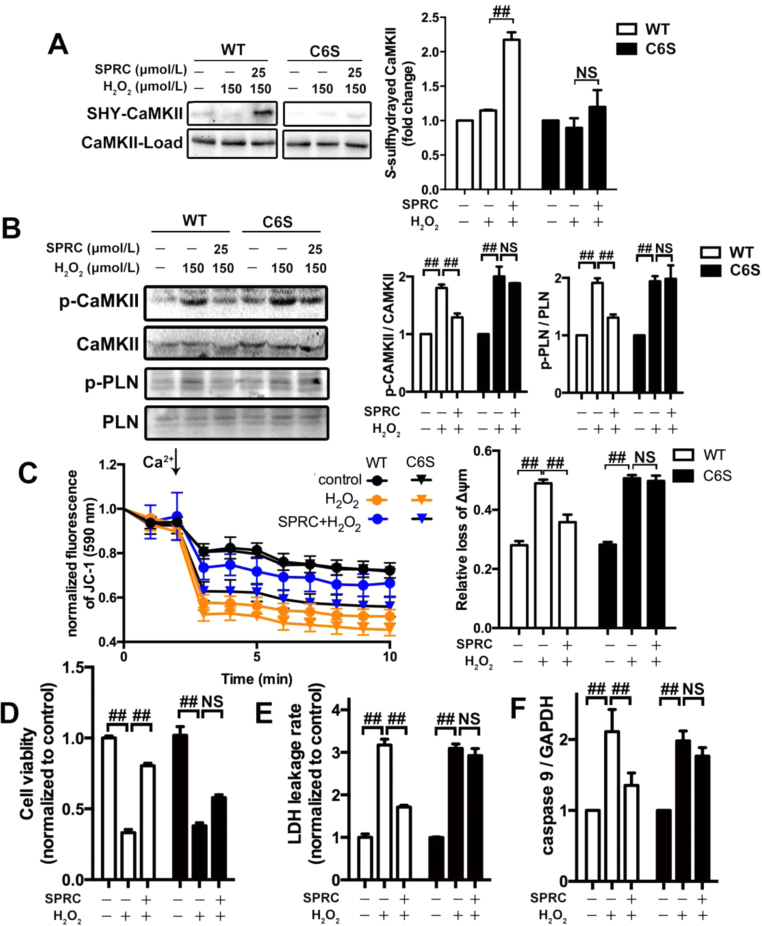
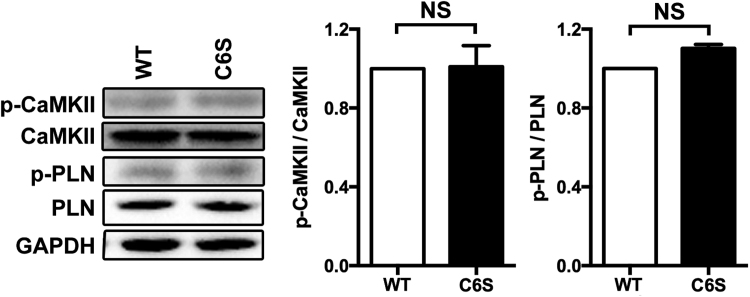
To identify potential target sites of S-sulfhydration on CaMKII, we mutated a key cysteine residue involved in CaMKII catalytic activity, and replaced this with the amino acid serine. In transfected cells no significant difference in the levels of p-CaMKII and p-PLN were found between the C6S-mutant-transfected or wild type-transfected cells. This data indicating that the substitution of cysteine-6 with a serine residue had no effect on catalytic activity (Fig. S6A). However, mutation of cysteine-6 was found to influence the basal level of S-sulfhydration of CaMKII and in SPRC treated C6S-mutant-transfected cells S-sulfhydration of CaMKII was abolished (Fig. 8A). This finding suggests that the cysteine-6 residue is an important site involved in S-sulfhydration. Interestingly, we found that in cells expressing that CaMKII C6S-mutation that the phosphorylation status of this protein was unchanged in the presence of SPRC (Fig. 8B). And the protection effect against mitochondria dysfunction of SPRC was attenuated in CaMKII C6S mutation cells (Fig. 8C). Furthermore, in C6S-mutant-transfected cells SPRC mediated cytoprotection and anti-apoptosis effect were partially abolished (Fig. 8D–F).
Fig. 8.
CaMKII Cys6mutant abolished the regulatory effect of H2S donor on CaMKII activity. (A) The mutant CaMKII (C6S) reduced H2S donor-induced CaMKII S-sulfhydration in H9c2 cardiomyocytes. (B) The levels of p-CaMKII and p-PLN in H9c2 cardiomyocytes with indicated treatments after transfected with mutation or WT plasmid. (C) The change of mitochondria membrane potential in H9c2 cardiomyocytes treated with mutation or WT plasmid. (D-F) The cell viability, LDH leakage rate and the activity of caspase 9 were measured after transfected with mutation or wild type plasmid. ##p < 0.01, NS: no significance.
3. Discussion
Growing evidence suggests that H2S plays an important role in many pathophysiologic conditions [32], [36], [37]. Yet, the cardioprotective mechanism relating to H2S in heart failure still remains unclear. In the present study a novel cardioprotective mechanism relating to the novel sulphur compound SPRC in heart failure is presented. It is demonstrated that SPRC protects against the mitochondrial dysfunction and apoptosis via increasing plasma H2S levels and the S-sulfhydrating CaMKII in heart failure. In vivo treatment with ISO induced cardiac dysfunction and left ventricular remodeling. SPRC, a chemical analogue of the natural product S-Allyl-L-cysteine found in Allium sativum, could increase plasma H2S level through increased expression of CSE protein in cardiac tissues. The combined effects of SPRC and the generation of H2S were found to have protective affects against ISO induced cardiac dysfunction and remodeling. Indeed, in the current study the severity of heart failure was far higher in CSE-/- mice as compared to their wild type counterparts. This is in agreement that CSE mediated H2S production is cardioprotective.
H2S is an endogenously gasotransmitter and it can mediated the regulation of nervous system, cardiovascular functions and so on. To identify whether H2S could protect against heart failure directly, we detected heart function by echocardiograph. In this study, we found that H2S could improve heart function. It also has been reported that the ratio of heart weight to body weight and the ratio of left ventricular weight to body weight were lower in NaHS + ISO group compared with that in ISO group. ISO treatment could decrease diastolic blood pressure and systolic blood pressure, and NaHS treatment could not increase diastolic blood pressure but it could increase systolic blood pressure significantly [18]. Therefore, H2S had protective effect against heart failure directly.
The pathophysiological process of heart failure is complex and many proteins and signaling pathways are involved during this process. The case for the current study stems from recent evidences that indicate that CaMKII plays a great role in heart failure [10], [26], [33]. For example, β-adrenergic receptor agonists and ROS can activate CaMKII, and activation of CaMKII appears to trigger heart failure [10], [35]. CaMKII is often elevated in heart failure and CaMKII overexpression in mice results in heart failure [42]. Recent evidence also links CaMKII to mPTP opening and to changes in Ca2+ homeostasis during ischemia reperfusion injury [13]. Moreover, CaMKII appears to be an important regulator of cellular processes associated with excitation-contraction coupling, Ca2+ homeostasis, and apoptosis [26], [33]. Thus, current evidence indicates a role for this protein in mediating stress responses within the heart. From a mechanistic perspective Ca2+/calmodulin-binding activates CaMKII via release of its regulatory domain leading to the induction of an auto-phosphorylation cascade and Ca2+/CaM-independent activity. It is speculated that the cardioprotective effect of SPRC in heart failure might relate to the regulation of CaMKII activity. Indeed, in the current work, CaMKII activity was elevated in ISO-induced heart failure whereas SPRC treatment dose-dependently decreased CaMKII activation in wild type mice. Importantly, this action was abolished in CSE-/- mice, and is suggestive that, 1) a functional CSE protein is required to mediate the biological effects of SPRC, 2) SPRC influences tissue H2S levels and in turn H2S regulates CaMKII activity and, 3) induction of sulfhydration of CaMKII is cardioprotective in the current model. In addition, adding the results of KN93 together with ISO suggested that H2S-CaMKII pathway existed in in vivo conditions.
ISO treatment was also found to promote oxidative stress as assessed by measurement of lipid peroxidation and the expression of the antioxidant enzyme SOD. ROS production is known to influence cell stress signaling and viability and has been reported to play a role in heart failure. It is demonstrated herein that H2O2 a major ROS species [39], can induce oxidative stress and lead to the activation of CaMKII in vitro. H2O2 induced CaMKII activation and changes to the phosphorylation status of its downstream target protein PLN were observed in the current study. Interestingly, NaHS and SPRC could inhibit CaMKII activation and also suppress MDA production, and increase SOD activity. Separate lines of evidence from previous studies have also shown that loss of mitochondrial CaMKII reduced cardiomyocyte damage in ischemia reperfusion injury via influencing the rate of mPTP opening [13]. The opening of mPTP results in the depolarization of ΔΨm, the release of cytochrome c from mitochondria to cytoplasm, and the mitochondrial pathway of apoptosis [14]. In this study, a decrease in mitochondrial swelling and the preservation of ΔΨm were observed following the use of NaHS and SPRC in isolated mitochondria and in cultured H9c2 cardiomyocytes. Furthermore, mitochondrial dysfunction observed in the current heart failure model was found to be inhibited by SPRC treatment and that these cytoprotective effects were abolished in CaMKII siRNA-mediated knockout cells. Therefore, SPRC could suppress the mPTP opening to protect against mitochondrial dysfunction and apoptosis in a CaMKII-dependent manner.
We hypothesized that SPRC mediates its inhibitory effects towards CaMKII by influencing critical cysteine residue required for its activity. Key cysteine residues in CaMKII have been determined to play important roles in redox-based regulation of CaMKII activity [17], [27], [41]. CaMKII contains 10 cysteine residues and studies have shown that S-nitrosylation of CaMKIIα at cysteine-6 is essential for the inactivation of CaMKII [30]. In a similar fashion to that of NO, H2S has recently been shown to mediate post-translational modification of proteins via the process of S-sulfhydration [45]. Cysteines in protein can be oxidized to form reactive cysteines, including glutathiolated cysteine, cysteine sulfenic acid and S-nitrosylated cysteine, which have low pKa and a positive electrostatic potential. Later, the sulfide anion could react with reactive cysteines to form a cysteine persulfide [6], [7], [15], [43]. Exposure of cells and tissues to SPRC was found to increase the level of S-sulfhydrated-CaMKII in vivo and in vitro. This observation indicated that CaMKII might be a cellular target protein to the S-sulfhydrating effects of H2S. To further prove this hypothesis, SPRC and NaHS were studied in wild type and CSE-/- mice. NaHS is an inorganic salt and it can release H2S through hydrolytic action. Interestingly, SPRC failed to induce the S-sulfhydration of CaMKII in CSE-/- mice. This is in stark contrast to the donor NaHS that was found to increase the level of S-sulfhydrated CaMKII. Thus, it is demonstrated that SPRC requires a functional CSE protein to mediate some of its biological effects in relation to H2S. Indeed, SPRC was found to only protect against heart failure in wild type but not in CSE-/- mice. And the suppression of CaMKII activity was related with H2S level in heart tissue. Furthermore, the reactive cysteine could also be oxidized to form sulfenyl and disulfide bridge, which would be a new mechanism for H2S to regulate the activity of protein.
As gasotransmitters, NO and H2S have numerous biological functions in common [1], [37], [46]. It is known that eNOS pathway play a great role in cardioprotective effect, and overexpression of eNOS could suppress cardiac hypertrophy [22]. Moreover, study showed that NO could inhibit CaMKII activity in vivo and in vitro [30]. The present study provided new evidence that there is a crosstalk between H2S and NO in generation and they exert biological effects through the same pathways. This led us to hypothesize that cysteine-6, a known target for S-nitrosylation, could be a potential site for H2S mediated S-sulfhydration. In the absence of H2S, the activity of the mutant CaMKII (C6S) was indistinguishable from that of the wild type CaMKII, indicating that the mutation at cysteine-6 did not influence the activity of CaMKII. SPRC treatment significantly increased S-sulfhydration of wild type CaMKII and reduces its activity. In contrast, in the mutant CaMKII (C6S), in which S-sulfhydration is abolished, phosphorylation could still occur and activity was maintained. In addition, the cytoprotective role of SPRC was significantly reduced since the mutant CaMKII (C6S) was still functional. Therefore, S-sulfhydration at cysteine-6 appeared to be necessary for the inactivation of CaMKII.
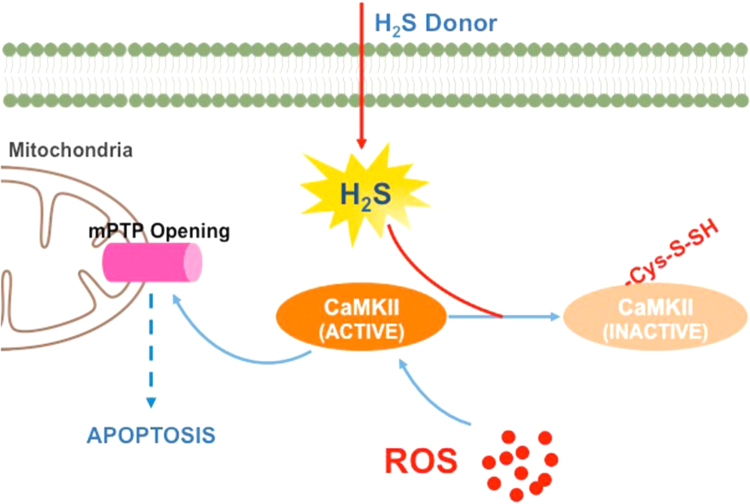
In conclusion, we demonstrated that H2S regulated the activation of CaMKII via S-sulfhydration at cysteine-6. The inhibition of CaMKII suppresses mitochondrial dysfunction and apoptosis and preserved cardiac function (Fig. 9). It must be noted that we cannot preclude that additional cysteine residue present on CaMKII are also susceptible to S-sulfhydrated by H2S and future work will hopefully explore this avenue of research. Ultimately, H2S-induced inactivation of CaMKII provides a new therapeutic strategy for the potential treatment of cardiovascular diseases.
Fig. 9.
Signaling pathway underlying the effects of H2S donor on CaMKII. Opening of mPTP induced by activated CaMKII results in mitochondrial dysfunction and apoptosis of myocardium in heart failure. H2S donor could release H2S in myocardium. The S-sulfhydration of CaMKII at cysteine-6 by H2S could restore the CaMKII activity and mitochondria dysfunction, to decrease apoptosis and oxidative stress in heart failure.
4. Innovation
H2S improves the cardiac function in the heart failure, however the cellular mechanisms for this remain largely unknown. In the current study, we found that [1] H2S protected against heart failure through inhibiting CaMKII excessive activation; [2] S-sulfhydrated CaMKII at cysteine 6 could suppress CaMKII phosphorylation; [3] S-sulfhydrated CaMKII inhibited mPTP opening and mitochondria dysfunction under oxidative stress. These results uncover a novel mechanism by which CaMKII is the molecular target of H2S and contributes to the heart failure therapeutic strategy.
5. Materials and methods
5.1. Mice and cell culture
Male C57BL/6 (6–8 weeks old) mice were maintained on standard conditions and free to receive food and water. The CSE-/- mice were a gift from Shanghai Research Center for Model Organisms. It is a conventional knockout model and has been published before [29]. All studies were performed 24 h after the last ISO administration. Mice were handled according to the Guide for the Care and Use of Laboratory Animals published by the National Research Council (United States) Committee. Experimental procedures were managed according to the local ethical committee of Fudan University. Animals were anaesthetized by isoflurane inhalations (2–3% for induction and 1.5–2% for maintenance). Animals were sacrificed by cervical dislocation under isoflurane anesthesia. Heart failure was induced by continuous subcutaneous injection of ISO (7.5 mg/kg/d) with or without KN93 (10 μM/kg/d), once a day for 4 weeks. S-propyl-L-cysteine (SPRC) (10, 25 mg/kg/d) was administered by gavage,D, L-propargylglycine (PAG) (37.5 mg/kg/d) and enalapril (20 mg/kg/d) by intraperitoneal injection, once a day for 4 weeks. The vehicle group received 0.2 ml normal saline by gavage, once a day for 4 weeks.
H9c2 cardiomyocytes (American Type Culture Collection) were cultured in complete Dulbecco's minimum essential medium (DMEM) (Gibco) supplemented with 10% fetal bovine serum (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco) in 5% CO2 at 37 °C. 150 μmol/L H2O2 was used to induce oxidative stress. NaHS and SPRC were added 4 h before H2O2 treatment.
The plasmids pAdeno-mCMv-CaMKII mutant and wild type were purchased from Obio Technology. The oligonucleotides used for mutagenesis were 5′-ATGATGACGACAAAGGATCCATGGCTTCGACCACCACCAGCACCCGGTTCACCGAC-3′ and 5′-TGTTCCAGACGCGGTCTA-3′ for cysteine-6. The altered nucleotides were inclined and underlined. The site-directed mutants were confirmed by DNA sequencing and termed as the mutant CaMKII (C6S). And then, the CaMKII mutant and wild type were constructed to recombinant adenoviruses. Concentration of the two viruses was determined to be at 1 × 1011 viral particles per ml.
5.2. Chemicals and antibodies
The following antibodies were used: anti-CaMKIIδ antibody (Santa Cruz Biotechnology) and anti-p-CaMKII (Thr286) antibody (Cell Signaling Technology); anti-ox-CaMKII (Met281/282) antibody (Millipore); anti-eNOS antibody (Cell Signaling Technology) and anti-p-eNOS (Ser1177) antibody (Cell Signaling Technology); anti-ATPase antibody (Cell Signaling Technology); anti-CSE antibody (Santa Cruz Biotechnology); anti-PLN antibody and anti-p-PLN (Thr17) antibody (Santa Cruz Biotechnology); anti-Bcl-2 antibody and anti-Bax antibody (Cell Signaling Technology); anti-Caspase 9 antibody (Cell Signaling Technology).
All drugs were purchased from Sigma unless otherwise stated.
5.3. Echocardiograph and cardiac parameters
After last ISO administration, echocardiograph was performed using Vevo770 (VisualSonics) and a 30 MHz transducer. Mice were anesthetized by isoflurane. The following parameters were measured from LV M-Mode tracings: left ventricular internal diameter-systole, left ventricular internal diameter-diastole, left ventricular anterior wall systole and left ventricular posterior wall systole. The other parameters were calculated from LV M-Mode derived dimensions: left ventricular end-systolic volume, left ventricular end-diastolic volume, ejection fraction and fraction shortening.
5.4. Concentration of hydrogen sulfide
The concentrations of H2S in plasma and heart tissue were measured using the modified monobromobimane (MBB) method as described previously [4], [28]. In brief, plasma was collected 24 h after last ISO administrating followed by centrifugation (3000 r/min, 30 min). And heart tissues were lysed in the lysis buffer (Pierce). 30 μl sample was mixed with 10 μl Tris-HCl (200 mmol/L, pH 8.5) and 70 μl MBB solution (3.5 mmol/L) at room temperature for 1 h. The reaction product sulfide dibimane was analyzed with HPLC system.
5.5. Histopathological observation
The isolated hearts were fixed in 10% formaldehyde solution and embedded in paraffin. Masson trichrome staining kit (Yuanye Biotech) was used to determine myocardial fibrosis according the manufacture's protocol. Images were taken using the microscope (Zeiss) and collagen content was calculated by IPP software.
5.6. Western blot
Homogenized tissue and H9c2 cardiomyocytes were lysed in the lysis buffer (Pierce). Lysates were used for Western Blot and immunoprecipitation as previously described [40]. Proteins were separated with SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blocked with 5% non-fat milk (0.1% Tween-20) for 1 h at room temperature and incubated with antibodies at 4 °C overnight. After washing with TBST (0.1% Tween-20), the membranes were incubated with an HRP-conjugated secondary antibody and detected with ECL (Millipore). GAPDH and β-actin were used as loading control. The quantitative results of protein level were analyzed by Alpha Ease software.
5.7. Mitochondrial ΔΨm measurement
H9c2 cardiomyocytes were loaded with JC-1 following manufacture's protocol (Beyotime). More details can be found in the Supplementary information.
5.8. ATP level, MDA level and SOD activity
The ATP level of H9c2 cells was measured with ATP Assay Kit (Beyotime) according to the manufacturer's protocol.
The MDA level and SOD activity of heart tissue were measured with MDA Assay Kit (Beyotime) and SOD Assay kit (Beyotime) respectively, according to the manufacturer's protocol.
5.9. TUNEL assay
Apoptosis in mouse myocardium was determined using TUNEL assay kit (Yeasen) according to the manufacturer's protocol. Samples were viewed in a dark room with fluorescence microscope (Zeiss). Images were taken from 3 randomly selected fields and the apoptotic cells were counted in each well to determine the average cell count.
5.10. Analysis of apoptosis
The activity of caspase-3 and caspase-9 in H9c2 cardiomyocytes were measured with caspase-3 fluorometric assay kit (BioVison) or caspase-9 fluorometric assay kit (BioVison) according to the manufacturer's protocol.
Apoptotic cells were also identified by staining with Hoechst 33258 (Beyotime) in H9c2 cells. Samples were viewed in a darkness room with fluorescence microscope (Zeiss). Images were taken from 3 randomly selected fields and the apoptotic cells were counted in each well to determine the average.
5.11. Mitochondrial isolation
Isolation of mitochondria from H9c2 cardiomyocytes was performed with Mitochondria Isolation Kit (Thermo Scientific) according the manufacturers’ protocol.
5.12. Measurement of intercellular Ca2+ ([Ca2+]i)
H9c2 cardiomyocytes were loaded with 5 μmol/L fluo-3/AM for 1 h at 37 °C. The fluorescence intensity was detected using the microplate reader with the excitation wavelength at 488 nm and the emission at 530 nm. The maximum intracellular Ca2+ elevation was calculated by (Fmax-F0)/F0 × 100%.
5.13. RNA interference
H9c2 cardiomyocytes were plated in DMEM with 10% FBS for 24 h in 6 wells plates. The culture medium was replaced by Opti-MEM (Invitrogen) without serum, then siRNA (50 nmol/L per well) was transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol for 48–72 h. Control siRNA containing a scrambled sequence was used as a negative control.
5.14. S-sulfhydration assay
The assay was performed as described previously [27]. In brief, cells were homogenized in HEN buffer (250 mmol/L Hepes-NaOH (pH 7.7), 1 mmol/L EDTA, and 0.1 mmol/L neocuproine) supplemented with 100 μmol/L deferoxamine and centrifuged at 13,000 r/min for 30 min at 4 °C. Cell lysates were added to blocking buffer (HEN buffer adjusted to 2.5% SDS and 20 mmol/L MMTS) at 50 °C for 20 min with frequent vortex. The MMTS was then removed by acetone and the proteins were precipitated at − 20 °C for 20 min. After acetone removal, the proteins were resuspended in HENS buffer (HEN buffer adjusted to 1% SDS). 4 mmol/L biotin-HPDP in dimethyl sulfoxide was added to the suspension without ascorbic acid. After incubation for 3 h at 25 °C, biotinylated proteins were precipitated by streptavidin-agarose beads, which were then washed with HENS buffer. The biotinylated proteins were eluted by SDS-PAGE sample buffer. Finally, the S-sulfhydration CaMKII was analyzed by Western Blot with anti-CaMKII antibody.
5.15. Statistical analysis
Statistical analysis was done using analysis of variance (ANOVA or Student's test). Post hoc pairwise comparisons were performed using GraphPad Prism. All the data were presented as means ± SEM. A p value < 0.05 was considered statistically significant.
Acknowledgments
We thank Drs. Xiaoyan Shen, Xinhua Liu and Lilong Pan for their technical assistance during the course of these studies.
Acknowledgments
Source of funding
This work was supported by the Key Laboratory Program of the Education Commission of Shanghai Municipality (ZDSYS14005); the National Natural Science Foundation of China (81703499); the Shanghai Sailing Program (16YF1410400); Shanghai Chenguang Program (17CG13).
Disclosures
None.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2018.08.008.
Contributor Information
Deqiu Zhu, Email: zdq_0726@163.com.
Yi Zhun Zhu, Email: yzzhu@must.edu.mo.
Appendix A. Supplementary material
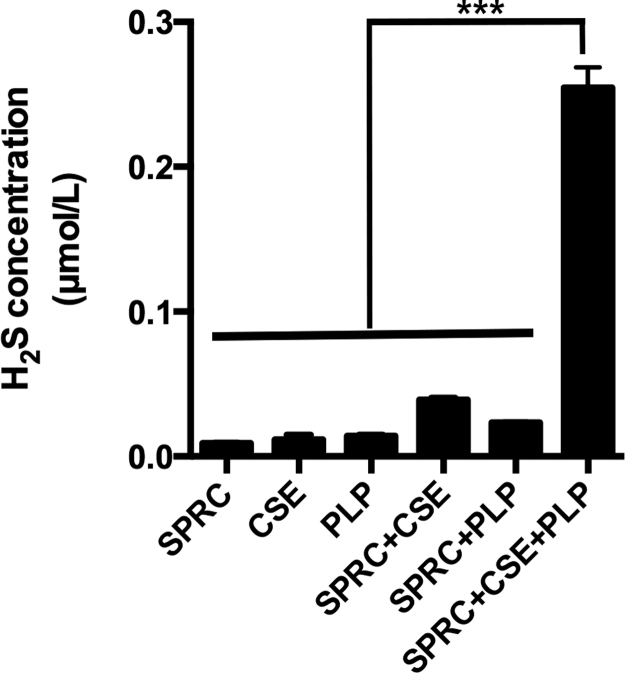
Fig. S1.
SPRC could release H2S depending on CSE. CSE is a H2S generating enzyme relies on pyridoxal 5′-phosphate (PLP). In cell free buffer, SPRC could release H2S only in the presence of CSE and PLP. Data represent means ± SEM, n = 6 for each group.
Fig. S2.
SPRC had protective effect against heart failure. (A) Administration of H2S donor SPRC (25 mg/kg/d) increased survival rate of mice compared with isoprenaline (ISO)-treated. n = 30. (B) H&E staining of sections from the left ventricular myocardium of ISO-induced heart failure mice subjected to SPRC, enalapril or PAG for 4 weeks.
Fig. S3.
H2S donor protected against oxidative stress and mitochondrial dysfunction in heart failure. (A-B) Activities of SOD and contents of MDA in left ventricular tissues of different groups treated with SPRC-L (10 mg/kg/d) or SPRC-H (25 mg/kg/d) with or without PAG in ISO-induced heart failure mice for 4 weeks. (C) The concentration of ATP in ISO-induced heart failure mice treated with SPRC or PAG for 4 weeks.
Fig. S4.
SPRC could not improve heart tissue injury induced by ISO in CSE-/- mice. (A) H&E staining of sections from the left ventricular myocardium with indicated treatments for 4 weeks in CSE-/- mice. (B) Masson trichrome stain showed fibrosis of the left ventricular endocardium in each group.
Fig. S5.
H2S donor regulated CaMKII activity to protect against oxidative stress-induced cell death. (A and B) After H2O2 treatment with or without H2S donor, the cell viability and the number of apoptotic cells were measured.
Fig. S6.
CaMKII (C6S) mutation did not change the activity of CaMKII. After transfected with mutation or wild type plasmid for 48 h, the protein were extracted and subjected to Western Blot.
References
- 1.Altaany Z., Ju Y., Yang G., Wang R. The coordination of S-sulfhydration, S-nitrosylation, and phosphorylation of endothelial nitric oxide synthase by hydrogen sulfide. Sci. Signal. 2014;7:ra87. doi: 10.1126/scisignal.2005478. [DOI] [PubMed] [Google Scholar]
- 2.Ariely R., Evans K., Mills T. Heart failure in China: a review of the literature. Drugs. 2013;73:689–701. doi: 10.1007/s40265-013-0057-8. [DOI] [PubMed] [Google Scholar]
- 3.Atkins B.Z., Hashmi Z.A., Ganapathi A.M., Harrison J.K., Hughes G.C., Rogers J.G., Milano C.A. Surgical correction of aortic valve insufficiency after left ventricular assist device implantation. J. Thorac. Cardiovasc. Surg. 2013;146:1247–1252. doi: 10.1016/j.jtcvs.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Bir S.C., Kolluru G.K., McCarthy P., Shen X., Pardue S., Pattillo C.B., Kevil C.G. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J. Am. Heart Assoc. 2012;1:e004093. doi: 10.1161/JAHA.112.004093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Modis K., Panopoulos P., Asimakopoulou A., Gero D., Sharina I., Martin E., Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. USA. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filipovic M.R. Persulfidation (S-sulfhydration) and H2S. Handb. Exp. Pharmacol. 2015;230:31. doi: 10.1007/978-3-319-18144-8_2. [DOI] [PubMed] [Google Scholar]
- 7.Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci. Signal. 2012;13:pe10. doi: 10.1126/scisignal.2002943. [DOI] [PubMed] [Google Scholar]
- 8.Gomez L., Paillard M., Thibault H., Derumeaux G., Ovize M. Inhibition of GSK3 beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- 9.Grambow E., Mueller-Graf F., Delyagina E., Frank M., Kuhla A., Vollmar B. Effect of the hydrogen sulfide donor GYY4137 on platelet activation and microvascular thrombus formation in mice. Platelets. 2014;25:166–174. doi: 10.3109/09537104.2013.786823. [DOI] [PubMed] [Google Scholar]
- 10.Grimm M., Brown J.H. Beta-adrenergic receptor signaling in the heart: role of CaMKII. J. Mol. Cell Cardiol. 2010;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Handy D.E., Loscalzo J. Redox regulation of mitochondrial function. Antioxid. Redox Signal. 2012;16:1323–1367. doi: 10.1089/ars.2011.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausenloy D.J., Maddock H.L., Baxter G.F., Yellon D.M. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning? Cardiovasc. Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 13.Joiner M.L., Koval O.M., Li J., He B.J., Allamargot C., Gao Z., Luczak E.D., Hall D.D., Fink B.D., Chen B., Yang J., Moore S.A., Scholz T.D., Strack S., Mohler P.J., Sivitz W.I., Song L.S., Anderson M.E. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joza N., Susin S.A., Daugas E., Stanford W.L., Cho S.K., Li C.Y., Sasaki T., Elia A.J., Cheng H.-Y.M., Ravagnan L. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- 15.Kabil O., Banerjee R. Redox biochemistry of hydrogen sulfide. J. Biol. Chem. 2010;285:21903–21907. doi: 10.1074/jbc.R110.128363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kan J., Guo W., Huang C., Bao G., Zhu Y., Zhu Y.Z. S-propargyl-cysteine, a novel water-soluble modulator of endogenous hydrogen sulfide, promotes angiogenesis through activation of signal transducer and activator of transcription 3. Antioxid. Redox Signal. 2014;20:2303–2316. doi: 10.1089/ars.2013.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishnan N., Fu C., Pappin D.J., Tonks N.K. H2S-Induced sulfhydration of the phosphatase PTP1B and its role in the endoplasmic reticulum stress response. Sci. Signal. 2011;4:ra86. doi: 10.1126/scisignal.2002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y.H., Lu M., Xie Z.Z., Hua F., Xie L., Gao J.H., Koh Y.H., Bian J.S. Hydrogen sulfide prevents heart failure development via inhibition of renin release from mast cells in isoproterenol-treated rats. Antioxid. Redox Signal. 2014;20:759–769. doi: 10.1089/ars.2012.4888. [DOI] [PubMed] [Google Scholar]
- 19.Mani S., Untereiner A., Wu L.Y., Wang R. Hydrogen sulfide and the pathogenesis of atherosclerosis. Antioxid. Redox Signal. 2014;20:805–817. doi: 10.1089/ars.2013.5324. [DOI] [PubMed] [Google Scholar]
- 20.Mustafa A.K., Sikka G., Gazi S.K., Steppan J., Jung S.M., Bhunia A.K., Barodka V.M., Gazi F.K., Barrow R.K., Wang R., Amzel L.M., Berkowitz D.E., Snyder S.H. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ. Res. 2011;109:1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nembhard W.N., Pathak E.B., Schocken D.D. Racial/ethnic disparities in mortality related to congenital heart defects AMONG children and adults in the United states. Ethn. Dis. 2008;18:442–449. [PubMed] [Google Scholar]
- 22.M. Ozaki, S. Kawashima, T. Fau - Yamashita, T. Yamashita, T. Fau - Hirase, T. Hirase, Y. Fau - Ohashi, Y. Ohashi, N. Fau - Inoue, N. Inoue, K.-i. Fau - Hirata, K. Hirata, M. Fau - Yokoyama, M. Yokoyama Overexpression of Endothelial Nitric Oxide Synthase Attenuates Cardiac Hypertrophy Induced by Chronic Isoproterenol infusion. [DOI] [PubMed]
- 23.Pan L.L., Liu X.H., Gong Q.H., Zhu Y.Z. S-Propargyl-cysteine (SPRC) attenuated lipopolysaccharide-induced inflammatory response in H9c2 cells involved in a hydrogen sulfide-dependent mechanism. Amino Acids. 2011;41:205–215. doi: 10.1007/s00726-011-0834-1. [DOI] [PubMed] [Google Scholar]
- 24.Polhemus D.J., Kondo K., Bhushan S., Bir S.C., Kevil C.G., Murohara T., Lefer D.J., Calvert J.W. Hydrogen sulfide attenuates cardiac dysfunction after heart failure via induction of angiogenesis. Circ. Heart Fail. 2013;6:1077–1086. doi: 10.1161/CIRCHEARTFAILURE.113.000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polhemus D.J., Lefer D.J. Emergence of hydrogen sulfide as an endogenous gaseous signaling molecule in cardiovascular disease. Circ. Res. 2014;114:730–737. doi: 10.1161/CIRCRESAHA.114.300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rokita A.G., Anderson M.E. New therapeutic targets in cardiology arrhythmias and Ca2+/calmodulin-dependent kinase II (CaMKII) Circulation. 2012;126:2125–2139. doi: 10.1161/CIRCULATIONAHA.112.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sen N., Paul B.D., Gadalla M.M., Mustafa A.K., Sen T., Xu R., Kim S., Snyder S.H. Hydrogen sulfide-linked sulfhydration of NF-kappaB mediates its antiapoptotic actions. Mol. Cell. 2012;45:13–24. doi: 10.1016/j.molcel.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen X., Pattillo C.B., Pardue S., Bir S.C., Wang R., Kevil C.G. Measurement of plasma hydrogen sulfide in vivo and in vitro. Free Radic. Biol. Med. 2011;50:1021–1031. doi: 10.1016/j.freeradbiomed.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen Y., Shen Z., Miao L., Xin X., Lin S., Zhu Y., Guo W., Zhu Y.Z. miRNA-30 family inhibition protects against cardiac ischemic injury by regulating cystathionine-gamma-lyase expression. Antioxid. Redox Signal. 2015;22:224–240. doi: 10.1089/ars.2014.5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Song T., Hatano N., Kambe T., Miyamoto Y., Ihara H., Yamamoto H., Sugimoto K., Kume K., Yamaguchi F., Tokuda M., Watanabe Y. Nitric oxide-mediated modulation of calcium/calmodulin-dependent protein kinase II. Biochem. J. 2008;412:223–231. doi: 10.1042/BJ20071195. [DOI] [PubMed] [Google Scholar]
- 31.Sun Y., Tian Y., Prabha M., Liu D., Chen S., Zhang R.Y., Liu X.Q., Tang C.S., Tang X.Y., Jin H.F., Du J.B. Effects of sulfur dioxide on hypoxic pulmonary vascular structural remodeling. Lab. Investig. 2010;90:68–82. doi: 10.1038/labinvest.2009.102. [DOI] [PubMed] [Google Scholar]
- 32.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat. Rev. Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 33.Toko H., Takahashi H., Kayama Y., Oka T., Minamino T., Okada S., Morimoto S., Zhan D.Y., Terasaki F., Anderson M.E., Inoue M., Yao A., Nagai R., Kitaura Y., Sasaguri T., Komuro I. Ca2+/Calmodulin-dependent kinase II delta causes heart failure by accumulation of p53 in dilated cardiomyopathy. Circulation. 2010;122:891–899. doi: 10.1161/CIRCULATIONAHA.109.935296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandiver M.S., Paul B.D., Xu R.S., Karuppagounder S., Rao F., Snowman A.M., Ko H.S., Il Lee Y., Dawson V.L., Dawson T.M., Sen N., Snyder S.H. Sulfhydration mediates neuroprotective actions of parkin. Nat. Commun. 2013;4 doi: 10.1038/ncomms2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wagner S., Ruff H.M., Weber S.L., Bellmann S., Sowa T., Schulte T., Anderson M.E., Grandi E., Bers D.M., Backs J., Belardinelli L., Maier L.S. Reactive oxygen species-activated Ca/calmodulin kinase IIdelta is required for late I(Na) augmentation leading to cellular Na and Ca overload. Circ. Res. 2011;108:555–565. doi: 10.1161/CIRCRESAHA.110.221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Q., Wang X.-L., Liu H.-R., Rose P., Zhu Y.-Z. Protective effects of cysteine analogues on acute myocardial ischemia: novel modulators of endogenous H2S production. Antioxid. Redox Signal. 2010;12:1155–1165. doi: 10.1089/ars.2009.2947. [DOI] [PubMed] [Google Scholar]
- 37.Wang R. Two's company, three'sa crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 38.WHO . World Health Organization; Geneva: 2014. The 10 Leading Causes of Death in the World, 2000 and 2012. [Google Scholar]
- 39.Whalen E.J., Foster M.W., Matsumoto A., Ozawa K., Violin J.D., Que L.G., Nelson C.D., Benhar M., Keys J.R., Rockman H.A., Koch W.J., Daaka Y., Lefkowitz R.J., Stamler J.S. Regulation of beta-adrenergic receptor signaling by S-nitrosylation of G-protein-coupled receptor kinase 2. Cell. 2007;129:511–522. doi: 10.1016/j.cell.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 40.Wu D., Hu Q., Liu X., Pan L., Xiong Q., Zhu Y.Z. Hydrogen sulfide protects against apoptosis under oxidative stress through SIRT1 pathway in H9c2 cardiomyocytes. Nitric Oxide. 2014 doi: 10.1016/j.niox.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 41.Xie Z.Z., Shi M.M., Xie L., Wu Z.Y., Li G., Hua F., Bian J.S. Sulfhydration of p66Shc at Cysteine59 mediates the antioxidant effect of hydrogen Sulfide. Antioxid. Redox Signal. 2014;21:2531–2542. doi: 10.1089/ars.2013.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xun Ai J.W.C., Thomas R. Shannon, Bers Donald M., Steven M. Ca2+/Calmodulin-dependent protein kinase modulates cardiac Ryanodine failure receptor phosphorylation and sarcoplasmic reticulum Ca 2+ leak in heart. Circ. Res. 2005 doi: 10.1161/01.RES.0000194329.41863.89. [DOI] [PubMed] [Google Scholar]
- 43.Yadav P.K., Martinov M., Vitvitsky V., Seravalli J., Wedmann R., Filipovic M.R., Banerjee R. Biosynthesis and reactivity of cysteine persulfides in signaling. J. Am. Chem. Soc. 2016;138:289–299. doi: 10.1021/jacs.5b10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang G., Wu L., Jiang B., Yang W., Qi J., Cao K., Meng Q., Mustafa A.K., Mu W., Zhang S. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang G., Zhao K., Ju Y., Mani S., Cao Q., Puukila S., Khaper N., Wu L., Wang R. Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid. Redox Signal. 2013;18:1906–1919. doi: 10.1089/ars.2012.4645. [DOI] [PubMed] [Google Scholar]
- 46.Yong Q.C., Cheong J.L., Hua F., Deng L.W., Khoo Y.M., Lee H.S., Perry A., Wood M., Whiteman M., Bian J.S. Regulation of heart function by endogenous gaseous mediators-crosstalk between nitric oxide and hydrogen sulfide. Antioxid. Redox Signal. 2011;14:2081–2091. doi: 10.1089/ars.2010.3572. [DOI] [PubMed] [Google Scholar]
- 47.Zhao K., Ju Y., Li S., Altaany Z., Wang R., Yang G. S-sulfhydration of MEK1 leads to PARP-1 activation and DNA damage repair. EMBO Rep. 2014;15:792–800. doi: 10.1002/embr.201338213. [DOI] [PMC free article] [PubMed] [Google Scholar]