Introduction: A key challenge in designing tissue repair strategies is knowing whether and how developmental mechanisms are used for successful repair of mature/adult tissues. Although it is known that developmental components are used in repair, it remains mostly unclear which ones are required and whether they act similarly as during development. This issue is further complicated by the fact that it is difficult to assess the similarities and differences between development and the repair of mature tissues, since the two contexts are highly dissimilar. A potentially useful yet underutilized approach is to understand developmental regrowth (defined here as the ability to compensate for missing tissues by restoring normal organ structures and function). An ideal model would have two key features: repair capacity in the organ of interest during development, and well-understood developmental mechanisms. This approach reduces the complexity of comparing mature repair processes to developmental ones. The African clawed frog, Xenopus laevis, has a high capacity to restore lost body structures, including the eye (Beck et al., 2009). It can regenerate the retina and lens after injury (reviewed in Barbosa-Sabanero et al. (2012) and Tseng (2017)), and Xenopus eye development is well characterized with known mechanisms (Viczian and Zuber, 2015).
We recently established an embryonic model of eye regrowth using Xenopus laevis. Our study showed that the developmental stage (st.) 27 tailbud embryo regrows its eye after tissue ablation (Kha et al., 2018). Regrowth is completed by 5 days and does not interrupt overall development. The regrown and age-appropriate eye contains the normal complement of eye structures and retinal cell types, connects to the brain via the optic nerve, and demonstrates visual preference. As this is a new model, here we further define this system by assessing the functionality of the regrown eye and determining whether the ability to regrow the eye shows age dependency.
Methods: Xenopus laevis embryos were obtained via in vitro fertilization and were raised in 0.1× Marc's Modified Ringer's (MMR) medium. The eye surgery and regrowth assay were performed as described (Kha et al., 2018).
For chemical treatments, concentrations of 28 µM of M50054 and 40 µM of NS3694 (Millipore, EMD Biosciences, Burlington, MA, USA) were used. For vehicle control, dimethyl sulfoxide (DMSO) treatment was used. After tissue removal surgery at st. 27, embryos were allowed to recover and then transferred to 0.1× MMR medium containing the chemical for 24 hours. Embryos were then washed with two changes of MMR and eye regrowth was assayed by 6 days post surgery (dps). To examine visual function, the visual preference assay was performed as previously described (Kha et al., 2018). The time that tadpoles spent in each colored background was measured within a two-minute time frame (n = 12 per condition). All sets of tadpoles were tested for two consecutive days. The results were similar for both days. For each condition, the average percentage of time spent in a white color background was calculated. Comparison of conditions was analyzed using a Student's t-test.
For eye regrowth assays, embryos at the following stages: 27, 28, 29–30, 31, 32, and 33–34 (Nieuwkoop and Faber, 1994), were anaesthetized prior to surgery with tricaine (n > 60 per stage(s)). Tissue removal surgery was performed using fine surgical forceps (Dumont No. 5). After surgery, embryos were allowed to recover, and then cultured at 22°C for 5 days. The contralateral eye of the embryo served as an internal control. Regrowth in operated eyes as compared to control unoperated eyes of sibling embryos were assessed using the Regrowth Index (RI) (Kha et al., 2018). The RI ranges from 0 to 300, where 0 indicates no regrowth in all embryos of a given condition and 300 indicates full regrowth in all embryos. Raw data from scoring was used to compare eye regrowth experiments. Comparison of regrowth under two different conditions was analyzed with Mann-Whitney U test for ordinal data with tied ranks, using normal approximation for large sample sizes.
Restoration of functionality in eye regrowth: When given the choice, young Xenopus tadpoles display a robust and consistent preference for swimming in a white background over a black background (Moriya et al., 1996). Blind tadpoles lose this ability for background preference. This natural visual preference has been used as an effective and efficient assay to investigate the visual response of tadpole eyes (Viczian and Zuber, 2014; Kha et al., 2018). In our previous work, we demonstrated that that tadpoles with only a single regrown eye showed strong preference to swim in the white background, similar to control tadpoles with only one unoperated eye (Kha et al., 2018). While the regrown eye showed robust background color preference, this tadpole behavior could potentially be due to contributions from the small proportion of st. 27 eye cells that remain after surgery. This is because surgery removes approximately 83% of eye cells on average (Kha et al., 2018). To confirm that functional recovery is due to the regrown tissues, and not pre-existing cells, it is crucial to show that visual behavior is restored in animals with eye regrowth as compared to eyes that are inhibited from regrowing.
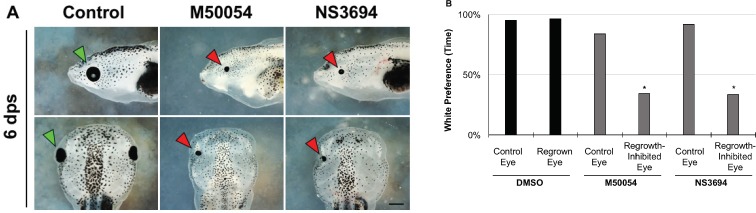
To assess whether remnant st. 27 eye cells could contribute to the white preference behavior shown by tadpoles with only one regrown eye, we used chemical methods to inhibit eye regrowth. We previously showed that eye regrowth required apoptosis (programmed cell death) (Kha et al., 2018). At 6 dps, a regrown eye is of similar size and structure to an uninjured eye (Figure 1A bottom left panel, compare regrown eye on the left side – as indicated by green arrowhead – to the uninjured eye on the contralateral right side). Chemical inhibition of apoptosis using either M50054 or NS3694 blocked eye regrowth resulting in the formation of small eyes (Figure 1A, compare control regrown eye (green arrowhead) to eyes treated with M50054 or NS3694 (red arrowheads)). Using the behavioral assay, we examined the visual preference of tadpoles carrying only one growth-inhibited eye. First, we performed surgeries to remove the left eye tissues at st. 27. The embryos were then treated with either vehicle only (DMSO) or with an apoptosis inhibitor. In order to test the tadpole visual preference of the operated eye, the uninjured contralateral (right) eye at st. 40 was removed (Figure 1A, bottom row: middle and right panels). (Eye removal at st. 40 does not induce replacement.) Six days after initial surgery, we performed the visual behavior assay.
Figure 1.
Restoration of visual function requires eye regrowth.
(A) Representative images showing effects on eye regrowth after apoptosis inhibitor treatments (M50054 or NS3694) at 6 days post surgery (dps) as compared to a DMSO-vehicle control (n = 12 per condition). Green arrowhead indicates a DMSO-treated regrowing eye. Red arrowheads indicate regrowth-inhibited eyes. To assay for visual function of a single eye, the right contralateral eye was removed for all conditions (shown in bottom middle and right panels). Top panels: dorsal is up, and anterior is to the left. Bottom panels: anterior is up. Scale bar: 500 µm. (B) DMSO-treated tadpoles with either one normal or one regrown eye display white background preference. Tadpoles with an uninjured eye treated with an apoptosis inhibitor also showed white background preference (n = 12 per condition, P> 0.05). Tadpoles with a regrowth-inhibited eye resulting from treatment with an apoptosis inhibitor lost white background preference (n = 12 per condition, *P < 0.01).
Consistent with our previous results, tadpoles with one regrown eye strongly preferred to swim in the white background, similar to control tadpoles with only one unoperated eye (Figure 1B, n = 12 per condition, P > 0.05) (Kha et al., 2018). As a second control, we also examined the uninjured eyes of tadpoles that were treated with an apoptosis inhibitor, M50054 or NS3694, for 24 hours after st. 27 (the normal course of treatment to successfully inhibit eye regrowth). There were no apparent morphological differences in the apoptosis inhibitor-treated eyes as compared to their age-matched siblings. These tadpoles showed a similar preference to swim in a white background as compared to those with a normal eye (n = 12, P > 0.05 for each inhibitor treatment as compared to control). In contrast, tadpoles with a single regrowth-inhibited eye showed a loss of preference for swimming in a white background as compared to those with a DMSO-treated regrown eye (Figure 1, n = 12 per condition, P < 0.01). Their behavior was similar to blind tadpoles. Together, our data showed that regrowth-inhibited eyes lack function when assessed using the visual preference assay. This indicated that presence of remnant eye cells is not sufficient to restore visual preference in tadpoles. Thus the restoration of visual function via eye regrowth is due to the regrown tissues.
Decline of eye regrowth ability with increasing age: Xenopus exhibits age-dependency in its regenerative abilities (Beck et al., 2009). This is a valuable and useful feature for studying mechanisms of regeneration as it allows for direct comparison and understanding of regenerative versus non-regenerative states within a single organism. For example, Xenopus tadpoles show decreased limb regeneration capacity as they near metamorphosis (Muneoka et al., 1986). We sought to examine whether eye regrowth ability changes with age.
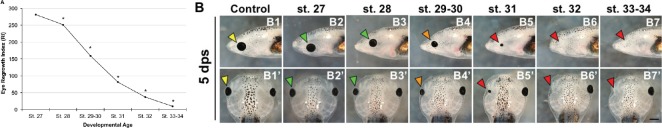
During Xenopus eye development, retinal histogenesis begins at st. 24 and is completed 2 days later at st. 41 (Holt et al., 1988). At st. 24, > 95% of the retinal cell population is mitotic. The proportion of mitotic cells in the developing eye gradually decreases, such that by st. 37/38, only about 5% of the retinal cells remain mitotic (Holt et al., 1988). We have shown that the tailbud embryo at st. 27 has robust and rapid eye regrowth ability (Kha et al., 2018). To determine whether eye regrowth ability is retained throughout development, we performed removal surgeries and assessed eye regrowth from st. 28 to 34 using the eye Regrowth Index (RI) (Kha et al., 2018). Our data showed a slight decline in Xenopus eye regrowth ability at st. 28 as compared to st. 27 (Figure 2A; st. 27 RI = 281, st. 28 RI = 251, n > 60, P < 0.01). In particular, 87% of st. 27 embryos regrew an eye that was morphologically indistinguishable from a corresponding normal eye at the same stage, and contained the expected structures including the lens, retina, and retinal pigmented epithelium (Figure 2A, compare control normal uninjured eye shown in Figure 2B1 and B1’, to st. 27 regrown eye shown in Figure 2B2 and B2’). Indicative of the decline in RI, only 66% of st. 28 embryos showed the ability for full eye regrowth (Figure 2B3 and B3’).
Figure 2.
Eye regrowth declines with increasing age.
The Regrowth Index (RI) is a measure of eye regrowth quality (Kha et al., 2018). Each regrown eye is assigned to one of four phenotypes: full (comparable to an unoperated control eye), partial (minor abnormalities with some reduction in size), weak (no lens and small size), and none (no regrowth). To obtain the RI value, the number of tadpoles in each category is multiplied by: 3 (full), 2 (partial), 1 (weak), or 0 (none), added together, and then divided by total number of tadpoles. A value of 300 denotes full regrowth in all individuals in the group whereas a value of 0 denotes no regrowth in all individuals. (A) At 5 days post surgery (dps): developmental stage. 27, RI = 281 (n> 100); st. 28 RI = 251 (n = 67); st. 29–30, RI = 159 (n = 142); st. 31, RI = 82 (n = 106); st. 32, RI = 37 (n =113); and st. 33–34, RI = 10 (n = 70). *P < 0.01, vs. st. 27. (B) Representative images showing resulting eyes at 5 dps. Corresponding top and bottom panel show side and top views of the same tadpole. (B1, B1’) Unoperated control eye indicated by yellow arrowheads. (B2, B2’) st. 27. (B3, B3’) st. 28. (B4, B4’) st. 29–30. (B5, B5’) st. 31. (B6, B6’) st. 32. (B7, B7’) st. 33–34. Green arrowheads: Full regrown eyes; orange arrowheads: partial eye regrowth; red arrowheads: no regrowth. Top panels: dorsal is up and anterior is to the left. Bottom panels: anterior is up. Scale bar: 500 µm.
There was a significantly greater decrease in regrowth ability during st. 29–30 (Figure 2A; RI = 159, n > 60, P < 0.01 when compared to st. 27). During these two stages, only 25% of embryos were capable of full eye regrowth (Figure 2B4 and B4’). Eye regrowth ability further declines with increasing developmental age. By st. 33–34, the RI has declined to 8 (n > 60, P < 0.01 when compared to st. 27). We also found that the ability for full eye regrowth is lost starting at st. 31; the regrown eyes are smaller and do not reach comparable size to normal, uninjured eye (Figure 2B5 and B5’). Together, our data showed that eye regrowth ability decreases with increasing developmental age. Furthermore, eye regrowth ability is mostly absent by st. 32 (Figure 2A and B6–B7’).
These findings are consistent with previous reports that removal of the late tailbud embryo eye at st. 33 failed to induce regrowth (Sedohara et al., 2003). However, it is somewhat surprising that the regrowth ability declined significantly by st. 31 (Figure 2), given that greater than 50% of retinal cell population in the uninjured embryonic eye remain mitotic at this time (Holt et al., 1988). Retinal histogenesis occurs within a short time window of approximately 2 days, as the optic vesicle transforms from being composed of mitotic retinal progenitor cells to a mature well-patterned eye containing the same structures found in the adult frog. During Xenopus eye regrowth, retinogenesis is delayed for over 24 hours but rapidly catches up to form a well-patterned and age-appropriate eye by 3 days, when overall development has reached st. 42 (Kha et al., 2018).
There are potential explanations for the loss of eye regrowth ability. During normal eye development, neural retina layer formation begins at st. 33/34 even as a third of retinal cells remain mitotic during that time (Holt et al., 1988). It could be that retinal layer formation changes the environment and properties of the retinal progenitor cells such that they are no longer able to respond and productively restore tissues after loss. Alternatively, there could be a developmental clock that regulates the timing of the initiation of retinal histogenesis. During eye regrowth, reparative retinal histogenesis is largely completed within 2 days, a similar time window as in the normally developing animal (Kha et al., 2018). Past a certain developmental stage (for example, st. 31), it is possible that retinal histogenesis could not be completed within the requisite time window and thus regrowth ability is lost. The decline of repair capacity with increasing age occurs in a number of animals, including humans, but remains little understood. Further studies will help to elucidate the mechanisms that regulate eye regrowth ability in Xenopus.
Conclusion and future perspectives: Xenopus laevis has unique advantages as a well-characterized developmental and regenerative model system. Furthermore, it displays age-dependent regenerative and regrowth abilities, facilitating the understanding of differences between endogenous regenerative and non-regenerative states. Our embryonic eye regrowth model can serve as a productive foundation for enabling an efficient and systematic dissection of developmental mechanisms in eye regrowth. In addition, it is known that apoptosis is required for this process, even though it is not required for normal eye development (Kha et al., 2018). Thus identifying repair-specific mechanisms that can interact with developmental pathways will also be critical for understanding eye regrowth. This knowledge can be applied towards testing eye repair strategies in adult and/or mature tissues. For example, there are Xenopus tadpole models (with mature eyes) of retinal degeneration (Choi et al., 2011). The genes identified from additional studies of eye regrowth can serve as validation targets for gain-of-function methods to prevent and/or repair diseased frog retinas. This approach can potentially reduce the time needed to generate viable candidates for mammalian studies.
This work was supported by grants from the National Institutes of Health (P20GM103440), and the University of Nevada, Las Vegas (a Faculty Opportunity Award and a doctoral dissertation graduate assistantship) to KAST.
Additional file: Open peer review report 1 (106.7KB, pdf) .
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Andrew Kaplan, McGill University, Canada.
References
- Barbosa-Sabanero K, Hoffmann A, Judge C, Lightcap N, Tsonis PA, Del Rio-Tsonis K. Lens and retina regeneration: new perspectives from model organisms. Biochem J. 2012;447:321–334. doi: 10.1042/BJ20120813. [DOI] [PubMed] [Google Scholar]
- Beck CW, Izpisúa Belmonte JC, Christen B. Beyond early development: Xenopus as an emerging model for the study of regenerative mechanisms. Dev Dyn. 2009;238:1226–1248. doi: 10.1002/dvdy.21890. [DOI] [PubMed] [Google Scholar]
- Choi RY, Engbretson GA, Solessio EC, Jones GA, Coughlin A, Aleksic I, Zuber ME. Cone degeneration following rod ablation in a reversible model of retinal degeneration. Invest Ophthalmol Vis Sci. 2011;52:364–373. doi: 10.1167/iovs.10-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the xenopus retina is independent of lineage and birth date. Neuron. 1988;1:15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Kha CX, Son PH, Lauper J, Tseng KA. A model for investigating developmental eye repair in Xenopus laevis. Exp Eye Res. 2018;169:38–47. doi: 10.1016/j.exer.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Moriya T, Kito K, Miyashita Y, Asami K. Preference for background color of the Xenopus laevis tadpole. J Exp Zool. 1996;276:335–344. doi: 10.1002/(SICI)1097-010X(19961201)276:5<335::AID-JEZ4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Muneoka K, Holler-Dinsmore G, Bryant SV. Intrinsic control of regenerative loss in Xenopus laevis limbs. J Exp Zool. 1986;240:47–54. doi: 10.1002/jez.1402400107. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD, Faber J. New York: Garland Pub; 1994. Normal Table of Xenopus Laevis (Daudin): A Systematical and Chronological Survey of the Development from the Fertilized Egg till the End of Metamorphosis. [Google Scholar]
- Sedohara A, Komazaki S, Asashima M. In vitro induction and transplantation of eye during early Xenopus development. Dev Growth Differ. 2003;45:463–471. doi: 10.1111/j.1440-169x.2003.00713.x. [DOI] [PubMed] [Google Scholar]
- Tseng AS. Seeing the future: using Xenopus to understand eye regeneration. Genesis. 2017;55:e23003. doi: 10.1002/dvg.23003. [DOI] [PubMed] [Google Scholar]
- Viczian AS, Zuber ME. A simple behavioral assay for testing visual function in Xenopus laevis. J Vis Exp. 2014:51726. doi: 10.3791/51726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viczian AS, Zuber ME. Retinal development. In: Moody SA, editor. Principles of Developmental Genetics. 2nd ed. London, UK: Elsevier; 2015. pp. 297–313. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.