Abstract
The sense of smell is important for human quality of life. This sophisticated sensorial system relies on the detection of odorant molecules that engage receptors expressed in the cilia of dedicated neurons that constitute the olfactory epithelium (OE). Importantly, the OE is a highly active site of adult neurogenesis where short-lived neurons are efficiently replenished, even after massive neuronal cell loss. It is suggested that the degree of olfactory function recovery after OE injury may depend on the nature of the lesion (traumatic, chemical, infectious or inflammatory), as well on the velocity of cellular regeneration. Topical steroidal anti-inflammatory drugs, such as glucocorticoids, are routinely prescribed for treating upper airway inflammatory conditions, such as chronic rhinosinusitis. While the therapeutic strategy aims to minimize the inflammatory damage and dysfunction to nasal air conduction, new evidences raise concerns if such drugs may impair neuronal regeneration in the OE. In consequence, new directions are necessary in terms of drug development or prescription, in order to preserve olfactory function through lifelong repeated episodes of chronic inflammation in the upper respiratory tract. Here we discuss mechanisms involved in glucocorticoid deleterious effects to OE regeneration and possible therapeutic alternatives considering relevant side effects.
Keywords: anti-inflammatory drugs, corticosteroids, hyposmia, inflammation, rhinosinusitis, olfactory epithelium, sensory neurons, cell proliferation
Introduction
The olfactory epithelium (OE) is mainly composed of specialized neurons that transduce signal from odorant molecules detection into action potentials relayed to the central nervous system (CNS). These neurons, called olfactory sensory neurons (OSNs), signal to the olfactory bulb according to a combinatorial code that allows humans to discriminate roughly one trillion olfactory stimuli (Ihara et al., 2013; Bushdid et al., 2014). The OSNs are strategically positioned in close proximity to the nasal cavity, which provides the means for odorants detection. However, such location also predisposes these cells to the exposure of toxic molecules, pathogens, allergens, and others. Chronic inflammation in the upper respiratory tract is often associated with nasosinus disease, including rhinosinusitis (Holbrook and Leopold, 2006). The recruitment of inflammatory cells is essential for microbe containment and elimination, a rationale formulated more than one century ago. However, the quite intuitive double-edged sword paradigm of inflammation also defines the destructive facet of this natural protective reaction, damaging bystander cells and eventually resulting in loss of function (Wyss-Coray and Mucke, 2002). The nasal mucosa is no exception, and is also severely injured when exposed to an inflammatory environment. Chronic rhinosinusitis can affect 5–15% of the general population, and a considerable fraction of these individuals may develop olfactory dysfunction due to inflammation (Holbrook and Leopold, 2006; Bachert et al., 2014). Hyposnomia and anosmia, the reduced and complete loss of sense of smell, respectively, underlie an important decrease of quality of life, including spoiled food consumption, depression and failure to detect potential harmful chemicals and danger signals. Raviv and Kern summarized that 25% of smell loss cases (more than 10 million people) could be attributed to chronic sinusitis. It is interesting to note some reports involving sinusitis describing human cases of olfactory function recovery after oral anti-inflammatory corticosteroid therapy that failed to be restored only by sinus surgery (Jafek et al., 1987; Raviv and Kern, 2004). This and other evidences contradicted the common belief that smell loss due to sinonasal disease was an obstruction problem analogous to ear wax as a cause of hearing deficits, wherein the end organs are preserved and normal. For instance, biopsies from patients that underwent nasal surgery revealed a high incidence of inflammatory cells influx in the olfactory mucosa of subjects with olfactory deficits (Kern, 2000). There is also strong support for the association between the beneficial effects of steroidal anti-inflammatory drugs (SAIDs) on olfactory function and drug-induced anti-inflammatory action (Wolfensberger and Hummel, 2002; Kim et al., 2017). Topical glucocorticoids (GCs) are considered first-line therapy for treating rhinosinusitis and nasal polyposis, and for this reason they have been highly used and evaluated (Rudmik et al., 2013). While proper control of inflammation is desirable, few attention have been focused on GCs effects on neuronal replacement in the OE. In fact, since the OE is a remarkable site of adult neurogenesis (Brann and Firestein, 2014), OSNs replacement after nasal mucosa injury have been taken for granted in GCs treated patients. How safe are GCs topically delivered in the nose for olfactory function recovery?
GCs Interfere with OE Neurogenesis
In order to answer the question above, we conducted experiments employing nasal infusion of the synthetic GC dexamethasone (DEX), a well characterized and potent GC receptor (GR) agonist, during the course of OSNs regeneration in murine OE (Crisafulli et al., 2018). The development of an acute and reversible inflammatory lesion in the OE allowed us to evaluate if our conditions reproduces the GC anti-inflammatory effect. In fact, DEX is able to prevent OSNs loss provoked by the infusion of gram-negative bacteria lipopolysaccharide (LPS), which robustly activates the innate immune response through Toll-like receptor 4. Although LPS dramatically damages the murine OE, the neuroepithelium is able to recover within few weeks. The regeneration profile is compatible with the recruitment of OE basal progenitors that proliferates and provides efficient replenish the neuronal loss after injury (Leung et al., 2007), as depicted in Figure 1. Importantly, if DEX is topically administrated once a day after the inflammatory OE lesion establishment for the first three consecutive days, the neuronal regeneration is hugely impaired (Crisafulli et al., 2018). We verified a very similar effect when DEX was employed after methimazole-induced lesion, a drug that kills mature OSNs without the involvement of inflammation. This prompted us to use an in vitro model to test if DEX directly interferes with the proliferative activity of OE progenitor cells. Neurospheres develop in culture when cells isolated from the OE of newborn pups are cultivated in non-adherent conditions in the presence of growth factors, and hence can be used for infering progenitor cells activity. We verified that DEX dose-dependently decreased OE neurosphere formation, an effect abolished in the presence of a GR antagonist. This suggests that DEX interferes with OE progenitors cell-cycling independently of its anti-inflammatory effect. A recent study showed that DEX delivered systemically also impairs OE regeneration after lesion promoted by genetically-driven tumor necrosis factor-α (TNF-α) production in the OE (Chen et al., 2017). However, the authors correlated DEX effects with its interference on the activity of nuclear factor κB (NF-κB), one of the transcription factors that masters the pro-inflammatory gene expression. Curiously, NF-κB activity was crucial in horizontal basal cells, a quiescent progenitor cell recruited after lesions. Accordingly, DEX would interfere with NF-κB as a potential link with a transient inflammatory response that is required for regeneration. While this hypothesis is attractive, our results obtained in vitro with neurosphere culture argues that DEX has direct effects on OE progenitor cells independently of the presence of inflammatory signaling (Figure 1). DEX can interfere with the mitotic activity of basal progenitor cells in vivo, without evidences that DEX promotes cell death or interferes with neuronal differentiation. Indeed, a recent study also supports our findings on proliferation and cellular viability, but using the GC methylprednisolone in brain neural stem cells (Al-Mayyahi et al., 2018). This indicates a general effect of GCs on neural stem and progenitor cells that is not restricted to a unique neurogenic niche.
Figure 1.
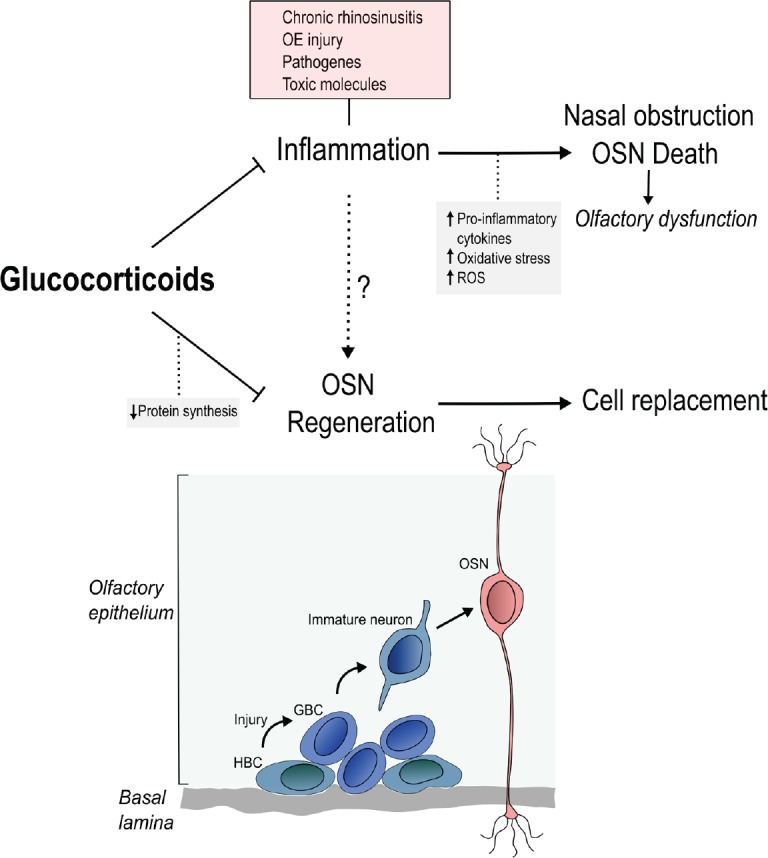
The paradox of glucocorticoid treatment for olfactory recovery during inflammation.
Several conditions activate inflammation, which recruits cells that release massive amounts of cytokines, lipid mediators and reactive oxygen species (ROS). This environment promotes nasal obstruction and olfactory sensory neurons (OSNs) cell death. Newborn OSNs are generated from basal cells (horizontal and globose basal progenitors; horizontal basal cells (HBCs) and globose basal cells (GBCs)) resulting in replacement of dead cells. The basal cells are the major cell population competent for the high proliferative activity necessary for generating the main olfactory cell types. While glucocorticoids (GCs) are drugs of choice for controlling inflammation, these molecules also inhibit olfactory progenitor cells proliferation and negatively impacts neurogenesis in the olfactory epithelium (OE). One proposed mechanism for GCs deleterious effects is the decreased protein synthesis rate, which is necessary for cell-cycle activity. This contradictory panorama defines the GC paradox.
Control of Protein Synthesis as A GC Signaling Target
In an effort to correlate a mechanism with the deleterious GC effect, we reasoned that DEX would mimic a general signal of unfavorable conditions, as those found during stress or prolonged fasting that leads to muscle wasting through the corticosteroid hormone. In fact, we observed a time-dependent effect in which mammalian target of rapamycin (mTOR) complex 1 (mTORC1) downstream signaling is disturbed by DEX. This involves decreased ribosomal S6 kinase activity, leading to insufficient activity of a required step for cell-cycling, namely protein synthesis. We were able to monitor this cellular process through nascent polypeptide chain labeling with puromycin, which was found to be a powerful method to evaluate DEX effects on protein synthesis in situ. At this moment it is not possible to know if decreased protein synthesis is cause or consequence of insufficient proliferative activity of progenitor cells in the OE. New approaches are necessary to establish the appropriate relationship. For instance, manipulations in mTORC1 activity could restore protein synthesis and revert DEX effects on neurogenesis. Unfortunately, accurate tools are lacking or not easily accessible in terms of restoring protein synthesis with reliable, stable, and specific interventions. It is also not clear whether regenerative improvements would be achieved after stimulating protein synthesis. Decreased activity of this cellular process could actually circumvent endoplasmic reticulum (ER) stress at the cost of insufficient neurogenesis, demanding caution before naming the putative culprits.
Alternative Strategies to Restrain Inflammation in the Nasal Mucosa
GCs are potent anti-inflammatory molecules, but this pharmacological property coexists with profound metabolic changes associated with transcriptional events target by GRs. Independently of determining the requirement of the ‘genomic’ or ‘nongenomic’ GR mechanisms, GCs negative impact on neurogenesis falls in the broad spectrum of corticosteroid side-effects. Although non-SAIDs (NSAIDs) represent a quite large catalog of options, SAIDs are preferred as topically-delivered intranasal drugs (see introduction). In the last decade, novel ligands with dissociated effects called selective GR agonists (SEGRAs) (also called dissociated GR ligands, and selective GR modulators; SEGRMs – especially in case of non-steroidal molecules) have been developed in order to circumvent GCs side-effects while retaining anti-inflammatory activity. These prototype drugs were designed in order to mimic GR interaction with pro-inflammatory transcription factors such as NF-κB. The literature regarding the effects of SEGRAs/SEGRMs in different immune cells remains quite scarce (Xavier et al., 2016), but it will be interesting to test how this promising compounds would impact OE regeneration. Nevertheless, these dissociated agonists can still impact OSNs regeneration since there are evidences that NF-κB signaling is required for horizontal basal cells repopulate the injured OE as mentioned above. Thus, it is possible that the development of other anti-inflammatory approaches will be necessary to tackle the paradox of GC treatment in nasal mucosa. For instance, the promotion of pro-resolution phenotype of immune cells.
Conclusions
Olfactory loss of function provokes a considerable decrease in quality of life. Chronic inflammation in the upper respiratory tract is one of the leading causes that results in damage to the neuronal elements of the OE. Failure in achieving resolution of inflammation requires pharmacological therapy based on GCs that interfere with the recruitment and proliferation of basal progenitor cells, resulting in reduced neuronal regeneration. Repeated cycles of inflammation and GC therapy may interfere with the normal cytoarchitecture of the OE, disturbing the organization of the signals relayed to the brain. The dissection of GCs’ molecular targets that negatively impact neuronal replenishment, such as protein synthesis pathways, may help to develop adjuvant treatments or design new strategies that do not promote considerable risk of olfactory disorder development.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: This work was supported by research grants to IG from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2007/53732-8), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 484869/2012-4), and CEPID Redoxoma (FAPESP 2013/07937-8).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Cristoforo Comi, Amedeo Avogadro University, Italy.
Funding: This work was supported by research grants to IG from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2007/53732-8), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 484869/2012-4), and CEPID Redoxoma (FAPESP 2013/07937-8).
References
- Al-Mayyahi RS, Sterio LD, Connolly JB, Adams CF, Al-Tumah WA, Sen J, Emes RD, Hart SR, Chari DM. A proteomic investigation into mechanisms underpinning corticosteroid effects on neural stem cells. Mol Cell Neurosci. 2018;86:30–40. doi: 10.1016/j.mcn.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Bachert C, Pawankar R, Zhang L, Bunnag C, Fokkens WJ, Hamilos DL, Jirapongsananuruk O, Kern R, Meltzer EO, Mullol J, Naclerio R, Pilan R, Rhee CS, Suzaki H, Voegels R, Blaiss M. ICON: chronic rhinosinusitis. World Allergy Organ J. 2014;7:25. doi: 10.1186/1939-4551-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brann JH, Firestein SJ. A lifetime of neurogenesis in the olfactory system. Front Neurosci. 2014;8:182. doi: 10.3389/fnins.2014.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushdid C, Magnasco MO, Vosshall LB, Keller A. Humans can discriminate more than 1 trillion olfactory stimuli. Science. 2014;343:1370–1372. doi: 10.1126/science.1249168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Reed RR, Lane AP. Acute inflammation regulates neuroregeneration through the NF-kappaB pathway in olfactory epithelium. Proc Natl Acad Sci U S A. 2017;114:8089–8094. doi: 10.1073/pnas.1620664114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisafulli U, Xavier AM, Dos Santos FB, Cambiaghi TD, Chang SY, Porcionatto M, Castilho BA, Malnic B, Glezer I. Topical dexamethasone administration impairs protein synthesis and neuronal regeneration in the olfactory epithelium. Front Mol Neurosci. 2018;11:50. doi: 10.3389/fnmol.2018.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook EH, Leopold DA. An updated review of clinical olfaction. Curr Opin Otolaryngol Head Neck Surg. 2006;14:23–28. doi: 10.1097/01.moo.0000193174.77321.39. [DOI] [PubMed] [Google Scholar]
- Ihara S, Yoshikawa K, Touhara K. Chemosensory signals and their receptors in the olfactory neural system. Neuroscience. 2013;254:45–60. doi: 10.1016/j.neuroscience.2013.08.063. [DOI] [PubMed] [Google Scholar]
- Jafek BW, Moran DT, Eller PM, Rowley JC, 3rd, Jafek TB. Steroid-dependent anosmia. Arch Otolaryngol Head Neck Surg. 1987;113:547–549. doi: 10.1001/archotol.1987.01860050093023. [DOI] [PubMed] [Google Scholar]
- Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope. 2000;110:1071–1077. doi: 10.1097/00005537-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Kim DH, Kim SW, Hwang SH, Kim BG, Kang JM, Cho JH, Park YJ, Kim SW. Prognosis of olfactory dysfunction according to etiology and timing of treatment. Otolaryngol Head Neck Surg. 2017;156:371–377. doi: 10.1177/0194599816679952. [DOI] [PubMed] [Google Scholar]
- Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- Raviv JR, Kern RC. Chronic sinusitis and olfactory dysfunction. Otolaryngol Clin North Am. 2004;37:1143–1157. doi: 10.1016/j.otc.2004.06.003. v-vi. [DOI] [PubMed] [Google Scholar]
- Rudmik L, Hoy M, Schlosser RJ, Harvey RJ, Welch KC, Lund V, Smith TL. Topical therapies in the management of chronic rhinosinusitis: an evidence-based review with recommendations. Int Forum Allergy Rhinol. 2013;3:281–298. doi: 10.1002/alr.21096. [DOI] [PubMed] [Google Scholar]
- Wolfensberger M, Hummel T. Anti-inflammatory and surgical therapy of olfactory disorders related to sino-nasal disease. Chem Senses. 2002;27:617–622. doi: 10.1093/chemse/27.7.617. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35:419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Xavier AM, Anunciato AK, Rosenstock TR, Glezer I. Gene expression control by glucocorticoid receptors during innate immune responses. Front Endocrinol (Lausanne) 2016;7:31. doi: 10.3389/fendo.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]


