Abstract
The use of autologous nerve grafts remains the gold standard for treating nerve defects, but current nerve repair techniques are limited by donor tissue availability and morbidity associated with tissue loss. Recently, the use of conduits in nerve injury repair, made possible by tissue engineering, has shown therapeutic potential. We manufactured a biodegradable, collagen-based nerve conduit containing decellularized sciatic nerve matrix and compared this with a silicone conduit for peripheral nerve regeneration using a rat model. The collagen-based conduit contains nerve growth factor, brain-derived neurotrophic factor, and laminin, as demonstrated by enzyme-linked immunosorbent assay. Scanning electron microscopy images showed that the collagen-based conduit had an outer wall to prevent scar tissue infiltration and a porous inner structure to allow axonal growth. Rats that were implanted with the collagen-based conduit to bridge a sciatic nerve defect experienced significantly improved motor and sensory nerve functions and greatly enhanced nerve regeneration compared with rats in the sham control group and the silicone conduit group. Our results suggest that the biodegradable collagen-based nerve conduit is more effective for peripheral nerve regeneration than the silicone conduit.
Keywords: nerve regeneration, biodegradable, decellularized, collagen, nerve conduit, growth factor, peripheral nerve injury, regeneration, silicone conduit, rat model
Introduction
Nerve autografts have been used widely for peripheral nerve regeneration (Belkas et al., 2004). Although only 50% of autograft patients regain useful function (Lee and Wolfe, 2000), autologous nerve grafting is currently the most effective technique for nerve repair. The success of autologous nerve grafts is attributable to the presence of Schwann cells; basal lamina endoneural tubes, which provide neurotrophic factors; and endoneural tube surface adhesion molecules to regenerate axons. The disadvantages of this technique include loss of function at the donor site, donor-site morbidity (including scarring and, occasionally, neuroma and pain), and need for multiple surgeries. In addition, some biological constraints such as infiltration of fibroblast into anastomosis site, and lack of ECM material cannot be overcome by developments in microsurgery (Ciardelli and Chiono, 2006).
Nerve guidance channels have been developed to overcome some of the disadvantages associated with autologous nerve grafting. This scaffold guides axonal regrowth, protects the injured nerve, prevents invasion of scar tissue, and concentrates neurotrophic factors (Seckel, 1990; Meek and Coert, 2008). Non-biodegradable and biodegradable biomaterials are used to create these scaffolds (Wang et al., 2005).
Silicone conduits have been used mostly for nerve regeneration (Wang-Bennett and Coker, 1990). These are non-biodegradable and non-permeable to large molecules and create an isolated environment for nerve regeneration. The disadvantages of non-biodegradable artificial nerve conduits include chronic foreign body reaction that causes excessive scar tissue formation, inflexibility, and lack of stability (Ciardelli and Chiono, 2006). All non-biodegradable materials have such disadvantages, thus requiring removal by a second surgical procedure.
Recently, synthetic polymers, including polyurethane, poly lactic acid, and polycaprolactone (PCL), have been used in biodegradable nerve conduits. Neurotube™ and Neurolac™, which are made of poly lactic acid and PCL, are commercially available products approved by the US Food and Drug Administration (FDA) or carrying the CE Mark indicating conformity with European regulatory requirements (Meek and Coert, 2008; Kehoe et al., 2012; Gaudin et al., 2016). Natural polymers, such as extracellular matrix (ECM), polysaccharides, and proteins, for neural regeneration conduits have been researched. Most of the FDA- or CE-approved commercially available products are made of collagen, e.g., Neurotube™, Neuroflex™, and Neuromend™ (Meek and Coert, 2008; Kehoe et al., 2012).
The hollow nerve regeneration conduit connects the damaged neurons to induce nerve regeneration, but neurons grow slowly along the conduit walls; and, thus, regeneration rates are very slow. To overcome these limitations, microstructure and multi-channel neural regeneration conduits have been proposed (de Ruiter et al., 2008; Hu et al., 2009; Yao et al., 2010). Decellularization is a method of removing impurities such as cells, DNA, and RNA from tissues, leaving only the ECM, which can then be used in artificial organ and tissue regeneration. Acellular ECM may serve as a tissue engineering scaffold because it has active components, such as growth factors, and a similar microenvironment that promotes tissue regeneration.
In this study, we developed and tested a new type of a biodegradable, decellularized sciatic nerve conduit. This conduit consists of a collagen hollow-fiber wall and acellular sciatic nerve ECM as an inner microstructure. Our hypothesis is that the outer collagen wall will block penetration of the surrounding fibrous tissue and the internal microstructure will promote axonal growth.
To confirm this hypothesis, we analyzed the remnant growth factors, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and laminin of acellular porcine sciatic nerve and tested our nerve conduit using a rat sciatic nerve defect model. We compared histological and functional results in sciatic nerve defect rats that did not receive a conduit, received a silicone conduit, and received a growth factor nerve conduit (GFNC).
Materials and Methods
Decellularization process
The dermis and sciatic nerve were harvested from crossbred pigs (12 months) (which were harvested from pigs at the market according to ISO 22442-2), such as landrace, yorkshire, and duroc; and the tissue was decellularized. Briefly, the porcine dermis was thoroughly washed with 70% ethanol and cut into circular pieces (100 mm in diameter). The samples were soaked in 70% ethanol with 0.5 N NaOH solution as a detergent for 24 hours with continuous agitation. Finally, the porcine skin was neutralized with 0.5 N HCl, then washed with distilled water. Decellularized sciatic nerve was also prepared in the same manner as the decellularized skin. The decellularized dermis and nerve were separately added to PBS after grinding to create a 2% and 5% solution, respectively. Hemotoxylin and eosin (H&E) tissue staining (Cat No. 3801698, Leica, Richmend, IL, USA) and DNA quantification (Cat No.1660001EDU, Bio-RAD, Hercules, California, USA) were performed to analyze the decellularization efficiency of porcine nerve tissue.
Production of GFNC
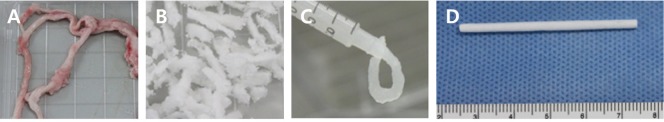
The decellularized dermis was dried on a Teflon mold to prepare a 150 µm thickness sheet, then rolled using a cylindrical mold having a 2-mm diameter hollow cylinder, and finally crosslinked using hexamethylene diisocyanate (Cat No. 52649; Sigma-Aldrich, St. Louis, USA) as a crosslinking agent to improve the physical properties and shorten degradation time. Finally, the decellularized nerve was inserted into a hollow collagen tube and then freeze-dried to prepare the GFNC (Figure 1).
Figure 1.
Production of biologically degradable nerve conduit.
The growth factor nerve conduit was manufactured by converting decellularized sciatic nerve to 5% gel solution and placing it into the collagen tube: (A) Harvested nerve; (B) decellularization; (C) 5% gel solution of decellularized nerve; and (D) conduit filled with decellularized nerve gel.
Component analysis of the GFNC
Growth factor (NGF and BDNF) analysis
The residual nerve growth factors in the acellular porcine sciatic nerve were analyzed by enzyme-linked immunosorbent assay (ELISA). The ELISA Kits (Cat No. K0332140 and EK0308; Komabiotech, Seoul, Korea) were used to analyze the growth factors of the decellularized sciatic nerve. The decellularized sciatic nerve was pulverized, and the specimens (100 mg/mL) were allowed to stand for 2 hours at room temperature with ELISA standard. After that, the analysis was performed according to the manufacturer's protocol.
Laminin analysis
Laminin is known to help axonal and peripheral nerve regeneration (Chen and Strickland S, 2003). Laminin ELISA Kit (Cat No. EK0435; Komabiotech, Seoul, Korea, Range 156–10,000 pg/mL, sensitivity < 10 pg/mL) was used to analyze the laminin from the decellularized sciatic nerve. The decellularized sciatic nerve was pulverized, and the specimens (100 mg/mL concentration) were allowed to stand for 2 hours at room temperature with laminin standard. After that, the analysis was performed according to the manufacturer's protocol.
Analysis of conduit morphologic structure
We used a scanning electron microscope (SEM, CX-100S, COXEM., Ltd., Daejeon, Korea) to analyze the microstructure, confirm the porous conduit structures for axonal regeneration, and scan three-dimensional structures. The samples were coated with gold and measured at 30× and 200× magnifications under 20 kV accelerating voltage by SEM.
In vivo study
This animal experiment was approved by the Institutional Animal Care and Use Committee of Genewel Co., Ltd. (GAP-AVAL-14015). The animals were maintained in compliance with all regulatory guidelines provided by the Ministry of Food and Drug Safety. The 64 Sprague-Dawley rats (male, 220–250 g, 7 weeks old, specific-pathogen-free) were randomly and evenly divided into four groups: normal control, sham, silicone conduit, and GFNC. In the sham group, sciatic nerve was cut into a 10 mm gap, and the muscle and skin were sutured without connecting the nerve.
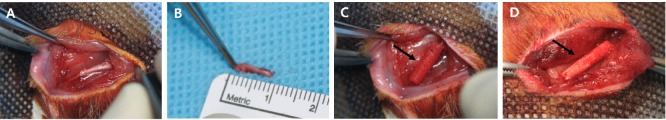
Briefly, the animals were anesthetized by 2% isoflurane inhalation (Hana Pharmaceutical Co., Ltd., Gyeonggi-do, Korea). The skin from the clipped right lateral thigh was scrubbed in the routine fashion with povidone iodine solution. An incision was made along the leg of the animal. The sciatic and posterior tibial nerves were exposed by a muscle-splitting incision. A 10 mm segment of right sciatic nerve was resected from the 5 mm point upward from the site where the proximal nerve was divided into the tibial nerve and the peroneal nerve. Afterward, the GFNC or silicone conduit (Silastic® RX-50; DOW CORNING, Midland, MI, USA) was placed into this nerve gap, and approximately 1 mm of each nerve end was sutured with 8-0 nylon into the conduit using a microsurgical technique. The muscle and skin were closed using 4-0 silk sutures (Figure 2).
Figure 2.
Surgical dissection of the right sciatic nerve and conduit implantation in rats.
The conduits were placed into this nerve gap using a microsurgical technique: (A) Exposed nerve; (B) dissected nerve; (C) implanted growth factor nerve conduit (arrow); and (D) growth factor nerve conduit sutured at both ends.
Walking track analysis
The rats were filmed while walking through a straight runway (10 × 50 cm) at 4, 8, 12, 18, and 24 weeks post-surgery. We measured the ankle stance angle (ASA) thrice each from the films by capturing the ankle angle in the mid-stance phase of walking and then calculated the mean of the three measurements. Because rats whose sciatic nerves have been transected tend to scratch and bite the anesthetic feet, sometimes resulting in amputation of toes and thus potentially unusable data, we categorized the degree of self-mutilation as no self-mutilation, mild, moderate, or severe using the autotomy stages described by Wall et al. (1979). Footprinting was evaluated and quantified, and the mean values of the footprinting were calculated. The Sciatic Functional Index (SFI), calculated from the measurements described above, is a tool to evaluate functional sciatic nerve recovery following injury. An SFI of 0% means normal, and 100% means complete damage (de Medinaceli et al., 1982; Bain et al., 1989; De Medinaceli, 1989; Dellon and Mackinnon, 1989; Tonge and Golding, 1993; Weber et al., 1993; Bervar, 2000; Dijkstra et al., 2000; Varejao et al., 2001; Sarikcioglu et al., 2009).
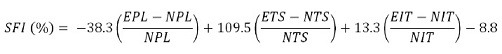
where EPL indicates experimental paw length, NPL indicates unoperated normal paw length, ETS indicates the distance between the first and fifth toes of operated experimental foot, NTS indicates the distance between the first and fifth toes of unoperated experimental foot; EIT indicates the distance between the second and forth toes of operated experimental foot, and NIT indicates the distance between the second and forth toes of unoperated experimental foot.
Electrophysiological test
The animals were anesthetized by 2% isoflurane inhalation, and their sciatic nerves were exposed using the previous incision line at 18 and 24 weeks post-surgery. A recording electrode was inserted 1 cm from the conduit end, and a testing electrode was inserted 0.5 cm from the conduit end to penetrate the nerve. A grounding electrode was inserted under the skin in the femoral region. We placed a bipolar-stimulating electrode 1 cm superior to the proximal end of the implanted conduit, and the direct peripheral nerve was stimulated with a nerve conduction tester (Keypoint®, 2-channel EMG/SEP system, Alpine Biomed Aps, Skovlunde, Denmark). The value was increased from the lowest stimulation time (ms), stimulation frequency (Hz) and stimulation intensity (mA) until the reaction occurred, and the value was fixed and measured 5 times per animal. The stimulation period, stimulation frequency, and stimulation strength were examined at 0.1 ms, 0.5 Hz, and 5 mA, respectively. We recorded the distal latency and amplitude to determine nerve conduction and compared the results between the groups (Choi et al., 1996).
Histological analysis
The sciatic nerves with implants were harvested at twice (18 and 24 weeks) and fixed in formalin. Afterward, the specimens were stained using hematoxylin & eosin (H&E) staining or immunohistochemical (IHC) staining for the S100 protein. Subsequently, we analyzed nerve tissue regeneration, regeneration degree, fibrotic tissue infiltration, and neuroma formation from H&E-stained specimens. To quantify the immunohistological response of S100 protein, we compared the area of the stained S100 protein with the tube area from IHC stained specimens. The area of the stained S100 protein was measured by image analysis software (Image J, Bethesda, Maryland, USA).
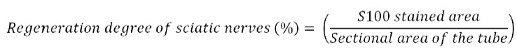
Fine structural analysis
The specimens harvested from each group at 24 weeks were sectioned by ultramicrotomy. We used a transmission electron microscope (TEM) system for biologic organisms (Bio-TEM, JEM-1400plus, JEOL Ltd., Tokyo, Japan) to obtain the photographs of the slides at room temperature. We compared the myelin, Schwann cells, and perineurium of the specimens with those of normal sciatic nerves. We checked the number of axonal myelination thrice each with a magnification power of 1000 and quantified the observations.
Statistical analysis
Data are expressed as the mean ± SD. All data were statistically analyzed using t-test and the chi-square test with SPSS 15.0 software for Windows (SPSS Inc., Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Results
Decellularization analysis results
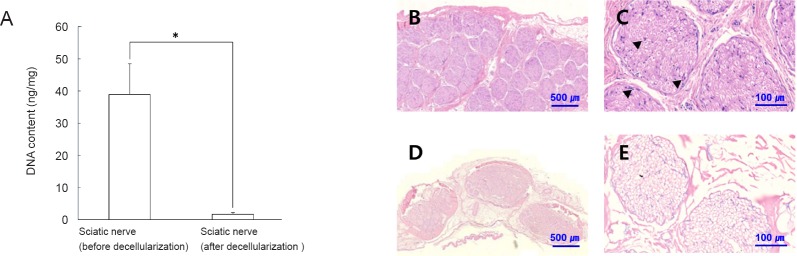
The H&E staining (Figure 3) confirmed that decellularization eliminated nucleic acid and cytoplasm from nerve tissue. Quantitative analysis of decellularization efficiency showed that the DNA content was decreased from 38.89 ng/mg to 1.73 ng/mg in dermis tissue (P = 0.05) (Figure 3). The DNA content of the decellularized sciatic nerve was considered satisfactory for tissue engineering applications and comparable to other decellularization studies (Wang et al., 2012; Jones et al., 2017).
Figure 3.
DNA assay and hematoxylin and eosin staining of decellularized sciatic nerve.
DNA assays were performed to confirm decellularization (A). *P = 0.05. In addition, cells of nerve tissue (arrows) were examined by hematoxylin and eosin staining. Nuclei of cells (arrows) were present in the tissues before decellularization (B, C), but no cells were observed in the tissues after decellularization (D, E). Scale bars: 500 µm in B and D, 100 µm in C and E.
Component of the GFNC
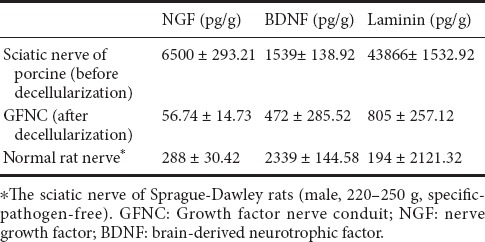
The mean values of the GFNC effective components are contained in Table 1. NGF, BDNF, and laminin were present in porcine sciatic nerve tissue at concentrations of 6500 ± 293.21, 1539 ± 138.92, and 43,866 ± 1532.92 pg/g, respectively. NGF, BDNF and laminin concentrations decreased to 56.74 ± 14.73 pg/g, 472 ± 285.52 pg/g, and 805 ± 257.12 pg/g, respectively, after decellularization. Compared with normal rat nerve tissue, GFNC NGF, BDNF and laminin concentrations were low but remained at a ratio of 1:5 (Table 1).
Table 1.
Mean value of effective components of the GFNC
Morphologic conduit structure
As shown in Figure 4, hollow collagen tubes with a diameter of approximately 1.5 mm and filled with acellular sciatic nerve ECM were prepared. SEM of the GFNC confirmed that the outer collagen wall of the conduit was approximately 20 µm thick and had a compact structure to prevent scar tissue infiltration. The internal ECM structure also had a pore size of approximately 0.5 to 20 μm, which provided a suitable environment for axonal growth and nerve regeneration (Figure 4).
Figure 4.
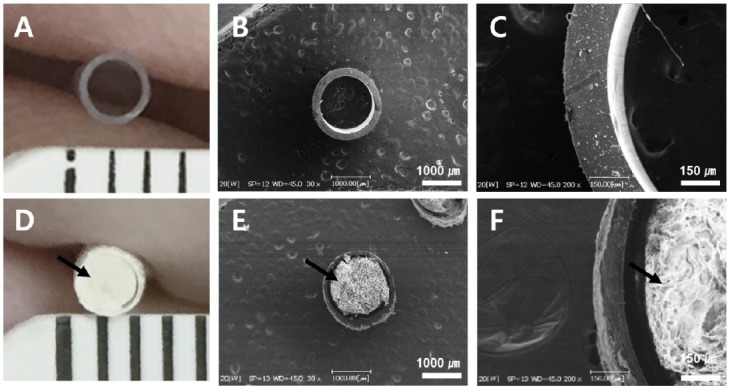
Morphologic structure of silicone conduit and GFNC.
(A) The silicone conduit had a hollow cylindrical shape with an inner diameter of 1.5 mm. (B, C) Scanning electron microscope (SEM) images. The porous structure of the GFNC was filled with nerve growth factor, brain-derived neurotrophic factor, and laminin (arrows), making the GFNC ideal for promoting axonal growth and nerve regeneration; inner diameter of the GFNC was 1.5 mm (D), SEM images (E, F). Scale bars: 1000 μm in B and E, 150 μm in C and F. GFNC: Growth factor nerve conduit.
In vivo study
Neurological function
SFI results are shown in Figure 5. As time passed, the GFNC group had better outcomes compared with the sham and silicone conduit groups, and at 18 and 24 weeks statistically significant results were achieved (P < 0.05). The GFNC group exhibited significantly improved outcomes at both 18 and 24 weeks compared to the sham control and silicone conduit groups (Figure 5).
Figure 5.
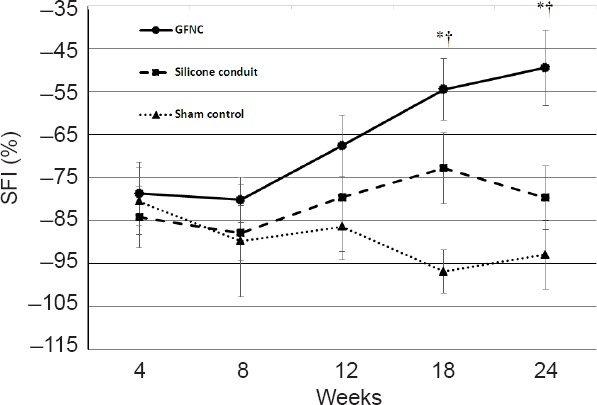
Sciatic Functional Index (SFI) of rats in each group.
The SFI index of normal rats was zero. At 4 weeks, the SFI was similar in all three groups. As time progressed, the GFNC group consistently demonstrated increased SFI and significantly better results at 18 weeks than the other groups. We suspect that the sham group SFI decreased with time because the nerve was not regenerated. The silicone conduit group exhibited a significantly lower SFI than the GFNC group. *P < 0.05, vs. the silicon conduit group, †P < 0.05, vs. sham group. n = 16 at 18 weeks, n = 8 at 24 weeks. GFNC: Growth factor nerve conduit.
The ASA measurements in all three groups were similar at 8 weeks. Rats in all three groups showed signs of tibial nerve injuries. After 18 weeks, all group subjects raised their right fifth toe from the runway surface, a behavior that is caused by tibial nerve injury. Additionally, the sham control and silicone conduit groups exhibited joint contractures due to fibular nerve neuropathy. A contracture phenomenon was observed in which the toe was contracted by the calf muscle neuropathy. On the other hand, in the GFNC group, the walking angle was maintained because the degree of abnormality of the fifth toe was low. The mean of the ASA measurements at 24 weeks was 56° in the normal control group, 35° in the sham group, 41° in the silicone conduit group, and 48° in the GFNC group. Rats in the GFNC group showed an increase in ASA compared with the silicon conduit and sham groups, and the difference was statistically significant (P = 0.037, vs. silicon conduit group; P = 0.025, vs. sham group). The ASA of the GFNC group was closest to the value of the normal control group (Figure 6).
Figure 6.
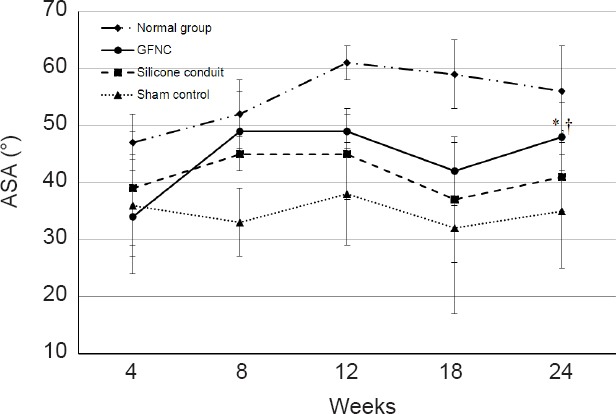
Ankle stance angle (ASA) in each group.
If the nerve is damaged, ASA decreases because the rat does not have a normal gait. The sham control group showed consistently low ASA values because the nerve was completely severed. The GFNC and silicone conduit groups that connected the nerve ends exhibited better ASA measurements than the sham group, but the results at 24 weeks were significant only in the GFNC group. *P < 0.05, vs. silicon conduit group (n = 8), †P < 0.05, vs. sham group (n = 8).
Following sciatic neurectomy, animals showed foot-biting and self-mutilation behaviors. Self-mutilation is likely pain-related behavior (Yoo, Na and Yoon, 2008). Based on our observations, the sham, silicone conduit, and GFNC groups experienced anesthesia dolorosa. Continuous high-grade self-mutilation behavior was observed in the sham group. Grade 3–4 (Moderate, Severe) and grade 2–3 (Mild, Moderate) self-mutilation behavior were observed in the silicone conduit and GFNC groups, respectively (P = 0.020) after 18 weeks. At 24 weeks, no statistically significant difference existed between the groups although we did find that the grade of self-mutilation was lower in the GFNC group than in the other groups. Minimal nerve regeneration induced pain reduction and caused self-mutilation (Figure 7).
Figure 7.
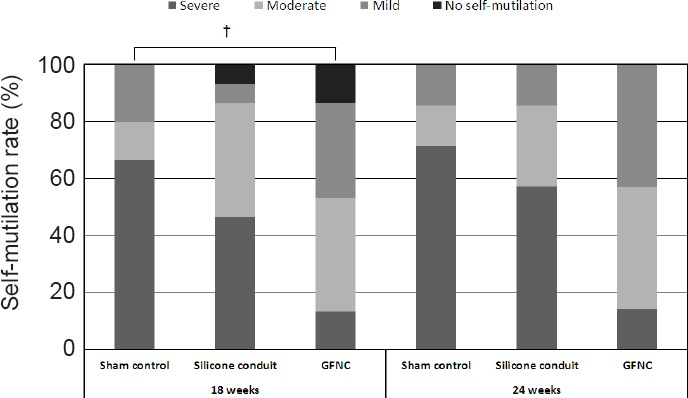
Self-mutilation in each group after 18 weeks.
Self-mutilation, which tends to occur in non-sensory areas after severing the sciatic nerve, was classified according to severity. The sham group showed a self-mutilation rate of 60% and a severe grade. The GFNC group had a rate greater than 80% but the lowest severity compared to the other groups. †P < 0.05, vs. sham group (n = 16). GFNC: Growth factor nerve conduit.
Electrophysiological function
The mean nerve conduction velocity (NCV) for each group is shown in Figure 8. At 18 weeks, the mean NCV in the normal control, sham, silicone conduit, and GFNC groups was 50.8, 18.2, 20.2, and 38.5 m/s, respectively. At 24 weeks, the mean NCV in the normal control, sham, silicone conduit, and GFNC groups was 49.8, 11.7, 25.9, and 38.8 m/s, respectively (P < 0.05, vs. the silicone conduit group; P < 0.05, vs. sham group). These results demonstrated that the GFNC group had a significantly higher NCV than the silicone conduit group (Figure 8).
Figure 8.
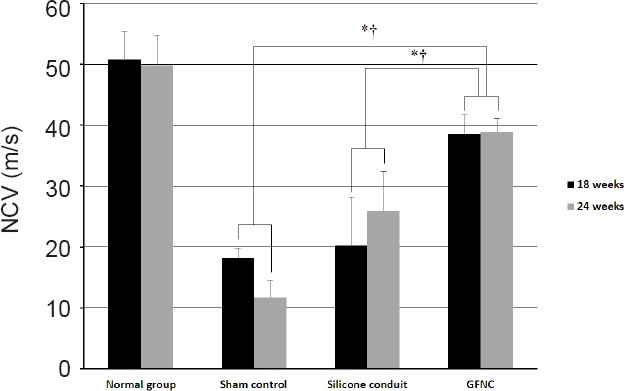
Electrophysiological testing in the sciatic nerve of rats at 18 weeks.
When the nerve regenerated, it approximated the nerve conduction velocity (NCV) of normal nerves. The GFNC group exhibited faster nerve regeneration than the other groups. The sham group exhibited a low value because the nerve did not regenerate. In the silicone conduit group, the NCV value increased as nerve regeneration progressed. *P < 0.05, vs. silicone conduit group; †P < 0.05, vs. sham group. n = 16 at 18 weeks, n = 8 at 24 weeks. GFNC: Growth factor nerve conduit.
Histological changes in the sciatic nerve
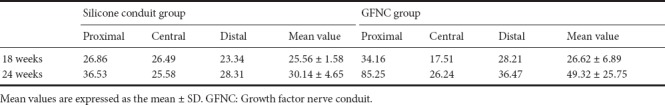
H&E staining revealed that, in the normal control group, fibrous tissue penetrated the peripheral nerve tissue. In contrast, no fibrous tissue penetration occurred in the silicone conduit and GFNC groups. H&E staining also showed that nerve tissue was growing inside the conduits in both the silicone conduit and GFNC groups. IHC staining demonstrated the regeneration rate of the silicone conduit group was 30.14 ± 4.65% and 49.32 ± 25.75% in the GFNC group at 24 weeks. Both groups demonstrated no adverse reactions, whereas IHC staining of the sham g roup demonstrated fibrous tissue penetration (Figures 9, 10, and Table 2).
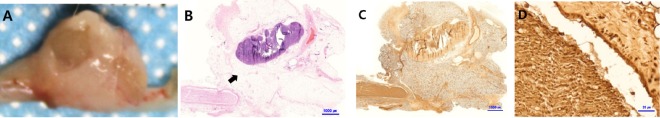
Figure 9.
Histological analysis of the sham group at 8 weeks.
In the image of the surgical site (A), the defective nerve area is rounded and appears to be connected. However, hematoxylin-eosin (B) and S100 (C) staining confirmed that the nerves did not regenerate and that other tissues formed (arrow). Also, fibrous tissue surrounded the nerve tissue (D). Scale bars: 1000 µm in B, C, and 20 µm in D.
Figure 10.
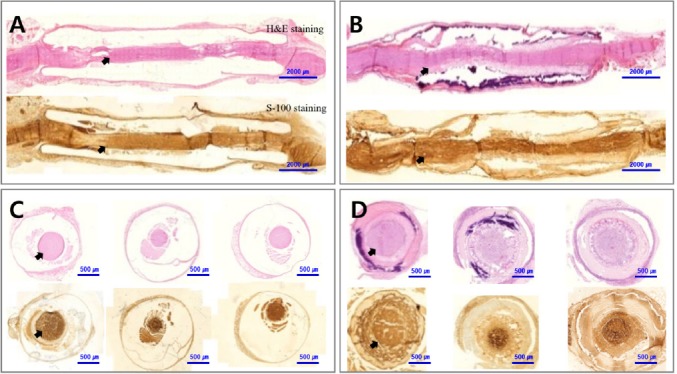
Histological analysis of the sciatic nerve in the silicone conduit and GFNC groups at 24 weeks.
In both the silicone conduit (A, C) and GFNC (B, D) groups, scar tissues did not penetrate and the nerve (arrows) regenerated at 24 weeks. Panels (C) and (D) depict the proximal, central, and distal aspects of the sciatic nerve from the left. The proximal part of the nerve tended to regenerate more than the distal end. Scale bars: 2000 µm in A, B and 500 µm in C, D. GFNC: Growth factor nerve conduit.
Table 2.
Nerve regeneration rate (%) per unit area in the silicone conduit and GFNC groups
Fine structure in the sciatic nerve
TEM at × 1000 magnification demonstrated that normal nerves contain myelin, Schwann cells, blood vessels, and perineurium and that Schwann cells and ECM were filled with well myelinated nerve fibers arranged evenly. At 24 weeks, the silicone conduit group demonstrated relatively less organized and more ECM, and the size of Schwann cells and degree of myelination were smaller than that of normal neurons. In the GFNC group, the degree of myelination, Schwann cell size, and the number of nerve cells were small compared to the normal control group, but the structure was similar to that of the normal nerve when compared with the silicone conduit group. Axonal myelination in the normal control, silicone conduit, and GFNC groups was 61, 24, and 41 units/image, respectively (Figure 11).
Figure 11.
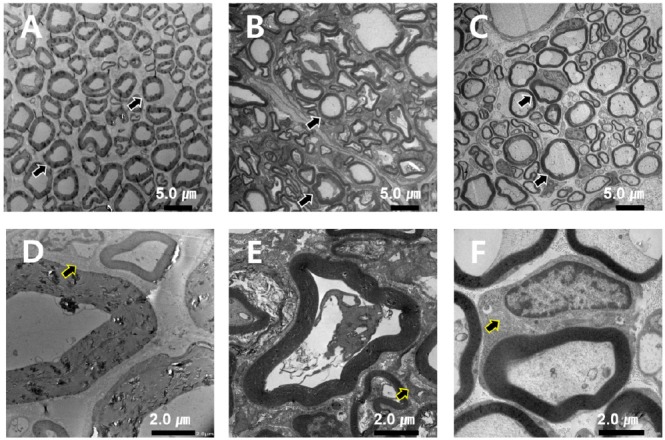
Transmission electron microscope images of the sciatic nerve in each group at 24 weeks.
The normal control group had uniform myelin distribution and structure (A). The silicone conduit group showed less homogenization of myelin and relatively more extracellular matrix (ECM) (B). The GFNC group also had decreased myelin thickness, Schwann cell size, and axon number than the normal control group than the silicone conduit group (C), but these indices were similar between the GFNC and the normal control groups. Myelination of the normal control, silicone conduit, and GFNC groups was 61, 24, and 41 units/image, respectively, with 1000x magnification at 24 weeks. Panels D, E, and F depict the Schwann cells (arrows) surrounding the myelin. Scale bars: 5 µm in A–C, and 2 µm in D–F. GFNC: Growth factor nerve conduit.
Discussion
Although autologous nerve grafts are widely used for the reconstruction of nerve defects, the grafts do not result in complete restoration of the nerve. To address this problem, research has focused on the development of nerve conduits made of polymer materials and on allogeneic or heterologous neurotransplantation. Initially, silicone (Rodriguez et al., 1999) or polytetrafluoroethylene (PTFE) (Stanec and Stanec, 1998) was used in the conduits to promote nerve regeneration. However, these materials did not disintegrate, and a second surgical procedure was required for removal. Thus, research shifted to biodegradable and biocompatible materials, including polyglycolic acid (PGA) (Rosson et al., 2009), poly vinyl alcohol (PVA) (Rosson et al., 2009), PVA (Stocco et al., 2018), and collagen (Taras et al., 2011). Recently, efforts have focused on adding growth factors to the biodegradable and biocompatible materials to promote more rapid neuronal regeneration. To make nerve fibers grow, induction of Schwann cells is important because Schwann cells secrete nerve growth factors, such as NGF and BDNF (Tonge and Golding, 1993). NGF and BDNF are effective for nerve regeneration (Utley et al., 1996; Lee et al., 2003) but have a short half-life and may lose their bioactivity when they interact with body fluids or enzymes. Several methods have been used to protect the bioactivity of these growth factors.
In this study, in order to produce an effective and biocompatible conduit for nerve regeneration, porcine dermis was decellularized to create a tube. To protect the bioactivity of growth factors, decellularized NGF and BDNF were loaded into the tube to create the GFNC. The purpose of this study was to evaluate the efficacy of the GFNC as compared to a silicone conduit on nerve regeneration and functional recovery following reconstruction of a 10 mm defect in a sciatic nerve.
In the rat peripheral nerve injury model, the regeneration process using a conduit results in clinical and physiological functional changes and produces morphological changes as well. In this study, nerve regeneration was measured by analysis of kinematic function, sensory function, and tissue changes.
To verify the interconnectivity of motor and sensory functions, we calculated the SFI and measured the ASA for all the groups. The SFI of the GFNC group demonstrated that the GFNC is approximately 1.6 times more effective than the silicone conduit at 24 weeks post-procedure. Additionally, the GFNC group had the ASA that was nearest to normal of the three groups.
Electrophysiological assessment results showed that the GFNC group had a similar NCV as the normal group. The NCV of the GFNC group was almost twice as fast as that of the silicone conduit group.
H&E and IHC staining confirmed that nerve regeneration occurred in both types of conduits; and we found no immune reaction, toxicity, and scar tissue infiltration in either conduit group. However, the silicone conduit contained no materials to induce axonal growth and nerve regeneration. Hence, the regeneration rate of the GFNC group was much faster than that of the silicone conduit group. The area of regeneration was larger in the GFNC group than the silicone conduit group at 18 and 24 weeks.
The GFNC group had a less-organized and thinner myelin than the normal group and relatively larger volume of ECM. But the GFNC group had more axonal myelination and Schwann cells than the silicone conduit group as observed by TEM at 24 weeks.
The limitation of this study is the small number of animals used to prove our hypothesis, therefore we get the statistically significant results of self-mutilating behavior in the silicone conduit and GFNC groups, respectively after 18 weeks. But at 24 weeks, no statistically significant difference existed between the groups although we did find that the grade of self-mutilation was lower in the GFNC group than in the other groups.
Based on these results, the GFNC is a more promising alternative for peripheral nerve reconstruction than the silicone conduit. Its superiority is attributable to the growth factors (NGF, BDNF, and laminin) contained in the decellularized sciatic nerve from which the conduit is made. The NGF and BDNF promote peripheral nerve regeneration, and laminin aides in axonal lengthening and peripheral nerve adhesion (Madison et al., 1987; Williams et al., 1987; Labrador et al., 1998).
In conclusion, these promising results warrant further investigation into the safety of the GFNC biodegradation post-regeneration and into other potential biological conduit materials.
Additional file: Open peer review report 1 (166KB, pdf) .
Footnotes
Conflicts of interest: We have received research grants from the Small and Medium Business Administration and collaborated with Genewel. We have used Genewel's laboratory and animal laboratories. There are no conflicts of interest.
Financial support: This study was supported by a grant from Small and Medium Business Administration (S2082152). The funder did not participate in data collection and analysis, article writing or submission of this paper.
Institutional review board statement: This animal experiment was authorized by the Institutional Animal Care and Use Committee of Genewel Co. Ltd. (GAP-AVAL-14015). The animals were maintained in compliance with all regulatory guidelines provided by the Ministry of Food and Drug Safety.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Michele Fornaro, Midwestern University, USA; Stuart Kyle, Bradford Teaching Hospitals NHS Foundation Trust, UK.
Funding: This study was supported by a grant from the Small and Medium Business Administration (S2082152).
(Copyedited by Li CH, Song LP, Zhao M)
References
- Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–138. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- Belkas JS, Shoichet MS, Midha R. Peripheral nerve regeneration through guidance tubes. Neurol Res. 2004;26:151–160. doi: 10.1179/016164104225013798. [DOI] [PubMed] [Google Scholar]
- Bervar M. Video analysis of standing--an alternative footprint analysis to assess functional loss following injury to the rat sciatic nerve. J Neurosci Methods. 2000;102:109–116. doi: 10.1016/s0165-0270(00)00281-8. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Strickland S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J Cell Biol. 2003;163:889–899. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.S, Jo JH, Seo SS, Son CM. A comparison study between autogenous nerve graft and silicone tubing method in segmental defect of sciatic nerve in rats. J Korean Orthop. 1996;31:833–843. [Google Scholar]
- Ciardelli G, Chiono V. Materials for peripheral nerve regeneration. Macromol Biosci. 2006;6:13–26. doi: 10.1002/mabi.200500151. [DOI] [PubMed] [Google Scholar]
- De Medinaceli L. Use of sciatic function index and walking track assessment. Microsurgery. 1989;11:191–192. doi: 10.1002/micr.1920110221. [DOI] [PubMed] [Google Scholar]
- de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–643. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- de Ruiter GC, Spinner RJ, Malessy MJ, Moore MJ, Sorenson EJ, Currier BL, Yaszemski MJ, Windebank AJ. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(lactic-co-glycolic acid) nerve tubes. Neurosurgery. 2008;63:144–153. doi: 10.1227/01.NEU.0000335081.47352.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellon AL, Mackinnon SE. Sciatic nerve regeneration in the rat. Validity of walking track assessment in the presence of chronic contractures. Microsurgery. 1989;10:220–225. doi: 10.1002/micr.1920100316. [DOI] [PubMed] [Google Scholar]
- Dijkstra JR, Meek MF, Robinson PH, Gramsbergen A. Methods to evaluate functional nerve recovery in adult rats: walking track analysis, video analysis and the withdrawal reflex. J Neurosci Methods. 2000;96:89–96. doi: 10.1016/s0165-0270(99)00174-0. [DOI] [PubMed] [Google Scholar]
- Gaudin R, Knipfer C, Henningsen A, Smeets R, Heiland M, Hadlock T. Approaches to peripheral nerve repair: generations of biomaterial conduits yielding to replacing autologous nerve grafts in craniomaxillofacial surgery. Biomed Res Int. 2016;2016:3856262. doi: 10.1155/2016/3856262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Huang J, Ye Z, Xia L, Li M, Lv B, Shen X, Luo Z. A novel scaffold with longitudinally oriented microchannels promotes peripheral nerve regeneration. Tissue Eng Part A. 2009;15:3297–3308. doi: 10.1089/ten.TEA.2009.0017. [DOI] [PubMed] [Google Scholar]
- Jones G, Herbert A, Berry H, Edwards JH, Fisher J, Ingham E. Decellularization and characterization of porcine superflexor tendon: a potential anterior cruciate ligament replacement. Tissue Eng Part A. 2017;23:124–134. doi: 10.1089/ten.tea.2016.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe S, Zhang XF, Boyd D. FDA approved guidance conduits and wraps for peripheral nerve injury: a review of materials and efficacy. Injury. 2012;43:553–572. doi: 10.1016/j.injury.2010.12.030. [DOI] [PubMed] [Google Scholar]
- Labrador RO, Buti M, Navarro X. Influence of collagen and laminin gels concentration on nerve regeneration after resection and tube repair. Exp Neurol. 1998;149:243–252. doi: 10.1006/exnr.1997.6650. [DOI] [PubMed] [Google Scholar]
- Lee AC, Yu VM, Lowe JB, 3rd, Brenner MJ, Hunter DA, Mackinnon SE, Sakiyama-Elbert SE. Controlled release of nerve growth factor enhances sciatic nerve regeneration. Exp Neurol. 2003;184:295–303. doi: 10.1016/s0014-4886(03)00258-9. [DOI] [PubMed] [Google Scholar]
- Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Surg. 2000;8:243–252. doi: 10.5435/00124635-200007000-00005. [DOI] [PubMed] [Google Scholar]
- Madison RD, da Silva C, Dikkes P, Sidman RL, Chiu TH. Peripheral nerve regeneration with entubulation repair: comparison of biodegradeable nerve guides versus polyethylene tubes and the effects of a laminin-containing gel. Exp Neurol. 1987;95:378–390. doi: 10.1016/0014-4886(87)90146-4. [DOI] [PubMed] [Google Scholar]
- Meek MF, Coert JH. US Food and Drug Administration/Conformit Europe-approved absorbable nerve conduits for clinical repair of peripheral and cranial nerves. Ann Plast Surg. 2008;60:110–116. doi: 10.1097/SAP.0b013e31804d441c. [DOI] [PubMed] [Google Scholar]
- Rodriguez FJ, Gomez N, Labrador RO, Buti M, Ceballos D, Cuadras J, Verdu E, Navarro X. Improvement of regeneration with predegenerated nerve transplants in silicone chambers. Restor Neurol Neurosci. 1999;14:65–79. [PubMed] [Google Scholar]
- Rosson GD, Williams EH, Dellon AL. Motor nerve regeneration across a conduit. Microsurgery. 2009;29:107–114. doi: 10.1002/micr.20580. [DOI] [PubMed] [Google Scholar]
- Sarikcioglu L, Demirel BM, Utuk A. Walking track analysis: an assessment method for functional recovery after sciatic nerve injury in the rat. Folia Morphol (Warsz) 2009;68:1–7. [PubMed] [Google Scholar]
- Seckel BR. Enhancement of peripheral nerve regeneration. Muscle Nerve. 1990;13:785–800. doi: 10.1002/mus.880130904. [DOI] [PubMed] [Google Scholar]
- Stanec S, Stanec Z. Ulnar nerve reconstruction with an expanded polytetrafluoroethylene conduit. Br J Plast Surg. 1998;51:637–639. doi: 10.1054/bjps.1998.9996. [DOI] [PubMed] [Google Scholar]
- Stocco E, Barbon S, Lora L, Grandi F, Sartore L, Tiengo C, Petrelli L, Dalzoppo D, Parnigotto PP, Macchi V, De Caro R, Porzionato A, Grandi C. Partially oxidized polyvinyl alcohol conduitfor peripheral nerve regeneration. Sci Rep. 2018;8:604. doi: 10.1038/s41598-017-19058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taras JS, Jacoby SM, Lincoski CJ. Reconstruction of digital nerves with collagen conduits. J Hand Surg Am. 2011;36:1441–1446. doi: 10.1016/j.jhsa.2011.06.009. [DOI] [PubMed] [Google Scholar]
- Tonge DA, Golding JP. Regeneration and repair of the peripheral nervous system. Semin Neurosci. 1993;5:385–390. [Google Scholar]
- Utley DS, Lewin SL, Cheng ET, Verity AN, Sierra D, Terris DJ. Brain-derived neurotrophic factor and collagen tubulization enhance functional recovery after peripheral nerve transection and repair. Arch Otolaryngol Head Neck Surg. 1996;122:407–413. doi: 10.1001/archotol.1996.01890160047009. [DOI] [PubMed] [Google Scholar]
- Varejao AS, Cabrita AM, Patricio JA, Bulas-Cruz J, Gabriel RC, Melo-Pinto P, Couto PA, Meek MF. Functional assessment of peripheral nerve recovery in the rat: gait kinematics. Microsurgery. 2001;21:383–388. doi: 10.1002/micr.21803. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7:103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Wang B, Tedder ME, Perez CE, Wang G, de Jongh Curry AL, To F, Elder SH, Williams LN, Simionescu DT, Liao J. Structural and biomechanical characterizations of porcine myocardial extracellular matrix. J Mater Sci Mater Med. 2012;23:1835–1847. doi: 10.1007/s10856-012-4660-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Bennett LT, Coker NJ. Analysis of axonal regeneration through the silicone regeneration chamber: a retrograde tracing study in the rabbit facial nerve. Exp Neurol. 1990;107:222–229. doi: 10.1016/0014-4886(90)90139-j. [DOI] [PubMed] [Google Scholar]
- Wang X, Hu W, Cao Y, Yao J, Wu J, Gu X. Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain. 2005;128:1897–1910. doi: 10.1093/brain/awh517. [DOI] [PubMed] [Google Scholar]
- Weber RA, Proctor WH, Warner MR, Verheyden CN. Autotomy and the sciatic functional index. Microsurgery. 1993;14:323–327. doi: 10.1002/micr.1920140507. [DOI] [PubMed] [Google Scholar]
- Williams LR, Danielsen N, Müller H, Varon S. Exogenous matrix precursors promote functional nerve regeneration across a 15‐mm gap within a silicone chamber in the rat. J Comp Neurol. 1987;264:284–290. doi: 10.1002/cne.902640211. [DOI] [PubMed] [Google Scholar]
- Yao L, Billiar KL, Windebank AJ, Pandit A. Multichanneled collagen conduits for peripheral nerve regeneration: design, fabrication, and characterization. Tissue Eng Part C Methods. 2010;16:1585–1596. doi: 10.1089/ten.TEC.2010.0152. [DOI] [PubMed] [Google Scholar]
- Yoo DJ, Na HS, Yoon YW. Behavioral manifestation of neuropathic pain following sciatic nerve section in rats. Exp Neurobiol. 2008;17:79–86. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.