Abstract
Alzheimer's disease (AD) is the most common form of dementia in the older population, however, the precise cause of the disease is unknown. The neuropathology is characterized by the presence of aggregates formed by amyloid-β (Aβ) peptide and phosphorylated tau; which is accompanied by progressive impairment of memory. Diverse signaling pathways are linked to AD, and among these the Wnt signaling pathway is becoming increasingly relevant, since it plays essential roles in the adult brain. Initially, Wnt signaling activation was proposed as a neuroprotective mechanism against Aβ toxicity. Later, it was reported that it participates in tau phosphorylation and processes of learning and memory. Interestingly, in the last years we demonstrated that Wnt signaling is fundamental in amyloid precursor protein (APP) processing and that Wnt dysfunction results in Aβ production and aggregation in vitro. Recent in vivo studies reported that loss of canonical Wnt signaling exacerbates amyloid deposition in a transgenic (Tg) mouse model of AD. Finally, we showed that inhibition of Wnt signaling in a Tg mouse previously at the appearance of AD signs, resulted in memory loss, tau phosphorylation and Aβ formation and aggregation; indicating that Wnt dysfunction accelerated the onset of AD. More importantly, Wnt signaling loss promoted cognitive impairment, tau phosphorylation and Aβ1–42 production in the hippocampus of wild-type (WT) mice, contributing to the development of an Alzheimer's-like neurophatology. Therefore, in this review we highlight the importance of Wnt/β-catenin signaling dysfunction in the onset of AD and propose that the loss of canonical Wnt signaling is a triggering factor of AD.
Keywords: Wnt signaling, Wnt target genes, Wnt/β-catenin, Alzheimer disease, amyloid-β, tau phosphorylation, memory loss, synaptic dysfunction
Alzheimer's Disease (AD) and Wnt Signaling
AD is the most prevalent dementia, increasing drastically with age (Mayeux and Stern, 2012). It is manifested by a progressive loss of learning and memory; as a result of the accumulation of senile plaques (SPs) and neurofibrillary tangles (NFTs) in the hippocampus and cerebral cortex (Selkoe and Hardy, 2016). The most studied hypotheses to explain the onset and development of AD is the amyloid hypothesis (Selkoe and Hardy, 2016). According to this cascade, amyloidogenic amyloid precursor protein (APP) processing lead to amyloid-β (Aβ) eptide formation; deregulating kinases, phosphatases, and several signaling pathways that promote tau phosphorylation, synaptic failure and neurodegeneration (Selkoe and Hardy, 2016). However, although the neuropathological signs, and potential protective mechanisms of AD have been described, the precise cause remains unclear. Interestingly, an important signaling pathway that is strongly related to AD is Wnt signaling (De Ferrari et al., 2014; Inestrosa and Varela-Nallar, 2014). This pathway is fundamental in the development of the Nervous Central System; but also plays important roles in the adult brain, regulating processes of synaptic plasticity and memory (Inestrosa and Varela-Nallar, 2014). Diverse reports have indicated that Wnt signaling is impaired in different AD mouse models (Scali et al., 2006; Toledo and Inestrosa, 2010). More important, familial AD patients with PS1 mutations have reduced β-catenin levels, strongly suggesting Wnt signaling loss (Zhang et al., 1998). More important, an allele of low-density lipoprotein receptor-related protein-6 (LRP6) associated with a loss of function of Wnt signaling is a susceptibility gene for late-onset AD (De Ferrari et al., 2007). In fact, a recent study showed that AD patients have a loss of Wnt activity, such as indicated by decreased β-catenin levels and increased tyrosine-216 phosphorylation of glycogen synthase kinase 3 (GSK-3β) compared with age-matched controls in the prefrontal cortical lobe structures of AD brains (Folke et al., 2018). Therefore, is possible that Wnt signaling activity plays a key role in the development of AD.
Wnt ligand binds to the Frizzled (Fz) receptor and LRP5/6 co-receptor, disassembling the destruction complex and inhibiting GSK-3β (Gao and Chen, 2010). This permits β-catenin to accumulate and translocate to the nucleus, where it binds to transcriptional factors T-cell factor/lymphoid enhancer factor (TCF/LEF) to induce the transcription of Wnt target genes (Nusse and Clevers, 2017). In contrast, in absence of Wnt proteins, GSK-3β becomes active, phosphorylating β-catenin and promoting its degradation via proteasome (Nusse and Clevers, 2017). The Wnt signaling family is composed of 19 ligands and 10 Fz receptors, which depending on the cellular context and ligand-receptor binding could activate Wnt/β-catenin dependent or independent signaling (Mikels and Nusse, 2006; Grumolato et al., 2010). In the neurons, the Wnt-Fz complexes are expressed spatially and temporally distinct in the cell (Varela-Nallar et al., 2012). Fz1 is located at the presynaptic region, promoting synaptic differentiation in hippocampal neurons (Varela-Nallar et al., 2009); Fz2 is mainly located at the neuronal soma (Varela-Nallar et al., 2012); Fz3 is expressed in dendrites (Varela-Nallar et al., 2012), regulating axonal outgrowth, guidance, and neural tube closure (Wang et al., 2002; Lyuksyutova et al., 2003); Fz5 control the survival of mature neurons in the thalamus (Liu et al., 2008), and it is localized in growth cones during development of hippocampal neurons (Varela-Nallar et al., 2012; Slater et al., 2013), where regulates synaptogenesis in the hippocampus (Sahores et al., 2010); Fz7 expression decreases during hippocampal development with a distribution in the soma and neurites (Zhao et al., 2005) and Fz9 is important for hippocampal development, due its enrichment at the growth cone (Varela-Nallar et al., 2012) and it is expressed along the processes of the post-synaptic region in mature hippocampal neurons (Ramirez et al., 2016).
Wnt signaling has important functions in the adult brain. Among these, it regulates the presynaptic structure (Inestrosa and Varela-Nallar, 2014), inducing the clustering of synapsin I (Lucas and Salinas, 1997; Hall et al., 2000) and the α7-nicotinic acetylcholine receptor (nAChR) (Farias et al., 2007); promoting the endocytosis and recycling of vesicles and increasing the pre-synaptic sites (Cerpa et al., 2008). Also, stimulated dendritogenesis (Ciani et al., 2011), induced neurotransmitter release (Cerpa et al., 2008), facilitates the induction of LTP (Chen et al., 2006) and its activity is modulated in spatial memory tasks (Tabatadze et al., 2012), playing a role in synaptic plasticity and cognition. The majority of the canonical Wnt pathway effects are mediated by the transcription of Wnt target genes; however several effects also could be occurring by a mechanism that did not required changes in the expression of Wnt target genes ref-jnc. An example is Wnt-7a in hippocampal neurons, which induces the clustering of presynaptic proteins without altering the expression of genes in response to β-catenin (Cerpa et al., 2008). But, independent of the mechanism, it is essential to highlight that synaptic failure is an early event in the AD pathology (Terry, 1991; Selkoe, 2002), supporting the possibility that Wnt signaling activity could be the key in AD pathogenesis.
Role of Wnt Signaling in the Pathogenesis of AD
Almost twenty years ago, our laboratory first proposed a relation between the Wnt signaling pathway and AD, suggesting that activation of Wnt/β-catenin signaling is protective against amyloid toxicity in vitro (De Ferrari and Inestrosa, 2000). Later, diverse reports have validated the neuroprotective role of the Wnt signaling pathway against the damage induced by Aβ species, preventing tau phosphorylation and preserving the cognitive abilities in vivo using mouse models of AD (Toledo and Inestrosa, 2010; Vargas et al., 2014; Rivera et al., 2016). We showed that activation of Wnt signaling by ANDRO (Andrographolide) (Tapia-Rojas et al., 2015a) ameliorates spatial memory loss, reduces the activation of inflammatory processes, and decreases the levels of Aβ species and tau phosphorylation in the hippocampus of a natural model of AD (Rivera et al., 2016). Similarly, in the triple transgenic (3×) transgenic (Tg)-AD mouse, activation of Wnt pathway reduces tau phosphorylation, Aβ formation, and prevents apoptosis (Jin et al., 2017). More importantly, our laboratory recently demonstrated that ANDRO improves learning and memory, recovery synaptic transmission, and reduces the levels of phosphorylated tau and Aβ aggregates in aged Octodon degus, a natural model of AD (Rivera et al., 2016). Thus, the protective role of Wnt signaling observed in vivo propose that activation of Wnt signaling pathways could be used as a potential treatment to impede the progression of AD.
Interestingly, studies have also suggested that the loss of Wnt signaling could be involved in the etiology of both familial AD (FAD) and sporadic AD (SAD). Concerning to FAD, mutations in the γ-secretase component Presenilin 1 (PS1) blocks the Wnt/β-catenin pathway (Zhang et al., 1998); promoting GSK-3β activity, tau hyper phosphorylation (Takashima et al., 1998), β-catenin degradation (Zhang et al., 1998) and repressing the transcriptional activities of the transcriptional co-factors of TCF/β-catenin (Francis et al., 2006). Also, conditional Lrp6 gene deletion in a mouse model of AD stimulates amyloid pathology (Liu et al., 2014), suggesting that Wnt signaling dysfunction could be involved in the early appearance and severity in genetic cases of AD (Baki et al., 2004). In SAD, the role of Wnt signaling in the initiation of AD is supported by studies that indicate that a polymorphism in the GSK-3 promoter increases its activity and is a risk factor for late-onset AD (Mateo et al., 2006). Also, an allele of the LRP6 co-receptor related to Wnt signaling dysfunction is a susceptibility gene for SAD (De Ferrari et al., 2007). Finally, chronic lithium medication reduces the prevalence of AD in patients with bipolar disorders (Forlenza et al., 2012), suggesting that activation of Wnt signaling might prevent processes necessary for the initiation and progression of SAD.
We recently demonstrated that loss of Wnt signaling activity could be a factor triggering the onset and progression of AD. In our last study, we were the first to demonstrate that inhibition of the Wnt signaling in the J20 Tg mouse model of AD, previous to the appearance of AD signs, accelerates the onset and development of the pathology, including accelerated Aβ production and aggregation, tau phosphorylation, and memory loss (Tapia-Rojas and Inestrosa, 2018). Interestingly, in the same study we reported that dysfunction of Wnt signaling triggered a neurodegenerative AD-like process in wild-type (WT) mice, leading to Aβ formation, tau phosphorylation, and alterations in learning and memory (Tapia-Rojas and Inestrosa, 2018). Thus, the loss of Wnt signaling could be considered a potential cause of AD and activation of Wnt signaling could prevent the development of the pathology.
In the following sections, we will describe studies that demonstrate the influence of canonical Wnt activity on the production and aggregation of Aβ, tau phosphorylation and synaptic failure that leads to memory loss, the main events associated to the AD neuropathology.
Wnt signaling promotes Aβ production
The Aβ peptide is produced by amyloidogenic APP processing, in which the APP protein is cleaved by the β-secretase enzyme and subsequently by the γ-secretase complex to generate products of 39–43 amino acids that are highly toxic to neurons (De Strooper et al., 2010). Amyloidogenic APP processing is abnormal and the causes that lead to this process in the brain are unidentified (Masters and Selkoe, 2012). Several studies have suggested that Wnt signaling could play a role in Aβ production. A Wnt agonist of the Wnt pathway in N2a cells decreases the Aβ peptide concentration and represses the transcription of the BACE (β-secretase enzyme β-site APP-cleaving enzyme) through the binding of β-catenin to TCF4 (Parr et al., 2015). In contrast, loss of Wnt function using a β-catenin shRNA significantly increases BACE1 mRNA and its protein expression (Parr et al., 2015). Similarly, LRP6 knockdown favors the formation of APP fragments via the amyloidogenic pathway, such as C-terminal fragment (CTF)β and the Aβ peptide (Liu et al., 2014).
Interestingly, we have demonstrated that inhibition of the Wnt/β-catenin pathway increases the Aβ1–42 concentration and the Aβ42/Aβ40 ratio, favoring the formation of Aβ oligomers in vitro (Tapia-Rojas et al., 2016). Specifically, our results show that blocking canonical Wnt signaling at different scales results in amyloidogenic proteolytic processing of APP, increasing the levels of C99, Aβ1–42 peptide, Aβ42/Aβ40 ratio, and low-molecular-weight Aβ oligomers such as trimers and tetramers (Tapia-Rojas et al., 2016). In contrast, we showed that activation of the Wnt pathway favors non-amyloidogenic APP processing, reducing Aβ1–42 peptide formation and aggregation, with the concomitant increment in Aβ1–40 peptide (Tapia-Rojas et al., 2016). The ability of canonical Wnt signaling to impede Aβ aggregation was also reported previously in our laboratory, using WASP-1 (Wnt-activating small molecule potentiator-1), a synergic molecule of the Wnt ligand Wnt-3a that enhances activation of the pathway, which blocks Aβ aggregation in in vitro assays (Vargas et al., 2015). More importantly, using mutant forms of β-catenin that are incapable of binding to the TCF/LEF transcription factor (β-cat ΔTCF), we showed for the first time that activation of canonical Wnt signaling requires of the transcription of Wnt target genes to switch APP processing to the non-amyloidogenic APP pathway, indicated by the formation of C83 fragment, and prevents Aβ1–42 production and formation of oligomers favoring the Aβ1–40 peptide generation (Tapia-Rojas et al., 2016). Finally, we proposed that Wnt signaling regulates APP processing, possibly by a mechanism that involves changes in the expression of BACE1 enzyme, because overexpression of β-catenin activate Wnt target genes and reduce BACE1 protein levels, in contrast to β-cat ΔTCF (Tapia-Rojas et al., 2016). This mechanism is relevant, because BACE1 inhibition also reduces Aβ in plasma, cerebrospinal fluid (CSF), and brain samples of rats, monkeys, and in AD patients (Kennedy et al., 2016). Therefore, our results indicate that inhibition of the Wnt/β-catenin signaling pathway promotes amyloidogenic APP processing, Aβ1–42 formation and aggregation.
It was previously reported that deletion of the Lrp6 gene in an APP-PS1 mouse model of AD aggravates amyloid deposition in the brain of these animals (Liu et al., 2014), suggesting that Wnt signaling also plays a role in the amyloid pathology in vivo. In addition, our laboratory demonstrated that activation of Wnt signaling in aged Octodon degus reduced Aβ1–40 and Aβ1–42 peptides, decreased the levels of Aβ oligomers, and the number of senile plaques in the hippocampus (Rivera et al., 2016), indicating that Wnt signaling activation prevents the formation of the Aβ peptide and the development of amyloid deposits. Interestingly and in contrast to the previously mentioned, we recently demonstrated that Wnt signaling dysfunction accelerates Aβ1–42 production in the hippocampus of a J20 Tg AD mouse model and promotes Aβ1–42 formation in the hippocampus of WT mice (Tapia-Rojas and Inestrosa, 2018). The increased concentrations of Aβ1–42 peptide in the hippocampus of these animals was accompanied by an increase in the Aβ42/Aβ40 ratio, suggesting higher toxicity in the brain of both Tg and WT animals only by inhibiting Wnt/β-catenin signaling (Tapia-Rojas and Inestrosa, 2018). This effect is similar to that previously reported in FAD patients, in which an increased Aβ42/Aβ40 ratio has been directly related with severe forms of the pathology (Hellstrom-Lindahl et al., 2009). We also reported that in treated mice, the levels of Aβ1–42 in the CSF were decreased (Tapia-Rojas and Inestrosa, 2018), suggesting altered clearance of Aβ possibly as consequence of Aβ aggregation. In fact, we observed a major presence of Aβ soluble species in the brain of animals exposed to Wnt inhibitors and in J20 Tg mice we detected the presence of Aβ species that could correspond to hexamers and do-decamer soluble aggregates (Tapia-Rojas and Inestrosa, 2018), which are considered synaptotoxic (Lesné et al., 2006, 2013). The latter is consistent with reports that have indicated that few alterations in the Aβ42/Aβ40 ratio promote Aβ aggregation forming oligomers (Yoshiike et al., 2003; Kuperstein et al., 2010). Additionally, in Tg mice with deficient function of Wnt signaling we observed increased number of senile plaques (Tapia-Rojas and Inestrosa, 2018), validating the in vitro studies that indicated that Wnt signaling loss lead to Aβ production and aggregation.
Importantly, we reveal that Wnt signaling inhibition trigger the formation Aβ1–42 and increase the Aβ42/Aβ40 ratio in the hippocampus of WT mice, effect that was accompanied of a reduction in Aβ1–42 concentration in the CSF. The Aβ pathology observed in WT mice is consistent with previous studies, in which we demonstrated that a high methionine diet induces dysfunction of canonical Wnt signaling at the same time that it promotes Aβ formation, oxidative stress and memory impairment (Tapia-Rojas et al., 2015b). Altogether, our in vitro and in vivo studies demonstrate that inhibition of Wnt/β-catenin signaling promotes amyloidogenic APP processing, Aβ1–42 production, increased Aβ42/Aβ40 ratio and formation of aggregates; which are fundamental for promoting toxicity in the AD pathology (McLean et al., 1999).
The amyloid accumulation is promoted by an imbalance between Aβ production and clearance. Microglia in the brain plays an important role in Aβ clearance by a variety of phagocytic and digestive mechanisms. Among these mechanisms, TREM2 (an innate immune receptor and a Type I transmembrane protein) regulates phagocytosis and promotes microglial survival, contributing to Aβ elimination (Ries and Sastre, 2016). Interestingly, TREM2 promotes microglial survival by activating the Wnt/β-catenin signaling pathway, suggesting that Wnt pathway is necessary to the correct clearance of amyloid deposits (Zheng et al., 2017). Another possibility to degrade Aβ is promoting the expression of Insulin degrading enzyme (IDE). Unpublished data of our laboratory suggest that IDE is a target gene of the Wnt pathway, therefore Wnt signaling also could modulates Aβ degradation. More studies are necessary to validate these possibilities.
Wnt signaling regulates tau phosphorylation
Tau phosphorylation is mediated by diverse protein kinases which can phosphorylate tau at different sites (Mandelkow and Mandelkow, 2012). Among these enzymes, GSK-3β phosphorylates practically all tau residues described in AD (Kremer et al., 2011; Hernandez et al., 2013). GSK-3β is a central component of Wnt signaling and its activity is mediated by the function of the Wnt/β-catenin pathway (Metcalfe and Bienz, 2011). GSK-3β inhibition impedes the formation of NFTs observed in a double transgenic of GSK-3β and tau protein (Engel et al., 2006). Our laboratory showed that Wnt signaling activation by ANDRO reduces tau phosphorylation in young and old APP-PS1 AD mouse model (Serrano et al., 2014). A similar effect was observed in aged Octodon degus treated with ANDRO, where the levels of phosphorylated Thr231, Ser235, and both Ser202 and Thr205 (AT8 epitope) were decreased (Rivera et al., 2016). Synergic activation of canonical Wnt signaling by WASP-1 in APP-PS1 mice also reduced PHF-1 (Ser396 and Ser404) tau phosphorylation (Vargas et al., 2015), strongly suggesting that Wnt activation prevent tau phosphorylation.
In contrast, mutations in PS1 promote GSK-3β activity and tau phosphorylation (Takashima et al., 1998). Conditional transgenic mice overexpressing GSK-3β in the adult brain showed decreased β-catenin levels in a nuclear fraction, increased tau phosphorylation, and neuronal death (Lucas et al., 2001). Inhibition of Wnt signaling favors GSK-3β-mediated tau hyper phosphorylation (Scali et al., 2006; Hooper et al., 2008). Local infusion of Dkk1, a canonical Wnt antagonist, leads to astrocyte activation, PHF1 tau phosphorylation, and neuronal death in the hippocampus of rats, effect that was blocked by lithium administration (Scali et al., 2006). In our last study we reported that attenuation of canonical Wnt signaling in J20 Tg mice accelerated the appearance of phosphorylated tau epitopes, including Thr231, Ser235, and AT8 (Tapia-Rojas et al., 2016). Surprisingly, we also have reported the presence of phosphorylated tau residues in WT mice blocking Wnt pathway. Inhibition of Wnt signaling by a high methionine diet also lead to increased phosphorylation at Ser235 and Thr231 sites in the hippocampus of WT mice (Tapia-Rojas et al., 2015b). This same effect was observed using specific inhibitors of the canonical Wnt signaling pathway, which increased phosphorylation at Ser235, Thr231, PHF1 and AT8 epitopes in the hippocampus of WT mice. Then, inhibition of Wnt signaling activates kinases that can phosphorylate tau, specifically to GSK-3β, and thus could favor its dissociation of the microtubules.
Memory loss mediated by canonical Wnt signaling dysfunction
Memory loss is the final consequence of AD pathology and according to the neurodegeration progress, memory impairment is more evident (Shankar and Walsh, 2009). The amyloid hypothesis proposes that loss of cognitive abilities is product of the synaptic failure mediated by soluble Aβ species (Selkoe and Hardy, 2016). Wnt signaling plays key roles regulating synaptic structure and function (Speese and Budnik, 2007; Oliva et al., 2013). Additionally, Wnt signaling activation occur in spatial learning (Tabatadze et al., 2012) and result in the transcription of Wnt target genes important to memory consolidation, such as calmodulin-dependent protein kinase type IV (CaMKIV) (Arrazola et al., 2009). Thus, activation of Wnt/β-catenin signaling recovers the hippocampus-dependent cognitive impairment in an APP-PS1 mouse model of AD (Toledo and Inestrosa, 2010; Serrano et al., 2014) and in Octodon degus (Rivera et al., 2016). The precise mechanism involved in the restoration of memory mediated by Wnt signaling activation in AD models is yet unknown, but possible mechanism could include the cholinergic system, because activation of canonical Wnt pathway promotes the pre-synaptic localization of α7-nAChRs (Farias et al., 2007) and the canonical ligand Wnt3a induce the differentiation of adipose-derived stem cells into cholinergic neurons, were the expression of Wnt3a increases and correlates with an increased expression choline acetyltransferase (Liu et al., 2012).
Interestingly, an increase in the levels of the Wnt/β-catenin antagonist Dkk-1 is necessary to produce Aβ-mediated synaptic loss (Purro et al., 2012), suggesting that Wnt signaling has a central role in synaptic failure and memory loss Aβ-induced. Thus, Wnt signaling loss accelerates the appearance of cognitive impairment in J20 Tg mouse model of AD. More important, Wnt signaling impairment leads to altered long-term potentiation (LTP) in WT mice, a neurophysiological correlate of learning and memory (Chen et al., 2006), and triggered severe defects in spatial learning and memory in WT mice. Only blocking canonical Wnt pathway is possible to promote spatial memory loss (Tapia-Rojas and Inestrosa, 2018) and impaired recognition memory in WT mice (Fortress et al., 2013), suggesting that Wnt signaling activity modulates process of learning and memory by a mechanism independent of Aβ. Therefore, canonical Wnt signaling dysfunction is a key factor inducing learning and memory impairment and could be relevant in neurodegenerative diseases such as AD.
Conclusions
It is now known that the canonical Wnt signaling pathway is not only a protective agent in AD. Wnt/β-catenin signaling activity is fundamental for the onset of AD (Figure 1); signaling inhibition can accelerate the appearance and development of AD neuropathology and memory loss in a Tg mouse model of AD. More importantly, Wnt signaling dysfunction is sufficient to promote a neuropathological process that involves the development of three AD hallmarks: i) production and aggregation of Aβ, ii) tau phosphorylation and iii) hippocampus-dependent cognitive impairment. Thus, loss of the canonical Wnt pathway could be considered a triggering factor of the AD pathogenesis and the use of activators of the Wnt pathway could be used to prevent the development of AD-like dementia. Currently, there are no public clinical trials testing the beneficial effects of Wnt activation in a therapeutical treatment of AD. The challenge is to prevent the loss of the function of the Wnt pathway during aging, in order to prevent the appearance and development of AD.
Figure 1.
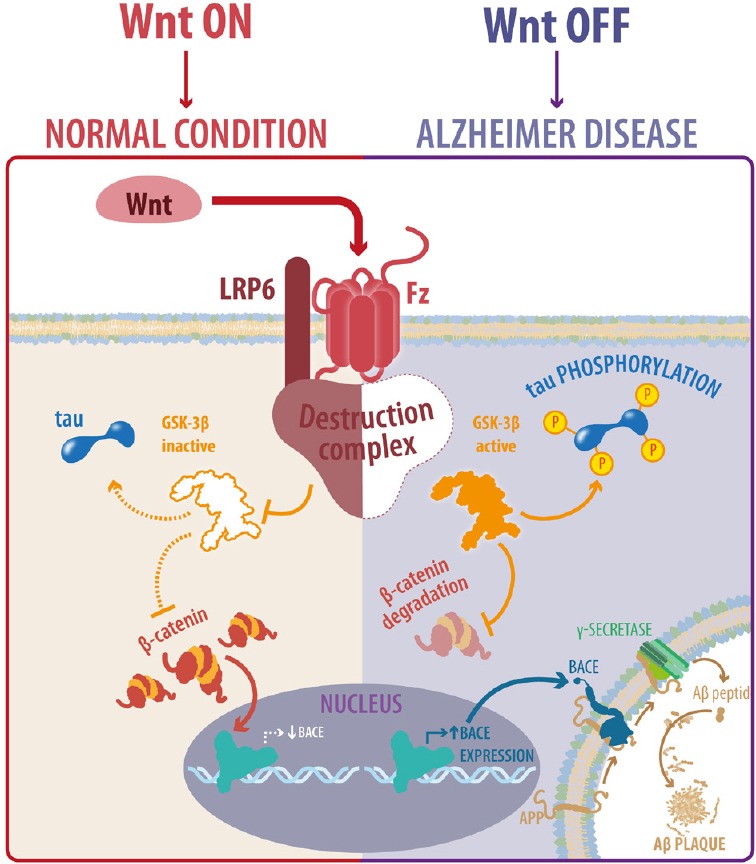
Wnt signaling activity is crucial in the pathogenesis of Alzheimer's disease (AD).
When Wnt signaling is active (Wnt ON), tau protein remains dephosphorylated and Aβ1–42 peptide is not produced preserving a normal condition; in contrast, loss of Wnt signaling function (Wnt OFF) leads to tau phosphorylation, Aβ1–42 production and aggregation and induces AD pathology. LRP6: Lipoprotein receptor-related protein-6; Fz: Frizzled; GSK-3β: glycogen synthase kinase 3.
Additional file: Open peer review reports 1 (104.4KB, pdf) , 2 (103.6KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that they have no conflicts of interest.
Financial support: This work was supported by grants PFB 12/2007 from the Basal Centre for Excellence in Science and Technology and FONDECYT, No. 1120156 (to NCI) and a pre-doctoral fellowship from the National Commission of Science and Technology of Chile (CONICYT) (to CTR).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Yun-Bae Kim, Chungbuk National University, Korea; Paulina Carriba, Cardiff University, UK.
Funding: This work was supported by grants PFB (Basal Financing Program) 12/2007 from the Basal Centre for Excellence in Science and Technology and FONDECYT, No. 1120156 (to NCI) and a pre-doctoral fellowship from the National Commission of Science and Technology of Chile (CONICYT) (to CTR).
References
- Arrazola MS, Varela-Nallar L, Colombres M, Toledo EM, Cruzat F, Pavez L, Assar R, Aravena A, Gonzalez M, Montecino M, Maass A, Martinez S, Inestrosa NC. Calcium/calmodulin-dependent protein kinase type IV is a target gene of the Wnt/beta-catenin signaling pathway. J Cell Physiol. 2009;221:658–667. doi: 10.1002/jcp.21902. [DOI] [PubMed] [Google Scholar]
- Baki L, Shioi J, Wen P, Shao Z, Schwarzman A, Gama-Sosa M, Neve R, Robakis NK. PS1 activates PI3K thus inhibiting GSK-3 activity and tau overphosphorylation: effects of FAD mutations. EMBO J. 2004;23:2586–2596. doi: 10.1038/sj.emboj.7600251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerpa W, Godoy JA, Alfaro I, Farias GG, Metcalfe MJ, Fuentealba R, Bonansco C, Inestrosa NC. Wnt-7a modulates the synaptic vesicle cycle and synaptic transmission in hippocampal neurons. J Biol Chem. 2008;283:5918–5927. doi: 10.1074/jbc.M705943200. [DOI] [PubMed] [Google Scholar]
- Chen J, Park CS, Tang SJ. Activity-dependent synaptic Wnt release regulates hippocampal long term potentiation. J Biol Chem. 2006;281:11910–11916. doi: 10.1074/jbc.M511920200. [DOI] [PubMed] [Google Scholar]
- Ciani L, Boyle KA, Dickins E, Sahores M, Anane D, Lopes DM, Gibb AJ, Salinas PC. Wnt7a signaling promotes dendritic spine growth and synaptic strength through Ca(2)(+)/Calmodulin-dependent protein kinase II. Proc Natl Acad Sci U S A. 2011;108:10732–10737. doi: 10.1073/pnas.1018132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari G, Papassotiropoulos A, Biechele T, Wavrant De-Vrieze F, Avila M, Major M, Myers A, Sáez K, Henríquez J, Zhao A, Wollmer M, Nitsch R, Hock C, Morris C, Hardy J, Moon R. Common genetic variation within the low-density lipoprotein receptor-related protein 6 and late-onset Alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:9434–9439. doi: 10.1073/pnas.0603523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ferrari GV, Inestrosa NC. Wnt signaling function in Alzheimer's disease. Brain Res Brain Res Rev. 2000;33:1–12. doi: 10.1016/s0165-0173(00)00021-7. [DOI] [PubMed] [Google Scholar]
- De Ferrari GV, Avila ME, Medina MA, Perez-Palma E, Bustos BI, Alarcon MA. Wnt/beta-catenin signaling in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2014;13:745–754. doi: 10.2174/1871527312666131223113900. [DOI] [PubMed] [Google Scholar]
- De Strooper B, Vassar R, Golde T. The secretases: enzymes with therapeutic potential in Alzheimer disease. Nat Rev Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel T, Goni-Oliver P, Lucas JJ, Avila J, Hernandez F. Chronic lithium administration to FTDP-17 tau and GSK-3beta overexpressing mice prevents tau hyperphosphorylation and neurofibrillary tangle formation, but pre-formed neurofibrillary tangles do not revert. J Neurochem. 2006;99:1445–1455. doi: 10.1111/j.1471-4159.2006.04139.x. [DOI] [PubMed] [Google Scholar]
- Farias GG, Valles AS, Colombres M, Godoy JA, Toledo EM, Lukas RJ, Barrantes FJ, Inestrosa NC. Wnt-7a induces presynaptic colocalization of alpha 7-nicotinic acetylcholine receptors and adenomatous polyposis coli in hippocampal neurons. J Neurosci. 2007;27:5313–5325. doi: 10.1523/JNEUROSCI.3934-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folke J, Pakkenberg B, Brudek T. Impaired Wnt signaling in the prefrontal cortex of Alzheimer's disease. Mol Neurobiol. 2018 doi: 10.1007/s12035-018-1103-z. doi: 10.1007/s12035-018-1103-z. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, de Paula VJ, Machado-Vieira R, Diniz BS, Gattaz WF. Does lithium prevent Alzheimer's disease? Drugs Aging. 2012;29:335–342. doi: 10.2165/11599180-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Fortress AM, Schram SL, Tuscher JJ, Frick KM. Canonical Wnt signaling is necessary for object recognition memory consolidation. J Neurosci. 2013;33:12619–12626. doi: 10.1523/JNEUROSCI.0659-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis YI, Stephanou A, Latchman DS. CREB-binding protein activation by presenilin 1 but not by its M146L mutant. Neuroreport. 2006;17:917–921. doi: 10.1097/01.wnr.0000220137.06542.a0. [DOI] [PubMed] [Google Scholar]
- Gao C, Chen YG. Dishevelled: The hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Grumolato L, Liu G, Mong P, Mudbhary R, Biswas R, Arroyave R, Vijayakumar S, Economides AN, Aaronson SA. Canonical and noncanonical Wnts use a common mechanism to activate completely unrelated coreceptors. Genes Dev. 2010;24:2517–2530. doi: 10.1101/gad.1957710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall AC, Lucas FR, Salinas PC. Axonal remodeling and synaptic differentiation in the cerebellum is regulated by WNT-7a signaling. Cell. 2000;100:525–535. doi: 10.1016/s0092-8674(00)80689-3. [DOI] [PubMed] [Google Scholar]
- Hellstrom-Lindahl E, Viitanen M, Marutle A. Comparison of Abeta levels in the brain of familial and sporadic Alzheimer's disease. Neurochem Int. 2009;55:243–252. doi: 10.1016/j.neuint.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Lucas J, Avila J. GSK3 and tau: two convergence points in Alzheimer's disease. J Alzheimers Dis. 2013;33:S141–144. doi: 10.3233/JAD-2012-129025. [DOI] [PubMed] [Google Scholar]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Varela-Nallar L. Wnt signaling in the nervous system and in Alzheimer's disease. J Mol Cell Biol. 2014;6:64–74. doi: 10.1093/jmcb/mjt051. [DOI] [PubMed] [Google Scholar]
- Jin N, Zhu H, Liang X, Huang W, Xie Q, Xiao P, Ni J, Liu Q. Sodium selenate activated Wnt/beta-catenin signaling and repressed amyloid-beta formation in a triple transgenic mouse model of Alzheimer's disease. Exp Neurol. 2017;297:36–49. doi: 10.1016/j.expneurol.2017.07.006. [DOI] [PubMed] [Google Scholar]
- Kennedy ME, Stamford AW, Chen X, Cox K, Cumming JN, Dockendorf MF, Egan M, Ereshefsky L, Hodgson RA, Hyde LA, Jhee S, Kleijn HJ, Kuvelkar R, Li W, Mattson BA, Mei H, Palcza J, Scott JD, Tanen M, Troyer MD, et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS beta-amyloid in animal models and in Alzheimer's disease patients. Sci Transl Med. 2016;8:363ra150. doi: 10.1126/scitranslmed.aad9704. [DOI] [PubMed] [Google Scholar]
- Kremer A, Louis JV, Jaworski T, Van Leuven F. GSK3 and Alzheimer's disease: facts and fiction. Front Mol Neurosci. 2011;4:17. doi: 10.3389/fnmol.2011.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperstein I, Broersen K, Benilova I, Rozenski J, Jonckheere W, Debulpaep M, Vandersteen A, Segers-Nolten I, Van Der Werf K, Subramaniam V, Braeken D, Callewaert G, Bartic C, D’Hooge R, Martins IC, Rousseau F, Schymkowitz J, De Strooper B. Neurotoxicity of Alzheimer's disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010;29:3408–3420. doi: 10.1038/emboj.2010.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S, Sherman M, Grant M, Kuskowski M, Schneider J, Bennett D, Ashe K. Brain amyloid-β oligomers in ageing and Alzheimer's disease. Brain. 2013;136:1383–1398. doi: 10.1093/brain/awt062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesné S, Koh MT, Kotilinek L, Kayed R, Glabe CG, Yang A, Gallagher M, Ashe KH. A specific amyloid-β protein assembly in the brain impairs memory. Nature. 2006;440:352. doi: 10.1038/nature04533. [DOI] [PubMed] [Google Scholar]
- Liu B, Deng C, Zhang Y, Zhang J. Wnt3a expression during the differentiation of adipose-derived stem cells into cholinergic neurons. Neural Regen Res. 2012;7:1463–1468. doi: 10.3969/j.issn.1673-5374.2012.19.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Wang Y, Smallwood PM, Nathans J. An essential role for Frizzled5 in neuronal survival in the parafascicular nucleus of the thalamus. J Neurosci. 2008;28:5641–5653. doi: 10.1523/JNEUROSCI.1056-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CC, Tsai CW, Deak F, Rogers J, Penuliar M, Sung YM, Maher JN, Fu Y, Li X, Xu H, Estus S, Hoe HS, Fryer JD, Kanekiyo T, Bu G. Deficiency in LRP6-mediated Wnt signaling contributes to synaptic abnormalities and amyloid pathology in Alzheimer's disease. Neuron. 2014;84:63–77. doi: 10.1016/j.neuron.2014.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas FR, Salinas PC. WNT-7a induces axonal remodeling and increases synapsin I levels in cerebellar neurons. Dev Biol. 1997;192:31–44. doi: 10.1006/dbio.1997.8734. [DOI] [PubMed] [Google Scholar]
- Lucas JJ, Hernandez F, Gomez-Ramos P, Moran MA, Hen R, Avila J. Decreased nuclear beta-catenin, tau hyperphosphorylation and neurodegeneration in GSK-3beta conditional transgenic mice. EMBO J. 2001;20:27–39. doi: 10.1093/emboj/20.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior-posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. doi: 10.1101/cshperspect.a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters CL, Selkoe DJ. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006262. doi: 10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo I, Infante J, Llorca J, Rodriguez E, Berciano J, Combarros O. Association between glycogen synthase kinase-3beta genetic polymorphism and late-onset Alzheimer's disease. Dement Geriatr Cogn Disord. 2006;21:228–232. doi: 10.1159/000091044. [DOI] [PubMed] [Google Scholar]
- Mayeux R, Stern Y. Epidemiology of Alzheimer disease. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling--two contrasting models. J Cell Sci. 2011;124:3537–3544. doi: 10.1242/jcs.091991. [DOI] [PubMed] [Google Scholar]
- Mikels AJ, Nusse R. Wnts as ligands: processing, secretion and reception. Oncogene. 2006;25:7461–7468. doi: 10.1038/sj.onc.1210053. [DOI] [PubMed] [Google Scholar]
- Nusse R, Clevers H. Wnt/beta-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Oliva C, Vargas J, Inestrosa N. Wnt signaling: Role in LTP, neural networks and memory. Ageing Res Rev. 2013;12:786–800. doi: 10.1016/j.arr.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Parr C, Mirzaei N, Christian M, Sastre M. Activation of the Wnt/beta-catenin pathway represses the transcription of the beta-amyloid precursor protein cleaving enzyme (BACE1) via binding of T-cell factor-4 to BACE1 promoter. FASEB J. 2015;29:623–635. doi: 10.1096/fj.14-253211. [DOI] [PubMed] [Google Scholar]
- Purro SA, Dickins EM, Salinas PC. The secreted Wnt antagonist Dickkopf-1 is required for amyloid beta-mediated synaptic loss. J Neurosci. 2012;32:3492–3498. doi: 10.1523/JNEUROSCI.4562-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez VT, Ramos-Fernandez E, Henriquez JP, Lorenzo A, Inestrosa NC. Wnt-5a/Frizzled9 receptor signaling through the Gαo-Gβγ complex regulates dendritic spine formation. J Biol Chem. 2016;291:19092–19107. doi: 10.1074/jbc.M116.722132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M, Sastre M. Mechanisms of Aβ clearance and degradation by glial cells. Front Aging Neurosci. 2016;8:160. doi: 10.3389/fnagi.2016.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera DS, Lindsay C, Codocedo JF, Morel I, Pinto C, Cisternas P, Bozinovic F, Inestrosa NC. Andrographolide recovers cognitive impairment in a natural model of Alzheimer's disease (Octodon degus) Neurobiol Aging. 2016;46:204–220. doi: 10.1016/j.neurobiolaging.2016.06.021. [DOI] [PubMed] [Google Scholar]
- Sahores M, Gibb A, Salinas PC. Frizzled-5, a receptor for the synaptic organizer Wnt7a, regulates activity-mediated synaptogenesis. Development. 2010;137:2215–2225. doi: 10.1242/dev.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scali C, Caraci F, Gianfriddo M, Diodato E, Roncarati R, Pollio G, Gaviraghi G, Copani A, Nicoletti F, Terstappen GC, Caricasole A. Inhibition of Wnt signaling, modulation of Tau phosphorylation and induction of neuronal cell death by DKK1. Neurobiol Dis. 2006;24:254–265. doi: 10.1016/j.nbd.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Hardy J. The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med. 2016;8:595–608. doi: 10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano FG, Tapia-Rojas C, Carvajal FJ, Hancke J, Cerpa W, Inestrosa NC. Andrographolide reduces cognitive impairment in young and mature AbetaPPswe/PS-1 mice. Mol Neurodegener. 2014;9:61. doi: 10.1186/1750-1326-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Walsh DM. Alzheimer's disease: synaptic dysfunction and Abeta. Mol Neurodegener. 2009;4:48. doi: 10.1186/1750-1326-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater PG, Ramirez VT, Gonzalez-Billault C, Varela-Nallar L, Inestrosa NC. Frizzled-5 receptor is involved in neuronal polarity and morphogenesis of hippocampal neurons. PLoS One. 2013;8:e78892. doi: 10.1371/journal.pone.0078892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speese S, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabatadze N, Tomas C, McGonigal R, Lin B, Schook A, Routtenberg A. Wnt transmembrane signaling and long-term spatial memory. Hippocampus. 2012;22:1228–1241. doi: 10.1002/hipo.20991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima A, Murayama M, Murayama O, Kohno T, Honda T, Yasutake K, Nihonmatsu N, Mercken M, Yamaguchi H, Sugihara S, Wolozin B. Presenilin 1 associates with glycogen synthase kinase-3beta and its substrate tau. Proc Natl Acad Sci U S A. 1998;95:9637–9641. doi: 10.1073/pnas.95.16.9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapia-Rojas C, Inestrosa NC. Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer's disease in J20-APP transgenic and wild-type mice. J Neurochem. 2018;144:443–465. doi: 10.1111/jnc.14278. [DOI] [PubMed] [Google Scholar]
- Tapia-Rojas C, Burgos PV, Inestrosa NC. Inhibition of Wnt signaling induces amyloidogenic processing of amyloid precursor protein and the production and aggregation of Amyloid-beta (Abeta)42 peptides. J Neurochem. 2016;139:1175–1191. doi: 10.1111/jnc.13873. [DOI] [PubMed] [Google Scholar]
- Tapia-Rojas C, Schuller A, Lindsay CB, Ureta RC, Mejias-Reyes C, Hancke J, Melo F, Inestrosa NC. Andrographolide activates the canonical Wnt signalling pathway by a mechanism that implicates the non-ATP competitive inhibition of GSK-3beta: autoregulation of GSK-3beta in vivo. Biochem J. 2015a;466:415–430. doi: 10.1042/BJ20140207. [DOI] [PubMed] [Google Scholar]
- Tapia-Rojas C, Lindsay CB, Montecinos-Oliva C, Arrazola MS, Retamales RM, Bunout D, Hirsch S, Inestrosa NC. Is L-methionine a trigger factor for Alzheimer's-like neurodegeneration? Changes in Abeta oligomers, tau phosphorylation, synaptic proteins, Wnt signaling and behavioral impairment in wild-type mice. Mol Neurodegener. 2015b;10:62. doi: 10.1186/s13024-015-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry R, Masliah E, Salmon DP, Butters N, DeTeresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol 1991. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Toledo EM, Inestrosa NC. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer's disease. Mol Psychiatry. 2010;15:272–285. doi: 10.1038/mp.2009.72. 228. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Ramirez VT, Gonzalez-Billault C, Inestrosa NC. Frizzled receptors in neurons: from growth cones to the synapse. Cytoskeleton (Hoboken) 2012;69:528–534. doi: 10.1002/cm.21022. [DOI] [PubMed] [Google Scholar]
- Varela-Nallar L, Grabowski CP, Alfaro IE, Alvarez AR, Inestrosa NC. Role of the Wnt receptor Frizzled-1 in presynaptic differentiation and function. Neural Dev. 2009;4:41. doi: 10.1186/1749-8104-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JY, Fuenzalida M, Inestrosa NC. In vivo activation of Wnt signaling pathway enhances cognitive function of adult mice and reverses cognitive deficits in an Alzheimer's disease model. J Neurosci. 2014;34:2191–2202. doi: 10.1523/JNEUROSCI.0862-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas JY, Ahumada J, Arrazola MS, Fuenzalida M, Inestrosa NC. WASP-1, a canonical Wnt signaling potentiator, rescues hippocampal synaptic impairments induced by Aβ oligomers. Exp Neurol. 2015;264:14–25. doi: 10.1016/j.expneurol.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Thekdi N, Smallwood PM, Macke JP, Nathans J. Frizzled-3 is required for the development of major fiber tracts in the rostral CNS. J Neurosci. 2002;22:8563–8573. doi: 10.1523/JNEUROSCI.22-19-08563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiike Y, Chui DH, Akagi T, Tanaka N, Takashima A. Specific compositions of amyloid-beta peptides as the determinant of toxic beta-aggregation. J Biol Chem. 2003;278:23648–23655. doi: 10.1074/jbc.M212785200. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, De Strooper B, He X, Yankner BA. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- Zhao C, Aviles C, Abel RA, Almli CR, McQuillen P, Pleasure SJ. Hippocampal and visuospatial learning defects in mice with a deletion of frizzled 9, a gene in the Williams syndrome deletion interval. Development. 2005;132:2917–2927. doi: 10.1242/dev.01871. [DOI] [PubMed] [Google Scholar]
- Zheng H, Jia L, Liu CC, Rong Z, Zhong L, Yang L, Chen XF, Fryer JD, Wang X, Zhang YW, Xu H, Bu G. TREM2 promotes microglial survival by activating Wnt/β-catenin pathway. J Neurosci. 2017;37:1772–1784. doi: 10.1523/JNEUROSCI.2459-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


