Keywords: peripheral nerve regeneration, crushed sciatic nerve, RNA-seq, adherens junctions, remodeling of adherens junctions, Venn diagram, ingenuity pathway analysis, differentially expressed genes, comprehensive transcript analysis, transcriptomics, heatmap, neural regeneration
Abstract
The neural regeneration process is driven by a wide range of molecules and pathways. Adherens junctions are critical cellular junctions for the integrity of peripheral nerves. However, few studies have systematically characterized the transcript changes in the adherens junction pathway following injury. In this study, a rat model of sciatic nerve crush injury was established by forceps. Deep sequencing data were analyzed using comprehensive transcriptome analysis at 0, 1, 4, 7, and 14 days after injury. Results showed that most individual molecules in the adherens junctions were either upregulated or downregulated after nerve injury. The mRNA expression of ARPC1B, ARPC3, TUBA8, TUBA1C, CTNNA2, ACTN3, MET, HGF, NME1 and ARF6, which are involved in the adherens junction pathway and in remodeling of adherens junctions, was analyzed using quantitative real-time polymerase chain reaction. Most of these genes were upregulated in the sciatic nerve stump following peripheral nerve injury, except for CTNNA2, which was downregulated. Our findings reveal the dynamic changes of key molecules in adherens junctions and in remodeling of adherens junctions. These key genes provide a reference for the selection of clinical therapeutic targets for peripheral nerve injury.
Introduction
Peripheral nerves have a striking capacity for regeneration compared with nerves in the central nervous system (Chen et al., 2007; Gu et al., 2011, 2014). Understanding the molecular events in peripheral nerve regeneration might offer strategies to promote axon regeneration in the central nervous system (Camara-Lemarroy et al., 2010; Bosse, 2012; Yu et al., 2015; Farkas and Monaghan, 2017; Li et al., 2017). After nerve injury, distal axons gradually degenerate and eventually disappear, a process known as Wallerian degeneration (Gaudet et al., 2011; Yu et al., 2016; Cheng et al., 2017; Yi et al., 2017). Schwann cells divide and align longitudinally along the basal lamina (Bhatheja and Field, 2006; Masaki and Matsumura, 2010; Webber and Zochodne, 2010; Morrissey and Sherwood, 2015). Schwann cells and basal lamina tubes promote regenerating axon extension (Keynes, 1987; Doherty and Walsh, 1994; Reichert et al., 1994; Walsh and Doherty, 1996; Zhang et al., 2008). Adhesion molecules are critical for axonal regrowth and axon-axon or axon-Schwann cell attachment (Fex Svennigsen and Dahlin, 2013). Numerous studies have reported changes in expression of adhesion molecules following sciatic injury (Ide, 1996; Fu and Gordon, 1997; Raivich and Makwana, 2007; Patodia and Raivich, 2012).
Adhesion receptors (such as, E-cadherin and N-cadherin) mediate homophilic recognition across the intercellular cleft, and they link to a cytoskeletal network via catenins. These core structural components form adherens junctions. Adherens junctions play a key role in cell attachment, growth, and migration (Niessen and Gottardi, 2008; Etienne-Manneville, 2011, 2012; Peglion et al., 2014; Woichansky et al., 2016; Malinova and Huveneers, 2017). The adherens junction is regulated by receptors and intracellular signaling (Padmanabhan et al., 2015). For example, endocytosis and recycling of cell surface E-cadherin are regulated by hepatocyte growth factor (HGF) and the receptor tyrosine kinase Met (Anderson et al., 2006). Met phosphorylates tyrosine residues in the cytoplasmic tail of E-cadherin, and thereby induces the internalization of E-cadherin. Subsequently, E-cadherin is recycled to sites of new cell-cell contacts. However, few studies, if any, have systematically analyzed transcript changes of molecules in the pathways of the adherens junction and in remodeling of adherens junctions following peripheral nerve injury.
In the current study, we analyzed the molecular events in adherens junctions and remodeling of adherens junctions following peripheral nerve regeneration based on high throughput RNA-seq data. We found distinct transcription changes in different molecules in the pathways of adherens junctions and remodeling of adherens junctions, which were further confirmed by quantitative real-time polymerase chain reaction (qRT-PCR). Our study aimed to reveal comprehensive transcript changes in the pathways of adherens junctions and remodeling of adherens junctions.
Materials and Methods
Animals
Thirty adult, 2-month-old, male Sprague-Dawley rats, weighing 180–220 g, were obtained from the Experimental Animal Center of Nantong University, China (animal license number: SCXK (Su) 2014-0001 and SYXK (Su) 2012-0031), and were used for deep sequencing. An additional 30 rats were used for qRT-PCR. This study was approved by the Administration Committee of Experimental Animals, Jiangsu, China (approval No. 20160229-007).
Establishment of sciatic nerve crush injury model
Thirty rats were randomly divided into five groups: 0, 1, 4, 7, and 14 days after sciatic nerve crush with 6 rats in each group. The 0 day group was an uninjured group used as a control. Rats were anesthetized and underwent one-side sciatic nerve crush surgery as described previously (Yi et al., 2015; Xing et al., 2017). In brief, the left sciatic nerve at mid-thigh level was crushed by forceps (RWD Life Science, Shenzhen, China) three times at a constant force of 54 N for 10 seconds each time. Rats were sacrificed by cervical dislocation at the respective time for each group.
Transcriptome sequencing and bioinformatic analysis
After 5-mm sciatic nerve segments were harvested from the crush site, total RNA was extracted using TRIzol. Transcriptome sequencing was performed as described previously (Yi et al., 2015). The abundances of genes at 0, 1, 4, 7, and 14 days after sciatic nerve crush were expressed as RPKM (reads per kilobase transcriptome per million mapped reads) (Mortazavi et al., 2008). Genes with a false discovery rate ≤0.001 and a fold change ≥ 2 (log2 ≥ 1) were defined as differentially expressed genes (DEGs). DEGs were further uploaded to QIAGEN's Ingenuity® Pathway Analysis (IPA®, QIAGEN, Valencia, CA, USA); the canonical signaling pathways adherens junctions and remodeling of adherens junctions were analyzed based on the Ingenuity Pathways Knowledge Base.
qRT-PCR
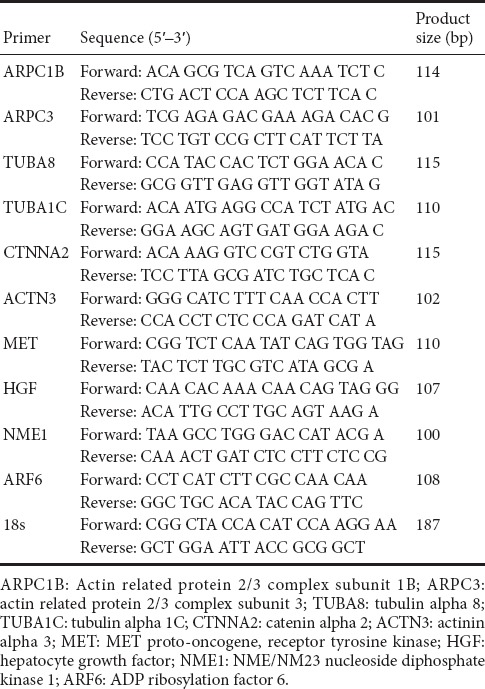
RNA samples from another set of 30 rats were extracted and reverse-transcribed to cDNA with the Prime-Script reagent Kit (TaKaRa, Dalian, China). The procedure was performed as previously described (Yi et al., 2015). Relative mRNA expression levels at 0, 1, 4, 7, and 14 days after sciatic nerve crush were calculated via the comparative 2−ΔΔCt method, in which 18s rRNA was used as a reference. The sequences of primers of target genes ARPC1B, ARPC3, TUBA8, TUBA1C, CTNNA2, ACTN3, MET, HGF, NME1, ARF6 and reference gene 18s were listed in Table 1.
Table 1.
Primer pairs for quantitative real-time polymerase chain reaction
Statistical analysis
Statistical analysis was performed with GraphPad Prism 6.0 software (GraphPad Software. Inc., La Jolla, CA, USA). Experimental outcomes were represented as the mean ± SEM. One-way analysis of variance and Dunnett's multiple comparisons were used to test differences among multiple groups. P < 0.05 was considered statistically significant.
Results
Adherens junction and remodeling of adherens junction canonical signaling pathways following nerve injury
We analyzed changes of gene expression profiles at 1, 4, 7, and 14 days following injury with a rat sciatic nerve crush injury model, and found 13,721, 14,321, 14,745, and 6979 DEGs compared with intact nerves, respectively (Yi et al., 2015; Qin et al., 2016). With these DEGs, ingenuity pathway analysis was applied to analyze diseases and functions. The DEGs of injured nerves were highly enriched in cell-to-cell signaling and interaction, cellular movement, and cellular growth and proliferation (Yi et al., 2015).
DEGs were enriched in the pathways of adherens junctions and remodeling of adherens junctions at all four time points (1, 4, 7, and 14 days after injury) (Table 2). The adherens junction is a structure linking adhesion receptors to the cytoplasmic skeleton, and is critical for peripheral nerve regeneration. E-cadherin forms homodimers and interacts with β-catenin, which further binds to α-catenin and links to actin and tubulin. Actin can be bound by Actn3 (α-actinin 3), an actin-binding protein (Figure 1). Actin branching is regulated by the ARP2/3 complex (Figure 1).
Table 2.
Significance of adherens junction signaling and remodeling of adherens junctions at each time point following peripheral nerve injury
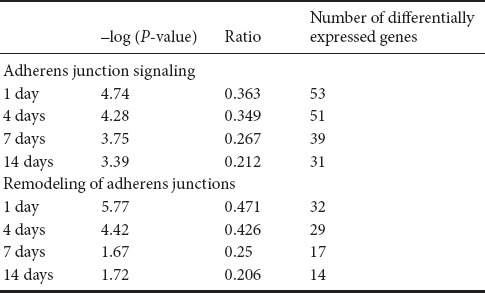
Figure 1.
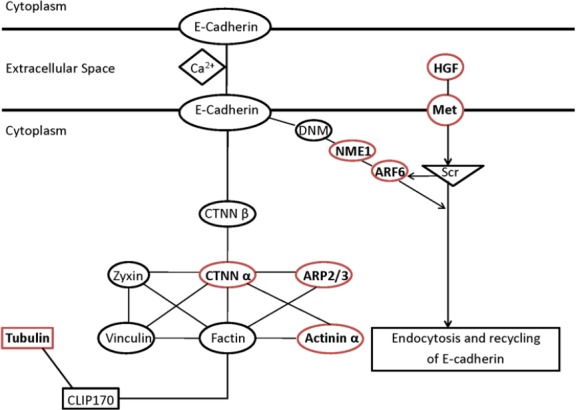
Schematic networks of adherens junction and remodeling of adherens junction pathways.
The molecules with red outlines are those verified by quantitative real-time polymerase chain reaction. HGF: Hepatocyte growth factor; Met: MET Proto-Oncogene, receptor tyrosine kinase; DNM: dynamin; NME1: NME/NM23 nucleoside diphosphate kinase 1; ARF6: ADP ribosylation factor 6; CTNN β: beta-catenin; CTNN α: alpha-catenin; ARP2/3: actin related protein 2/3 Complex; CLIP170: CAP-Gly domain containing linker protein 170.
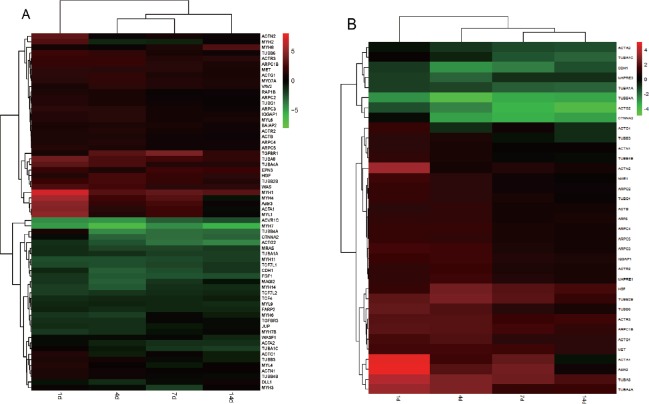
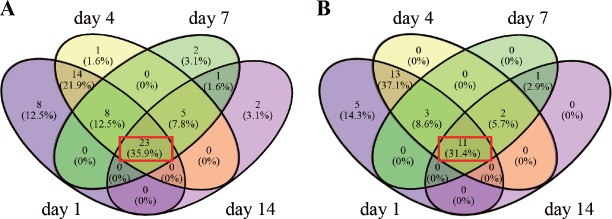
Transcription levels of most molecules in the adherens junction are either continuously upregulated, downregulated, or unaffected (Figure 2A). When analyzing the DEGs at different time points, 35.9% of genes were continuously upregulated or downregulated at all time points (see the intersection in red, Figure 3A; genes are listed in Table 3). These indicate that an individual molecule in the pathway of the adherens junction may have a consistent role during nerve recovery.
Figure 2.
Heatmap of differentially expressed genes (DEGs) in the adherens junction (A) and remodeling of adherens junction (B) pathways at 1, 4, 7, and 14 days post injury.
Red indicates upregulation and green indicates downregulation. Fold changes of DEGs were labeled on the right.
Figure 3.
Pattern of differentially expressed genes (DEGs) in the adherens junction (A) and remodeling of adherens junction (B) pathways at 1, 4, 7, and 14 days after injury shown as Venn diagrams.
The intersection shows the overlapped DEGs. The red rectangle represents common DEGs at all different time points.
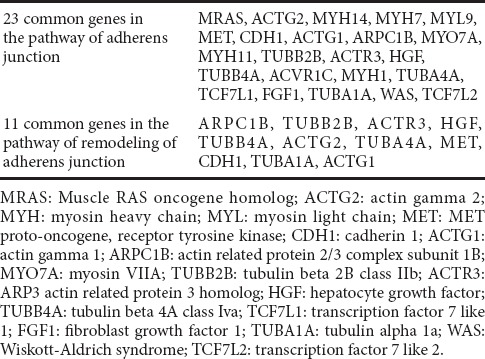
Table 3.
Overlapped differentially expressed genes (34 common genes) in the adherens junction and remodeling of adherens junction pathways
Adherens junctions are highly regulated by molecules involved in remodeling of adherens junctions. HGF and the receptor Met promote the process of endocytosis and recycling of E-cadherin (Anderson et al., 2006). ADP-ribosylation factor-6 (ARF6) activated by Src also promotes internalization of E-cadherin (Peglion et al., 2014). Additionally, NME1 regulates ARF6-mediated endocytosis of E-cadherin (Figure 1). Similar to molecules in the adherens junction pathway, molecules in the remodeling of adherens junctions pathway also displayed continuous upregulation or downregulation (Figure 2B); 31.4% genes were upregulated or downregulated in the pathway of remodeling of adherens junctions at all time points (see the intersection in red, Figure 3B; genes are listed in Table 3).
qRT-PCR validation of genes in adherens junctions and remodeling of adherens junction pathways
qRT-PCR was performed to further validate RNA-seq results for individual genes. Studies have shown that N-cadherin and E-cadherin are upregulated following injury in different injury models (Hasegawa et al., 1996; Ide, 1996; Hatoko et al., 2001; Tada et al., 2001a, b). We analyzed mRNA expression of multiple key molecules based on the significant biological functions. The ARP2/3 complex has seven subunits, of which ARPC1B and ARPC3 were upregulated from day 1 to day 14 post injury (Figure 4A, B). TUBA8 (a subtype of α-tubulin) was also continuously upregulated, although the degree of fold change was decreased (Figure 4C). However, mRNA levels of TUBA1C (a subtype of α-tubulin) declined from day 4 postinjury compared with intact nerves (Figure 4D). Additionally, CTNNA2 (α-catenin) declined on day 1 postinjury and remained decreased (Figure 4E). ACTN3 was upregulated on days 1 and 7 (Figure 4F).
Figure 4.
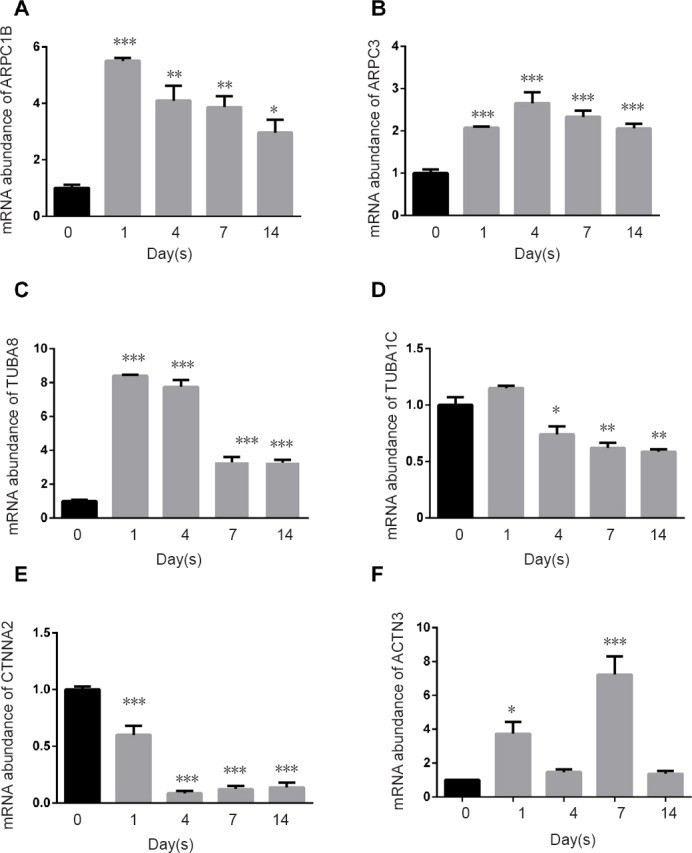
Quantitative real-time polymerase chain reaction results for molecules in the adherens junction pathway.
(A–F) Relative mRNA expression of ARPC1B, ARPC3, TUBA8, TUBA1C, CTNNA2, and ACTN3, respectively. *P < 0.05, **P < 0.01, ***P < 0.001, vs. 0 day control. Data are expressed as the mean ± SEM (n = 3, one-way analysis of variance and Dunnett's multiple comparisons). 18s rRNA was used as an internal control. ARPC1B: Actin related protein 2/3 complex subunit 1B; ARPC3: actin related protein 2/3 complex subunit 3; TUBA8: tubulin alpha 8; TUBA1C: tubulin alpha 1C; CTNNA2: catenin alpha 2; ACTN3: actinin alpha 3.
Multiple molecules were further identified in the pathway of remodeling of adherens junctions. MET expression was upregulated on day 1 postinjury, but no significant difference was observed on day 4 or later (Figure 5A). The mRNA levels of HGF, NME1, and ARF6 were increased at all time points following injury (Figure 5B–D). The qPCR results of most genes matched the RNA-seq results. These results confirm the presence of distinct transcript changes in the pathways of adherens junctions and remodeling of adherens junctions.
Figure 5.
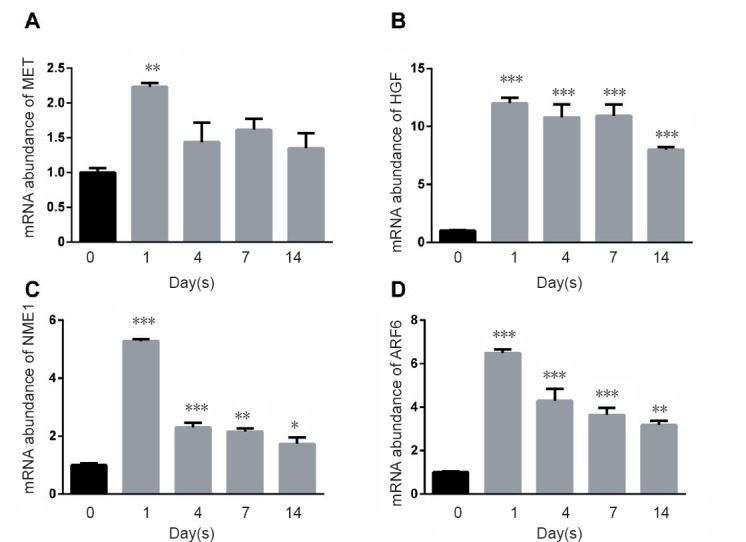
Quantitative real-time polymerase chain reaction results for molecules involved in the remodeling of adherens junction pathway.
(A–D) Relative mRNA expression of MET, HGF, NME1, and AFR6, respectively. *P < 0.05, **P < 0.01, ***P < 0.001, vs. 0 day control. Data are expressed as the mean ± SEM (n = 3, one-way analysis of variance and Dunnett's multiple comparisons). 18s rRNA was used as an internal control. MET: MET proto-oncogene, receptor tyro-sine kinase; HGF: hepatocyte growth factor; NME1: NME/NM23 nucleoside diphos-phate kinase 1; ARF6: ADP ribosylation factor 6.
Discussion
This study systematically analyzed gene expression changes involved in the pathways of adherens junctions and remodeling adherens junctions following peripheral nerve injury and recovery. We found dissimilar expression patterns of molecules in adherens junction and remodeling of adherens junction pathways, and found that increases or decreases in mRNA levels of most individual molecules in adherens junctions were persistent compared with levels in intact nerves, suggesting a sustained role of specific molecules during nerve recovery. Our study suggests that the adherens junction may play an important role in nerve regeneration, based on the robust transcript alterations of individual molecules in adherens junctions. Molecules in the adherens junction pathway may be potential therapeutic targets for axon regeneration.
Interestingly, most adhesion molecules that were upregulated or downregulated in the peripheral nerve injury in this study are also expressed in the central nervous system, indicating that a single molecule cannot account for the different regeneration capacities in the central and peripheral nervous systems. Studies have shown expression changes of adhesion molecules following sciatic nerve injury (Ide, 1996; Fu and Gordon, 1997; Raivich and Makwana, 2007; Patodia and Raivich, 2012). However, few, if any, studies have reported transcript or protein changes of adhesion molecules following the spinal cord injury. It would be interesting to systematically compare the changes of adhesion molecules in the spinal cord versus in peripheral nerves in future studies.
Based on the transcript changes after injury, peripheral recovery was divided into three phases: acute, sub-acute, and post-acute stages (Yi et al., 2015). Following axon degeneration, Schwann cells divide and align along the basal lamina. Regrowing axons extend from the proximal site to the distal site in the tubes provided by Schwann cells, a process lasting until the post-acute stage. This process is consistent with our finding that 34 upregulated or downregulated genes (23 genes in the adherens junction pathway and 11 genes in the remodeling of adherens junctions pathway) were observed at all of the different time points, indicating that these genes might share similar functions throughout the whole process for axon-axon and axon-Schwann cell interaction.
As a functional unit, adherens junctions play a vital role in cell interaction, growth, and migration (Niessen and Gottardi, 2008; Etienne-Manneville, 2012). During regeneration, axons attach to Schwann cells through multiple adhesion molecules, including but not limited to the cadherin proteins N-cadherin and E-cadherin. N-cadherin is upregulated during peripheral nerve regeneration, which might mediate adhesion of Schwann cells to axons (Letourneau et al., 1991; Ide, 1996; Fu and Gordon, 1997). E-cadherin, catenins, and F-actin in Schwann cells might maintain the growth and function of compact myelin layers (Menichella et al., 2001; Young et al., 2002; Tricaud et al., 2005; Perrin-Tricaud et al., 2007; Gess et al., 2008; Lewallen et al., 2011; Basak et al., 2015). A previous study has shown that the protein levels of E-cadherin start to increase on day 3 after nerve suture (Tada et al., 2001b). Tada et al. (2001a) have found that E-cadherin expression is upregulated during nerve regeneration after nonvascularized and vascularized nerve grafts. However, our current study found that a cytoplasmic partner of cadherins, α-catenin, is downregulated during nerve injury and regeneration, revealing distinct transcript changes in adherens junctions. Distinct responses of transcription in adherens junctions suggest that individual molecules might serve other functions besides intercellular adhesion. In addition, this might also indicate that a single molecule might be regulated by multiple signaling pathways. For example, cytosolic β-catenin is also regulated by the canonical Wnt signaling pathway (Klinke et al., 2015). Hasegawa et al. (1996) have shown that E-cadherin is enriched in proliferated Schwann cells after nerve crush injury. Future studies will be needed to further characterize the distribution of individual molecules in adherens junctions and investigate their specific roles during peripheral nerve regeneration.
Our transcriptome analysis identified comprehensive changes of adherens junctions during nerve regeneration. The robust changes of molecules in adherens junctions may be critical for cell-cell attachment, offering a basis for axon regrowth.
Additional file: Open peer review report 1 (89.4KB, pdf) .
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
Financial support: This study was supported by the National Natural Science Foundation of China, No. 31700926; the Priority Academic Program Development of Jiangsu Higher Education Institutions of China. The funders did not participant in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, and in the decision to submit the paper for publication.
Institutional review board statement: This study was approved by the Administration Committee of Experimental Animals, Jiangsu, China (approval No. 20160229-007). The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1985).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Nikolai A. Sopko, Johns Hopkins School of Medicine, Department of Urology, USA.
Funding: This study was supported by the National Natural Science Foundation of China, No. 31700926; the Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
(Copyedited by McCollum L, Norman C, Wang J, Li CH, Qiu Y, Song LP, Zhao M)
References
- Anderson MR, Harrison R, Atherfold PA, Campbell MJ, Darnton SJ, Obszynska J, Jankowski JA. Met receptor signaling: a key effector in esophageal adenocarcinoma. Clin Cancer Res. 2006;12:5936–5943. doi: 10.1158/1078-0432.CCR-06-1208. [DOI] [PubMed] [Google Scholar]
- Basak S, Desai DJ, Rho EH, Ramos R, Maurel P, Kim HA. E-cadherin enhances neuregulin signaling and promotes Schwann cell myelination. Glia. 2015;63:1522–1536. doi: 10.1002/glia.22822. [DOI] [PubMed] [Google Scholar]
- Bhatheja K, Field J. Schwann cells: origins and role in axonal maintenance and regeneration. Int J Biochem Cell Biol. 2006;38:1995–1999. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Bosse F. Extrinsic cellular and molecular mediators of peripheral axonal regeneration. Cell Tissue Res. 2012;349:5–14. doi: 10.1007/s00441-012-1389-5. [DOI] [PubMed] [Google Scholar]
- Camara-Lemarroy CR, Guzman-de la Garza FJ, Fernandez-Garza NE. Molecular inflammatory mediators in peripheral nerve degeneration and regeneration. Neuroimmunomodulation. 2010;17:314–324. doi: 10.1159/000292020. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Wang YX, Yu J, Yi S. Critical signaling pathways during Wallerian degeneration of peripheral nerve. Neural Regen Res. 2017;12:995–1002. doi: 10.4103/1673-5374.208596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P, Walsh FS. Signal transduction events underlying neurite outgrowth stimulated by cell adhesion molecules. Curr Opin Neurobiol. 1994;4:49–55. doi: 10.1016/0959-4388(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Control of polarized cell morphology and motility by adherens junctions. Semin Cell Dev Biol. 2011;22:850–857. doi: 10.1016/j.semcdb.2011.07.023. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S. Adherens junctions during cell migration. Subcell Biochem. 2012;60:225–249. doi: 10.1007/978-94-007-4186-7_10. [DOI] [PubMed] [Google Scholar]
- Farkas JE, Monaghan JR. A brief history of the study of nerve dependent regeneration. Neurogenesis. 2017;4:e1302216. doi: 10.1080/23262133.2017.1302216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fex Svennigsen A, Dahlin LB. Repair of the peripheral nerve-remyelination that works. Brain Sci. 2013;3:1182–1197. doi: 10.3390/brainsci3031182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SY, Gordon T. The cellular and molecular basis of peripheral nerve regeneration. Mol Neurobiol. 1997;14:67–116. doi: 10.1007/BF02740621. [DOI] [PubMed] [Google Scholar]
- Gaudet AD, Popovich PG, Ramer MS. Wallerian degeneration: gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 2011;8:110. doi: 10.1186/1742-2094-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gess B, Halfter H, Kleffner I, Monje P, Athauda G, Wood PM, Young P, Wanner IB. Inhibition of N-cadherin and beta-catenin function reduces axon-induced Schwann cell proliferation. J Neurosci Res. 2008;86:797–812. doi: 10.1002/jnr.21528. [DOI] [PubMed] [Google Scholar]
- Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–6156. doi: 10.1016/j.biomaterials.2014.04.064. [DOI] [PubMed] [Google Scholar]
- Gu X, Ding F, Yang Y, Liu J. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93:204–230. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Seto A, Uchiyama N, Kida S, Yamashima T, Yamashita J. Localization of E-cadherin in peripheral glia after nerve injury and repair. J Neuropathol Exp Neurol. 1996;55:424–434. doi: 10.1097/00005072-199604000-00005. [DOI] [PubMed] [Google Scholar]
- Hatoko M, Tada H, Tanaka A, Kuwahara M, Yurugi S. The differential expression of N-cadherin in vascularized and nonvascularized nerve grafts: a study in a rat sciatic nerve model. Ann Plastic Surg. 2001;47:322–327. doi: 10.1097/00000637-200109000-00017. [DOI] [PubMed] [Google Scholar]
- Ide C. Peripheral nerve regeneration. Neurosci Res. 1996;25:101–121. doi: 10.1016/0168-0102(96)01042-5. [DOI] [PubMed] [Google Scholar]
- Keynes RJ. Schwann cells during neural development and regeneration: leaders or followers? Trends Neurosci. 1987;10:137–139. [Google Scholar]
- Klinke DJ, 2nd, Horvath N, Cuppett V, Wu Y, Deng W, Kanj R. Interlocked positive and negative feedback network motifs regulate beta-catenin activity in the adherens junction pathway. Mol Biol Cell. 2015;26:4135–4148. doi: 10.1091/mbc.E15-02-0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneau PC, Roche FK, Shattuck TA, Lemmon V, Takeichi M. Interactions of Schwann cells with neurites and with other Schwann cells involve the calcium-dependent adhesion molecule, N-cadherin. J Neurobiol. 1991;22:707–720. doi: 10.1002/neu.480220706. [DOI] [PubMed] [Google Scholar]
- Lewallen KA, Shen YA, De la Torre AR, Ng BK, Meijer D, Chan JR. Assessing the role of the cadherin/catenin complex at the Schwann cell-axon interface and in the initiation of myelination. J Neurosci. 2011;31:3032–3043. doi: 10.1523/JNEUROSCI.4345-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Qian T, Wang X, Liu J, Gu X. Noncoding RNAs and their potential therapeutic applications in tissue engineering. Engineering. 2017;3:3–15. [Google Scholar]
- Malinova TS, Huveneers S. Sensing of cytoskeletal forces by asymmetric adherens junctions. Trends Cell Biol. 2017;28:328–341. doi: 10.1016/j.tcb.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Masaki T, Matsumura K. Biological role of dystroglycan in Schwann cell function and its implications in peripheral nervous system diseases. J Biomed Biotechnol. 2010;2010:740403. doi: 10.1155/2010/740403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menichella DM, Arroyo EJ, Awatramani R, Xu T, Baron P, Vallat JM, Balsamo J, Lilien J, Scarlato G, Kamholz J, Scherer SS, Shy ME. Protein zero is necessary for E-cadherin-mediated adherens junction formation in Schwann cells. Mol Cell Neurosci. 2001;18:606–618. doi: 10.1006/mcne.2001.1041. [DOI] [PubMed] [Google Scholar]
- Morrissey MA, Sherwood DR. An active role for basement membrane assembly and modification in tissue sculpting. J Cell Sci. 2015;128:1661–1668. doi: 10.1242/jcs.168021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5:621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Gottardi CJ. Molecular components of the adherens junction. Biochim Biophys Acta. 2008;1778:562–571. doi: 10.1016/j.bbamem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan A, Rao MV, Wu Y, Zaidel-Bar R. Jack of all trades: functional modularity in the adherens junction. Curr Opin Cell Biol. 2015;36:32–40. doi: 10.1016/j.ceb.2015.06.008. [DOI] [PubMed] [Google Scholar]
- Patodia S, Raivich G. Downstream effector molecules in successful peripheral nerve regeneration. Cell Tissue Res. 2012;349:15–26. doi: 10.1007/s00441-012-1416-6. [DOI] [PubMed] [Google Scholar]
- Peglion F, Llense F, Etienne-Manneville S. Adherens junction treadmilling during collective migration. Nat Cell Biol. 2014;16:639–651. doi: 10.1038/ncb2985. [DOI] [PubMed] [Google Scholar]
- Perrin-Tricaud C, Rutishauser U, Tricaud N. P120 catenin is required for thickening of Schwann cell myelin. Mol Cell Neurosci. 2007;35:120–129. doi: 10.1016/j.mcn.2007.02.010. [DOI] [PubMed] [Google Scholar]
- Qin J, Zha GB, Yu J, Zhang HH, Yi S. Differential temporal expression of matrix metalloproteinases following sciatic nerve crush. Neural Regen Res. 2016;11:1165–1171. doi: 10.4103/1673-5374.187059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raivich G, Makwana M. The making of successful axonal regeneration: genes, molecules and signal transduction pathways. Brain Res Rev. 2007;53:287–311. doi: 10.1016/j.brainresrev.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Reichert F, Saada A, Rotshenker S. Peripheral nerve injury induces Schwann cells to express two macrophage phenotypes: phagocytosis and the galactose-specific lectin MAC-2. J Neurosci. 1994;14:3231–3245. doi: 10.1523/JNEUROSCI.14-05-03231.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada H, Hatoko M, Tanaka A, Kuwahara M, Mashiba K, Yurugi S. The difference in E-cadherin expression between nonvascularized and vascularized nerve grafts: study in the rat sciatic nerve model. J Surg Res. 2001a;100:57–62. doi: 10.1006/jsre.2001.6212. [DOI] [PubMed] [Google Scholar]
- Tada H, Hatoko M, Tanaka A, Kuwahara M, Mashiba K, Yurugi S. Detection of E-cadherin expression after nerve repair in a rat sciatic nerve model. Ann Plastic Surg. 2001b;47:178–182. doi: 10.1097/00000637-200108000-00012. [DOI] [PubMed] [Google Scholar]
- Tricaud N, Perrin-Tricaud C, Bruses JL, Rutishauser U. Adherens junctions in myelinating Schwann cells stabilize Schmidt-Lanterman incisures via recruitment of p120 catenin to E-cadherin. J Neurosci. 2005;25:3259–3269. doi: 10.1523/JNEUROSCI.5168-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh FS, Doherty P. Cell adhesion molecules and neuronal regeneration. Curr Opin Cell Biol. 1996;8:707–713. doi: 10.1016/s0955-0674(96)80113-x. [DOI] [PubMed] [Google Scholar]
- Webber C, Zochodne D. The nerve regenerative microenvironment: early behavior and partnership of axons and Schwann cells. Exp Neurol. 2010;223:51–59. doi: 10.1016/j.expneurol.2009.05.037. [DOI] [PubMed] [Google Scholar]
- Woichansky I, Beretta CA, Berns N, Riechmann V. Three mechanisms control E-cadherin localization to the zonula adherens. Nat Commun. 2016;7:10834. doi: 10.1038/ncomms10834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing L, Cheng Q, Zha G, Yi S. Transcriptional profiling at high temporal resolution reveals robust immune/inflammatory responses during rat sciatic nerve recovery. Mediators Inflamm. 2017;2017:3827841. doi: 10.1155/2017/3827841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Tang X, Yu J, Liu J, Ding F, Gu X. Microarray and qPCR analyses of wallerian degeneration in rat sciatic nerves. Front Cell Neurosci. 2017;11:22. doi: 10.3389/fncel.2017.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Zhang H, Gong L, Wu J, Zha G, Zhou S, Gu X, Yu B. Deep Sequencing and bioinformatic analysis of lesioned sciatic nerves after crush injury. PLoS One. 2015;10:e0143491. doi: 10.1371/journal.pone.0143491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young P, Boussadia O, Berger P, Leone DP, Charnay P, Kemler R, Suter U. E-cadherin is required for the correct formation of autotypic adherens junctions of the outer mesaxon but not for the integrity of myelinated fibers of peripheral nerves. Mol Cell Neurosci. 2002;21:341–351. doi: 10.1006/mcne.2002.1177. [DOI] [PubMed] [Google Scholar]
- Yu B, Zhou S, Yi S, Gu X. The regulatory roles of non-coding RNAs in nerve injury and regeneration. Prog Neurobiol. 2015;134:122–139. doi: 10.1016/j.pneurobio.2015.09.006. [DOI] [PubMed] [Google Scholar]
- Yu J, Gu X, Yi S. Ingenuity pathway analysis of gene expression profiles in distal nerve stump following nerve injury: insights into wallerian degeneration. Front Cell Neurosci. 2016;10:274. doi: 10.3389/fncel.2016.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yeh J, Richardson PM, Bo X. Cell adhesion molecules of the immunoglobulin superfamily in axonal regeneration and neural repair. Restor Neurol Neurosci. 2008;26:81–96. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.