Abstract
Portal vein thrombosis (PVT) is a frequent complication in the natural history of patients with liver cirrhosis (LC). The prevalence of PVT in LC is highly variable, ranging from 0.6% to 25% according to different reports. The impact of PVT on the natural history of LC is unclear, but it seems to negatively affect the prognosis of patients undergoing liver transplantation (LT) by increasing post-LT mortality and delaying waiting time. The antithrombotic treatment of PVT is still challenging as PVT may often remain asymptomatic and incidentally diagnosed, and a spontaneous partial/total regression of PVT is observed in an important proportion of patients, even in the absence of anticoagulation. Recent evidence suggested that the anticoagulant treatment for PVT may favorably affect both ischemic and bleeding outcomes in LC patients. Anticoagulant therapies so far available include unfractioned heparin, low molecular weight heparins (LMWHs) and fondaparinux for acute treatment, and LMWHs and vitamin K antagonists (VKAs) for long-term treatment. No robust data currently support the use of direct oral anticoagulants (DOACs) in patients with LC and PVT, as the safety and efficacy of DOACs in this setting is still unclear. This review summarizes current evidence for the evaluation and management of patients with LC and PVT.
Keywords: DOACs, fondaparinux, liver cirrhosis, LMWH, portal vein thrombosis, VKAs
Introduction
Liver cirrhosis (LC) has been long considered a clinical condition associated with an elevated risk of bleeding. However, bleeding complications occur essentially in the gastrointestinal tract and are related to the hyperdynamic flux of the portal vein.1 Conversely, systemic bleeding is rare as documented by the very low incidence of intracerebral hemorrhage, as compared to the general population.2 In accordance with this, we recently demonstrated that during a 2-year follow up, >90% of bleeding events were localized in the gastrointestinal tract, while bleeding in other sites was rare.3 Conversely, there is a growing body of evidence indicating that LC may be complicated by venous thrombosis in the systemic and portal circulation.4 This association is supported by previous data showing that LC is characterized by a prothrombotic state, particularly related to increased platelet and clotting function.5 Low-grade endotoxemia may represent an interesting factor contributing to thrombosis as indicated by the fact that bacterial lipopolysaccharides may be responsible for either platelet or clotting system activation.5–8
In this setting, predictors and clinical presentation of patients with portal vein thrombosis (PVT) are still not well characterized, and PVT, especially if partial, is only incidentally diagnosed in patients with LC. Consequently, there is a lack of agreement on the most appropriate anticoagulant treatment for PVT in LC patients.
Our review will summarize current evidence on: (1) prevalence of PVT in LC and (2) anticoagulant treatment options for patients with LC and PVT.
Portal vein thrombosis in liver cirrhosis
PVT is defined as the formation of a thrombus within the main portal vein or intrahepatic portal branches. PVT can be occlusive/complete or partial/incomplete, eventually extending to the superior mesenteric vein (SMV) that is a harmful and life-threatening complication.9,10
The prevalence and incidence of PVT vary among different studies due to heterogeneous diagnostic methods and target populations.11–15 In particular, autopsy studies reported a prevalence of PVT of 6–64%, while the prevalence ranged from 0.6 to 26% by angiography, surgery or LT studies.12 Finally, ultrasonography studies reported a prevalence of PVT of 5–24.2%.12
One of the largest study by Nery and colleagues15 reported a cumulative incidence of PVT, among Child–Turcotte–Pugh (CTP) A or B patients (n = 1234) enrolled in the Thrombocir study, of 4.6%, 8.2% and 10.7% at 1, 3 and 5 years, respectively.
Recently, the multicenter prospective study ‘Portal vein thrombosis relevance on liver cirrhosis: Italian venous thrombotic events registry’ (PRO-LIVER), including 753 LC patients, reported a prevalence of ultrasonography (US)-documented PVT of 17%.16
Several predictors of PVT have been reported: decreased portal vein blood flow velocity (<15 cm/s),14 low prothrombin time and grade ⩾2 esophageal varices15 and decompensated cirrhosis, older age, the presence of concomitant hepatocellular carcinoma (HCC), a prior PVT or previous gastrointestinal (GI) bleeding.16
Concerning the clinical presentation, PVT is almost asymptomatic, a common finding during imaging studies performed for HCC surveillance.11,15 In the PRO-LIVER study, the percentage of asymptomatic PVT at baseline was 43%.16
The impact of PVT on the natural history of patients with LC is not well characterized. Some evidence suggested that PVT may unfavorably influence the prognosis of candidates for liver transplantation (LT). Thus, PVT would contribute to a two-fold higher risk of post-LT mortality. The occurrence of PVT affected approximately 2–26% of the patients awaiting LT and was classically considered an absolute contraindication to LT.17 A retrospective analysis, including 48,570 LC patients undergoing LT, showed that 6.8% had PVT at LT and that PVT was independently associated with increased 90-day mortality and graft failure.18 In contrast, data regarding PVT and mortality in LC patients provided equivocal results15,19 depending on patient selection, PVT diagnosis and small sample size.
Several studies have shown that the natural history of PVT is quite variable, including spontaneous resolution, unchanged appearance (both features seem to occur in 33–75% of untreated patients)20 and worsening (i.e. progression from partial to complete thrombosis). Identification of characteristics of patients predicting progression or recanalization of PVT remains a critical issue to resolve if anticoagulation should be considered or not. Until new evidence become available, it would be reasonable to assume that some risk factors for new-onset PVT, may also facilitate PVT recurrences; they include cirrhosis severity, presence of hepatocellular carcinoma or other malignancies, acquired or inherited thrombophilia, previous thrombotic events, local endotoxemia and immobilization.7,21–23 A recent study showed that cirrhosis etiology may also have a role in the onset of PVT, and in turn of PVT recurrence; thus, patients with cirrhosis due to nonalcoholic steatohepatitis seem to be at higher risk of developing PVT compared with other etiologies.19
Anticoagulant therapy
Robust data on the optimal management of LC patients with PVT are lacking and current guidelines do not propose definitive evidence-based treatment strategies
Anticoagulation is usually considered as the first choice for the treatment of patients with PVT unrelated to cirrhosis.24 Conversely, in LC patients this issue remains still unclear. The efficacy and safety of low molecular weight heparins (LMWHs), fondaparinux and vitamin K antagonists (VKAs) were studied in this regard (Table 1).
Table 1.
Characteristics of studies of liver cirrhosis patients treated with LMWH/fondaparinux (Panel A) or warfarin (Panel B).
| Author/ year | Study population | AC / NO-AC patients (n) | Months of AC | Type of AC | PVT recanalization |
|---|---|---|---|---|---|
| Panel A. LMWH/fondaparinux | |||||
| Francoz25 | LC patients candidate to LT | 19 / 10 | 8.1 | Nadroparin 5700 UI/day followed by acenocoumarol | AC: 8/19 NO-AC: 0/10 |
| Garcovich26 | LC patients with PVT | 15 / 15 | 3–6 | LMWH | AC: 7/15 NO-AC: 5/15 |
| Senzolo27 | LC patients with PVT | 35 / 21 | 6.0 | Nadroparin 95 antiXa U/kg body weight td | AC: 12/33 complete; 9/33 partial (>50%) NO-AC: 1/21 |
| Cui28 | LC patients with hepatitis B | 65 | 6.0 | Enoxaparin once-daily (1.5 mg/kg) or twice-daily (1 mg/kg) | 78.5% of patients achieved complete/partial recanalization |
| Amitrano29 | LC patients with PVT | 28 | 6.0 | Enoxaparin 200 U/kg/day | 33% had complete and 50% partial recanalization |
| Villa30 | LC patients without PVT | 34 / 36 | 12.0 | Enoxaparin 4000 IU/day | AC: no PVT NO-AC: 27.7% developed PVT |
| Zhang31 | LC patients | 7 | 7–21 days | Fondaparinux 2.5 mg/day | All patients had recanalization |
| Panel B. Warfarin (other) | |||||
| Chung32 | LC patients with PVT | 14 / 14 | 3.7 | Warfarin | AC: 11/14 (6 complete, 5 partial) NO-AC: 5/14 (3 complete and 2 partial) |
| Risso33 | LC patients with PVT and LT | 50 / 20 | NR | NR | AC: 35/50 NO-AC: 8/20 |
| Chen34 | LC patients with PVT | 30 / 36 | 7.6 | Warfarin | AC: 15/22 NO-AC: 4/16 |
| Wang35 | LC patients with PVT and TIPS placement | 31 / 33 | 12.0 | Warfarin | AC: 31/31 NO-AC: 30/32 |
| Delgado36 | LC patients with PVT | 55 | 6.8 | LMWH/VKAs | 33 patients had recanalization (60%; complete in 25) |
| Werner37 | LC patients with PVT | 28 | 10.0 | Warfarin | complete or partial resolution of PVT in 39% and 43% |
AC, anticoagulation; INR, international normalized ratio; LC, liver cirrhosis; LMWH, low molecular weight heparin; NR, not reported; PVT, portal vein thrombosis; TIPS, transjugular intrahepatic portosystemic shunt; VKAs, vitamin K antagonists.
Retrospective studies explored the role of anticoagulants in LC patients. Delgado and colleagues36 found that 60% of patients with LC [model of end-life disease (MELD): 12.8 ± 3.8] and acute/subacute thrombosis or progression of previous thrombosis of the spleno-porto-mesenteric axis treated with LMWHs or VKAs, achieved partial or complete recanalization after 6.8 (range: 1–56) months of anticoagulation therapy. Overall, 5 out of 55 patients developed bleeding complications related to anticoagulation and platelet count <50 × 109/l and use of VKAs were the only factors more frequently observed in these patients. Moreover, 38.5% of patients who achieved complete recanalization had recurrent thrombosis a median of 1.3 months after stopping anticoagulation therapy. Another retrospective study, by Werner and colleagues,37 confirmed the efficacy of warfarin in patients with LC (MELD: 7–29) awaiting LT who were diagnosed with PVT. These authors found a complete or partial resolution of PVT in 39% and 43%, respectively, of the 28 patients who were treated during the study period with warfarin (Mean: 302, range 54–1213 days). Bleeding was reported only in 4% of treated patients.
The role of anticoagulation treatment in patients with cirrhosis and PVT was also analyzed by prospective studies. Safety and efficacy of LMWH was studied by Amitrano and colleagues29 in 28 cirrhotic patients with PVT. After a 6-month enoxaparin treatment (at the dosage of 200 U/kg/d) complete recanalization of portal vein occurred in 33% and partial recanalization in 50% of patients, and no bleeding events were observed during the treatment. Moreover, Senzolo and colleagues27 confirmed the efficacy of LMWHs treating 33 LC and PVT with nadroparin (95 antiXa U/kg body weight td) for 6 months. Complete recanalization of portal vein was observed in 36% of treated patients and 5% of the 21 LC and PVT no anticoagulated patients (control group). Additionally, thrombus progression occurred in 15/21 non-anticoagulated patients and in 5/33 anticoagulated patients. No significant differences in bleeding rate was observed between the two groups. Another study examined the efficacy and safety of LMWH therapy with different doses of enoxaparin for acute PVT in LC patients with hepatitis B.28 Of the 65 patients, the 78.5% of patients achieved complete/partial recanalization of PVT after 6 months of anticoagulation therapy. Nevertheless, enoxaparin in once-daily (1.5 mg/kg) versus twice-daily (1 mg/kg) was associated with a higher risk for nonvariceal bleeding suggesting that 1 mg/kg enoxaparin subcutaneously every 12 h is a better anticoagulation regimen in the treatment of PVT in cirrhotic patients. In an international registry of splanchnic vein thrombosis in cirrhotic (n = 167) and noncirrhotic patients, anticoagulant treatment was administered in the acute phase in 465 out of 604 patients (77.0%). The incidence of thrombotic events was 5.6 per 100 patient-years during anticoagulant treatment and 9.2 per 100 patient-years in the subgroup of patients who remained untreated.21 The rate of major bleedings was 3.9 per 100 patient-years during anticoagulation, and 5.8 per 100 patient-years in the subgroup of never-treated patients. However, the rate of thrombotic and bleeding events according to anticoagulation in LC group was not reported. Moreover, it is unknown the severity of liver disease in patients with and without events at follow up.
Francoz and colleagues25 analyzed the role of VKAs [international normalized ratio (INR) values: 2–3] in LC patients (mean MELD: 12.8) with splanchnic vein thrombosis awaiting transplantation. They found that the proportion of partial/complete recanalization was significantly higher in those who received (n = 19) than in those who did not receive (n = 10) anticoagulation (42% versus 0%) after 8.1 months of follow up. No difference in bleeding rate was observed between the two groups.
Finally, a recent meta-analysis38 summarized the effects of anticoagulant therapy (LMWHs or VKAs) in patients with cirrhosis and PVT analyzing eight studies that reported rates of recanalization. The authors showed, among 353 included patients, a significantly higher proportion of PVT recanalization (71%) in patients treated with anticoagulants than in patients who did not receive anticoagulants (42%). Overall, six studies (257 patients) reported rates of any bleeding; there was no difference in the proportion of patients with major or minor bleeding between groups that did versus did not receive anticoagulants (11% for both groups). In addition, analyzing data extracted from six studies (comprising 225 patients) they showed that PVT progressed in 9% of patients treated with anticoagulants versus 33% of patients who did not receive these drugs. In particular, LMWHs but not warfarin, was significantly associated with a complete PVT resolution as compared with untreated patients, whereas both LMWHs and warfarin were effective in reducing PVT progression.
This meta-analysis also showed that data regarding safety were independent from liver failure degree and are consistent with a previous report30 in cirrhosis without PVT in which anticoagulant treatment did not increase the bleeding risk. Regarding this latter issue, Villa and colleagues30 performed a controlled study in 70 LC patients, with demonstrated patent portal veins, assigned randomly to groups that were given enoxaparin (4000 IU/day, subcutaneously for 48 weeks; n = 34) or no treatment (controls, n = 36). At the end of the follow up (58 ± 37 weeks in the control and 89 ± 57 weeks in the treated group), no PVT was observed in the group of patients treated with enoxaparin; conversely, in the control group 27.7% developed PVT. Moreover, liver decompensation (ascites, encephalopathy, bacterial peritonitis, portal hypertensive bleeding) rate was less frequent among patients treated with enoxaparin (11.7%) than controls (59.4%). Only one case-series study investigated the effect of fondaparinux in acute PVT in patients with decompensated LC.31 In this study, seven LC patients were treated with fondaparinux 2.5 mg/day. All patients were CTP class B–C, six with ascites and two with hepatic encephalopathy. All patients had a recanalization of the portal vein after 7–21 days of treatment, and no side effects were reported.31
In patients with LC, direct oral anticoagulants (DOACs) could represent an alternative treatment to VKAs or to LMWHs.39,40 DOACs are desirable as they do not require routine monitoring and can be taken orally. Unfortunately, patients with chronic liver disease were excluded from clinical trials that demonstrated efficacy and safety when compared with traditional anticoagulation.
However, there is no interventional study that explored the efficacy and safety of DOACs in LC patients until now. The only available data derive from a case report41 and three observational studies with small sample sizes42–44 that evaluated the effect of DOACs in cirrhotic patients (Table 2). Recently, the efficacy and safety of DOACs, compared with warfarin, has been investigated in patients with atrial fibrillation (AF) and advanced liver fibrosis.45,46 Thus, in 2330 AF outpatients of whom 1297 were treated with VKAs and 1033 with DOACs (276 dabigatran, 365 apixaban, 358 rivaroxaban and 34 edoxaban), liver fibrosis was significantly associated with major bleedings in patients receiving VKAs (14.3% versus 5.6%, p < 0.001) but not in those on DOACs.45,46 However, the results should be interpreted with caution, given the relatively low number of patients with advanced liver fibrosis in this study.
Table 2.
Studies investigating DOACs in patients with liver cirrhosis.
| Author (year) | Study design | Setting | Anticoagulant | Main findings |
|---|---|---|---|---|
| Intagliata44 | Retrospective | 39 patients with LC treated for PVT or nonsplanchnic VTE | 20 on DOACs (11 apixaban, 9 rivaroxaban) 19 on VKAs (n = 13)/ LMWH (n = 6) |
Two major bleedings in the VKAs/LMWH group and one major bleeding in the DOAC group |
| Yang41 | Case report | Patient with LC and recurrent PVT | Rivaroxaban | Resolution of PVT after 3 months of Rivaroxaban |
| Hum42 | Retrospective | LC patients anticoagulated over 3 years for PVT/DVT or stroke prevention in patients with atrial fibrillation | 27 patients on DOACs and 18 on VKAs or LMWH | Less major bleedings in the DOACs group (4% versus 28%, p = 0.03). Similar recurrent thrombosis in the two groups |
| De Gottardi43 | Prospective/ survey | 36 patients with LC anticoagulated for splanchnic vein thrombosis (75%), deep vein thrombosis (5%), atrial fibrillation (14%) and others (6%) | DOACs: rivaroxaban (83%), dabigatran (11%), apixaban (6%) |
1 case of recurrent PVT (DOAC replaced by LMWH) and 5 cases of bleedings (4 minor and 1 major) |
DOACs, direct oral anticoagulants; DVT, deep venous thrombosis; LC, liver cirrhosis; LMWH, low molecular weight heparin; PVT, portal vein thrombosis; VTE, venous thromboembolism.
Hence, current evidence do not support the routine use of DOACs in patients with LC and PVT, as the safety and efficacy of DOACs in this setting are still unclear.
A summary of anticoagulant options for the treatment of LC patients with symptomatic PVT47,48 is reported in Table 3.
Table 3.
Anticoagulant treatment options for the treatment of symptomatic portal vein thrombosis in patients with liver cirrhosis.
| Treatment of symptomatic PVT |
|---|
|
Acute phase/waiting for invasive procedures: heparin (LMWH or fondaparinux at therapeutic doses preferred over UFH*) Specific situations: – Severe renal impairment (<30 ml/min): UFH preferred (LMWH and fondaparinux have substantial renal excretion and should be avoided); – Thrombocytopenia: Fondaparinux preferred (UFH and LMWH may favor platelet count decrease). |
|
Chronic phase: LMWH or VKAs (INR target 2.0–3.0) for 6 months. Specific situations: – VKAs preferred over LMWH for long-term anticoagulation (such as PVT in presence of thrombophilia**, personal/familial history of thrombosis or PVT extended to SMV. |
|
*Enoxaparin: 1.5 mg/kg/od or 1 mg/kg/bid. Dalteparin: 200 U/kg/od or 100 U/kg/bid. Fondaparinux 7.5 mg/day (5.0 mg/day when <50 kg and 10.0 mg/day when >100 kg) for at least 5 days and until INR > 2.0.
Caution with elderly patients (>75 years), moderate renal impairment (30–50 ml/min) and underweight <50 kg), platelet count <100,000 mmc and presence of esophageal varices. Measurement of anti-Xa levels may be considered for enoxaparin monitoring in underweight, obese, pregnant, or patients with kidney disease. |
Thrombophilia: (1) Myeloproliferative disorders, (2) Factor V Leiden or prothrombin mutation, (3) Antiphospholipid antibodies, (4) PNH.
INR, international normalized ratio; LMWH, low molecular weight heparin; PNH, paroxysmal nocturnal hemoglobinuria; PVT, portal vein thrombosis; SMV, superior mesenteric vein; UFH, unfractioned heparin; VKA, vitamin K antagonist.
Conclusion
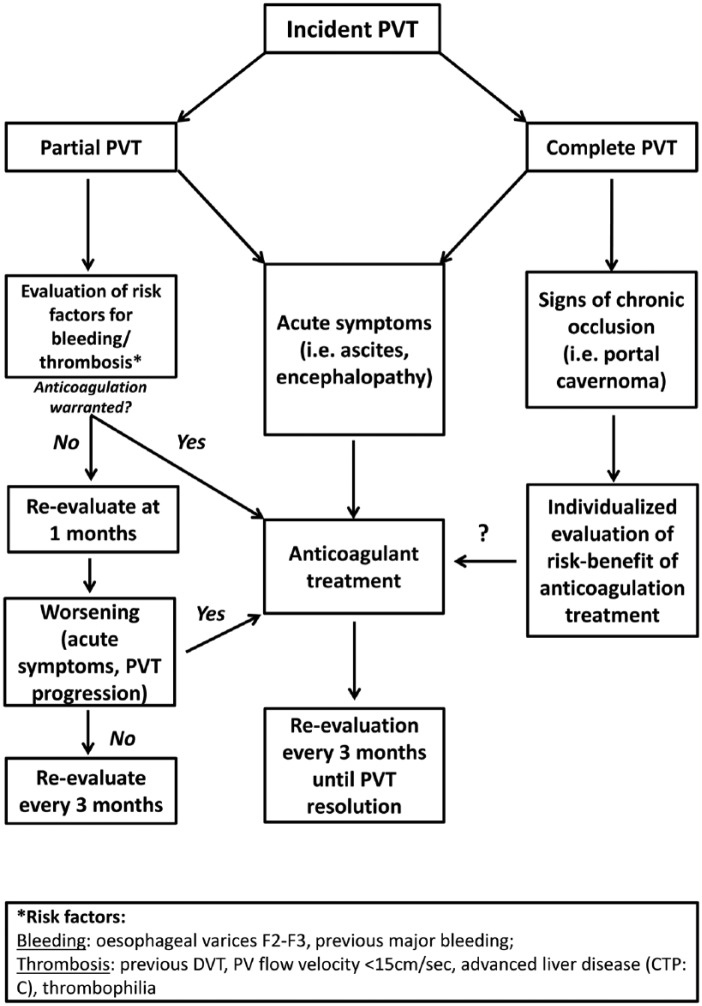
Despite a lack of agreement on whether AC is an appropriate treatment for PVT in cirrhosis, anticoagulation could be considered in patients with LC and PVT who: (1) are candidates for LT, (2) are waiting for invasive procedures (3) have a PVT extended to SMV, (4) develop acute symptoms of hepatic decompensation (regardless if partial or complete PVT) and (5) have thrombophilia (Figure 1).49
Figure 1.
Evaluation and management of patients with incident PVT.
PVT, portal vein thrombosis.
However, effort should be made to identify and remove modifiable risk factors for bleeding before the initiation of anticoagulation, such as the presence of esophageal or gastric varices.
In addition, to these specific situations, a still challenging scenario is represented by patients presenting with partial, asymptomatic PVT, considering that a significant proportion of patients with partial PVT shows a spontaneous resolution even in absence of anticoagulation.50 Thus, nearly 40% of patients with LC may have a ‘transient PVT’ with a spontaneous resolution without anticoagulant treatment; however, two studies reported a prevalence of recurrent PVT after spontaneous recanalization ranging from 21.3% to 45%.50 Studies investigating risk factors for recurrent PVT are therefore needed.
In the absence of guidelines regarding this specific setting, a diagnostic-therapeutic algorithm is proposed (Figure 1). Otherwise, as trials which investigated the impact of low or full doses of anticoagulants are still lacking, it could be arguable a short-term follow up to assess if PVT progresses or less; in case of PVT progression the use of anticoagulation could be considered.
In conclusion, the use of anticoagulants (mostly LMWHs) for the treatment of PVT in patients with LC seems to be well tolerated and effective but larger prospective studies are needed to further identify patient candidates for anticoagulation.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Stefania Basili, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome, Italy.
Daniele Pastori, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome, Italy.
Valeria Raparelli, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome, Italy.
Francesco Violi, I Clinica Medica, Department of Internal Medicine and Medical Specialties, Sapienza University of Rome, Viale del Policlinico 155, Rome, 00161, Italy.
References
- 1. Ferro D, Angelico F, Caldwell SH, et al. Bleeding and thrombosis in cirrhotic patients: what really matters? Dig Liver Dis 2012; 44: 275–279. [DOI] [PubMed] [Google Scholar]
- 2. Lai CH, Cheng PY, Chen YY. Liver cirrhosis and risk of intracerebral hemorrhage: a 9-year follow-up study. Stroke 2011; 42: 2615–2617. [DOI] [PubMed] [Google Scholar]
- 3. Basili S, Raparelli V, Napoleone L, et al. Platelet count does not predict bleeding in cirrhotic patients: results from the PRO-LIVER study. Am J Gastroenterol 2018; 113: 368–375. [DOI] [PubMed] [Google Scholar]
- 4. Lisman T, Violi F. Cirrhosis as a risk factor for venous thrombosis. Thromb Haemost 2017; 117: 3–5. [DOI] [PubMed] [Google Scholar]
- 5. Raparelli V, Basili S, Carnevale R, et al. Low-grade endotoxemia and platelet activation in cirrhosis. Hepatology 2017; 65: 571–581. [DOI] [PubMed] [Google Scholar]
- 6. Violi F. Should the term coagulopathy in cirrhosis be abandoned? JAMA Intern Med 2015; 175: 862–863. [DOI] [PubMed] [Google Scholar]
- 7. Violi F, Ferro D, Basili S, et al. Ongoing prothrombotic state in the portal circulation of cirrhotic patients. Thromb Haemost 1997; 77: 44–7. [PubMed] [Google Scholar]
- 8. Nocella C, Carnevale R, Bartimoccia S, et al. Lipopolysaccharide as trigger of platelet aggregation via eicosanoid over-production. Thromb Haemost 2017; 117: 1558–1570. [DOI] [PubMed] [Google Scholar]
- 9. Yerdel MA, Gunson B, Mirza D, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000; 69: 1873–1881. [DOI] [PubMed] [Google Scholar]
- 10. Sarin SK, Philips CA, Kamath PS, et al. Toward a comprehensive new classification of portal vein thrombosis in patients with cirrhosis. Gastroenterology 2016; 151: 574–577 e3. [DOI] [PubMed] [Google Scholar]
- 11. Amitrano L, Guardascione MA, Brancaccio V, et al. Risk factors and clinical presentation of portal vein thrombosis in patients with liver cirrhosis. J Hepatol 2004; 40: 736–741. [DOI] [PubMed] [Google Scholar]
- 12. Fimognari FL, Violi F. Portal vein thrombosis in liver cirrhosis. Intern Emerg Med 2008; 3: 213–218. [DOI] [PubMed] [Google Scholar]
- 13. Maruyama H, Okugawa H, Takahashi M, et al. De novo portal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes. Am J Gastroenterol 2013; 108: 568–574. [DOI] [PubMed] [Google Scholar]
- 14. Zocco MA, Di Stasio E, De Cristofaro R, et al. Thrombotic risk factors in patients with liver cirrhosis: correlation with MELD scoring system and portal vein thrombosis development. J Hepatol 2009; 51: 682–689. [DOI] [PubMed] [Google Scholar]
- 15. Nery F, Chevret S, Condat B, et al. Causes and consequences of portal vein thrombosis in 1,243 patients with cirrhosis: results of a longitudinal study. Hepatology 2015; 61: 660–667. [DOI] [PubMed] [Google Scholar]
- 16. Violi F, Corazza RG, Caldwell SH, et al. Portal vein thrombosis relevance on liver cirrhosis: Italian Venous Thrombotic Events Registry. Intern Emerg Med 2016; 11: 1059–1066. [DOI] [PubMed] [Google Scholar]
- 17. Chen H, Turon F, Hernandez-Gea V, et al. Nontumoral portal vein thrombosis in patients awaiting liver transplantation. Liver Transpl 2016; 22: 352–365. [DOI] [PubMed] [Google Scholar]
- 18. Englesbe MJ, Schaubel DE, Cai S, et al. Portal vein thrombosis and liver transplant survival benefit. Liver Transpl 2010; 16: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stine JG, Shah PM, Cornella SL, et al. Portal vein thrombosis, mortality and hepatic decompensation in patients with cirrhosis: a meta-analysis. World J Hepatol 2015; 7: 2774–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Qi X, Han G, Fan D. Management of portal vein thrombosis in liver cirrhosis. Nat Rev Gastroenterol Hepatol 2014; 11: 435–46. [DOI] [PubMed] [Google Scholar]
- 21. Ageno W, Riva N, Schulman S, et al. Long-term clinical outcomes of splanchnic vein thrombosis: results of an International Registry. JAMA Intern Med 2015; 175: 1474–1480. [DOI] [PubMed] [Google Scholar]
- 22. Qi X, Ren W, De Stefano V, et al. Associations of coagulation factor V Leiden and prothrombin G20210A mutations with Budd-Chiari syndrome and portal vein thrombosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2014; 12: 1801–1812 e7. [DOI] [PubMed] [Google Scholar]
- 23. Quintarelli C, Ferro D, Valesini G, et al. Prevalence of lupus anticoagulant in patients with cirrhosis: relationship with beta-2-glycoprotein I plasma levels. J Hepatol 1994; 21: 1086–1091. [DOI] [PubMed] [Google Scholar]
- 24. Plessier A, Darwish-Murad S, Hernandez-Guerra M, et al. Acute portal vein thrombosis unrelated to cirrhosis: a prospective multicenter follow-up study. Hepatology 2010; 51: 210–218. [DOI] [PubMed] [Google Scholar]
- 25. Francoz C, Belghiti J, Vilgrain V, et al. Splanchnic vein thrombosis in candidates for liver transplantation: usefulness of screening and anticoagulation. Gut 2005; 54: 691–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcovich M, Zocco MA, Ainora ME, et al. Clinical outcome of portal vein thrombosis (PVT) in cirrhotic patients: observe or treat? Hepatology (Baltimore, Md) 2011; 54: 1261A–1262A. [Google Scholar]
- 27. Senzolo M, Sartori TM, Rossetto V, et al. Prospective evaluation of anticoagulation and transjugular intrahepatic portosystemic shunt for the management of portal vein thrombosis in cirrhosis. Liver Int 2012; 32: 919–927. [DOI] [PubMed] [Google Scholar]
- 28. Cui SB, Shu RH, Yan SP, et al. Efficacy and safety of anticoagulation therapy with different doses of enoxaparin for portal vein thrombosis in cirrhotic patients with hepatitis B. Eur J Gastroenterol Hepatol. 2015; 27: 914–919. [DOI] [PubMed] [Google Scholar]
- 29. Amitrano L, Guardascione MA, Menchise A, et al. Safety and efficacy of anticoagulation therapy with low molecular weight heparin for portal vein thrombosis in patients with liver cirrhosis. J Clin Gastroenterol 2010; 44: 448–451. [DOI] [PubMed] [Google Scholar]
- 30. Villa E, Camma C, Marietta M, et al. Enoxaparin prevents portal vein thrombosis and liver decompensation in patients with advanced cirrhosis. Gastroenterology 2012; 143: 1253–1260 e1–4. [DOI] [PubMed] [Google Scholar]
- 31. Zhang ZH, Zhang JW, He P, et al. Fondaparinux is effective for acute portal vein thrombosis in decompensated cirrhotic patients. Medicine 2017; 96: e8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chung JW, Kim GH, Lee JH, et al. Safety, efficacy, and response predictors of anticoagulation for the treatment of nonmalignant portal-vein thrombosis in patients with cirrhosis: a propensity score matching analysis. Clin Mole Hepatol 2014; 20: 384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Risso A, Stradella D, Martini S, et al. Liver transplantation in cirrhotic patients with portal vein thrombosis: a single centre experience. Dig Liver Dis 2014; 46: e40. [Google Scholar]
- 34. Chen H, Liu L, Qi X, et al. Efficacy and safety of anticoagulation in more advanced portal vein thrombosis in patients with liver cirrhosis. Eur J Gastroenterol Hepatol 2016; 28: 82–89. [DOI] [PubMed] [Google Scholar]
- 35. Wang Z, Jiang MS, Zhang HL, et al. Is post-TIPS anticoagulation therapy necessary in patients with cirrhosis and portal vein thrombosis? A randomized controlled trial. Radiology 2016; 279: 943–951. [DOI] [PubMed] [Google Scholar]
- 36. Delgado MG, Seijo S, Yepes I, et al. Efficacy and safety of anticoagulation on patients with cirrhosis and portal vein thrombosis. Clin Gastroenterol Hepatol 2012; 10: 776–783. [DOI] [PubMed] [Google Scholar]
- 37. Werner KT, Sando S, Carey EJ, et al. Portal vein thrombosis in patients with end stage liver disease awaiting liver transplantation: outcome of anticoagulation. Dig Dis Sci 2013; 58: 1776–1780. [DOI] [PubMed] [Google Scholar]
- 38. Loffredo L, Pastori D, Farcomeni A, et al. Effects of anticoagulants in patients with cirrhosis and portal vein thrombosis: a systematic review and meta-analysis. Gastroenterology 2017; 153: 480–487 e1. [DOI] [PubMed] [Google Scholar]
- 39. Lisman T, Kamphuisen PW, Northup PG, et al. Established and new-generation antithrombotic drugs in patients with cirrhosis - possibilities and caveats. J Hepatol 2013; 59: 358–366. [DOI] [PubMed] [Google Scholar]
- 40. Intagliata NM, Maitland H, Caldwell SH. Direct oral anticoagulants in cirrhosis. Curr Treat Options Gastroenterol 2016; 14: 247–256. [DOI] [PubMed] [Google Scholar]
- 41. Yang H, Kim SR, Song MJ. Recurrent acute portal vein thrombosis in liver cirrhosis treated by rivaroxaban. Clin Mol Hepatol 2016; 22: 499–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hum J, Shatzel JJ, Jou JH, et al. The efficacy and safety of direct oral anticoagulants vs traditional anticoagulants in cirrhosis. Eur J Haematol 2017; 98: 393–397. [DOI] [PubMed] [Google Scholar]
- 43. De Gottardi A, Trebicka J, Klinger C, et al. Antithrombotic treatment with direct-acting oral anticoagulants in patients with splanchnic vein thrombosis and cirrhosis. Liver Int. Epub ahead of print 19 November 2016. DOI: 10.1111/liv.13285. [DOI] [PubMed] [Google Scholar]
- 44. Intagliata NM, Henry ZH, Maitland H, et al. Direct oral anticoagulants in cirrhosis patients pose similar risks of bleeding when compared to traditional anticoagulation. Dig Dis Sci 2016; 61: 1721–1727. [DOI] [PubMed] [Google Scholar]
- 45. Pastori D, Lip GYH, Farcomeni A, et al. Incidence of bleeding in patients with atrial fibrillation and advanced liver fibrosis on treatment with vitamin K or non-vitamin K antagonist oral anticoagulants. IntJ Cardiol 2018; 264: 58–63. [DOI] [PubMed] [Google Scholar]
- 46. Pastori D, Lip GYH, Farcomeni A, et al. Data on incidence of bleeding in patients with atrial fibrillation and advanced liver fibrosis on treatment with vitamin K or non-vitamin K antagonist oral anticoagulants. Data Brief 2018; 17: 830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Senzolo M, Riggio O, Primignani M; Italian Association for the Study of the Liver (AISF) ad hoc. Vascular disorders of the liver: recommendations from the Italian Association for the Study of the Liver (AISF) ad hoc committee. Dig Liver Dis 2011; 43: 503–514. [DOI] [PubMed] [Google Scholar]
- 48. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141: e419S–e496S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Turon F, Hernandez-Gea V, Garcia-Pagan JC. Portal vein thrombosis: yes or no on anticoagulation therapy. Curr Opin Organ Transplant 2018; 23: 250–256. [DOI] [PubMed] [Google Scholar]
- 50. Qi X, Guo X, Yoshida EM, et al. Transient portal vein thrombosis in liver cirrhosis. BMC Med 2018; 16: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]