Abstract
Inhaled iloprost is an effective therapy for patients with pulmonary arterial hypertension (PAH); however, some patients experience extended inhalation times when using the V10 formulation (10.0 µg/mL) to deliver a 5 -µg dose (at mouthpiece) and are at risk of incomplete inhalations and reduced inhalation frequency. VENTASWITCH was an observational, case-crossover study to evaluate inhalation behavior in patients with PAH switched from iloprost V10 to V20 (20.0 µg/mL) formulation for delivering a 5 -µg dose using the I-Neb® AAD® device. Adults with PAH participating in a German Ventavis® (iloprost) patient-support program, who were switched from the V10 to V20 formulation, were enrolled. The co-primary endpoints were mean daily proportion of complete inhalations and mean daily inhalation frequency. The secondary endpoint was mean daily inhalation duration. Data were collected for three months before and after switching. Overall, 63 patients were included. Switching from V10 to V20 resulted in a significant increase in the mean daily proportion of complete inhalations (92% vs. 97%, P < 0.0001) and inhalation frequency (4.6 vs. 4.9 inhalations/day, P = 0.0430), and reduction in mean inhalation duration (11.8 vs. 6.5 min; P < 0.0001). Greater increases in daily proportions of complete inhalations were observed in older patients (≥ 65 vs. < 65 years) and those receiving more (3 vs. < 3) concomitant PAH medications. Switching from V10 to V20 iloprost formulation significantly improved inhalation behavior in patients with PAH and may facilitate improved adherence to therapy.
Keywords: pulmonary arterial hypertension, nebulizers, pharmacotherapy
Introduction
Pulmonary arterial hypertension (PAH) is a rare, severe disease characterized by endothelial dysfunction and remodeling of the small pulmonary vessels resulting in increased pulmonary arterial pressure (PAP) and pulmonary vascular resistance (PVR), which can lead to progressive right heart failure and death.1–3
Several prostacyclin analogs are approved for treatment of PAH; these agents act by increasing intracellular levels of cyclic guanosine monophosphate, thereby promoting arterial vasodilation and inhibiting cell proliferation, inflammation, and platelet aggregation.4,5 Iloprost is a chemically stable prostacyclin analog that can be administered intravenously, orally, or by inhalation; it has a relatively short half-life of 20–30 min, thus requiring frequent dosing.6–10 In patients with PAH, inhaled iloprost has been shown to significantly increase exercise capacity, improve symptoms and PVR, and reduce the incidence of clinical events compared with placebo,2,11 and is approved for the treatment of PAH in patients with New York Heart Association (NYHA) functional class (FC) III symptoms in Europe and NYHA FC III or IV symptoms in the USA.8,10
The I-Neb® AAD® is one of the most commonly used nebulizer systems for iloprost inhalation and is routinely used within the Ventavis® (inhaled iloprost) patient-support program (VENTAPLUS) in Germany. The I-Neb® AAD® optimizes drug delivery by analyzing the patient’s breathing pattern and adjusting the delivered dose for each breath, providing drug delivery only during inspiration and reducing waste during expiration.12,13 Furthermore, it digitally records inhalation data, including completeness of inhalations, number of inhalations per day, frequency and duration of inhalation sessions, and provides feedback to the patient to improve treatment adherence. Delivery of iloprost via the I-Neb® AAD® was shown to reduce mean PAP and PVR, and to increase cardiac index in patients with PAH.14
The recommended administration schedule of iloprost is 6–9 inhalations per day, with a starting dose of 2.5 µg and a target maintenance dose of 5 µg per administration.8,10 Iloprost nebulizer solution is available as 10 µg/mL (V10) and 20 µg/mL (V20) formulations for administration via the I-Neb® AAD®. The V10 formulation can deliver both approved doses (iloprost 2.5 µg or 5 µg) at the mouthpiece, whereas the V20 formulation delivers iloprost 5 µg at the mouthpiece. However, some patients experience extended inhalation times when inhaling the 5 µg dose using the V10 formulation, putting them at risk of incomplete inhalations, reduced inhalation frequency, and potentially suboptimal treatment. Recent studies have shown that inhalation time is reduced for patients inhaling iloprost 5 µg using the V20 formulation, which contains a higher concentration of iloprost compared with the V10 formulation.15,16 This study (VENTASWITCH) evaluated the effect on inhalation behavior of switching from V10 to V20 in patients with PAH enrolled in the VENTAPLUS German patient-support program for inhaled iloprost.
Methods
Study design
VENTASWITCH was an observational, non-blinded, case-crossover study conducted in Germany between September 2015 and November 2016 in patients with PAH who were receiving V10 at an iloprost dose of 5 µg via the I-Neb® AAD® and switched to the V20 formulation on the recommendation of the treating physician (ClinicalTrials.gov ID: NCT02826252). Data were collected retrospectively for three months before and prospectively or retrospectively for three months after switching from V10 to V20. Switching involved changing the formulation disc and drug chamber only, with the I-Neb® device remaining the same. The study was approved by the Institutional Ethics Committee of Justus Liebig University, Giessen, Germany (Approval No. 132/16).
Patients
Adults (aged ≥18 years) diagnosed with Group 1 PAH participating in an inhaled iloprost (using the I-Neb® AAD®) patient-support program (VENTAPLUS) were included in VENTASWITCH if they had either been switched, or had agreed to be switched, from V10 to V20 according to their physician’s decision. Patients were required to provide written informed consent and to have received V10 therapy for ≥2 weeks. Patients participating in any other clinical or interventional study were excluded.
Data sources and assessments
Data sources included patient interviews and inhalation data downloaded from the patient’s I-Neb® AAD® device (automated collection independent of investigator involvement) during routine visits in the context of the patient-support program, and adverse-event (AE) data transferred to the VENTAPLUS database. Inhalation and AE data were collected for three months before and three months after the switching date. Inhalation data were routinely transferred from the device to a central database at ContraCare GmbH (Nuremberg, Germany), and the pseudonymized data were transferred to the study database. Patients were instructed to actively report any AEs and were also asked about AEs during patient interviews in their home by ContraCare. This data collection method allowed the study to be conducted without clinical investigator involvement and thus represented a new digital study design approach. National and international data protection laws, as well as regulations on observational studies, were followed.
Primary and secondary endpoints
The co-primary endpoints were the mean daily proportion of complete inhalations and inhalation frequency on V20 compared with V10. These were calculated using all inhalations with none, partial, or full dose delivered in the respective time frame. Days without any inhalation records (missing data) were not considered for calculation of mean daily proportion of complete inhalations and were considered as zero for inhalation frequency. The secondary endpoint was mean daily inhalation duration per session. Safety was assessed over the study period based on AE data transferred from the VENTAPLUS database. Quality review, including source data verification, was not conducted for the study as the variables determining the primary and secondary endpoints were taken from the automatic tracking and read-out of the I-Neb® AAD® system and were therefore regarded as unbiased.
Statistical analyses
A total sample size of 50–100 patients was planned to examine inhalation behavior and safety in patients maintained on the iloprost 5 -µg single-inhalation dose (delivered at mouthpiece) who switched from V10 to V20, with all statistical tests being exploratory.
The study endpoints and patient-wise mean differences were analyzed descriptively. Exploratory P values were calculated at α = 0.05 level with no multiple test correction using a two-sided sign test for comparison of continuous variables and a two-sided McNemar’s test for comparison of dichotomous variables. The incidence of AEs was summarized descriptively by Medical Dictionary for Regulatory Activities System Organ Class and Preferred Term. Post hoc analyses of the efficacy endpoints stratified by age group (< 65 vs. ≥ 65 years) and number of concomitant PAH therapies (< 3 vs. 3) were performed to investigate potentially different treatment effects in these subgroups.
Results
Patients
In total, 64 patients were enrolled, 63 patients were included in the analysis (one patient did not switch formulations and was excluded), and 62 patients completed the study.
Table 1 shows patient characteristics at the time of switching from V10 to V20. Most patients (59%) were women, 22 patients (35%) were aged ≥ 65 years, and 39 patients (62%) were receiving triple combination PAH therapy.
Table 1.
Patient characteristics at time of switching.
| Characteristic | Patients (n = 63) |
|---|---|
| Age (years) | 57.8 ± 16.0 |
| Female sex (n (%)) | 37 (59) |
| Time since PAH diagnosis (years) | 1.1 ± 0.5 |
| Concomitant medications (n (%)) | |
| Sildenafil 20 mg | 31 (49) |
| Macitentan 10 mg | 28 (44) |
| Bosentan 125 mg | 12 (19) |
| Tadalafil 20 mg | 11 (17) |
| Ambrisentan 10 mg | 6 (10) |
| Riociguat 2.5 mg | 5 (8) |
| Ambrisentan 5 mg | 3 (5) |
Data are presented as mean ± standard deviation unless otherwise stated.
PAH, pulmonary arterial hypertension.
Inhalation behavior
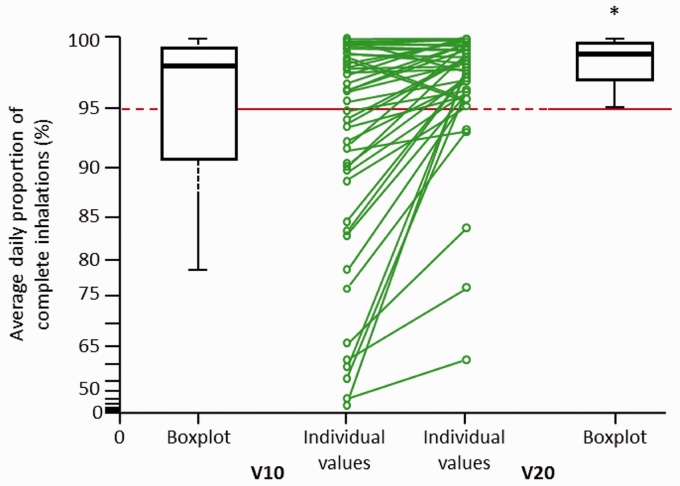
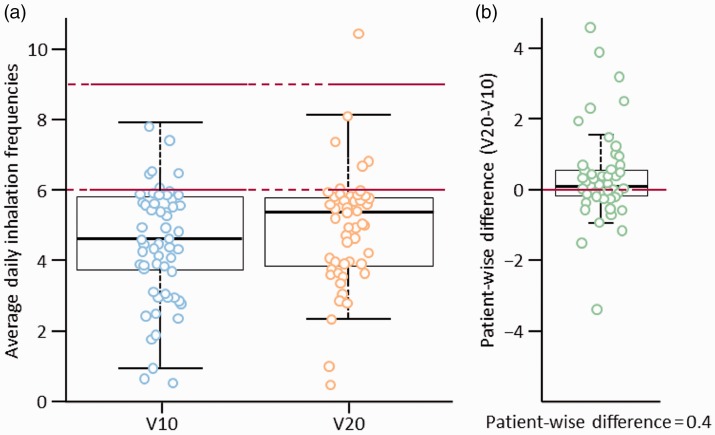
Switching from V10 to V20 led to significant improvements in co-primary endpoints. The mean (± standard deviation) daily proportion of complete inhalations increased from 92 ± 14% with V10 to 97 ± 6% with V20 (patient-wise mean difference of 6%; P < 0.0001; Fig. 1). In addition, the mean daily inhalation frequency increased from 4.6 ± 1.6 with V10 to 4.9 ± 1.5 with V20 (P = 0.0430; Fig. 2).
Fig. 1.
Average daily proportion of complete inhalations on V10 and V20. Line graph shows individual patient changes. V10, iloprost 10 µg/mL solution; V20, iloprost 20 µg/mL solution.
Fig. 2.
Average daily inhalation frequencies on (a) V10 and V20 and (b) patient-wise difference between formulations. V10, iloprost 10 µg/mL solution; V20, iloprost 20 µg/mL solution.
Switching from V10 to V20 led to a significant decrease in the secondary endpoint of mean daily inhalation duration per session, from 11.8 ± 4.7 min to 6.5 ± 2.8 min (patient-wise mean difference of –5.3 min; P < 0.0001).
Switching from V10 to V20 significantly increased the proportion of patients achieving a mean daily proportion of complete inhalations ≥ 95% (P < 0.0001) and decreased the proportion of patients with incomplete inhalations (Table 2). All patients with a daily proportion of complete inhalations < 90% still had improvements in their inhalation behavior (Fig. 1).
Table 2.
Patients with inhalation behavior ≥95% and incomplete inhalations while on V10 and V20 (n = 63).
| Subgroup (n (%)) | V10 | V20 | P value |
|---|---|---|---|
| Patients with mean daily complete inhalations ≥95% | 40 (63) | 58 (92) | < 0.0001 |
| Patients with ≥ 1 incomplete inhalation | 62 (98) | 54 (86) | < 0.0001 |
| Proportion of total inhalations that were incomplete | 2,350/27,763 (8) | 886/28,872 (3) | – |
V10, iloprost 10 µg/mL solution; V20, iloprost 20 µg/mL solution.
In total, 56 patients (89%) did not achieve ≥ 95% of days at the recommended inhalation frequency of 6–9 times per day with either formulation. Only one patient (2%) used the device more frequently than recommended (i.e. > 9 times per day) when taking V20. The proportion of patients achieving the recommended inhalation frequency for ≥ 95% of study days increased from 5% with V10 to 11% with V20.
Subgroup analyses
Stratification by age suggested greater improvements when switching older patients than younger patients. Switching from V10 to V20 increased the mean daily proportion of complete inhalations to a greater extent in patients aged ≥ 65 years than in those aged < 65 years (patient-wise mean differences of 10% [P < 0.0001] and 3% [P = 0.0001], respectively; Table 3), while mean daily inhalation frequency increased to a similar extent in both older and younger patients with PAH (patient-wise mean differences of 0.4 and 0.3 inhalations, respectively; Table 4). Switching also increased the mean daily proportion of complete inhalations ≥ 95% in patients regardless of age (exploratory McNemar’s test, P = 0.0020 and P = 0.0078 for patients ≥ 65 and < 65 years, respectively; e-Table 1), but did not appear to significantly increase the proportion of patients with recommended inhalation frequency on ≥ 95% of days in either subgroup (data not shown).
Table 3.
Daily proportion of complete inhalations according to age and number of concomitant PAH medications.
| Patient subgroup | Patients (n) | Proportion of complete inhalations
(%) |
|||
|---|---|---|---|---|---|
| V10 | V20 | Patient-wise difference | P value* | ||
| Aged ≥ 65 years | 22 | 85.4 ± 17.9 | 95.7 ± 8.3 | 10.3 | < 0.0001 |
| Aged < 65 years | 41 | 95.1 ± 9.2 | 98.1 ± 3.9 | 2.9 | 0.0001 |
| 3 concomitant PAH medications | 39 | 90.1 ± 16.0 | 97.0 ± 7.0 | 6.9 | < 0.0001 |
| <3 concomitant PAH medications | 24 | 94.4 ± 7.9 | 97.6 ± 3.5 | 3.2 | < 0.0001 |
Data are presented as mean ± standard deviation unless otherwise stated.
Exploratory P value (two-sided sign test).
PAH, pulmonary arterial hypertension; V10, iloprost 10 µg/mL solution; V20, iloprost 20 µg/mL solution.
Table 4.
Mean daily inhalation frequencies with V10 and V20, stratified by age group and number of concomitant PAH medications.
| Patient subgroup | Patients (n) | Mean daily inhalations (n) |
|||
|---|---|---|---|---|---|
| V10 | V20 | Patient-wise difference | P value* | ||
| Aged ≥ 65 years | 22 | 4.1 ± 1.68 | 4.6 ± 1.34 | 0.4 ± 0.94 | 0.0182 |
| Aged < 65 years | 41 | 4.8 ± 1.52 | 5.1 ± 1.63 | 0.3 ± 1.27 | 0.0340 |
| 3 concomitant PAH medications | 39 | 4.4 ± 1.70 | 4.9 ± 1.30 | 0.5 ± 1.13 | 0.0014 |
| <3 concomitant PAH medications | 24 | 4.8 ± 1.41 | 4.9 ± 1.91 | 0.2 ± 1.19 | 0.3793 |
Data are presented as mean ± standard deviation unless otherwise stated.
Exploratory P value (two-sided sign test).
PAH, pulmonary arterial hypertension; V10, iloprost 10 µg/mL solution; V20, iloprost 20 µg/mL solution.
Greater improvements with switching were observed in patients receiving a higher number of concomitant PAH medications. Switching from V10 to V20 increased the mean daily proportion of complete inhalations by 7% in patients receiving triple combination therapy (P < 0.0001) and by 3% in those receiving < 3 concomitant PAH medications (P < 0.0001; Table 3). Patients receiving triple combination therapy also had a greater increase in mean daily inhalation frequency than those receiving < 3 concomitant PAH medications (patient-wise mean differences of 0.5 and 0.2 inhalations, respectively; Table 4). Switching increased the mean daily proportion of complete inhalations ≥ 95% in patients regardless of the number of concomitant PAH medications (<3 or 3; e-Table 2) but did not significantly increase the proportion of patients with recommended inhalation frequency on ≥ 95% of days in either subgroup (data not shown).
Safety
One AE was recorded during the study observation period. This was a serious AE (hospitalization due to worsening of underlying disease), deemed unrelated to study drug, which led to cessation of V20 treatment.
Discussion
VENTASWITCH provides real-world data that supply evidence of patient inhalation behavior through automatic data capture into a central database. The findings demonstrate that switching from the V10 to the V20 formulation of iloprost increases the daily proportion of complete inhalations and decreases daily inhalation duration per session in patients with PAH, while also substantially shortening inhalation time. Although switching increased the mean daily inhalation frequency from 4.6 inhalations with V10 to 4.9 inhalations with V20, this still remained below the recommended frequency of 6–9 inhalations per day; thus, the improvement was relatively small. The reasons for this are unclear and elucidation falls outside the scope of this study. However, even a small improvement in inhalation behavior may have real clinical value. Time-consuming inhalation periods, in combination with a high inhalation frequency, have been described as one of the major limitations of inhaled iloprost in daily clinical practice;17 this was improved by changing the formulation, as shown in our study. The impact of rapid iloprost inhalation on pulmonary hemodynamics, functional capacity, and adherence, in combination with a more advanced inhaler might, in general, improve inhalation therapy and outcome in patients with PAH, and should be explored in future studies.
The observed small improvement in inhalation frequency was achieved by simply changing the iloprost formulation, without increasing the incidence of side effects. Indeed, switching from V10 to V20 appeared to help patients better manage their PAH and use of the I-Neb® AAD® in daily life, as reflected by the improved inhalation behavior observed in this study. Furthermore, findings from VENTASWITCH suggest that switching also provides greater improvement in inhalation behavior in older patients (aged ≥ 65 years) and in patients receiving a greater number of concomitant PAH medications. This is of clinical importance and relevant to patients, as those with severe disease may have more difficulty in performing inhalations of ≥ 10 min duration.18 Nevertheless, the findings of this study indicate that there remains a need for continued advances in technology and nebulizer development to further improve inhalation behavior, reduce inhalation durations, and improve daily inhalation frequency in patients with PAH.
Iloprost via the I-Neb® AAD® appeared to be well tolerated in this observational study, with few AEs reported. However, there was no direct physician involvement in the collection of AE data and this may have led to underreporting compared with other clinical studies.11,17,19
The potential benefit of the V20 formulation over the V10 formulation with respect to inhalation behavior was suggested by a retrospective analysis of nine patients enrolled in the US-based Ventavis registry, RESPIRE (Registry to Prospectively Evaluate Use of Ventavis in Patients with Pulmonary Arterial Hypertension), which found that switching to V20 was associated with significantly lower inhalation time and decrease in percent incomplete doses compared with the V10 formulation.16 However, before the VENTASWITCH study, the impact of switching from V10 to V20 on inhalation behavior had only been evaluated in a small number of patients. US study CONVERT analyzed inhalation data in 19 patients retrospectively collected from the I-Neb® device 28 days before and after switching. CONVERT found that switching to V20 was associated with shortened treatment times and a decrease in the proportion of incomplete doses compared with V10.15 The VENTASWITCH study has corroborated these findings in a much larger patient population (n = 63), over an extended observation time (three months before and after switching), and using prospective as well as retrospective data capture.
A limitation of the current study is that its non-interventional, observational design precluded direct comparison of V20 and V10 behavior; however, as patients were switched from V10 to V20, inter-patient variability was minimized. In addition, this was a local German study undertaken in a cohort of patients regularly supported, trained, and visited by medical-device specialists, in whom switching was based on the discretion of the physician and patient; thus, the findings may not be representative of V10 and V20 therapy in general. Further studies are also warranted to evaluate inhalation behavior with V20 using the latest nebulizer, BREELIB®,17 which has recently been approved for use with inhaled iloprost in Germany and some other countries, compared with using the I-Neb®.
The post hoc subgroup analyses differentiating the inhalation behavior of older patients or those receiving several concomitant PAH-targeted therapies was biased by the arbitrary cut-offs chosen. Nevertheless, the exploratory data on the effect of treatment switch on inhalation behavior in these subgroups provide useful insights for these vulnerable patient populations, which may help inform decisions in daily clinical practice. Assessment of iloprost efficacy in treating PAH and detailed patient baseline characteristics as well as PAH and medical history were not included as part of the study protocol, as our study sought solely to investigate changes in inhalation behavior.
Underreporting of AEs is another potential limitation of the study. Because safety was not a primary objective of the study, and as no clinical investigators were involved in the study, investigator-led exploration and assessment of AEs was not conducted. However, to minimize underreporting of AEs/complications, all patients were enrolled in the VENTAPLUS patient program and instructed to report any AEs. Patients were also interviewed at home by ContraCare employees. All patients were closely followed via the patient-support program to minimize a bias in AE reporting. In addition, the relatively low rate of AEs might be influenced by the fact that all patients were receiving stable iloprost inhalation therapy and were tolerating inhalation with V10 well; therefore, tolerability was not influenced by changing the formulation. As a consequence, patients experiencing side effects even at lower doses (2.5 µg) and those with early discontinuation of iloprost inhalation due to side effects were not captured by our analysis. Despite these limitations, a study strength is that inhalation data were obtained directly from the patient’s I-Neb®, thus reducing bias associated with self-reporting and investigator involvement.
In conclusion, due to a shortening of inhalation time, switching from the V10 to V20 formulation significantly improved inhalation behavior in patients with PAH, and may facilitate improved adherence to therapy. Subgroup analyses of complete inhalations and average daily inhalation frequency by age have shown that patients aged ≥ 65 years seem to gain particular benefit from switching to V20. The significant improvement in mean daily proportion of complete inhalations and inhalation frequency with V20 due to a shortening of inhalation time may suggest improved adherence to therapy. The clinical impact of rapid iloprost inhalation warrants exploration in future studies.
Acknowledgments
Medical writing assistance was provided by Adelphi Communications Ltd (Bollington, UK), funded by Bayer AG (Berlin, Germany). Posters were presented at the ERS International Congress, September 9–13, 2017, Milan, Italy, and at the German Congress for Health Research, October 4–6, 2017, Berlin, Germany.
Conflict of interest
MJR reports grants, personal fees, and non-financial support from Bayer AG during the study, grants from United Therapeutics, and personal fees from Actelion, Mundi Pharma, Roche, and OMT outside the submitted work. BS, AR, SB, and CM are employees of Bayer Vital GmbH. FK is an employee of Bayer AG. VG reports grants from Bayer Vital GmbH during the study and is an employee of ContraCare GmbH. H-AG reports grants from DFG (German Research Foundation), honoraria from Actelion, Bayer, Ergonex, Gilead, GSK, Novartis, and Pfizer, consultancy fees from AbbVie, Actelion, Bayer, Bellerophon Pulse Technologies, Ergonex, Gilead, GSK, Medscape, Merck Sharp & Dohme, Novartis, OMT, Pfizer, and Web MD Global, and speaker’s bureau fees from Actelion, Bayer, Ergonex, Gilead, GSK, Novartis, and Pfizer. Bayer AG was the trial sponsor and contributed to the study design and data analysis and interpretation.
Funding
The VENTASWITCH study was funded by Bayer Vital GmbH. The VENTAPLUS patient-support program was funded by Bayer Vital GmbH (Leverkusen, Germany) and conducted by ContraCare GmbH (Nuremberg, Germany).
References
- 1.Rosenkranz S. Pulmonary hypertension 2015: current definitions, terminology, and novel treatment options. Clin Res Cardiol 2015; 104: 197–207. [DOI] [PubMed] [Google Scholar]
- 2.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 3.Harrison A, Hatton N, Ryan JJ. The right ventricle under pressure: evaluating the adaptive and maladaptive changes in the right ventricle in pulmonary arterial hypertension using echocardiography (2013 Grover Conference series). Pulm Circ 2015; 5: 29–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeVarge BL. Prostanoid therapies in the management of pulmonary arterial hypertension. Ther Clin Risk Manag 2015; 11: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J 2008; 31: 891–901. [DOI] [PubMed] [Google Scholar]
- 6.Grant SM, Goa KL. Iloprost. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs 1992; 43: 889–924. [DOI] [PubMed] [Google Scholar]
- 7.Malekmohammad M, Sharif-Kashani B, Monjazebi F, et al. Intermittent intravenous administration of Iloprost in patients with idiopathic pulmonary arterial hypertension. Int J Cardiovac Acad 2016; 2: 114–118. [Google Scholar]
- 8.Bayer AG. Ventavis (iloprost), US prescribing information. Available at: https://www.4ventavis.com/pdf/Ventavis_PI.pdf2017.
- 9.Olschewski H, Walmrath D, Schermuly R, et al. Aerosolized prostacyclin and iloprost in severe pulmonary hypertension. Ann Intern Med 1996; 124: 820–824. [DOI] [PubMed] [Google Scholar]
- 10.Bayer AG. Ventavis (iloprost). Summary of Product Characteristics. 2017: 1–65. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000474/WC500048691.pdf.
- 11.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. [DOI] [PubMed] [Google Scholar]
- 12.Dhand R. Intelligent Nebulizers in the Age of the Internet: The I-neb Adaptive Aerosol Delivery (AAD) System. J AerosolMed Pulm Drug Deliv 2010; 23: iii–v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardaker LEA, Hatley RHM. In vitro characterization of the I-neb Adaptive Aerosol Delivery (AAD) system. J Aerosol Med Pulm Drug Deliv 2010; 23: S11–S20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richter MJ, Ghofrani HA, Voswinckel R, et al. Acute hemodynamic effects of nebulized iloprost via the I-neb Adaptive Aerosol Delivery system in pulmonary hypertension. Pulm Circ 2015; 5: 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Studer SM, Kawut SM, Benton W, et al. CONVERT: study of patients with pulmonary arterial hypertension treated with iloprost (inhalation), evaluating inhalation times, compliance, safety, and tolerability when converting from the iloprost inhalation solution 10 µg/mL to the 20 µg/mL with the I-neb® AAD® system. Am J Respir Crit Care Med 2012; 185: A4788.
- 16.McConnell J, Hobbs K, Elwing J, et al. RESPIRE Registry: Inhalation times and treatment adherence of iloprost delivered at 10µg/mL and 20µg/mL concentrations in patients with pulmonary arterial hypertension. Presentation at 9th International PHA Conference and Scientific Sessions, Garden Grove, California, USA, 25–27 June 2010.
- 17.Gessler T, Ghofrani HA, Held M, et al. The safety and pharmacokinetics of rapid iloprost aerosol delivery via the BREELIB nebulizer in pulmonary arterial hypertension. Pulm Circ 2017; 7: 505–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respir Care 2015; 60: 794–802. [DOI] [PubMed] [Google Scholar]
- 19.Olschewski H, Hoeper MM, Behr J, et al. Long-term therapy with inhaled iloprost in patients with pulmonary hypertension. Respir Med 2010; 104: 731–740. [DOI] [PubMed] [Google Scholar]