Abstract
In this issue of Blood, Burch et al addressed the challenge of how erythroid cells acquire sufficient carbon for heme synthesis during erythropoiesis.1
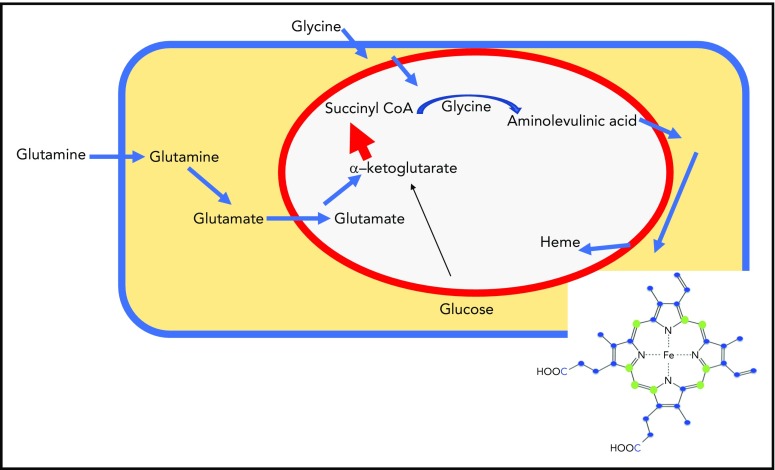
Erythroid cells use carbon atoms derived from glutamine metabolism as heme building blocks. Glutamine enters from the bloodstream through a known transporter, is converted to glutamate by glutaminase, enters the mitochondrial matrix, and is converted to α-ketoglutarate by glutamate dehydrogenase. Increased amounts of α-ketoglutarate dehydrogenase generate large amounts of succinyl-CoA, which condenses with glycine to form ALA, the first building block of heme. ALA is transported into the cytosol, where multiple heme biosynthetic enzymes generate heme intermediates. Ultimately, heme precursors re-enter the mitochondrial matrix, where iron is added to the porphyrin ring. Although glucose gives rise to α-ketoglutarate in the citric acid cycle, most of the carbons in heme in experiments by Burch et al were derived from labeled glutamine rather than glucose (blue spheres represent carbon derived from heme). A large increase in α-ketoglutarate dehydrogenase may enable the citric acid cycle in the mitochondrial matrix (within the red circle) to maintain full function, even though significant amounts of succinyl-CoA are diverted into heme synthesis in developing erythroid cells.
Because the citric acid cycle generates succinyl-coenzyme A (succinyl-CoA), which condenses with glycine to form aminolevulinic acid (the first product of heme biosynthesis), it was logical to assume that the carbons of succinyl-CoA were derived from metabolism of glucose through the usual source of succinyl-CoA, the citric acid cycle (see figure). However, siphoning off large amounts of succinyl-CoA from the citric acid cycle to briefly support the synthesis of up to 1.2 × 109 molecules of heme, each of which incorporates 8 molecules of succinyl-CoA, could severely distort other metabolic pathways that depend on the full activity of the citric acid cycle past the midway point of an α-ketoglutarate formation, the source for succinyl-CoA. By using glucose or glutamine nutrients uniformly labeled with 13C (a natural stable isotope of carbon that differs in molecular weight from the commonly found carbon-12), the authors followed incorporation of these labeled carbon sources into differentiating erythroid cells and subsequently used liquid chromatography and mass spectrometry to analyze the heme carbon masses. They concluded that glutamine supplied much more carbon to heme than glucose, whereas a succinate compound downstream of succinyl-CoA in the citric acid cycle contributed almost no carbon. Notably, both glucose and glutamine are abundant in the bloodstream, and either carbon source might theoretically be sufficient. Their discoveries explain how developing erythroid cells can maintain a functional citric acid cycle, even during periods when metabolic activity is heavily skewed toward heme synthesis.
Previously published work showed the importance of glutamine uptake in hematopoietic stem cells, suggesting that blocking glutamine use during differentiation prevented erythroid differentiation and promoted myeloid differentiation, possibly by interfering with nucleoside synthesis.2 To address the role of glutaminolysis, which generates glutamate in the cytosol, the authors treated cells with 6-diazo-5-oxo-L-norleucine, an inhibitor of glutaminolysis and also of many other enzymatic reactions. To more specifically block glutamine synthetase and formation of the α-ketoglutarate precursor glutamate, the authors treated cells with aminooxyacetic acid, a broad-spectrum inhibitor of pyridoxal-5′-phosphate–dependent transaminases. However, many side reactions are still potentially affected by this inhibitor, whereas a specific glutamate synthetase inhibitor, CB839, developed to treat cancers that are thought to depend on glutamine to support anabolic growth, could provide greater specificity in this experimental setting.3 Interestingly, high rates of anemia have not yet been reported to be associated with use of CB839 in experimental trials.
Glutamate enters the mitochondrial matrix through the transporter SLC25A22, whereupon the enzyme glutamate dehydrogenase converts it to α-ketoglutarate. The enzyme α-ketoglutarate dehydrogenase consists of 3 enzymatic subunits; the E1 subunit is responsible for decarboxylating α-ketoglutarate and converting it to the 4-carbon succinate molecule that enzymatically binds to CoA. The enzyme aminolevulinic acid synthase condenses succinyl-CoA with glycine in the mitochondrial matrix, forming the first heme precursor, aminolevulinic acid (ALA). ALA is exported to the cytosol, where several enzymes gradually build the porphyrin ring, which reenters the mitochondria to undergo the 2 final heme biosynthetic steps, which include insertion of iron at the center of the porphyrin ring. Because the pathway from exogenous glutamine to succinate CoA needs large amounts of the enzyme α-ketoglutarate dehydrogenase to direct glutamate into succinyl-CoA, the authors checked levels of the E1 subunit of α-ketoglutarate, and they found that levels increased about fourfold during differentiation, indicating that the cell was able to regulate and remodel important metabolic steps to maintain metabolic functions of the full citric acid cycle despite diversion of large amounts of succinyl-CoA into heme synthesis.
Similar to other studies that alter a widely accepted paradigm, the article by Burch et al raises several questions that merit further study. How does the erythroid cell remodel expression of α-ketodehydrogenase to more efficiently funnel succinyl-CoA into heme biosynthesis? Are levels of the plasma membrane glutamine importer, the mitochondrial glutamate importer, or the plasma membrane and mitochondrial glycine importers also increased in heme-synthesizing erythroid cells? The authors conclude that glutamine plays another undefined role in promoting early erythropoiesis that extends beyond its role in serving as a precursor to heme and nucleotide synthesis. Notably, enzymatic activities of aconitase and isocitrate dehydrogenase were high in erythroid cells, which is interesting because these enzymes are important in a process known as reductive carboxylation, in which α-ketoglutarate is carboxylated by isocitrate dehydrogenase and converted to citrate by aconitase.4 A mitochondrial citrate exporter could export a precursor of fatty acid synthesis to the cytosol, which could alter metabolism in other yet unrecognized ways.
The heme biosynthetic pathway has been an unending source of wonder for decades. Here, the article by Burch et al reminds us that much remains to be learned, and many assumptions may be overturned by experimental interrogation in the future. Newer methodologies such as metabolomics have opened the way to fresh insights into the process of heme biosynthesis.
Footnotes
Conflict-of-interest disclosure: The author declares no competing financial interests.
REFERENCES
- 1.Burch JS, Marcero JR, Maschek JA, et al. Glutamine via α-ketoglutarate dehydrogenase provides succinyl-CoA for heme synthesis during erythropoiesis. Blood. 2018;132(10):987-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oburoglu L, Tardito S, Fritz V, et al. Glucose and glutamine metabolism regulate human hematopoietic stem cell lineage specification. Cell Stem Cell. 2014;15(2):169-184. [DOI] [PubMed] [Google Scholar]
- 3.Choi YK, Park KG. Targeting glutamine metabolism for cancer treatment. Biomol Ther (Seoul). 2018;26(1):19-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 2017;36(10):1302-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]