Key Points
PITPs facilitate TGF-β1 secretion in megakaryocytes regulating hematopoiesis.
Pitpα−/−/β−/− megakaryocytes have a defect in α-granule morphology and function.
Abstract
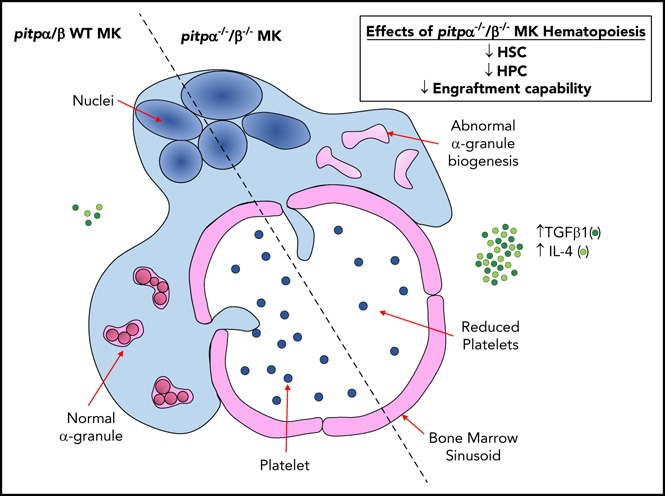
We hypothesized that megakaryocyte (MK) phosphoinositide signaling mediated by phosphatidylinositol transfer proteins (PITPs) contributes to hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) regulation. Conditional knockout mice lacking PITPs specifically in MKs and platelets (pitpα−/− and pitpα−/−/β−/−) bone marrow (BM) manifested decreased numbers of HSCs, MK-erythrocyte progenitors, and cycling HPCs. Further, pitpα−/−/β−/− BM had significantly reduced engrafting capability in competitive transplantation and limiting dilution analysis. Conditioned media (CM) from cultured pitpα−/− and pitpα−/−/β−/− BM MKs contained higher levels of transforming growth factor β1 (TGF-β1) and interleukin-4 (IL-4), among other myelosuppressive cytokines, than wild-type BM MKs. Correspondingly, BM flush fluid from pitpα−/− and pitpα−/−/β−/− mice had higher concentrations of TGF-β1. CM from pitpα−/− and pitpα−/−/β−/− MKs significantly suppressed HPC colony formation, which was completely extinguished in vitro by neutralizing anti–TGF-β antibody, and treatment of pitpα−/−/β−/− mice in vivo with anti–TGF-β antibodies completely reverted their defects in BM HSC and HPC numbers. TGF-β and IL-4 synergized to inhibit HPC colony formation in vitro. Electron microscopy analysis of pitpα−/−/β−/− MKs revealed ultrastructural defects with depleted α-granules and large, misshaped multivesicular bodies. Von Willebrand factor and thrombospondin-1, like TGF-β, are stored in MK α-granules and were also elevated in CM of cultured pitpα−/−/β−/− MKs. Altogether, these data show that ablating PITPs in MKs indirectly dysregulates hematopoiesis in the BM by disrupting α-granule physiology and secretion of TGF-β1.
Visual Abstract
Introduction
Understanding how hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) are maintained is essential for determining normal and abnormal blood cell production regulation.1,2 Megakaryocytes (MKs) are regulators of HSC proliferation through the production and release of growth factors and cytokines, such as transforming growth factor β1 (TGF-β1) and platelet factor 4 (PF4).3-7 Intracellular signaling pathways within MKs that regulate growth factor secretion are still being defined. Many potential intracellular pathways use a rare membrane phospholipid, phosphatidylinositol, which possesses a head group that is readily phosphorylated in different configurations by lipid kinases.8 These phosphoinositides (PtdIns) serve different functions depending on location of the phosphorylation, providing diversity in signaling capacities.6 Critical to PtdIn signaling are phosphatidylinositol transfer proteins (PITPs), which bind phosphatidylinositol monomers and transfer them from endoplasmic reticulum to different cellular compartments in vitro.9,10 Production of specific PtdIns at a particular organelle depends on availability of specific precursor substrates that are shuttled by PITPs between organelles, as lipids cannot readily cross the aqueous cytosol. Since PtdIn distribution and function are organelle specific, PITPs are important regulators of PtdIn metabolism in space and time.11 While platelet PtdIn pathways contribute to hemostasis by affecting platelet shape and secretion of hemostatic substances,11-14 the role of PITPs in megakaryopoiesis and hematopoietic regulation is not clear.
One major role of MKs is creating stores of secretory vesicles, including α-granules, dense granules, and lysosomes containing growth and other bioactive factors.15 PtdIns in other cell types initiate intracellular trafficking by recruiting effector proteins involved in vesicular fusion and plasma membrane budding. PtdIn–protein interactions coordinate various membrane trafficking pathways and direct proteins into multivesicular bodies (MVBs), thought to be intermediates in formation of the 3 classes of MK granules.16 We hypothesized that PtdIn signaling facilitates biogenesis of MK granules and thus cytokine secretion. We evaluated whether conditional knockout mice lacking pitpα or both pitpα and pitpß specifically in MKs and platelets demonstrate altered hematopoietic regulation, and we unearthed a surprisingly new role for MK PITPs and the PtdIns pathway in regulating MK α-granule biogenesis, thus modulating MK TGF-β release and affecting hematopoiesis.
Methods
Mice
C57Bl/6J, Boy/J, and B6xBoy/J F1 (F1) mice (8- to 14-week-old males and females) were obtained from a breeding core facility at Indiana University School of Medicine. Pitpα (pitpαfl/fl pf4 Cre+) and pitpα/β (pitpαfl/fl/βfl/fl pf4 Cre+) knockout mice plus age- and sex-matched pitpαfl/fl and pitpαfl/flβfl/fl littermate (wild-type [WT]) control mice (10-16 weeks old, either males or females) were bred at the University of Pennsylvania in accordance with Institutional Animal Care and Use Committee guidelines. Conditional pitpα−/− mice were described previously.17 See supplemental Methods (available on the Blood Web site) for description of pitpα−/− and pitpα−/−/β−/− mice. These mice lack class I pitpα or both pitpα and pitpß gene isoforms specifically in MKs and platelets. These mice were grossly normal, with no changes in weight, organ morphology, or survival (data not shown). Mice (mouse (m) αII+/+/human (h) αII−/−) that lack mouse αII but express human αII on their platelets were described previously18 and were kindly provided by Morty Poncz of Children’s Hospital of Pennsylvania. These animals were housed at the Children’s Hospital of Philadelphia animal facility. All procedures were performed after approval by the Children’s Hospital of Philadelphia’s Animal Care and Use Committee. All further animal procedures were approved by the Indiana University Use and Care of Animals Committee. Animals were maintained under temperature- and light-controlled conditions (21-24°C, 12 hour light/12 hour dark cycle); group-housed according to age, sex, and genotype; and fed ad libitum.
Production of CM and collection of BM flush fluid
Bone marrow (BM) was flushed from pitpα−/−, pitpα−/−/β−/− and WT mice. Equal numbers of BM cells (7 × 107cells/mouse) were cultured in Iscove modified Dulbecco medium (Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum (Sigma-Aldrich, St. Louis, MO) and 50 ng/mL recombinant mouse thrombopoietin (rmTPO; R&D Systems, Minneapolis, MN) for 72 hours. MKs were purified by bovine serum albumin (BSA; Fisher Scientific, Pittsburgh, PA) gradient centrifugation, washed, and recultured with 50 ng/mL rmTPO for 48 hours followed by conditioned media (CM) collection. Cultured pitpα/β WT and pitpα−/−/β−/− BM yielded equivalent number of MKs (data not shown). BM flush fluid was collected by repeatedly flushing 1 mL sterile cold PBS through femurs, followed by centrifugation (3000 rpm) for 15 minutes at 4°C.
ELISA determination of protein concentrations
An TGF-β1 enzyme-linked immunosorbent assay (ELISA) was performed according to the manufacturer’s instruction (DuoSet Mouse TGF-β1; R&D Systems). See supplemental Methods for more details. Similar procedures were used to determine PF4 concentrations using the DuoSet Mouse CXCL4/PF4 kit (R&D Systems), serum TPO concentrations using the DuoSet Mouse TPO kit (R&D Systems), thrombospondin-1 (TSP-1) using the Mouse TSP-1 ELISA kit (catalog no. EL-M1137; Elabscience, Houston, TX), and von Willebrand factor (vWF) using the Mouse vWF ELISA kit (catalog no. LS-F22891; LifeSpan BioSciences, Seattle, WA).
Western blot
Tissue-cultured MKs were purified by BSA gradient centrifugation then lysed in RIPA buffer containing a proteinase inhibitor and phosphatase inhibitor cocktail. For TPO stimulation, the purified MKs were TPO starved for 1 hour followed by stimulation with TPO for the indicated amount of time and concentrations. Reactions were stopped by adding 2× complete RIPA buffer. Lysates were collected after 13 000 rpm centrifugation for 15 minutes at 4°C. The protein concentration in total MK lysate was quantified by BCA protein assay (Fisher Scientific). An equal amount of total protein was loaded into the sodium dodecyl sulfate gel. After electrophoresis, the gel was transferred to a polyvinylidene difluoride membrane, which was incubated with primary antibody followed by corresponding horseradish peroxidase–conjugated secondary antibodies. Bands were developed by incubation with ECL reagent (GE Healthcare Bio-Sciences, Pittsburgh, PA) and imaged with a KwikQuant Imager (Kindle Biosciences). Expression of each protein was quantified by ImageJ for densitometry. See supplemental Methods for further details.
Transmission electron microscopy
TPO cultured BM MKs were harvested and purified by BSA gradient centrifugation on day 6, fixed with 2.5% glutaraldehyde, 2.0% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) overnight at 4°C, and processed as described in supplemental Methods. Sections were examined with a JEOL 1010 electron microscope fitted with a Hamamatsu digital camera and AMT Advantage image capture software at the Electron Microscopy Resource Laboratory at the University of Pennsylvania.
Reverse-transcription PCR
Messenger RNA expression of TGF-β1 and TGF-β2 in tissue cultured MKs was analyzed by conventional semiquantitative reverse-transcription polymerase chain reaction (PCR; QIAGEN, Hilden, Germany) using primer sets 5′-GAACCCGTGTTGCTCTCCCG-3′ (forward), 5′-TCACAGGAGCAGTGGGCGCT-3′ (reverse) for TGF-β1 and 5′-GTTGGGAACGCGTTGCATTT-3′ (forward), 5′-GCGCATAAACTGATCCATGT-3′ (reverse) for TGF-β2.19
Flow cytometry immunophenotyping of BM HSCs and HPCs
This was performed by flushing femurs of pitpα−/−, pitpα−/−/β−/−, and WT mice and incubating BM cells with fluorochrome conjugated antimouse antibodies (1 μg/106 cells) in PBS at room temperature for 20 minutes. See supplemental Methods for details on antibodies. Long-term HSCs (LT-HSCs) were defined as Lin−Sca1+c-Kit+CD34−Flt3−, short-term HSCs (ST-HSCs) as Lin−Sca1+c-Kit+CD34+Flt3−, multipotent progenitors as Lin−Sca1+c-Kit+CD34+Flt3+, common myeloid progenitors as Lin−Sca1−c-Kit+CD34intFcγRlo, granulocyte-macrophage progenitors (GMPs) as Lin−Sca1−c-Kit+CD34hiFcγRhi, and megakaryocyte-erythrocyte progenitors (MEPs) as Lin−Sca1−c-Kit+CD34−/loFcγR− using an LSRII flow cytometer (BD Biosciences, San Diego, CA).
HPC assays
BM cells flushed from femurs of C57Bl/6, pitpα WT, pitpα−/−, pitpα/β WT, and pitpα−/−/β−/− mice were plated at 5 × 104 or 2 × 105 BM cells/mL in 1% methylcellulose culture medium with 30% fetal bovine serum (Fisher Scientific), 0.1 mM hemin (Sigma-Aldrich), 1 U/mL recombinant human erythropoietin (R&D Systems), 10 ng/mL recombinant mouse granulocyte-macrophage colony-stimulating factor (R&D Systems), 10 ng/mL rmTPO (R&D Systems), 50 ng/mL recombinant mouse stem cell factor (R&D Systems), and/or 5% vol/vol pokeweed mitogen spleen conditioned medium. Colonies were scored after 6 days of incubation at 5% CO2 and 5% O2 in a humidified chamber. Colony-forming unit (CFU) granulocyte-macrophage (CFU-GM), burst-forming unit erythroid (BFU-E), CFU-MK, and CFU granulocyte, erythrocyte, macrophage, and megakaryocyte (CFU-GEMM) were distinguished, and colonies per femur calculated.20,21 See supplemental Methods for further details.
Hematopoietic stem and progenitor cell engrafting studies
Competitive repopulating units (CRUs) were calculated using limiting dilution analysis.22 See supplemental Methods for details.
In vivo TGF-β neutralization
Pitpα−/−/β−/− and WT mice were given intraperitoneal injections of either 0.5 mg/kg unconjugated anti-mouse TGF-β(1,2,3) neutralizing antibody (clone 1D11; R&D Systems) or rat immunoglobulin G1 isotype control (clone 43414; R&D Systems) once per day for 2 days. BM was flushed 24 hours following the last injection and used for immunophenotyping of HSC/HPC by flow cytometry and for HPC colony assays.
Statistical analysis
BioPlex, flow cytometry, and colony assay data are expressed as mean ± standard error of the mean (SEM). ELISAs and luciferase activity assay data are expressed as mean ± standard deviation. Student 2-tailed t tests compared indicated experimental groups. P values <.05 were considered significant.
Results
HSC and HPC numbers are reduced in pitpα−/− and pitpα−/−/β−/− mouse BM
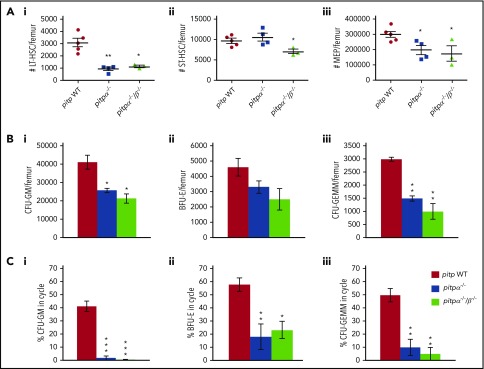
The role of PITP in MKs on hematopoietic regulation was assessed by deleting predominant PITP isoforms in MKs and platelets, PITPα (pitpαfl/fl pf4cre+; referred to as pitpα−/−) or both PITPα and PITPβ (pitpαfl/flβfl/fl pf4cre+; referred to as pitpα−/−/β−/−).17 As the pf4 promoter is activated earlier in MK development, we confirmed by western blot that PITPs were deleted in MKs without compensatory upregulation of the complementary isoform (supplemental Figure 1A). To confirm that this depletion was specific to MK and platelets, we examined PITPα and PITPβ levels in lineage- and CD61-negative (Lin−CD61−) BM cells in pitpα WT, pitpα−/−, pitpα/β WT, and pitpα−/−/β−/− mice. We found that Lin−CD61− BM cells did not express PITPα (confirmed using a splenocyte positive control), and PITPβ levels were equivalent in all animals examined (supplemental Figure 1B). Next, we analyzed phenotypically defined HSCs and HPCs (Figure 1A; supplemental Figure 2) in pitpα−/− and pitpα−/−/β−/− BM. Compared with littermates, pitpα−/− BM manifested significantly decreased LT-HSCs (∼65% decrease) and MEPs (∼54% decrease). Pitpα−/−/β−/− BM also demonstrated reduced LT-HSCs (∼58% decrease), ST-HSCs (∼28% decrease), and MEPs (∼43% decrease; Figure 1A), with decreasing trends in GMPs (supplemental Figure 2A). Multipotent progenitors and common myeloid progenitor numbers were normal even when both PITPs isoforms were deleted in MKs and platelets (supplemental Figure 2B-C). Pitpα−/− and pitpα−/−/β−/− BM had decreased CFU-GMs, BFU-Es, and CFU-GEMMs (Figure 1B) when cultured in vitro, with a decreased percentage of HPCs in S phase of the cell cycle (Figure 1C), suggesting that MK and platelet PITPs influence HPC proliferation.
Figure 1.
Deletion of pitpα and pitpα/pitpß in MKs results in decreased HSC and HPC numbers in the BM. BM was collected from WT littermate control (n = 5), pitpα−/− (n = 4), or pitpα−/−/β−/− (n = 3) mice and analyzed for HSC and HPC numbers. (A) The number of LT-HSCs (Ai), ST-HSCs (Aii), and MEPs (Aiii) per femur was determined by flow cytometry. (B) Progenitor cell numbers and function were analyzed utilizing a functional HPC colony assay examining CFU-GMs (Bi), BFU-Es (Bii), and CFU-GEMMs (Biii) per femur. (C) The percentage of CFU-GMs (Ci), BFU-Es (Cii), and CFU-GEMMs (Ciii) in the S phase of the cell cycle was determined using the high-specific-activity tritiated thymidine kill technique. For the colony assays, each mouse was plated in triplicate. All data are presented as mean ± SEM. *P < .05, **P < .005, and ***P < .0005 when compared with WT as determined by Student t tests.
Pitpα−/−/β−/− BM demonstrates poor engrafting efficiency
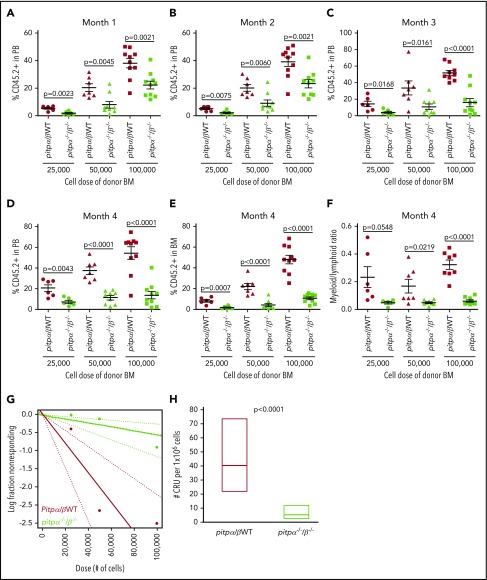
We performed competitive BM transplantation and limiting dilution analysis to examine if knocking out both PITPα and PITPβ in MKs and platelets changes engrafting efficiency and CRUs (measure of functional HSC numbers) of donor BM cells. The percentage of donor CD45.1− CD45.2+ cells in the peripheral blood (PB) and BM of host mice 1 to 4 months posttransplantation demonstrated that pitpα−/−/β−/− BM had significant dose-dependent defects in engraftment at all time points (Figure 2A-E). Pitpα−/−/β−/− donor cells also demonstrated myeloid/lymphoid ratio skewing toward the lymphoid lineage in the PB 4 months posttransplantation (Figure 2F). Poisson distribution of donor BM 4 months following transplantation revealed CRU frequencies of 1/24 744 in WT and 1/177 984 in pitpα−/−/β−/− (Figure 2G; supplemental Table 1), an ∼7.19-fold decrease in pitpα−/−/β−/− BM CRUs (Figure 2H). Therefore, there is a severe competitive disadvantage to BM containing MKs and platelets that lack both PITPα and PITPβ.
Figure 2.
Engrafting efficiency of pitpα/β WT and pitpα−/−/β−/−BM as assessed by competitive BM transplantation and limiting dilution analysis. Donor BM cells (CD45.1− CD45.2+) from pitpα/β WT and pitpα−/−/β−/− mice at various doses (25 000, 50 000, and 100 000) were mixed with 1 × 105 competitor Boy/J BM cells (CD45.1+CD45.2−) and injected IV into lethally irradiated F1 host mice (CD45.1+CD45.2+). Following 1 (A), 2 (B), 3 (C), and 4 months (D), the percentage of donor-derived cells (referred to as CD45.2+) in the PB was determined by flow cytometry. (E) The percentage of donor-derived cells in the BM was determined at 4 months. (F) The myeloid (CD11b+)/ lymphoid (CD3+ and B220+) ratio in the PB at 4 months was determined by flow cytometry. (G-H) Limiting dilution analysis was performed. (G) Poisson statistical analysis of data from the limiting dilution transplantation. Each circle represents the percentage of negative mice for a specific dose of cells. Each solid line indicates best-fit linear model for the data set. Dotted lines represent 95% confidence intervals. (H) The number of CRUs in 1 × 106 cells was calculated. See supplemental Table 1 for more details. Data represent the mean ± SEM of 6 to 10 host/recipient mice per group.
PITP is not required for MK differentiation, proplatelet formation, and platelet survival
To further characterize pitpα−/− and pitpα−/−/β−/− mice, we analyzed their blood parameters. Mice with MKs and platelets that lacked PITPα had mild thrombocytopenia (76% of normal), while mice with platelets that lacked both isoforms had more pronounced thrombocytopenia (∼59% of normal; supplemental Figure 3A). Further, we noted that pitpα−/−/β−/− platelets had a significantly larger mean volume (6.425 ± 0.329 fL; P < .007, Student t test) than controls (5.800 ± 0.110 fL; supplemental Table 2). However, these mice demonstrated no spontaneous thrombosis or hemorrhaging. Other blood parameters of these mice were comparable to littermate controls (supplemental Table 2).
Given that platelets are produced by MKs and conditional deletion of PITPs was initiated in MKs, we examined if the observed macrothrombocytopenia is caused by dysregulation of MK development in absence of PITPs. We measured MKs in pitpα−/−/β−/− BM by flow cytometry staining for CD41 (integrin αIIb, a cell-surface marker for platelets and MK). MK frequency in pitpα−/−/β−/− BM was identical to WT controls (supplemental Figure 3B). Nucleated BM cells per femur were equivalent (supplemental Figure 3C). Histological analysis also demonstrated CD41+ MK numbers in femurs of pitpα−/−/β−/− mice (401 ± 36.37) were comparable to littermate controls (445 ± 48.70, P = .073) (supplemental Figure 3D-E).
Since decreased platelet counts cannot be attributed to MKs numbers, we hypothesized that PITP-deficient MKs may be developmentally stunted and not produce platelets normally. We assessed TPO-induced megakaryopoiesis from fetal liver tissue (embryonic day 13.5) by measuring DNA ploidy expansion, and no differences in ploidy distribution were seen between pitpα−/−/β−/− MKs and controls (supplemental Figure 3F). Another possible cause of platelet reduction is the inability of pitpα−/−/β−/− MKs to extend proplatelet projections from MKs that break off to form platelets, but numbers of pitpα−/−/β−/− MKs that could form proplatelets were comparable to controls after 24 hours (supplemental Figure 3G).
Platelet turnover (observed as a reduction in circulating platelets) might reflect shortening of platelet lifespan due to PITP deletions. We measured pitpα−/−/β−/− platelet lifespan by injecting WT or pitpα−/−/β−/− MKs into the retro-orbital sinus of genetically labeled mice expressing human αIIb integrin on their platelets. Injected MKs quickly became lodged in the lung capillary bed and released platelets in the host mouse circulation. These platelets can be distinguished from host platelets by discriminating for mouse (donor) or human (host) αIIb integrin markers. Pitpα−/−/β−/− platelets survived normally (supplemental Figure 3H). Thus, PITP does not impact MK differentiation or platelet turnover.
CM from cultured pitpα−/− and pitpα−/−/β−/− MK suppress HPC colony formation
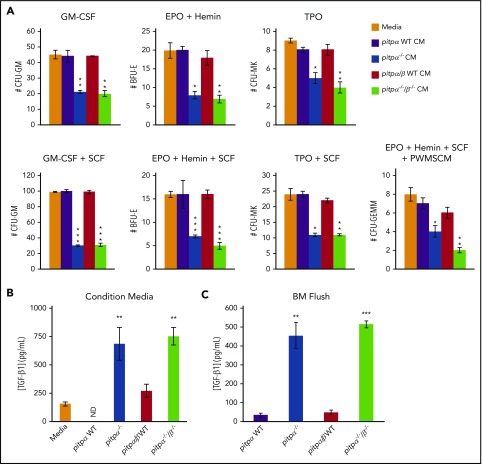
MKs can regulate hematopoiesis through the production and secretion of cytokines.3,4,14,23 Therefore, we evaluated effects of TPO-culture–expanded WT, pitpα−/−, and pitpα−/−/β−/− MK CM on C57Bl/6 BM HPC colony formation. CM from pitpα−/− and pitpα−/−/β−/− MKs significantly suppressed CFU-GM, BFU-E, CFU-GEMM, and CFU-MK colony formation of WT BM compared with WT MKs CM (Figure 3A). Thus, pitpα−/− and pitpα−/−/β−/− MKs released factors that suppressed HPC proliferation. Since large numbers of cytokines and growth factors are stored in MKs, we examined cytokine and growth factor levels in the WT, pitpα−/−, and pitpα−/−/β−/− MK CM by bioplex analysis. Multiple differences in protein concentrations were noted (supplemental Table 3). Noteworthy among these are interleukin-4 (IL-4), interferon-γ–induced protein-10, and monokine induced by γ interferon, which all have myelosuppressive effects.24-30 However, only IL-4 was increased in pitpα−/− and pitpα−/−/β−/− MK CM (∼91% and ∼88%, respectively). As PF4 and TGF-β1 are known to be stored and secreted by MK31-34 but were not part of our screen, we analyzed TGF-β1 and PF4 levels in the CM by ELISA. Pitp−/− MKs CM had modestly decreased PF4 (∼21% to 30% decreased; supplemental Figure 4A) but elevated TGF-β1 levels (∼64% to 100% increase; Figure 3B). TGF-β1 levels were also significantly higher in BM flush fluid of pitpα−/− and pitpα−/−/β−/− femurs (Figure 3C). To test if the increase in BM TGF-β1 concentrations was due to altered TPO expression (a major MK growth and development factor), we examined serum TPO concentrations. TPO levels were similar in sera of all groups (supplemental Figure 4B). Thus, TPO concentration was steady despite pitp deletion and was not likely influencing cytokine and growth factor concentrations.
Figure 3.
CM from expanded pitpα−/−and pitpα−/−/β−/−MKs suppressed colony formation. (A) HPC colony assays were performed utilizing BM from WT C57Bl/6 mice in the presence of CM from pitpα WT littermate, pitpα−/−, pitpα/β WT littermate, and pitpα−/−/β−/− MKs cultures and the indicated growth factors. CFU-GM, BFU-E, CFU-MK, and CFU-GEMM colony numbers were determined by morphology of the cells and colony formation. Each test was plated in triplicate. Data are the average of 2 different batches of CM ± SEM. *P < .05, **P < .005, and ***P < .0005 when compared with media alone control as determined by Student t tests. (B) TGF-β1 levels in pitpα WT, pitpα−/−, pitpα/β WT, and pitpα−/−/β−/− MKs CM was examined by ELISA. Samples were plated in triplicate. The data are representative of 2 different batches of samples ± standard deviation. ND indicates that TGF-β1 was not detected in sample. **P < .005 when compared with either media alone or WT control as determined by Student t tests. (C) TGF-β1 levels in pitpα WT, pitpα−/−, pitpα/β WT, and pitpα−/−/β−/− BM flush fluid was examined by ELISA. Samples were plated in triplicate. Data are the average levels of 5 mice per group. **P < .005 and ***P < .0005 when compared with WT BM flush as determined by Student t tests. EPO, erythropoietin; PWMSCM, pokeweed mitogen spleen conditioned media; SCF, stem cell factor.
Neutralizing TGF-β reverses pitpα−/− and pitpα−/−/β−/− MK suppressive effects on hematopoiesis
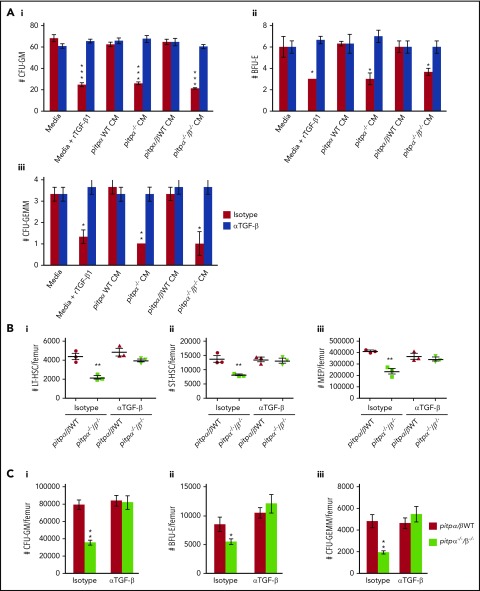
As TGF-β concentrations were significantly greater in the CM of both pitpα−/− and pitpα−/−/β−/− MKs, we first determined if TGF-β had a role in the myelosuppressive effects of the CM by pretreating the CM with a neutralizing anti-TGF-β antibody prior to its use in a HPC colony assay. Anti-TGF-β neutralizing antibody completely ablated the myelosuppressive effects of recombinant TGF-β1 (rTGF-β1) or the CM (Figure 4A), demonstrating that myelosuppression of pitpα−/− and pitpα−/−/β−/− MKs CM was due primarily to TGF-β. To determine whether TGF-β had an in vivo role regulating hematopoiesis in pitpα−/−/β−/− mice, we administered neutralizing anti-TGF-β or isotype control antibody once a day for 2 consecutive days (Figure 4B-C; supplemental Figure 4C). Treatment of littermate control mice with anti-TGF-β antibody had no effect on HSC and HPC numbers, but administering anti-TGF-β to pitpα−/−/β−/− mice restored phenotypically defined LT-HSC, ST-HSC, MEP (Figure 4B), and GMP numbers (which were significantly suppressed in pitpα−/−/β−/− mice in this experiment; supplemental Figure 4C). HPC numbers in pitp−/− BM returned to littermate control levels following in vivo treatment with neutralizing TGF-β antibodies (Figure 4C). Thus, myelosuppression in pitpα−/−/β−/− mice was likely through TGF-β acting alone or with other myelosuppressive cytokines.
Figure 4.
Neutralizing TGF-β with antibodies in vitro and in vivo reversed the myelosuppressive effect of pitpα−/−/β−/−MKs. (A) HPC colony assays were performed in the presence of media with 10 ng/mL rTGF-β1 or CM from pitp WT and pitpα−/−/β−/− MKs that was pretreated with 1 µg/mL anti-TGF-β neutralizing antibody or an isotype antibody control for one hour at 4°C prior to being placed into the HPC colony assays. BM from WT C57Bl/6 mice was used for the HPC colony assays. The number of CFU-GM (Ai), BFU-E (Aii) and CFU-GEMM (Aiii) was counted per 5 × 104 nucleated BM cells. Each group was plated in triplicate. Data are presented as mean ± SEM and are representative of 2 separate experiments. ***P < .0005 when compared with media isotype control group as determined by Student t tests. (B-C) Pitpα−/−/β−/− and WT mice were treated with intraperitoneal injections once per day for 2 days of either 0.5 mg/kg anti-TGF-β or isotype control antibodies. (B) Twenty-four hours following the final injection of antibodies, BM was collected and analyzed phenotypically for LT-HSC (Bi), ST-HSC (Bii), and MEP (Biii) numbers per femur by flow cytometry. (C) BM was also collected and analyzed by HPC colony assay. The number of CFU-GMs (Ci), BFU-Es (Cii), and CFU-GEMMs (Ciii) per femur was counted (n = 3 per group). For the colony assays, each mouse sample was plated in triplicate. For the in vivo data, data represent mean ± SEM. *P < .05 and **P < .005 when compared with pitpα/β WT isotype control as determined by Student t test.
IL-4 synergized with TGF-β1 to suppress HPC colony formation
Since pitpα−/− and pitpα−/−/β−/− MKs release increased IL-4, and IL-4 has inhibitory effects on HPCs,25,27,28 we assessed whether IL-4 synergized with TGF-β1 to mediate HPC suppression (Figure 5A). Significant suppression of colony formation occurred with concentrations as low as 1 ng/mL rTGF-β1 (P = .0006) or recombinant IL-4 (rIL-4) (P = .0001) alone. However, combination of both at only 0.001 to 0.01 ng/mL each significantly suppressed colony numbers. To examine roles for IL-4 alone or with TGF-β, pitp−/− MKs CM was pretreated with either neutralizing antibodies against TGF-β, IL-4, or TGF-β + IL-4 or isotype control antibodies (Figure 5B). While TGF-β antibodies completely blocked pitp−/− MK CM suppression, IL-4 antibodies were only partially neutralizing, suggesting that TGF-β is likely the primary suppressive agent produced or released by pitp−/− MK.
Figure 5.
TGF-β1 and IL-4 worked synergistically to inhibit HPC colony formation. WT C57Bl/6 BM (5 × 104 cells per plate) was used for the colony assays. (A) HPC colony assays were performed where rTGF-β1 and rIL-4 were added to the plates at the indicated concentrations prior to incubation. When both cytokines were added, they were added in equal amounts. *P < .05, **P < .005, and ***P < .0005 when compared with media alone group as determined by Student t test. (B) CM obtained from pitpα−/−/β−/− MKs was pretreated with 1 µg/mL neutralizing TGF-β and/or IL-4 antibodies or their isotype controls for 1 hour at 4°C prior to being added to the HPC colony assays. Media alone, media with 10 ng/mL rTGF-β1, and media with 10 ng/mL rIL-4 were used as controls, each condition being treated with control or neutralizing antibody. *P < .05 and **P < .005 when compared with group with media vehicle pretreated with isotype control. ǂP = .019 when compared with group with pitpα−/−/β−/− MK CM pretreated with isotype control. (A-B) Total number of colonies was counted. Each group was plated in triplicate. Data are the mean colony numbers ± SEM. Ab, antibody.
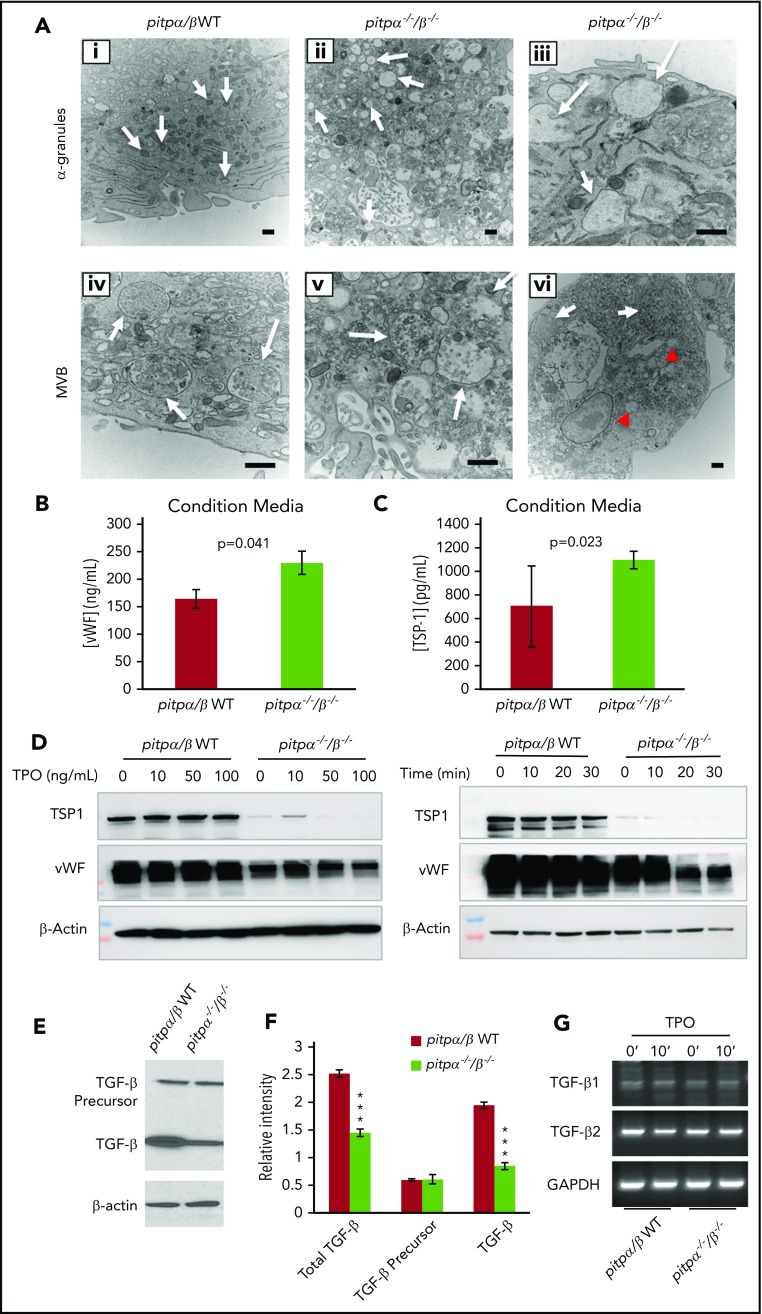
Pitpα−/−/β−/− MKs have abnormal α-granule ultrastructure
Since Pitps are emerging as regulators of PtdIn signaling and help coordinate membrane trafficking,9 we examined MK morphology via section electron microscopy (Figure 6A; supplemental Figure 5). Pitpα−/−/β−/− MKs were enriched in large, granular compartments that were less electron dense than typical α-granules (white arrows, Figure 6Ai-iii) and abnormally large and misshaped MVBs (intermediates in α-granule biogenesis) that contained a less electron-dense matrix (white arrows, Figure 6Aiv-vi).16 Many pitpα−/−/β−/− MKs presented with highly vacuolated depleted cytoplasm regions, suggesting that they might be continuous with the extracellular space (supplemental Figure 5); similar profiles were rarely observed in WT MKs. Pitpα−/−/β−/− MKs accumulated abnormal autophagic-like compartments with internal membranes (red arrows, supplemental Figure 5F). These data suggests that α-granule formation is compromised in pitpα−/−/β−/− MKs and might reflect constitutive secretion or mispackaging of α-granule components.
Figure 6.
Pitpα−/−/β−/−MKs have abnormal α-granule biogenesis and defects in α-granule storage. (A) Electron microscope analysis of pitpα−/−/β−/− MKs showing α-granules (Ai-iii). WT MKs have electron-dense α-granules at 15 000× (Ai), while pitpα−/−/β−/− MKs have enlarged, empty α-granules at 15 000× (Aii) and 40 000× (magnified view; Aiii). Arrows point to α-granules. WT MKs contain electron-dense MVBs (Aiv), while pitpα−/−/β−/− MKs have enlarged, less electron-dense MVBs (Av). An example of massively enlarged MVBs (arrows) in pitpα−/−/β−/− MKs (Avi). While not representative, MVBs of this size were noted occasionally. These are significantly larger compared with a typical enlarged pitpα−/−/β−/− MK MVB (red arrowheads). All scale bars represent 500 nm. (B-C) ELISA quantification for TSP1 (B) and vWF (C) in MK CM. *P < .05, **P < .005, and ***P < .0005 when compared with control group as determined by Student t test. (D) Western blot analysis of pitpα−/−/β−/− MKs lysates probing for major α-granule components, TSP-1 and vWF. (E) Levels of TGF-β precursor propeptide and cleaved TGF-β monomers in pitpα−/−/β−/− and pitpα/β WT littermate control MKs lysates were examined by western blot. (F) Densitometry quantification of TGF-β expression detected by western blot (n = 3). (G) Semiquantitative reverse-transcription PCR analysis of TGF-β1 and TGF-β2 expression in littermate control and pitpα−/−/β−/− MKs.
To examine potential defects in α-granule storage, 2 major contents of α-granules, TSP-1 and vWF, were analyzed in CM by ELISA and MK lysate by western blot. Pitpα−/−/β−/− MKs CM had increased TSP-1 and vWF concentrations (Figure 6B-C). This corresponded with significantly lower TSP-1 and vWF protein levels in pitpα−/−/β−/− MKs independent of TPO stimulation (Figure 6D). These data further demonstrate that pitpα−/−/β−/− MKs have intracellular trafficking or secretion defects resulting in constitutive, uncontrolled release of typical α-granule contents and/or an inability to recycle proteins into their α-granule.
Normally, TGF-β is sequestered in a latent complex, stored in the MK α-granules and secreted in extracellular matrix in vivo or culture media in vitro through controlled degranulation. TGF-β is synthesized as a large precursor protein containing a propeptide region that gets cleaved prior to cell secretion.32 While western blotting cannot distinguish between the 3 isoforms of TGF-β, cleaved TGF-β (all isoforms) was decreased by 50% in cultured pitpα−/−/β−/− MKs compared with WT controls, while uncleaved TGF-β levels were comparable (Figure 6E-F). This indicates that translation of TGF-β is similar, but does not address if transcriptional rates are also similar. To address this, messenger RNA for both TGF-β1 and TGF-β2 were compared and the data indicate normal transcription of TGF-β1 and TGF-β2 in pitpα−/−/β−/− MKs at both resting or stimulated conditions (TPO for 10 minutes; Figure 6G). Thus, increased TGF-β levels in pitpα−/−/β−/− MKs CM possibly suggests a defect in TGF-β cleavage and/or TGF-β is trafficked, stored, or secreted improperly.
Discussion
Intracellular membrane trafficking is critical in MKs for packaging and release of bioactive secretory vesicles. However, little was known about how this trafficking is regulated. PITPs are emerging as important regulators of PtdIns signaling that help coordinate trafficking. PtdIns recruit proteins facilitating vesicular budding and release in yeast, fibroblasts, and neurons9 and may serve a similar role in MK. BM of mice with pitpα−/− and pitpα−/−/β−/− MK/platelets had impaired hematopoiesis with decreased HSCs, MEPs, and cycling HPCs. CM of pitpα−/− and pitpα−/−/β−/− BM MKs suppressed colony formation of WT C57Bl/6 BM HPCs through elevated TGF-β1 and IL-4 levels. As the TGF-β antibody neutralized suppression by pitpα−/− and pitpα−/−/β−/− MK CM in vitro and TGF-β antibody treatment restored pitpα−/−/β−/− BM HSCs and HPCs in vivo, TGF-β was implicated as the primary cytokine involved in hematopoietic suppression. Ultrastructural analysis of pitpα−/−/β−/− MKs showed depleted α-granules and misshaped MVBs suggesting defects in biogenesis, storage, and/or regulated secretion of α-granule contents.
Deletion of MK PITPs decreased HSCs and HPCs numbers and function in vivo and in vitro. These impairments were even more dramatic when hematopoiesis of pitpα−/−/β−/− BM was driven beyond steady-state conditions in transplantation assays. Despite the fact that under “steady-state conditions” MEPs and platelet numbers were decreased in pitpα−/−/β−/− mice, MK number and ploidy (endomitosis) were relatively normal. Therefore, reduction of HSC/HPC numbers and function by deletion of MK PITPs is likely an indirect effect due to changes in MK physiology (ie, changes in secretion of cytokines due to abnormal α-granule biogenesis), not changes in MK numbers. Interestingly, the pitp knockout MKs within the BM release platelets into the circulation less efficiently than WT MKs, yet pitp knockout MKs are capable of releasing normal quantities of platelets when the MKs are directly injected into the bloodstream. In addition, examination of the BM morphology suggests that pitpα−/−/β−/− MKs localize to the vasculature similarly to WT MKs (supplemental Figure 3D; data not shown), suggesting that pitpα−/−/β−/− MKs migrate normally to BM vasculature. The mechanism by which PITP enzymes enable MKs within the BM to efficiently generate circulating platelets is an area of investigation.
As MK-derived factors are important for maintenance of hematopoiesis, it is important to understand MK intracellular signaling pathways regulating secretion of these molecules. TGF-β is a potent regulator of hematopoiesis,3,4,14,23 but it is unclear how TGF-β levels in the MK CM are regulated by PITP. Concurrently, levels of TGF-β messenger RNA and TGF-β precursor protein levels in pitpα−/−/β−/− MKs were normal, yet levels of the cleaved TGF-β were decreased. This suggests a retention problem after proteolytic maturation of TGF-β. This could reflect either disruption of TGF-β uptake from extracellular stores or constitutive release of TGF-β and consequent depletion of α-granule contents. Given morphological defects in α-granules and MVB in pitpα−/−/β−/− MK, we favor the latter.
We hypothesized that disrupting PtdIns signaling pathways by deleting PITPs caused excessive release of MK α-granules contents. This is supported by the decrease of cleaved TGF-β in pitpα−/−/β−/− MKs lysates and concomitant increase of TGF-β in the CM. Also, other α-granule components such as vWF and TSP-1 were elevated in pitpα−/−/β−/− MK CM. Thus, for these α-granule components, it is likely that the elevated release from pitpα−/−/β−/− MKs is due to constitutive secretion. Alternatively, improper sorting or packaging of TGF-β and other typical α-granule components could bypass the MVB and α-granules entirely, and this material could get passively released into the extracellular BM environment. The slight decrease in PF4 levels in the pitpα−/− and pitpα−/−/β−/− MK CM was unexpected given that TGF-β1 and PF4 both reside within α-granules of the MK. However, this may be a consequence of heterogeneity found in α-granule contents and divergence of endocytosis and exocytosis pathways.35-37 PF4 is secreted and then endocytosed into α-granules.38 Thus, differences in PF4 release may indicate that PF4 packaging/secretion pathways are independent of PITPs regulation or that PF4 packaging/secretion pathways are regulated differently by PITPs then TGF-β. It is not known if α-granules store IL-4; thus, the mechanism of IL-4 oversecretion is not clear.
In this study, we conclude that the lack of PITP impairs proper intracellular membrane trafficking processes, disrupting α-granules biogenesis and/or impairing proper secretion of TGF-β, leading to dysregulated hematopoiesis. MKs α-granule contents are normally released in a coordinated, controlled fashion to ensure contents are secreted at proper times. A number of studies have previously shown TGF-β to be myelosuppressive and critical in regulation of hematopoiesis.3-7 We believe this is the first study linking release of TGF-β to MK PtdIn signaling. We show PITPs to be a prominent regulator of α-granule biogenesis and maintenance. Since PITP shuttles phosphatidylinositol from one membrane to another, where it can subsequently be phosphorylated to generate PtdIn species,9-11 it is possible that a specific pool of PtdIns is necessary for the formation and maintenance of MK α-granules. It has been reported that TGF-β is excessively secreted in an active (not latent) form by MKs in BM of mice with MLL-AF9–induced acute myeloid leukemia, resulting in impaired hematopoiesis due to a decreased HSC proliferation.39 Future experiments to examine whether PtdIn signaling pathways can be pharmacologically targeted to develop new therapeutics for patients with disorders of normal hematopoiesis may be of interest.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
These studies were supported by the National Institutes of Health (US Public Health Service grants PO1 HL120846 and PO1 HL40387 from the National Heart, Lung, and Blood Institute; C.S.A.), (R01 HL056416, R01 HL112669, and R35 HL139599 from the National Heart, Lung, and Blood Institute, U54 DK106846 from the National Institute of Diabetes, Digestive, and Kidney Diseases; H.E.B.), and (R01 HL121323 from the National Heart, Lung, and Blood Institute; M.M.). M.C. was supported by the National Institutes of Health (training grant T32 DK07519 from the National Institute of Diabetes, Digestive, and Kidney Disease; H.E.B.).
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.C., L.Z., S.C., C.T., A.S., A.L.D., C.S.A., X.H., and H.E.B. designed and performed the experiments; M.C., L.Z., M.S.M., C.S.A., and H.E.B. analyzed the data; M.C., L.Z., A.S., C.S.A. and H.E.B. wrote the manuscript; and M.C., L.Z., S.C., C.T., A.L.D., C.S.A., A.S., M.S.M., and H.E.B. reviewed and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hal E. Broxmeyer, Department of Microbiology and Immunology, Indiana University School of Medicine, 950 W Walnut St, R2-302, Indianapolis, IN 46202-5181; e-mail: hbroxmey@iupui.edu; and Charles S. Abrams, Department of Medicine, University of Pennsylvania School of Medicine, Room 622, Perelman Center for Advanced Medicine, 3400 Spruce St, Philadelphia, PA 19104-6160; e-mail: abrams@mail.med.upenn.edu.
REFERENCES
- 1.Shaheen M, Broxmeyer HE. Hematopoietic Cytokines and Growth Factors. In: Broxmeyer HE, ed. Cord Blood Biology, Transplantation, Banking, and Regulation. Bethesda, MD: AABB Press; 2011:35-74. [Google Scholar]
- 2.Shaheen M, Broxmeyer HE. Cytokine/receptor families and signal transduction. In: Hoffman R, Benz E, Silberstein L, et al, eds. Hematology: Basic Principles and Practice, 7th ed Philadelphia, PA: Elsevier; 2018:163-175. [Google Scholar]
- 3.Bruns I, Lucas D, Pinho S, et al. . Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med. 2014;20(11):1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Day RB, Link DC. Megakaryocytes in the hematopoietic stem cell niche. Nat Med. 2014;20(11):1233-1234. [DOI] [PubMed] [Google Scholar]
- 5.Malara A, Currao M, Gruppi C, et al. . Megakaryocytes contribute to the bone marrow-matrix environment by expressing fibronectin, type IV collagen, and laminin. Stem Cells. 2014;32(4):926-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Min SH, Abrams CS. Regulation of platelet plug formation by phosphoinositide metabolism. Blood. 2013;122(8):1358-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu VW, Scadden DT. Heterogeneity of the bone marrow niche. Curr Opin Hematol. 2016;23(4):331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Craene JO, Bertazzi DL, Bär S, Friant S. Phosphoinositides: major actors in membrane trafficking and lipid signaling pathways. Int J Mol Sci. 2017;18(3):E634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cockcroft S. The diverse functions of phosphatidylinositol transfer proteins. Curr Top Microbiol Immunol. 2012;362:185-208. [DOI] [PubMed] [Google Scholar]
- 10.Grabon A, Khan D, Bankaitis VA. Phosphatidylinositol transfer proteins and instructive regulation of lipid kinase biology. Biochim Biophys Acta. 2015;1851(6):724-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ile KE, Schaaf G, Bankaitis VA. Phosphatidylinositol transfer proteins and cellular nanoreactors for lipid signaling. Nat Chem Biol. 2006;2(11):576-583. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Delaney MK, O’Brien KA, Du X. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol. 2010;30(12):2341-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakir-Tamang L, Gerst JE. A phosphatidylinositol-transfer protein and phosphatidylinositol-4-phosphate 5-kinase control Cdc42 to regulate the actin cytoskeleton and secretory pathway in yeast. Mol Biol Cell. 2009;20(15):3583-3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao M, Perry JM, Marshall H, et al. . Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med. 2014;20(11):1321-1326. [DOI] [PubMed] [Google Scholar]
- 15.Koseoglu S, Flaumenhaft R. Advances in platelet granule biology. Curr Opin Hematol. 2013;20(5):464-471. [DOI] [PubMed] [Google Scholar]
- 16.Heijnen HF, Debili N, Vainchencker W, Breton-Gorius J, Geuze HJ, Sixma JJ. Multivesicular bodies are an intermediate stage in the formation of platelet alpha-granules. Blood. 1998;91(7):2313-2325. [PubMed] [Google Scholar]
- 17.Zhao L, Thorsheim CL, Suzuki A, et al. . Phosphatidylinositol transfer protein-α in platelets is inconsequential for thrombosis yet is utilized for tumor metastasis. Nat Commun. 2017;8(1):1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thornton MA, Zhang C, Kowalska MA, Poncz M. Identification of distal regulatory regions in the human alpha IIb gene locus necessary for consistent, high-level megakaryocyte expression. Blood. 2002;100(10):3588-3596. [DOI] [PubMed] [Google Scholar]
- 19.Roelen BA, Lin HY, Knezević V, Freund E, Mummery CL. Expression of TGF-betas and their receptors during implantation and organogenesis of the mouse embryo. Dev Biol. 1994;166(2):716-728. [DOI] [PubMed] [Google Scholar]
- 20.Broxmeyer HE, Hoggatt J, O’Leary HA, et al. . Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat Med. 2012;18(12):1786-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Broxmeyer HE, Orschell CM, Clapp DW, et al. . Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mantel CR, O’Leary HA, Chitteti BR, et al. . Enhancing hematopoietic stem cell transplantation efficacy by mitigating oxygen shock. Cell. 2015;161(7):1553-1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamazaki S, Ema H, Karlsson G, et al. . Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146-1158. [DOI] [PubMed] [Google Scholar]
- 24.Broxmeyer HE, Williams DE. The production of myeloid blood cells and their regulation during health and disease. Crit Rev Oncol Hematol. 1988;8(3):173-226. [DOI] [PubMed] [Google Scholar]
- 25.Jansen JH, Wientjens GJ, Fibbe WE, Willemze R, Kluin-Nelemans HC. Inhibition of human macrophage colony formation by interleukin 4. J Exp Med. 1989;170(2):577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rusten LS, Smeland EB, Jacobsen FW, et al. . Tumor necrosis factor-alpha inhibits stem cell factor-induced proliferation of human bone marrow progenitor cells in vitro. Role of p55 and p75 tumor necrosis factor receptors. J Clin Invest. 1994;94(1):165-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sonoda Y, Kuzuyama Y, Tanaka S, et al. . Human interleukin-4 inhibits proliferation of megakaryocyte progenitor cells in culture. Blood. 1993;81(3):624-630. [PubMed] [Google Scholar]
- 28.Sonoda Y, Okuda T, Yokota S, et al. . Actions of human interleukin-4/B-cell stimulatory factor-1 on proliferation and differentiation of enriched hematopoietic progenitor cells in culture. Blood. 1990;75(8):1615-1621. [PubMed] [Google Scholar]
- 29.Vellenga E, de Wolf JT, Beentjes JA, Esselink MT, Smit JW, Halie MR. Divergent effects of interleukin-4 (IL-4) on the granulocyte colony-stimulating factor and IL-3-supported myeloid colony formation from normal and leukemic bone marrow cells. Blood. 1990;75(3):633-637. [PubMed] [Google Scholar]
- 30.Youn BS, Mantel C, Broxmeyer HE. Chemokines, chemokine receptors and hematopoiesis. Immunol Rev. 2000;177(1):150-174. [DOI] [PubMed] [Google Scholar]
- 31.Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB. Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem. 1983;258(11):7155-7160. [PubMed] [Google Scholar]
- 32.Blakytny R, Ludlow A, Martin GE, et al. . Latent TGF-beta1 activation by platelets. J Cell Physiol. 2004;199(1):67-76. [DOI] [PubMed] [Google Scholar]
- 33.Coppinger JA, Cagney G, Toomey S, et al. . Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood. 2004;103(6):2096-2104. [DOI] [PubMed] [Google Scholar]
- 34.Piersma SR, Broxterman HJ, Kapci M, et al. . Proteomics of the TRAP-induced platelet releasate. J Proteomics. 2009;72(1):91-109. [DOI] [PubMed] [Google Scholar]
- 35.Chatterjee M, Huang Z, Zhang W, et al. . Distinct platelet packaging, release, and surface expression of proangiogenic and antiangiogenic factors on different platelet stimuli. Blood. 2011;117(14):3907-3911. [DOI] [PubMed] [Google Scholar]
- 36.Italiano JE Jr, Richardson JL, Patel-Hett S, et al. . Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sehgal S, Storrie B. Evidence that differential packaging of the major platelet granule proteins von Willebrand factor and fibrinogen can support their differential release. J Thromb Haemost. 2007;5(10):2009-2016. [DOI] [PubMed] [Google Scholar]
- 38.Lambert MP, Meng R, Xiao L, et al. . Intramedullary megakaryocytes internalize released platelet factor 4 and store it in alpha granules. J Thromb Haemost. 2015;13(10):1888-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gong Y, Zhao M, Yang W, et al. . Megakaryocyte-derived excessive transforming growth factor beta1 inhibits proliferation of normal hematopoietic stem cells in acute myeloid leukemia. Exp Hematol. 2018;60:40-46.e42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.