Abstract
Purpose
Randomized evidence for extreme hypofractionation in prostate cancer is lacking. We aimed to identify differences in toxicity and quality-of-life outcomes between standard fractionation and extreme hypofractionated radiation in a phase 3 randomized trial.
Methods and materials
We analyzed the results of the first 75 patients in our phase 3 trial, comparing 38 Gy relative biologic effectiveness (RBE) in 5 fractions (n = 46) versus 79.2 Gy RBE in 44 fractions (n = 29). Patients received proton radiation using fiducials and daily image guidance. We evaluated American Urological Association Symptom Index (AUASI), adverse events (AEs), and Expanded Prostate Index Composite (EPIC) domains. The primary endpoint of this interim analysis was the cumulative incidence of grade 2 (G2) or higher AEs. The randomized patient allocation scheme was a 2:1 ratio favoring the 38 Gy RBE arm.
Results
The median follow-up was 36 months; 30% of patients reached 48-month follow-up. AUASI scores differed <5 points (4.4 vs 8.6; P = .002) at 1 year, favoring the 79.2 Gy arm. Differences in AUASI were not significant at ≥18 months. EPIC urinary symptoms favored the 79.2 Gy arm at 1 year (92.3 vs 84.5; P = .009) and 18 months (92.3 vs 85.3; P = .03); bother scores were not significant at other time points. Cumulative ≥G2 genitourinary toxicity was similar between the 79.2 Gy and 38 Gy arms (34.5% vs 30.4%; P = .80). We found no differences in the EPIC domains of bowel symptoms, sexual symptoms, or bowel ≥G2 toxicities. Bladder V80 (79.2 Gy arm; P = .04) and V39 (38 Gy arm; P = .05) were predictive for cumulative G2 genitourinary AEs.
Conclusions
Low AE rates were seen in both study arms. Early temporary differences in genitourinary scores disappeared over time. Bladder constraints were associated with genitourinary AEs.
Summary.
We report on the results of a phase 3 trial of the initial 75 patients treated with hypofractionated or standard fractionated proton radiation, including adverse events, quality of life, and dosimetry.
Alt-text: Unlabelled box
Background
Several phase 3 randomized trials have shown a benefit of conventionally fractionated, high-dose radiation for localized prostate cancer.1, 2, 3, 4 However, an increase in dose has greatly increased the duration of radiation treatments, with a consequent increase in cost and radiation utilization. Brenner and Hall5 postulated that relatively high doses of radiation were necessary to maximize tumor control with standard fractionation. However, lower doses could be used with a hypofractionated approach because of the low α-to-β ratio (α/β) for prostate cancer. This approach is attractive because fewer fractions (fx) can decrease radiation equipment use and improve access to care. Furthermore, relatively high biologic doses could be delivered with a hypofractionated approach because of the relatively higher α/β of normal tissue compared with prostate cancer.
Several randomized trials have tested the use of moderately hypofractionated regimens based on the low α/β for prostate cancer.6, 7, 8, 9, 10, 11, 12 These trials compared moderate hypofractionation using a dose of 2.4 to 3.4 Gy versus 1.8 to 2 Gy per fx and have shown the safety of this approach.
We have proposed an extreme hypofractionated approach using proton beam for the treatment of low-risk prostate cancer. On the basis of the assumption of an α/β of 3.5 for late-responding normal tissue,13, 14, 15 we proposed that the equivalent dose in 2 Gy fx (EQD2Gy) for the bladder and genitourinary system and the rectum would be 76 Gy using 79.2 Gy relative biologic effectiveness (RBE) over 44 fx or 38 Gy RBE over 5 fx. Our hypothesis is that the biologic late normal-tissue dose for the rectum and bladder is similar for 79.2 Gy (RBE) in 44 treatments and for 38 Gy (RBE) in 5 treatments. Thus, late adverse events (AEs), late Expanded Prostate Index Composite (EPIC) scores, and late American Urological Association Symptom Index (AUASI) should be similar for both arms. Herein, we demonstrate the safety of aggressive hypofractionation for prostate cancer.
Methods and materials
The present study was approved by the Western Institutional Review Board (protocol 20101536). All patients provided written informed consent. This trial was registered with ClinicalTrials.gov (NCT01230866).
The initial 75 patients treated in our randomized, phase 3, low-risk prostate cancer trial were included in the present analysis. For inclusion, low-risk was defined as clinical stage T1 to T2a, Gleason score 6, and prostate-specific antigen level <10 ng/mL. The total projected study accrual is 150 patients, and an interim analysis was planned at 50% accrual. Patients were enrolled from July 18, 2011 through August 20, 2014. Three patients withdrew consent, and 75 patients were evaluable. A prepopulated block randomization sheet was used for the study arm assignment by the protocol research office. Forty-six patients were randomly assigned to receive 38 Gy RBE over 5 fx, and 29 received 79.2 Gy RBE over 44 fx. The randomized patient allocation scheme was a 2-to-1 ratio favoring the 38 Gy RBE arm. No major violations of this protocol were seen in any patient.
We limited inclusion to patients with an AUASI score of ≤17. Although prostate stereotactic body radiation therapy can be used to treat patients with higher AUASI scores, we defined our cohort in this manner to establish a more homogeneous cohort with only mild to moderate urinary bother and to better define differences between study arms.
Radiation therapy
Briefly, planning for proton therapy involved the fusion of diagnostic 1.5-T magnetic resonance images to computed tomography images. Patients were positioned supine. The clinical target volume contained the prostate only; planning target volumes were 2 mm posteriorly and 3 mm elsewhere. Margins were determined on the basis of prior publications,16, 17 which indicated that margins of approximately 2 mm are necessary when using a rectal balloon and taking images before beam delivery. The constructed optimization target volume included an additional 5 mm in the beam direction distally and proximally.
Proton-specific expansions accommodated changes in dose deposition and improved treatment delivery robustness. Proton beams were oriented laterally left and right. The plan was optimized, normalized, and evaluated on the basis of the optimization target volume. A rectal balloon was used every day before treatment and for all cases. The rectum was defined from the ischial tuberosity to the sigmoid flexure. The whole bladder was contoured, and patients drank 12 to 16 ounces of water approximately 60 minutes before treatment.
Daily kV imaging with matching to coil gold fiducials was performed before delivery of each beam. For the purposes of this study, standard fractionation was defined as 79.2 Gy (RBE) in 1.8 Gy (RBE) fractions and hypofractionation was defined as 38 Gy (RBE) in 7.6 Gy (RBE) fx. Patients included in the current analysis were treated with a double-scatter proton technique.
Biologic considerations
In accordance with published data, the dose to achieve rectal isotoxicity between the 2 arms was defined on the basis of a 3.5 α/β for late-responding normal tissue. In this manner, 38 Gy RBE in 5 fx was equivalent to 79.2 Gy RBE in 44 fx.13, 14, 15 If the late normal tissue α/β is <3.5 Gy RBE, the resulting biologic equivalent dose will be higher for the 38 Gy RBE arm and toxicity should be higher; the opposite should also be true. The dose of 38 Gy was similar to the dose used with hypofractionated high-dose-rate brachytherapy and has shown good results and low toxicity.18, 19
Toxicity assessment
Protocol toxicity was measured using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Statistical calculations for toxicity used a double-sided α < .05 threshold for significance. Use of CTCAE for grading urinary toxicity might be misleading. Patients are commonly prescribed α-2 blockers to decrease urinary frequency and urgency, but most patients will not have urinary retention or any threatening complication if the medication is not used. Other publications have modified the CTCAE grade 2 (G2) AEs to accommodate this inconsistency. In the present study, we used the strictest definition. Any use of a prescription or over-the-counter medication greater than baseline was considered a G2 AE.
Use of CTCAE may also be misleading for grade 3 (G3) toxicity because the definition usually includes need for a medical intervention, transfusion, or hospitalization. However, given the ambiguity in hospitalization criteria across the United States, we defined a G3 event as an intervention or transfusion.
Quality-of-life measures
Patients completed the EPIC-16 and AUASI-17 before treatment and during routine follow-up visits at 3, 6, 12, 18, and 24 months and yearly after completion of treatment. EPIC, a validated instrument, measures urinary, bowel, and sexual function and bother.
Statistical analysis
To statistically evaluate change over time in EPIC scores, responses were grouped by system and assigned a numeric score. The difference in mean scores for EPIC and AUASI was assessed with t test. Multi-item scale scores were transformed linearly to a 0-to-100 scale in accordance with the scoring instructions for EPIC. Lower numbers corresponded with worsening function and increased bother. To assess changes in health-related quality of life (QoL) from baseline, a clinically significant difference was defined as one-half a standard deviation and at least a 10-point change. A clinically significant change in AUASI scores was defined as a change of ≥5 points.
The primary endpoint of the current interim analysis was the cumulative incidence of ≥G2 AEs. Gastrointestinal and urinary tract AEs were analyzed by incidence and prevalence. Prevalence was calculated at 3, 6, 12, 18, and 24 months after radiation therapy. For incidence, we considered ≥G2 AEs occurring in each arm for 3 years. All AE analyses were conducted in the intention-to-treat population using the Fisher exact test and 2-sided .05 significance levels. Data and safety monitoring board reports, which included AEs, treatment failures, and accrual goals, were submitted every 6 months. The primary endpoint of the overall study is freedom from failure, which will be reported at the time of the final analysis.
Results
Patients
We included in the present analysis the initial 75 patients treated. We currently have accrued 110 patients of the 130 patients needed to complete the trial. However, we will accrue a total of 150 patients to account for a 15% potential loss to follow-up. The median follow-up is 36 months, and 30% have reached a follow-up of 4 years or longer. All patients had low-risk prostate cancer and were balanced between study arms. Three quartiles of patients in each arm had a prostate-specific antigen level between 4 and 10 ng/mL (P = .99), and the disease of more than 80% of patients in each arm was staged T1c (P = .51).
AEs
Urinary and bowel G2 AEs are shown in Table 1 and the Figure 1. No patient required intervention or transfusion for urinary or bowel symptoms. No G3 AEs were seen.
Table 1.
Cumulative grade 2 adverse events
| Adverse event | Cumulative events |
P-value | |
|---|---|---|---|
| 44 fraction (arm 1), no. (n = 29) | 5 fraction (arm 2), no. (n = 46) | ||
| Urinary tract grade 2 | |||
| 6 mo | 0 | 9 | .01 |
| 12 mo | 4 | 11 | .38 |
| 18 mo | 5 | 12 | .41 |
| 24 mo | 8 | 13 | > .99 |
| 36 mo | 9 | 14 | > .99 |
| 48 mo | 10 | 14 | .80 |
| Overall, n (%) | 10 (34.5) | 14 (30.4) | .80 |
| Bowel grade 2 | |||
| 6 mo | 1 | 5 | .4 |
| 12 mo | 1 | 6 | .24 |
| 18 mo | 3 | 7 | .73 |
| 24 mo | 4 | 9 | .76 |
| 36 mo | 5 | 9 | > .99 |
| 48 mo | 5 | 9 | > .99 |
| Overall, n (%) | 5 (17.2) | 9 (19.6) | > .99 |
Fig 1.
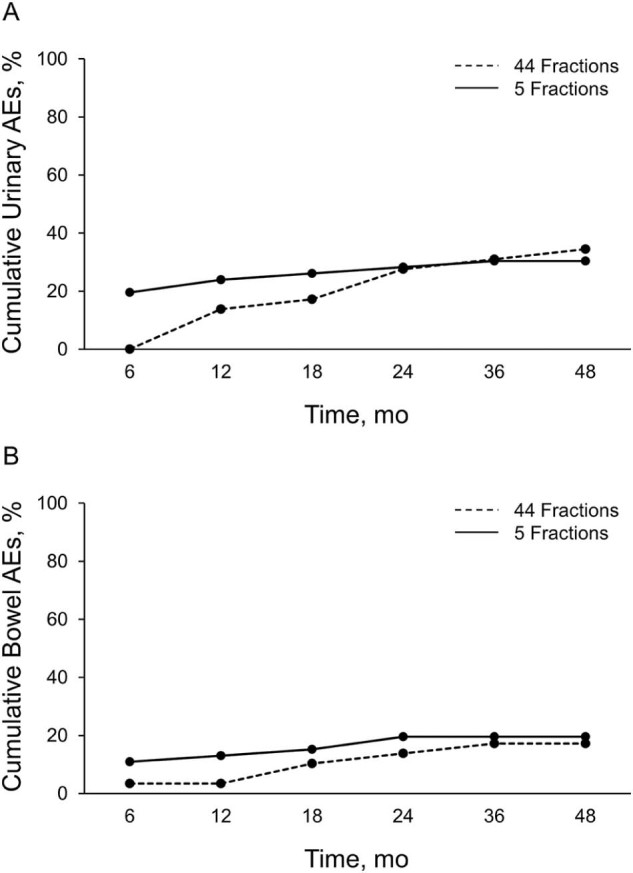
Cumulative incidence of urinary (A) and bowel (B) grade2 adverse events.
Of urinary symptoms, G2 AEs consisted of increased frequency that was managed with medical therapy for most cases. More patients in the 38 Gy arm took medications to help urinary function at 6 months than in the 79.2 Gy arm (P = .01). However, by 1 year, approximately the same proportion of patients in either arm had used medication to help with urinary function. During the first 4 years after treatment, a similar proportion of patients in either arm had used medication to help urinary function.
Among bowel symptoms, G2 AEs consisted of blood with bowel movements that was managed with medical therapy for most patients. During the first 4 years, the same proportion of patients used medications for bowel symptoms.
AUASI
AUASI scores remained low during the first 4 years and reflect the low utilization of urinary medications in this trial (Table 2). The largest difference between the 2 arms was seen at 1 year. However, the difference was smaller than the 5 points determined for clinical relevance.
Table 2.
AUASI and EPIC scores for standard fractionation and hypofractionation
| Score | Timepoint | 44 fractions (arm 1) |
5 fractions (arm 2) |
P-value | ||
|---|---|---|---|---|---|---|
| n | Mean (SD) AUASI | n | Mean (SD) AUASI | |||
| AUASI | ||||||
| Baseline | 29 | 4.28 (3.741) | 46 | 4.50 (3.613) | .80 | |
| 3 mo | 27 | 5.22 (4.022) | 45 | 6.51 (4.378) | .21 | |
| 6 mo | 25 | 5.20 (4.183) | 43 | 7.05 (6.583) | .16 | |
| 12 mo | 20 | 4.40 (3.218) | 41 | 8.58 (6.868) | .002a | |
| 18 mo | 23 | 4.96 (4.051) | 41 | 7.08 (6.591) | .12 | |
| 24 mo | 26 | 5.19 (5.254) | 43 | 6.54 (6.058) | .34 | |
| 36 mo | 18 | 4.94 (3.134) | 21 | 6.36 (6.337) | .34 | |
| 48 mo | 9 | 4.33 (3.428) | 8 | 7.08 (6.702) | .22 | |
| EPIC urinary | ||||||
| Baseline | 29 | 91.9 (8.210) | 46 | 91.6 (7.771) | .88 | |
| 3 mo | 26 | 90.3 (11.150) | 45 | 86.6 (11.679) | .20 | |
| 6 mo | 25 | 89.8 (11.133) | 43 | 87.2 (14.109) | .40 | |
| 12 mo | 20 | 92.3 (8.555) | 39 | 84.5 (13.800) | .009a | |
| 18 mo | 23 | 92.3 (10.874) | 39 | 85.3 (13.646) | .03a | |
| 24 mo | 26 | 91.7 (11.300) | 41 | 89.0 (12.313) | .36 | |
| 36 mo | 18 | 92.6 (6.593) | 24 | 87.2 (13.432) | .10 | |
| 48 mo | 9 | 90.2 (9.910) | 13 | 85.4 (12.467) | .32 | |
| EPIC bowel | ||||||
| Baseline | 29 | 95.5 (5.391) | 46 | 96.7 (3.866) | .32 | |
| 3 mo | 27 | 94.4 (6.652) | 45 | 92.8 (8.419) | .37 | |
| 6 mo | 25 | 90.7 (11.748) | 43 | 88.0 (14.083) | .40 | |
| 12 mo | 19 | 91.8 (7.739) | 40 | 87.1 (13.126) | .09 | |
| 18 mo | 23 | 90.8 (8.812) | 39 | 90.3 (9.536) | .84 | |
| 24 mo | 26 | 92.7 (6.193) | 41 | 91.5 (9.928) | .57 | |
| 36 mo | 18 | 94.3 (4.760) | 25 | 93.4 (7.565) | .64 | |
| 48 mo | 9 | 95.2 (4.282) | 12 | 92.1 (13.903) | .47 | |
| EPIC sexual | ||||||
| Baseline | 29 | 59.2 (22.409) | 45 | 59.8 (22.845) | .90 | |
| 3 mo | 27 | 56.9 (20.702) | 45 | 56.7 (24.474) | .98 | |
| 6 mo | 24 | 56.9 (20.105) | 43 | 55.3 (26.970) | .78 | |
| 12 mo | 19 | 55.3 (21.711) | 38 | 51.0 (24.849) | .51 | |
| 18 mo | 23 | 52.4 (24.919) | 39 | 45.7 (24.200) | .31 | |
| 24 mo | 23 | 56.1 (21.701) | 41 | 48.5 (25.713) | .22 | |
| 36 mo | 18 | 49.8 (19.763) | 25 | 47.1 (23.492) | .69 | |
| 48 mo | 9 | 61.3 (19.531) | 12 | 47.3 (21.660) | .14 | |
AUASI, American Urological Association Symptom Index; EPIC, Expanded Prostate Index Composite; SD, standard deviation.
Statistically significant.
EPIC scores
EPIC urinary scores were similar for the hypofractionated and standard arms during the first 4 years (Table 2). A small difference in the urinary domain was seen at 12 and 18 months that corresponded to a small decline in AUASI score in the hypofractionated arm. However, these changes were smaller than needed to determine clinical significance. Overall, urinary domain scores stayed high during the first 4 years.
The bowel domain showed no difference during the first 4 years. Scores for the bowel domain remained high as well. The sexual domain did not show a statistical difference during the first 4 years either. A small decline in the sexual domain was seen over time for both arms, and there was a small, nonsignificant difference favoring the 79.2 Gy arm at 4 years (Table 2).
Normal tissue doses
Table 3 describes the dose delivered to different organs at risk. For example, the volume of the bladder receiving ≥80 Gy was 4 cm3 on average for all patients treated in the 44 fx arm. The number in parentheses describes the standard deviation.
Table 3.
Normal tissue dose volume values
| Structure | Goal | Mean (SD) dose, Gy |
|---|---|---|
| 44 fractions (arm 1) | ||
| Rectum, % | V50 < 35 | 15.095 (3.914) |
| Rectum, % | V70 < 10 | 8.055 (2.366) |
| Bladder, cc | V80 < 8 | 4.095 (2.691) |
| Femoral heads, cc | V45 < 1 | 0 (0) |
| 5 fractions (arm 2) | ||
| Rectum, % | V24 < 35 | 13.961 (4.003) |
| Rectum, % | V33.6 < 10 | 7.391 (2.564) |
| Bladder, cc | V39 < 8 | 0.349 (0.923) |
| Femoral heads, cc | V23 < 1 | 0 (0) |
SD, standard deviation.
Doses delivered to the optimization target volume were similar for both study arms. Doses to normal tissue met pre-established constraints (Table 3). Doses to the bladder were predictive for long-term toxicity (Table 4). Similar to findings reported in prior publications,20, 21 small volumes of the bladder treated with high doses were predictive for G2 genitourinary AEs.
Table 4.
Relation between dose volume values and AEs
| Patient AEs | Volume value | P-value | |
|---|---|---|---|
| 44 fractions (arm 1) | |||
| V50 ≤ 15% | V50 > 15% | ||
| With bowel AE, n | 1 | 3 | |
| Without bowel AE, n | 10 | 11 | |
| Rate bowel AEs, % | 9.1 | 21.4 | .60 |
| V70 ≤ 8% | V70 > 8% | ||
| With bowel AE, n | 1 | 3 | |
| Without bowel AE, n | 10 | 11 | |
| Rate bowel AEs, % | 9.1 | 21.4 | .60 |
| V80 ≤ 4 cc | V80 > 4 cc | ||
| With urinary AE, n | 2 | 8 | |
| Without urinary AE, n | 10 | 5 | |
| Rate urinary AEs, % | 16.7 | 61.5 | .04a |
| 5 fractions (arm 2) | |||
| V24 ≤ 14% | V24 > 14% | ||
| With bowel AE, n | 5 | 4 | |
| Without bowel AE, n | 19 | 17 | |
| Rate bowel AEs, % | 20.8 | 19.0 | > .99 |
| V33.6 ≤ 7% | V33.6 > 7% | ||
| With bowel AE, n | 4 | 5 | |
| Without bowel AE, n | 18 | 18 | |
| Rate bowel AEs, % | 18.2 | 21.7 | > .99 |
| V39 ≤ 0.349 cc | V39 > 0.349 cc | ||
| With urinary AE, n | 10 | 6 | |
| Without urinary AE, n | 26 | 3 | |
| Rate urinary AEs, % | 27.8 | 66.7 | .05a |
AE, adverse event.
Statistically significant.
No dose relationship was predictive for G2 rectal toxicity. Rectal V70 (79.2 Gy arm) and the corresponding rectal V33.4 (38 Gy arm) were <10%, and prior studies have shown low rates of G2 rectal toxicity with these values.22 The average rectal dose for either arm was approximately 8%. However, the rates of >G2 toxicity were similar among patients with rectal V70 or V33.4 of more or less than 8%.
Discussion
Dose fractionation for the treatment of prostate cancer has been an important topic of clinical debate. When standard fractionation is used, improved results have been seen with dose escalation.4 However, treatment duration with some regimens approaches 9 weeks, and concerns about resource utilization and access to care have been raised. Furthermore, dose response curves define the ideal EQD2Gy for prostate cancer as >90 Gy, a dose never reached before, even in the most aggressive dose escalation trials.5 Because the α/β for prostate cancer has been defined as approximately 1.5, hypofractionated regimens can deliver relatively high biologic doses to the prostate for tumor control while maintaining adequately low biologic doses to normal tissue.5
In our trial, we defined the α/β for late AEs for normal tissue at 3.5 on the basis of existing literature.14, 23, 24 Using this α/β, the standard fractionated 79.2 Gy regimen corresponds to an EQD2Gy of 76.4 Gy for late normal tissue effects. Assuming a constant α/β, the hypofractionated 38 Gy regimen would correspond to an EQD2Gy of 76.7 for late normal tissue effects. Thus, both arms would be expected to have similar toxicity. The hypofractionated 38 Gy regimen is also in agreement with our previous experience using hypofractionated radiation in high-dose-rate brachytherapy. For cancer control, the 38 Gy regimen would have an EQD2Gy of 99 Gy compared with 74.7 Gy with the 79.2 Gy regimen. An EQD2Gy of 99 Gy should be more than adequate for cancer control for various risk groups.
Clinical data have shown little difference in α/β for various risk groups or for use of androgen suppression,25 and 38 Gy in 5 fx dose should maximize local tumor control for patients with prostate cancer.5 For comparison, the Conventional or Hypofractionated High-Dose Intensity Modulated Radiotherapy for Prostate Cancer (CHHiP) randomized trial had a similar proportion of patients who were biochemical or clinical failure-free at 5 years in the 74 Gy (88 ⋅ 3%) and 60 Gy (90 ⋅ 6%) groups.8 The 74 Gy and 60 Gy groups correspond to EQD2Gy values of 74 Gy and 77 Gy, respectively, for an α/β of 1.5. This is consistent with the results from Brenner and others.5
Several randomized trials have explored the use of moderately hypofractionated radiation therapy.6, 8, 9, 10, 11, 12, 26 Five of these trials compared moderately hypofractionated radiation with dose-escalated radiation6, 8, 9, 11, 26 and 2 trials compared hypofractionated regimens with doses that are lower than currently considered standard.10, 12 These 7 trials reported similar rates of long-term toxicity between the arms except for the Hypofractionated Versus Conventionally Fractionated Radiotherapy for Patients with Prostate Cancer (HYPRO) trial by Aluwini et al.6 In the HYPRO trial, ≥G3 genitourinary AEs were higher in the hypofractionated arm, and noninferiority for ≥G2 gastrointestinal AEs was not achieved.6 In their trial, the EQD2Gy (α/β of 3.5) for late damage to normal tissue in the hypofractionated arm was 81 Gy. This is the highest EQD2Gy for normal tissues among all 7 randomized trials. The EQD2Gy of 81 Gy for late normal tissue effects may have accounted for the increase in AEs in this trial.
In our trial, cumulative rates of AEs were similar between the 2 arms during the first 4 years. The α/β of 3.5 used in our trial was consistent with the data from 6 moderately hypofractionated randomized trials.6, 8, 9, 10, 11, 26 On basis of the evidence from all 7 randomized trials and including the present report, it seems safe to suggest that hypofractionated regimens should use an α/β between 3.0 and 4.0 to define normal tissue (rectum and bladder) dose constraints and the risk of late AEs for prostate cancer treatment.
Using a low α/β and excluding repopulation time, it is possible to predict late AEs and cancer control, as has been seen in the published literature.5, 6, 8, 9, 10, 11, 12, 25, 26 However, acute changes may be more affected by repopulation. In this manner, AEs may occur earlier in the hypofractionated arm than in the standard arm, although overall AEs may be similar.
The linear quadratic model used to define survival fx after radiation therapy is based on the premise of repair, reoxygenation, repopulation, and redistribution. Repopulation of normal cells within the irradiated tissue should increase the risk of acute and long-term toxicity for hypofractionated regimens received over a shorter time. Late damage may not be as dependent on radiation duration because normal tissue healing can continue for months or years after completion of radiation therapy. Yet, for an acute normal tissue response, repopulation may have a more prominent role. Thus, late events may be similar, but events may be seen earlier in the hypofractionated arm. In this manner, the cumulative incidence of AEs may follow distinctly different time-to-event curves for the hypofractionated and standard fractionated arms.
In our trial, overall AEs were similar. In the hypofractionated arm, 85% of urinary and 78% of bowel AEs occurred within 18 months; in the standard fractionated arm, these were 50% and 60%, respectively. Similarly, an increase in genitourinary AEs was seen at 6 months in the hypofractionated arm (Table 1). The Canadian trial found that acute toxicity was higher in the hypofractionated arm (11% vs 4%), although overall toxicity remained the same.10 The Australian trial12 also showed increased gastrointestinal symptoms at 1 month in the hypofractionated arm (total gastrointestinal symptom score of 5 vs 2.5); however, late gastrointestinal symptoms were the same. Lastly, in the CHHip trial,8 urinary toxicity appeared earlier and peak acute bowel toxicity was higher in the hypofractionated arm.
Similar to the early time to event seen for these AEs, we saw an early, small, non–clinically significant difference in urinary QoL and AUASI scores favoring the standard arm that disappeared after 18 months. Surprisingly, the 79.2 Gy arm actually had better sexual domain scores at 4 years than at 3 years (61.3 vs 49.8, respectively). It is unlikely that the EPIC sexual score improved over time in the 79.2 Gy arm. Rather, this improvement between the 3- and 4-year time points was likely related to the small sample size at 4 years in the 79.2 Gy arm (n = 9). Longer follow-up and a larger sample size may help to determine if this nonsignificant difference between the 2 arms persists. AE data, QoL scores, and AUASI scores are similar to the findings in the Canadian,10 Australian,12 and CHHip8 trials, and the current trial shows that overall AE rates are similar. However, AEs tended to happen sooner with hypofractionated RT.
In our trial, we did not find a relationship between bowel toxicity and rectal dose. However, our rectal V50 and V70 and corresponding values in the hypofractionated arm were <10%, and we used daily prebeam image guidance and rectal balloons for all patients. Possibly, any benefit of lower rectal doses may be difficult to see given the strict rectal dose criteria used. With image guidance, rectal balloons, and our lateral beam arrangement, a small volume of the rectum was likely consistently radiated. We plan to reanalyze the rectal dose relationship with a larger cohort, and a relationship may be found.
With regard to urinary damage, a significant relation was seen between high doses delivered to a small bladder volume and urinary AEs. Because bladder filling is somewhat inconsistent, and with image guidance only a small volume by the bladder neck is treated, it may be reasonable to think that high doses to small volumes affect urinary symptoms. In our study, small volumes of the bladder treated with high doses were predictive for G2 genitourinary AEs. Given the tight margins in the bladder (3 mm), only the bladder muscle at the bladder neck received doses that were predictive for genitourinary toxicity. This suggests that the bladder neck area is responsible for most of the urinary symptoms in this study.
Few papers have been able to show a relationship between bladder dose and late bladder AEs.27 We previously reported a dose-to-volume relationship and AEs for bladder tissue with 3-dimensional radiation therapy, which was still true in our present report of extreme hypofractionation or standard fractionation with proton therapy.20 Similarly, the Dutch dose-escalation randomized trial showed that bladder surface ≥80 Gy and dose to the trigone region were predictive for bladder neck obstruction28, 29 Low-dose-rate brachytherapy doses to the bladder neck have been associated with acute and chronic toxicity as well.
Other published experiences using extreme hypofractionation for prostate cancer have suggested the safety of this approach with acceptable urinary and bowel toxicity.30, 31, 32, 33, 34 However, existing published reports on extreme hypofractionation have been either nonrandomized or retrospective. Our study shows the safety of extreme hypofractionation for prostate cancer in a prospective, randomized phase 3 trial.
Limitations of this study include a small sample size. Thus, small differences in AEs, EPIC scores, and AUASI could have been missed. A larger sample size may also be helpful to better analyze the EPIC sexual domain, considering the large variability seen in the scores with a large standard deviation (Table 2). A follow-up of 3 years would not be adequate to accurately determine the overall study primary endpoint of freedom from failure for both arms. For this reason, the primary endpoint of the overall study will not be reported until adequate median follow-up is reached. However, a median follow-up of 3 years should be adequate to determine differences in AEs because more than half of the AEs occurred within 2 years. Similarly, our median follow-up should be long enough to describe changes in the EPIC bowel, EPIC urinary, and AUASI scores because, for the most part, they were already returning to baseline before 3 years.
Another limitation of the extrapolation of the data to clinical practice is the strict planning and treatment criteria used in the current study. Because both arms were treated with proton therapy with image guidance, prostate immobilization (rectal balloon), magnetic resonance imaging registration, and central quality assurance, AE rates could be higher without these technical assurances.
However, even though AE rates may be higher without the technical rigor of a prospective protocol, the trends found with regard to AE rates between the 2 fractionation schedules should still apply for patients treated with different technical standards. Lastly, all patients were treated with proton therapy. Therefore, our data may not be directly applicable to patients treated with x-rays. However, because both arms were treated with protons, the differences between the 2 fractionation schedules should be reflected for patients treated with x-rays as well.
Conclusions
Extreme hypofractionated radiation and standard radiation with daily prebeam fiducial-based image guidance, magnetic resonance imaging registration, and rectal balloons are associated with low rates of AEs. The AEs, bother, and QoL scores are similar overall for hypofractionation and standard fractionation. However, normal tissue changes tend to appear earlier in the hypofractionated arm. Normal tissue α/β for late events is approximately 3.5 Gy; as shown in this trial and prior publications, dose constraints using an adjusted α/β can be used safely.
Footnotes
Sources of support: None.
Conflicts of interest: The authors report no conflicts of interest.
References
- 1.Dearnaley D.P., Jovic G., Syndikus I. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: Long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014;15:464–473. doi: 10.1016/S1470-2045(14)70040-3. [DOI] [PubMed] [Google Scholar]
- 2.Kuban D.A., Levy L.B., Cheung M.R. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys. 2011;79:1310–1317. doi: 10.1016/j.ijrobp.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Peeters S.T., Heemsbergen W.D., Koper P.C. Dose-response in radiotherapy for localized prostate cancer: Results of the Dutch multicenter randomized phase III trial comparing 68 Gy of radiotherapy with 78 Gy. J Clin Oncol. 2006;24:1990–1996. doi: 10.1200/JCO.2005.05.2530. [DOI] [PubMed] [Google Scholar]
- 4.Zietman A.L., Bae K., Slater J.D. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: Long-term results from Proton Radiation Oncology group/American College of Radiology 95-09. J Clin Oncol. 2010;28:1106–1111. doi: 10.1200/JCO.2009.25.8475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brenner D.J., Hall E.J. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 6.Aluwini S., Pos F., Schimmel E. Hypofractionated versus conventionally fractionated radiotherapy for patients with prostate cancer (HYPRO): Late toxicity results from a randomised, non-inferiority, phase 3 trial. Lancet Oncol. 2016;17:464–474. doi: 10.1016/S1470-2045(15)00567-7. [DOI] [PubMed] [Google Scholar]
- 7.Arcangeli S., Strigari L., Gomellini S. Updated results and patterns of failure in a randomized hypofractionation trial for high-risk prostate cancer. Int J Radiat Oncol Biol Phys. 2012;84:1172–1178. doi: 10.1016/j.ijrobp.2012.02.049. [DOI] [PubMed] [Google Scholar]
- 8.Dearnaley D., Syndikus I., Mossop H. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol. 2016;17:1047–1060. doi: 10.1016/S1470-2045(16)30102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman K.E., Voong K.R., Pugh T.J. Risk of late toxicity in men receiving dose-escalated hypofractionated intensity modulated prostate radiation therapy: Results from a randomized trial. Int J Radiat Oncol Biol Phys. 2014;88:1074–1084. doi: 10.1016/j.ijrobp.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Lukka H., Hayter C., Julian J.A. Randomized trial comparing two fractionation schedules for patients with localized prostate cancer. J Clin Oncol. 2005;23:6132–6138. doi: 10.1200/JCO.2005.06.153. [DOI] [PubMed] [Google Scholar]
- 11.Pollack A., Walker G., Horwitz E.M. Randomized trial of hypofractionated external-beam radiotherapy for prostate cancer. J Clin Oncol. 2013;31:3860–3868. doi: 10.1200/JCO.2013.51.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeoh E.E., Botten R.J., Butters J., Di Matteo A.C., Holloway R.H., Fowler J. Hypofractionated versus conventionally fractionated radiotherapy for prostate carcinoma: Final results of phase III randomized trial. Int J Radiat Oncol Biol Phys. 2011;81:1271–1278. doi: 10.1016/j.ijrobp.2010.07.1984. [DOI] [PubMed] [Google Scholar]
- 13.Fowler J.F. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005;44:265–276. doi: 10.1080/02841860410002824. [DOI] [PubMed] [Google Scholar]
- 14.Marzi S., Saracino B., Petrongari M.G. Modeling of alpha/beta for late rectal toxicity from a randomized phase II study: Conventional versus hypofractionated scheme for localized prostate cancer. J Exp Clin Cancer Res. 2009;28:117. doi: 10.1186/1756-9966-28-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tucker S.L., Thames H.D., Michalski J.M. Estimation of α/β for late rectal toxicity based on RTOG 94-06. Int J Radiat Oncol Biol Phys. 2011;81:600–605. doi: 10.1016/j.ijrobp.2010.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vargas C., Falchook A., Indelicato D. Proton therapy for prostate cancer treatment employing online image guidance and an action level threshold. Am J Clin Oncol. 2009;32:180–186. doi: 10.1097/COC.0b013e3181841f13. [DOI] [PubMed] [Google Scholar]
- 17.Vargas C., Saito A.I., Hsi W.C. Cine-magnetic resonance imaging assessment of intrafraction motion for prostate cancer patients supine or prone with and without a rectal balloon. Am J Clin Oncol. 2010;33:11–16. doi: 10.1097/COC.0b013e31819fdf7c. [DOI] [PubMed] [Google Scholar]
- 18.Martinez A.A., Demanes J., Vargas C., Schour L., Ghilezan M., Gustafson G.S. High-dose-rate prostate brachytherapy: An excellent accelerated-hypofractionated treatment for favorable prostate cancer. Am J Clin Oncol. 2010;33:481–488. doi: 10.1097/COC.0b013e3181b9cd2f. [DOI] [PubMed] [Google Scholar]
- 19.Vargas C., Ghilezan M., Hollander M. A new model using number of needles and androgen deprivation to predict chronic urinary toxicity for high or low dose rate prostate brachytherapy. J Urol. 2005;174:882–887. doi: 10.1097/01.ju.0000169136.55891.21. [DOI] [PubMed] [Google Scholar]
- 20.Harsolia A., Vargas C., Yan D. Predictors for chronic urinary toxicity after the treatment of prostate cancer with adaptive three-dimensional conformal radiotherapy: dose-volume analysis of a phase II dose-escalation study. Int J Radiat Oncol Biol Phys. 2007;69:1100–1109. doi: 10.1016/j.ijrobp.2007.04.076. [DOI] [PubMed] [Google Scholar]
- 21.Ghadjar P., Zelefsky M.J., Spratt D.E. Impact of dose to the bladder trigone on long-term urinary function after high-dose intensity modulated radiation therapy for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2014;88:339–344. doi: 10.1016/j.ijrobp.2013.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vargas C., Martinez A., Kestin L.L. Dose-volume analysis of predictors for chronic rectal toxicity after treatment of prostate cancer with adaptive image-guided radiotherapy. Int J Radiat Oncol Biol Phys. 2005;62:1297–1308. doi: 10.1016/j.ijrobp.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 23.Vargas C., Mahajan C., Fryer A. Rectal dose-volume differences using proton radiotherapy and a rectal balloon or water alone for the treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;69:1110–1116. doi: 10.1016/j.ijrobp.2007.04.075. [DOI] [PubMed] [Google Scholar]
- 24.Brenner D.J. Fractionation and late rectal toxicity. Int J Radiat Oncol Biol Phys. 2004;60:1013–1015. doi: 10.1016/j.ijrobp.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Miralbell R., Roberts S.A., Zubizarreta E., Hendry J.H. Dose-fractionation sensitivity of prostate cancer deduced from radiotherapy outcomes of 5969 patients in seven international institutional datasets: α/β = 1.4 (0.9-2.2) Gy. Int J Radiat Oncol Biol Phys. 2012;82:e17–e24. doi: 10.1016/j.ijrobp.2010.10.075. [DOI] [PubMed] [Google Scholar]
- 26.Arcangeli G., Fowler J., Gomellini S. Acute and late toxicity in a randomized trial of conventional versus hypofractionated three-dimensional conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2011;79:1013–1021. doi: 10.1016/j.ijrobp.2009.12.045. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan A.N., Yorke E.D., Marks L.B., Eifel P.J., Shipley W.U. Radiation dose-volume effects of the urinary bladder. Int J Radiat Oncol Biol Phys. 2010;76:S116–S122. doi: 10.1016/j.ijrobp.2009.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heemsbergen W.D., Al-Mamgani A., Marnix Y., White M.G. Urinary obstruction in prostate cancer patients from the Dutch Trial (68 Gy vs. 78 Gy): Relationships with local dose, acute effects, and baseline characteristics. Int J Radiat Oncol Biol Phys. 2010;78:19–25. doi: 10.1016/j.ijrobp.2009.07.1680. [DOI] [PubMed] [Google Scholar]
- 29.Hathout L., Folkert M.R., Kollmeier M.A., Yamada Y., Cohen G.N., Zelefsky M.J. Dose to the bladder neck is the most important predictor for acute and late toxicity after low-dose-rate prostate brachytherapy: Implications for establishing new dose constraints for treatment planning. Int J Radiat Oncol Biol Phys. 2014;90:312–319. doi: 10.1016/j.ijrobp.2014.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King C.R., Brooks J.D., Gill H., Pawlicki T., Cotrutz C., Presti J.C., Jr Stereotactic body radiotherapy for localized prostate cancer: Interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009;73:1043–1048. doi: 10.1016/j.ijrobp.2008.05.059. [DOI] [PubMed] [Google Scholar]
- 31.Freeman D.E., King C.R. Stereotactic body radiotherapy for low-risk prostate cancer: Five-year outcomes. Radiat Oncol. 2011;6:3. doi: 10.1186/1748-717X-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loblaw A., Cheung P., D'Alimonte L. Prostate stereotactic ablative body radiotherapy using a standard linear accelerator: Toxicity, biochemical, and pathological outcomes. Radiother Oncol. 2013;107:153–158. doi: 10.1016/j.radonc.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 33.King C.R., Collins S., Fuller D. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: Results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys. 2013;87:939–945. doi: 10.1016/j.ijrobp.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 34.Katz A.J., Kang J. Quality of life and toxicity after SBRT for organ-confined prostate cancer, a 7-year study. Front Oncol. 2014;4:301. doi: 10.3389/fonc.2014.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]


