Abstract
Purpose
The purpose of this study was to determine the impact of splenic and thoracic bone marrow irradiation on hematologic toxicity in the setting of chemoradiation therapy for esophageal cancer.
Methods and materials
We analyzed 60 patients with carcinoma of the distal esophagus or gastroesophageal junction who received concurrent chemoradiation in the preoperative or definitive setting. Dosimetric and volumetric parameters were calculated for the spleen, thoracic spine, and posterior ribs. The primary endpoint was grade ≥3 hematologic toxicity (HT3+). Associations were assessed using logistic and linear regression models.
Results
Twenty-one patients (35%) experienced HT3+, including 18 patients with leukopenia and 5 with thrombocytopenia. Higher spleen V5-V20 was correlated with a lower risk of HT3+ on multivariable analysis (odds ratio: 0.83 per 10 cm3 increase in V10; P = .013). A dose-dependent decrease in spleen volume was observed after radiation therapy, and a greater decrease was independently associated with a lower risk of HT3+ (odds ratio: 0.93 per 1% volume decrease; P = .014). Dosimetric parameters of the thoracic spine were not significantly associated with HT3+.
Conclusions
A greater decrease in spleen size after radiation therapy and a higher spleen V5-V20 were independently associated with a lower risk of severe hematologic toxicity. Splenic irradiation may mitigate leukopenia associated with chemoradiation therapy.
Summary.
Splenic irradiation results in a dose-dependent decrease in spleen volume. A greater decrease in spleen volume after radiation therapy and a higher spleen V5-V20 were independently associated with a lower risk of severe hematologic toxicity. Our results suggest that splenic irradiation may mitigate leukopenia associated with chemoradiation therapy, possibly through a process that involves the release of sequestered cells.
Alt-text: Unlabelled box
Introduction
Since the publication of the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study, neoadjuvant chemoradiation therapy has been firmly established as the standard of care for locally advanced, resectable esophageal and gastroesophageal junction (GEJ) cancer.1 However, morbidity associated with chemoradiation can be significant, particularly with respect to hematologic toxicities. In the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study, leukopenia (60%) and thrombocytopenia (54%) were quite common, and 8% of patients experienced a grade ≥3 hematologic toxic effect of any type.2 Several potential factors contribute to these findings, including chemotherapy-related and radiation-related effects on hematopoiesis.
Several studies have previously investigated radiation dose to the bone marrow and its effect on acute hematologic toxicity for various disease sites, including anal, cervical, and thoracic.3, 4, 5 However, radiation therapy for esophageal cancer is unique from the aforementioned sites because the spleen, which is an additional hematopoietic organ, is often within the radiation field. The spleen is known to serve several functions, including serving as a reservoir for lymphocytes, platelets, and potentially other cell types.6
The effects of radiation on the normal functions of the spleen remain largely unknown, and the organ is not routinely designated as an organ at risk with applicable dosimetric constraints. One recent study found a dose-dependent decrease in spleen size after postoperative radiation therapy for gastric cancer.7 However, the clinical significance of this finding has not been clearly elucidated. The current study investigates the relationship between changes in blood cell counts and dosimetric parameters of the spleen and thoracic bone marrow, specifically among patients who received treatment to the distal esophagus or GEJ, given the anatomic level of the spleen. An improved understanding of the role of the spleen in acute hematologic toxicity and its relation to bone marrow suppression after radiation therapy may allow for improved design of radiation therapy fields to minimize adverse hematologic events.
Methods and materials
Patient selection
In this study, which was approved by the Institutional Review Board of Stanford University, we conducted a retrospective analysis of 60 patients with stage IB-IV adenocarcinoma or squamous cell carcinoma of the distal esophagus or GEJ who received concurrent chemoradiation in the preoperative or definitive setting at our institution between January 2007 and February 2015. Patients with metastatic disease were included if they received high-dose radiation therapy to the primary tumor, as per our institutional practice described later. Patients underwent computed tomography (CT) of the thorax, whole-body positron emission tomography/CT, and upper endoscopy with endoscopic ultrasound as part of the routine staging process. Treatment planning CT imaging from the T1 to L1 vertebral levels and encompassing the entire spleen was required for the analysis. Follow-up data were collected from the first 2 surveillance CT scans performed for each patient after the initial treatment planning scan.
Treatment planning
Radiation treatment was delivered using a 3-dimensional conformal or intensity modulated radiation therapy (IMRT) technique, primarily via volumetric modulated arc therapy. Our institutional practice for IMRT is to treat the elective regional nodal basins to a dose of 45 Gy at 1.8 Gy per fraction with a simultaneous integrated boost to the primary tumor and to treat any gross nodal disease to 50 to 54 Gy at 2 Gy per fraction. The initial clinical target volume (CTV) receiving 45 Gy includes the primary tumor with a 3 to 4 cm margin along the mucosa, elective periesophageal nodes, and any grossly involved lymph nodes. For tumors in the distal esophagus or GEJ, the celiac axis and perigastric nodes within the 3 to 4 cm mucosal margin are also included. The CTV receiving the boost includes the primary tumor and grossly involved nodes with a 1 to 2 cm margin. Our normal tissue constraints for IMRT plans typically include a total lung mean dose <12 Gy, total lung V20 <15%, and heart mean dose <25 Gy. Chemotherapy was delivered concurrently with radiation therapy in all cases. The majority of patients received weekly carboplatin and paclitaxel2 but additional drug regimens included 5-fluorouracil, capecitabine, cisplatin, oxaliplatin, and trastuzumab. Respiratory gating was typically used for distal esophageal tumors to account for any significant organ motion.
Dosimetric parameters
For each patient, the thoracic spine, posterior ribs, and spleen were delineated on the treatment planning CT scan by a trained radiation oncologist. The thoracic spine was contoured as the vertebral bodies, transverse processes, and posterior elements from the T1 to L1 vertebral levels, inclusive. The posterior ribs included the T1 to T12 ribs from the costovertebral joint to the midscapular line bilaterally. Dose volume histogram data were generated in MIM (version 6.5.7, MIM Software Inc.; Cleveland, OH) and the following data were recorded for each structure: volume; mean dose; and absolute and relative volume receiving at least 5 (V5), 10 (V10), 15 (V15), 20 (V20), 30 (V30), and 40 Gy (V40).
Laboratory data
Complete blood counts with differential, including white blood cell (WBC), absolute neutrophil, hemoglobin, and platelet counts, were obtained within 1 week prior to the start of radiation therapy and at weekly intervals throughout the treatment course as part of routine clinical care for each patient. Laboratory results were also recorded for up to 2 months after completion of radiation therapy to observe potential delayed changes in counts. Hematologic toxicity was graded in accordance with the Common Terminology Criteria for Adverse Events, version 4.0, as per the established cutoff values.8
Follow-up spleen size assessment
The spleen was contoured on available follow-up CT scans for each patient, and organ volume was recorded. Our institutional practice is to obtain the first follow-up scan approximately 1 month after completion of radiation therapy, whether treatment is delivered with definitive or neoadjuvant intent. Should no further local therapy be performed, we typically obtain surveillance imaging every 3 months thereafter for at least the first year. The spleen was contoured on each of the follow-up scans, and the change in spleen volume was expressed as a percentage of the pretreatment spleen volume on the radiation treatment planning CT scan.
Statistical analysis
The primary endpoint was any grade ≥3 hematologic toxicity (HT3+). Secondary endpoints included maximum relative and absolute changes in blood cell laboratory values between baseline and nadir. Associations between baseline characteristics and HT3+ were evaluated using Fisher's exact test. Differences between means were analyzed using paired or independent samples t testing. The associations between dosimetric parameters and study endpoints were assessed using logistic and linear regression models. Parameters with P < .10 on univariable analyses were included in multivariable regression models. All tests were 2-sided with an alpha level of 0.05. Change in spleen size was modeled in a mixed effects model, which accounts for within-patient correlation. Analyses were performed using SPSS (version 22, IBM; Armonk, NY) and SAS software (version 9.4, SAS Institute; Cary, NC).
Results
Patient characteristics
Baseline demographics and tumor characteristics by severity of hematologic toxicity are presented in Table 1. The median age at time of radiation therapy was 65 years (range, 28-91 years). The median dose and dose per fraction delivered to the primary planning target volume were 50 Gy (range, 44-59 Gy) and 2 Gy (range, 1.8-2.2 Gy), respectively. Nearly all treatments (97%) were delivered using IMRT, and the majority of patients (68%) received concurrent carboplatin and paclitaxel.
Table 1.
Patient and treatment characteristics by severity of hematologic toxicity
| Characteristic | No HT3+ |
HT3+ |
P-valuea | ||
|---|---|---|---|---|---|
| n | (%) | n | (%) | ||
| Total | 39 | 100.0 | 21 | 100.0 | |
| Sex | .606 | ||||
| Female | 2 | 5.1 | 2 | 9.5 | |
| Male | 37 | 94.9 | 19 | 90.5 | |
| Histology | .039 | ||||
| Adenocarcinoma | 39 | 100.0 | 18 | 85.7 | |
| Squamous cell carcinoma | 0 | 0.0 | 2 | 9.5 | |
| Adenosquamous | 0 | 0.0 | 1 | 4.8 | |
| Stage | .326 | ||||
| IB | 2 | 5.1 | 0 | 0.0 | |
| IIA | 6 | 15.4 | 2 | 9.5 | |
| IIB | 7 | 17.9 | 4 | 19.0 | |
| IIIA | 20 | 51.3 | 9 | 42.9 | |
| IIIB | 2 | 5.1 | 2 | 9.5 | |
| IIIC | 1 | 2.6 | 0 | 0.0 | |
| IV | 0 | 0.0 | 3 | 14.3 | |
| N/A | 1 | 2.6 | 1 | 4.8 | |
| Treatment intent | .143 | ||||
| Neoadjuvant | 35 | 89.7 | 15 | 71.4 | |
| Definitive | 4 | 10.3 | 6 | 28.6 | |
| Radiation therapy modality | .119 | ||||
| IMRT | 39 | 100.0 | 19 | 90.5 | |
| 3-dimensional CRT | 0 | 0.0 | 2 | 9.5 | |
| Concurrent chemotherapy | .154 | ||||
| Carboplatin and paclitaxel | 29 | 74.4 | 12 | 57.1 | |
| 5-FU/capecitabine with platinum agent | 8 | 20.5 | 9 | 42.9 | |
| 5-FU/capecitabine alone | 2 | 5.1 | 0 | 0.0 | |
5-FU, 5-flurouracil; CRT, conformal radiation therapy; HT3+, grade ≥3 hematologic toxicity; IMRT, intensity modulated radiation therapy.
Fisher's exact test P-value.
Hematologic toxicity
The mean pretreatment and nadir laboratory values are reported in Table 2. There was a statistically significant difference between the pretreatment and nadir values for each blood cell line (P < .001 for each). Twenty-one patients (35%) experienced HT3+, the majority of which were attributed to grade ≥3 leukopenia (18 patients). Five patients (8%) experienced grade ≥3 thrombocytopenia. No grade ≥3 neutropenia or anemia was observed in our study cohort.
Table 2.
Change in blood cell counts
| Blood cell parameter | Value |
|---|---|
| Overall HT3+, n (%) | 21 patients (35.0%) |
| WBC | |
| Baseline, mean (SD) | 7.2 (3.0) k/uL |
| Nadir, mean (SD) | 2.3 (0.7) k/uL |
| Grade 3 + , n (%) | 18 patients (30.0%) |
| ANC | |
| Baseline, mean (SD) | 5.0 (2.8) k/uL |
| Nadir, mean (SD) | 2.0 (0.9) k/uL |
| Grade 3 + , n (%) | 0 patients (0%) |
| Hemoglobin | |
| Baseline, mean (SD) | 12.7 (2.4) g/dL |
| Nadir, mean (SD) | 11.4 (1.7) g/dL |
| Grade 3+, n (%) | 0 patients (0%) |
| Platelets | |
| Baseline, mean (SD) | 234 (87) k/uL |
| Nadir, mean (SD) | 107 (43) k/uL |
| Grade 3 + , n (%) | 5 patients (8.3%) |
ANC, absolute neutrophil count; HT3+, grade ≥3 hematologic toxicity; SD, standard deviation; WBC, white blood cells.
Spleen volume
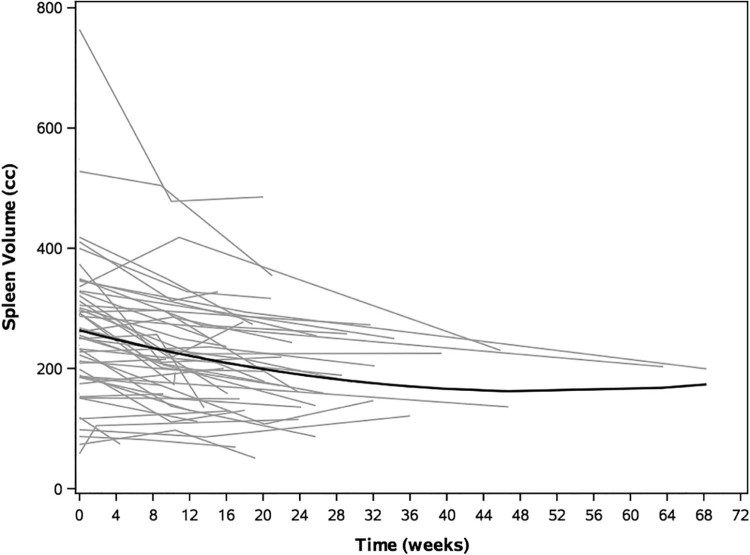
The mean baseline volume of the spleen was 266 ± 117 cm3. The data were approximately normally distributed, with a median volume of 257 cm3. Forty-eight patients had follow-up imaging available for review with a median time to first imaging of 10 weeks after the start of radiation (range, 2-64 weeks) and a median time to second imaging of 22 weeks (range, 10-68 weeks). The mean spleen volume at first follow-up was 213 ± 95 cm3, representing an average change of −14.8% from baseline (P < .001). At second follow-up, the mean spleen volume was 200 ± 91 cm3 (−23.2% change from baseline; P < .001; Fig 1). A higher absolute spleen V5-V30 was associated with a greater decrease in spleen volume (P < .05 for each; Supplementary Fig S1).
Figure 1.
Decrease in absolute spleen volume over time. Black line depicts a mixed-effects model of change in spleen volume. Gray lines depict individual patient data.
Dosimetric and volumetric predictors of HT3+
Dosimetric and volumetric data of the organs at risk are presented in Table 3 with respect to HT3+ status. The overall mean doses to the spleen, thoracic spine, and posterior ribs were 23.4 ± 4.5, 20.4 ± 5.2, and 12.4 ± 4.7 Gy, respectively. There was no statistically significant difference in mean dose to any of the 3 structures among patients who experienced any grade ≥3 hematologic toxicity compared with those who did not. The absolute and relative volume of the spine and ribs that received between 5 and 40 Gy was also not different between groups. However, those who did not experience HT3+ were found to have significantly larger volumes of spleen receiving at least 5 to 30 Gy of total radiation over the course of their treatments (P < .05 for each). These patients also had a larger baseline spleen volume by an average of 95 cm3 (P = .002), as well as a greater percentage decrease in spleen volume at first follow-up (22.9% vs 0.3%; P = .009).
Table 3.
Organ and dosimetry characteristics by severity of hematologic toxicity
| Parameter | No HT3+ |
HT3+ |
P-valuea | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Baseline spleen volume (cm3) | 299 | 119 | 204 | 85 | .002 |
| Percent decrease in spleen volume at first follow-up (%) | 22.9 | 14.5 | 0.3 | 30.0 | .009 |
| Percent decrease in spleen volume at second follow-up (%) | 28.8 | 19.3 | 11.6 | 40.4 | .118 |
| Spleen dosimetry | |||||
| Mean dose (Gy) | 23.8 | 6.5 | 22.7 | 9.2 | .593 |
| V5 (cm3) | 276.9 | 116.5 | 165.1 | 82.0 | < .001 |
| V10 (cm3) | 247.3 | 106.8 | 153.1 | 77.2 | .001 |
| V15 (cm3) | 216.7 | 104.7 | 136.5 | 72.1 | .003 |
| V20 (cm3) | 179.2 | 103.8 | 105.5 | 57.5 | .004 |
| V30 (cm3) | 92.7 | 76.0 | 54.6 | 41.5 | .038 |
| V40 (cm3) | 35.9 | 32.4 | 26.2 | 31.3 | .267 |
| Thoracic spine dosimetry | |||||
| Mean dose (Gy) | 20.2 | 5.5 | 21.0 | 4.7 | .581 |
| V5 (cm3) | 377.7 | 85.8 | 372.6 | 79.6 | .824 |
| V10 (cm3) | 355.2 | 86.0 | 350.8 | 75.0 | .842 |
| V15 (cm3) | 335.1 | 86.6 | 324.8 | 71.1 | .643 |
| V20 (cm3) | 303.3 | 91.8 | 288.5 | 72.6 | .525 |
| V30 (cm3) | 205.9 | 101.6 | 199.0 | 89.4 | .794 |
| V40 (cm3) | 92.9 | 58.2 | 90.7 | 69.3 | .895 |
| Posterior rib dosimetry | |||||
| Mean dose (Gy) | 12.4 | 5.1 | 12.5 | 4.2 | .932 |
| V5 (cm3) | 91.1 | 33.8 | 88.1 | 34.7 | .744 |
| V10 (cm3) | 79.1 | 28.6 | 74.2 | 27.4 | .526 |
| V15 (cm3 cm3) | 65.3 | 26.0 | 57.1 | 21.6 | .218 |
| V20 (cm3 cm3) | 47.1 | 26.1 | 38.1 | 18.0 | .167 |
| V30 (cm3) | 16.4 | 21.3 | 13.3 | 14.6 | .555 |
| V40 (cm3) | 3.6 | 7.9 | 3.3 | 8.3 | .909 |
HT3+, grade ≥3 hematologic toxicity; SD, standard deviation; Vx, volume in cc that receives at least x Gy.
Independent samples t test P-value.
Demographic and treatment-related predictors of hematologic toxicity were investigated using logistic regression models. On univariable analysis, baseline spleen volume, percentage change in spleen volume at first follow-up, and absolute volume of the spleen receiving at least 5 to 20 Gy (V5-V20) were significantly correlated with both overall HT3+ and grade ≥3 leukopenia (Table 4). On multivariable analysis, only the percentage change in spleen volume at first follow-up and absolute spleen V5 to V20 remained correlated with incidence of overall HT3+ (Table 5). Relative spleen dosimetry parameters were not significantly associated with HT3+ on univariable or multivariable analysis. Additional factors, including chemotherapy type and dosimetric parameters of the thoracic spine and posterior ribs, were not predictive of HT3+ or leukopenia.
Table 4.
Univariate logistic regression models of predictors of hematologic toxicity
| Parameter | Grade ≥3 hematologic toxicity |
Grade ≥3 leukopenia |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age | 1.02 | 0.97-1.08 | .461 | 0.99 | 0.94-1.05 | .832 |
| Male | 0.51 | 0.07-3.94 | .521 | 1.31 | 0.13-13.49 | .822 |
| Chemotherapy type | ||||||
| Carboplatin/paclitaxel | 0.46 | 0.15-1.42 | .175 | 0.63 | 0.20-2.01 | .433 |
| Other | Reference | — | — | Reference | — | — |
| Baseline spleen volume | 0.89a | 0.83-0.96 | .004 | 0.91a | 0.85-0.98 | .014 |
| Percent decrease in spleen volume at first follow-up | 0.95 | 0.91-0.98 | .006 | 0.96 | 0.93-1.00 | .029 |
| Percent decrease in spleen volume at second follow-up | 0.98 | 0.94-1.01 | .182 | 0.97 | 0.93-1.01 | .129 |
| Spleen dosimetry | ||||||
| Mean dose | 0.98 | 0.91-1.05 | .587 | 1.00 | 0.92-1.07 | .885 |
| V5 | 0.87a | 0.80-0.94 | .001 | 0.89a | 0.82-0.96 | .003 |
| V10 | 0.88a | 0.81-0.95 | .002 | 0.89a | 0.83-0.96 | .004 |
| V15 | 0.89a | 0.82-0.96 | .004 | 0.90a | 0.83-0.97 | .008 |
| V20 | 0.88a | 0.79-0.96 | .005 | 0.88a | 0.80-0.97 | .010 |
| V30 | 0.89a | 0.78-1.00 | .049 | 0.90a | 0.79-1.01 | .083 |
| V40 | 0.90a | 0.75-1.08 | .267 | 0.92a | 0.76-1.12 | .405 |
| Thoracic spine dosimetry | ||||||
| Mean dose | 1.03 | 0.93-1.14 | .575 | 1.00 | 0.90-1.11 | .988 |
| Posterior rib dosimetry | ||||||
| Mean dose | 1.01 | 0.90-1.12 | .931 | 0.96 | 0.84-1.09 | .492 |
CI, confidence interval; OR, odds ratio; Vx, volume in cm3 that receives at least x Gy.
ORs reported per 10 cm3 increase in volume.
Table 5.
Multivariate logistic regression models of predictors of hematologic toxicity
| Parameter | Grade ≥3 hematologic toxicity |
||
|---|---|---|---|
| Odds ratio | 95% CI | P-value | |
| Baseline spleen volume | 1.16a | 0.90-1.51 | .264 |
| Percent decrease in spleen volume at first follow-up | 0.94 | 0.89-0.99 | .014 |
| Spleen dosimetry | |||
| V5 | 0.75a | 0.57-0.98 | .037 |
| V10 | 0.83a | 0.72-0.96 | .014 |
| V15 | 0.86a | 0.75-0.98 | .029 |
| V20 | 0.87a | 0.76-0.99 | .040 |
| V30 | 0.89a | 0.74-1.06 | .181 |
| V40 | 0.94a | 0.75-1.21 | .649 |
CI, confidence interval; Vx, volume in cc that receives at least x Gy.
Spleen Vx parameters were added individually to separate multivariate models. Odds ratios for nondosimetric parameters represent results that control for spleen V5.
Odds ratios reported per 10 cm3 increase in volume.
With respect to individual blood count values, when controlling for baseline WBC count, a 10% decrease in spleen volume at first follow-up was associated with a 0.1 k/uL higher WBC nadir (P = .020), but a 10 cm3 increase in absolute spleen V5, V10, and V15 was each associated with a 0.02 k/uL higher nadir (P = .002, .005, and .032, respectively; Supplementary Fig S2). No additional associations were found between spleen dose and other blood count values. Furthermore, dose to the thoracic spine or ribs was not significantly associated with changes in individual laboratory values.
Discussion
Previous studies have found that a higher radiation dose volume to the bone marrow is associated with acute cytopenias,3, 4, 5, 9 but the impact of splenic irradiation on hematologic toxicity remains relatively unknown. In the current study, we did not note any relationship between radiation dose to the thoracic spine or ribs and the development of hematologic toxicity for patients treated with chemoradiation therapy to the distal esophagus. However, our data revealed that splenic irradiation was associated with a lower rate of hematologic toxicity. Furthermore, we demonstrated a dose-dependent change in spleen volume after radiation therapy, which inversely correlated with the development of hematologic toxicity.
A dose-dependent change in spleen size has been observed previously after chemoradiation therapy for gastric cancer.7 Similar to Trip et al, we demonstrated an average spleen volume reduction of 15% measured at a median of 10 weeks from the start of radiation therapy. However, a relationship between change in spleen size and hematologic outcomes had not been shown previously. Trip et al observed evidence of hyposplenism (ie, infectious events) after high-dose splenic irradiation, but the risk of infection was not correlated with any spleen dosimetric parameters.7
In our study, both change in spleen volume after radiation therapy and radiation dose to the spleen were significantly correlated with the risk of HT3+ (ie, greater decrease in spleen volume and higher absolute spleen V5-V20 were independently associated with a lower risk of toxicity). Larger baseline spleen volume was associated with lower risk of HT3+ on univariable analysis, but the effect did not remain significant after controlling for change in spleen volume and dosimetric parameters. Interestingly, relative spleen dosimetry parameters were not significantly associated with hematologic toxicity on univariable or multivariable analysis. Our results suggest that the absolute volume of spleen irradiated may have a greater effect on blood cell counts than the relative volume, even after controlling for baseline spleen volume.
Radiation therapy has been used for many decades in the palliative setting for symptomatic splenomegaly, including for cytopenias in the setting of myeloproliferative disorders.10 In contrast, no patients in our cohort demonstrated a pathologically enlarged spleen or known history of myeloproliferative disorder. Our data suggest that irradiation of the normal spleen may have physiologic effects similar to those of irradiation for symptomatic splenomegaly and may limit cytopenias associated with other etiologies.
A large multi-institutional retrospective study from Germany found that low-dose splenic irradiation ranging from 30 to 1600 cGy resulted in a 74% response rate with respect to improvement in blood cell counts in the setting of symptomatic splenomegaly.11 Although the biological mechanisms that lead to cytopenia may be different, irradiation of a non-pathologically-enlarged spleen in the setting of therapeutic radiation therapy to the abdomen or lower thorax may serve to mitigate decreased hematopoiesis due to radiation-induced bone marrow suppression. In our study, hematologic toxicity was primarily driven by leukopenia, with 18 of 21 patients with HT3+ experiencing grade ≥3 leukopenia.
The mechanism of the spleen's effect on peripheral WBC count remains unclear. One possible mechanism of action relates to the release of sequestered cells, including lymphocytes, from the spleen and other organ tissues.6 This hypothesis is supported by data that suggest that splenic irradiation is potentially effective in palliating hypersplenism-induced cytopenias due to congestion12, 13 and is consistent with the observed reduction in spleen size after radiation therapy. The concept of radiotherapeutic splenectomy, with high radiation doses over 20 Gy leading to functional asplenism, has also been discussed in the past.14 Furthermore, a study of blood cell migration in mice suggests that there is a shift in the proportion of WBCs from tissue cavities to the blood stream after surgical removal of the spleen.15 Radiotherapeutic splenectomy may possibly affect peripheral WBC count via a similar mechanism. Another hypothesis is that the spleen acts as a negative regulator of WBC production in the bone marrow or as a positive regulator of peripheral WBC apoptosis via humoral effects, as also evidenced by studies of leukocytosis after surgical splenectomy15, 16, 17 and data that support improved hematologic tolerance to radiation therapy in splenectomized patients.18
Prior studies investigating the impact of radiation dose on the spleen have found varying effects on hematologic outcomes.7, 19, 20 Chadha et al found that lymphopenia after definitive chemoradiation therapy for locally advanced pancreatic cancer was associated with a higher mean spleen dose (MSD) and higher spleen V10-V20.19 The median MSD was 6.8 Gy among patients in their cohort, compared with 23.1 Gy in our study. Higher radiation doses to the spleen and larger volumes irradiated could have a paradoxical effect on WBC counts by reducing the functional splenic volume. This is supported by the findings of Trip et al, which demonstrate that average leukocyte count was significantly higher than baseline 4 years after high-dose splenic irradiation (median MSD, 40 Gy).7 However, the relatively high rate of severe infectious events that they observed in their cohort suggests that WBC count may not be an adequate marker of immune function after radiation. The relationship between splenic dose and functional outcomes requires further investigation.
It is also possible that splenic irradiation affects separate blood cell lines differently. Thrombocytopenia after palliative splenic irradiation has previously been reported, albeit primarily in the setting of extramedullary hematopoiesis due to myelofibrosis.21, 22 In our study, we did not find a significant association between radiation dose to the spleen and change in platelet count or nadir. However, the lack of an observed effect may be related to the small sample size or minimal effect of extramedullary hematopoiesis in our cohort.
Although the impact of the spleen was unexpected, the lack of correlation of dose to the thoracic bones and ribs on the development of hematologic toxicity was equally surprising and suggests that chemotherapy may have been the predominant driver of hematologic toxicity in our series. Most patients received concurrent weekly carboplatin and paclitaxel, and no significant differences in hematologic toxicity were observed between the chemotherapy types.
Nevertheless, there are several limitations to our study, including its retrospective nature and relatively small sample size. There is inherent heterogeneity in the study sample, and the effects of potential confounding variables, including chemotherapy type, cannot be eliminated completely. Furthermore, follow-up was limited for many of our patients, and we were unable to demonstrate long-term persistence of effect or any recovery of spleen size.
Conclusions
In our study, radiation to the thoracic spine and ribs did not correlate with the development of hematologic toxicity during chemoradiation for esophageal cancer. However, splenic irradiation appeared to be associated with a lower rate of severe hematologic toxicity. Our results demonstrated that patients who did not experience hematologic toxicity had a larger baseline spleen volume, greater absolute volume of spleen irradiated, and greater decrease in spleen volume after radiation. Given the association with these factors, we hypothesize a possible mechanism that may involve the release of sequestered cells from the spleen into the circulation after irradiation. Although our study did not include patients with a pathologically enlarged spleen, prior studies examining the hematologic impact of palliative radiation therapy for symptomatic splenomegaly have shown similar effects on blood cell counts.
Before recommending routine irradiation of the spleen, further studies will be required to confirm these findings and to investigate the underlying mechanism of dynamic changes in blood cell counts with respect to splenic irradiation, as well as its effect on immune system function. Although the spleen is not routinely considered an organ at risk for the purposes of radiation therapy treatment planning, our results suggest that further attention should be paid to this organ and its relationship with hematologic toxicity.
Footnotes
Meeting information: The results of this study were presented at the 2016 Annual Meeting of the American Society of Radiation Oncology in Boston, MA; September 25 to 28, 2016.
Conflicts of interest: The authors have no conflicts of interest to disclose.
Supplementary material for this article (https://doi.org/10.1016/j.adro.2018.02.005) can be found at www.practicalradonc.org.
Supplementary data
The following is the supplementary data to this article:
Change in absolute spleen volume between baseline and first follow-up with respect to A) V5 of the spleen, B) V10 of the spleen, and C) V15 of the spleen. Solid line depicts line of best fit. Dotted lines depict 95% confidence interval.
White blood cell count nadir with respect to A) V5 of the spleen, B) V10 of the spleen, and C) V15 of the spleen. Solid line depicts line of best fit. Dotted lines depict 95% confidence interval. WBC, white blood cell.BSOnlineOnly>
References
- 1.Shapiro J., van Lanschot J.J.B., Hulshof M.C.C.M. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015;16:1090–1098. doi: 10.1016/S1470-2045(15)00040-6. [DOI] [PubMed] [Google Scholar]
- 2.van Hagen P., Hulshof M.C., van Lanschot J.J.B. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 3.Bazan J.G., Luxton G., Mok E.C., Koong A.C., Chang D.T. Normal tissue complication probability modeling of acute hematologic toxicity in patients treated with intensity-modulated radiation therapy for squamous cell carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2012;84:700–706. doi: 10.1016/j.ijrobp.2011.12.072. [DOI] [PubMed] [Google Scholar]
- 4.Mell L.K., Kochanski J.D., Roeske J.C. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:1356–1365. doi: 10.1016/j.ijrobp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Deek M.P., Benenati B., Kim S. Thoracic vertebral body irradiation contributes to acute hematologic toxicity during chemoradiation therapy for non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2016;94:147–154. doi: 10.1016/j.ijrobp.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swirski F.K., Nahrendorf M., Etzrodt M. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trip A.K., Sikorska K., van Sandick J.W. Radiation-induced dose-dependent changes of the spleen following postoperative chemoradiotherapy for gastric cancer. Radiother Oncol. 2015;116:239–244. doi: 10.1016/j.radonc.2015.07.036. [DOI] [PubMed] [Google Scholar]
- 8.National Cancer Institute . U.S. Department of Health and Human Services; Bethesda, MD: 2010. Common Terminology Criteria for Adverse Events v.4.0. [Google Scholar]
- 9.Cheng J.C., Bazan J.G., Wu J.K., Koong A.C., Chang D.T. Lumbosacral spine and marrow cavity modeling of acute hematologic toxicity in patients treated with intensity modulated radiation therapy for squamous cell carcinoma of the anal canal. Pract Radiat Oncol. 2014;4:198–206. doi: 10.1016/j.prro.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 10.Zaorsky N.G., Williams G.R., Barta S.K. Splenic irradiation for splenomegaly: A systematic review. Cancer Treat Rev. 2017;53:47–52. doi: 10.1016/j.ctrv.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kriz J., Micke O., Bruns F. Radiotherapy of splenomegaly: A palliative treatment option for a benign phenomenon in malignant diseases. Strahlenther Onkol. 2011;187:221–224. doi: 10.1007/s00066-011-2252-4. [DOI] [PubMed] [Google Scholar]
- 12.Kenawi M.M., el Ghamrawi K.A., Mohammad A.A., Kenawi A., el Sadek A.Z. Splenic irradiation for the treatment of hypersplenism from congestive splenomegaly. Br J Surg. 1997;84:860–861. [PubMed] [Google Scholar]
- 13.Bruns F., Bremer M., Dettmer A., Janssen S. Low-dose splenic irradiation in symptomatic congestive splenomegaly: Report of five cases with literature data. Radiat Oncol. 2014;9:86. doi: 10.1186/1748-717X-9-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinmann M., Becker G., Einsele H., Bamberg M. Clinical indications and biological mechanisms of splenic irradiation in chronic leukaemias and myeloproliferative disorders. Radiother Oncol. 2001;58:235–246. doi: 10.1016/s0167-8140(00)00316-9. [DOI] [PubMed] [Google Scholar]
- 15.Djaldetti M., Bergman M., Salman H., Cohen A.M., Fibach E., Bessler H. On the mechanism of post-splenectomy leukocytosis in mice. Eur J Clin Invest. 2003;33:811–817. doi: 10.1046/j.1365-2362.2003.01197.x. [DOI] [PubMed] [Google Scholar]
- 16.Palmer J.G., Kemp I., Cartwright G.E., Wintrobe M.M. Studies on the effect of splenectomy on the total leukocyte count in the albino rat. Blood. 1951;6:3–15. [PubMed] [Google Scholar]
- 17.Bessler H., Bergman M., Salman H., Beilin B., Djaldetti M. The relationship between partial splenectomy and peripheral leukocyte count. J Surg Res. 2004;122:49–53. doi: 10.1016/j.jss.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Salzman J.R., Kaplan H.S. Effect of prior splenectomy on hematologic tolerance during total lymphoid radiotherapy of patients with Hodgkin's disease. Cancer. 1971;27:471–478. doi: 10.1002/1097-0142(197102)27:2<471::aid-cncr2820270236>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Chadha A.S., Liu G., Chen H.-C. Does unintentional splenic radiation predict outcomes after pancreatic cancer radiation therapy? Int J Radiat Oncol Biol Phys. 2017;97:323–332. doi: 10.1016/j.ijrobp.2016.10.046. [DOI] [PubMed] [Google Scholar]
- 20.Zschaeck S., Blümke B., Wust P. Dose-escalated radiotherapy for unresectable or locally recurrent pancreatic cancer: Dose volume analysis, toxicity and outcome of 28 consecutive patients. PLoS ONE. 2017;12 doi: 10.1371/journal.pone.0186341. e0186341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott M.A., Chen M.G., Silverstein M.N., Tefferi A. Splenic irradiation for symptomatic splenomegaly associated with myelofibrosis with myeloid metaplasia. Br J Haematol. 1998;103:505–511. doi: 10.1046/j.1365-2141.1998.00998.x. [DOI] [PubMed] [Google Scholar]
- 22.Ishibashi N., Maebayashi T., Aizawa T. Myelosuppression toxicity of palliative splenic irradiation in myelofibrosis and malignant lymphoma. Hematology. 2015;20:203–207. doi: 10.1179/1607845414Y.0000000192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Change in absolute spleen volume between baseline and first follow-up with respect to A) V5 of the spleen, B) V10 of the spleen, and C) V15 of the spleen. Solid line depicts line of best fit. Dotted lines depict 95% confidence interval.
White blood cell count nadir with respect to A) V5 of the spleen, B) V10 of the spleen, and C) V15 of the spleen. Solid line depicts line of best fit. Dotted lines depict 95% confidence interval. WBC, white blood cell.BSOnlineOnly>