Abstract
Background
The “Avolition-apathy” domain of the negative symptoms was found to include different symptoms by factor analytic studies on ratings derived by different scales. In particular, the relationship of anhedonia with this domain is controversial. Recently introduced negative symptom rating scales provide a better assessment of anhedonia, allowing the distinction of anticipatory and consummatory aspects, which might be related to different psychopathological dimensions. The study of associations with external validators, such as electrophysiological, brain imaging or cognitive indices, might shed further light on the status of anhedonia within the Avolition-apathy domain.
Objectives
We used brain electrical microstates (MSs), which represent subsecond periods of quasi-stable scalp electrical field, associated with resting-state neural networks (and thus with global patterns of functional connectivity), to test whether the component symptoms of Avolition-apathy share the same correlates.
Method
We analyzed multichannel resting EEGs in 142 individuals with schizophrenia (SCZ) and in 64 healthy controls (HC), recruited within the add-on EEG study of the Italian Network for Research on Psychoses. Relative time contribution, duration and occurrence of four MS classes (MS-A/-B/-C/−D) were calculated. Group differences on MS parameters (contribution and duration) and their associations with negative symptom domains (assessed using the Brief Negative Symptoms Scale) were investigated.
Results
SCZ, in comparison to HC, showed increased contribution and duration of MS-C. The contribution of MS-A positively correlated with Avolition-apathy, but not with Expressive deficit. Within the Avolition-apathy domain, anticipatory anhedonia, avolition and asociality, but not consummatory anhedonia, showed the same correlations with MS-A contribution.
Conclusion
Our findings support the existence of distinct electrophysiological correlates of Avolition-apathy with respect to Expressive deficit, and lend support to the hypothesis that only the anticipatory component of anhedonia shares the same pathophysiological underpinnings of the Avolition-apathy domain.
Keywords: Schizophrenia, Avolition-apathy, Anhedonia, Resting-EEG, Brain electrical microstates
Highlights
-
•
Microstate C contribution and duration were increased in SCZ compared to HC.
-
•
Avolition-apathy was correlated with the contribution of microstate A.
-
•
Avolition-apathy might be associated with sensory processing deficit.
1. Introduction
Negative symptoms have long been considered a core feature of schizophrenia (Kraepelin, 1919; Bleuler, 1959). They represent a separate domain of the disease (Strauss et al., 1974; Kirkpatrick and Fischer, 2006) and have been associated with poor functional outcome, worse quality of life and poor response to pharmacological treatment (Lysaker and Davis, 2004; Orsel et al., 2004; Wegener et al., 2005; Harvey et al., 2012; Galderisi et al., 2013a; Galderisi et al., 2013b; Mucci et al., 2014; Mucci et al., 2017).
According to the literature, negative symptoms include blunted affect (reduced intensity and range of emotional expression), alogia (reduced spontaneous speech and loss of conversational fluency), avolition (reduced interest and motivation for productive activities, or sense of purpose), asociality (diminished interest in social drive or interest and desire for affiliation) and anhedonia (reduced ability to experience or anticipate pleasure) (Kirkpatrick and Fischer, 2006; Galderisi et al., 2016; Galderisi et al., 2018).
Several factor analytic studies have reported that negative symptoms can be divided into two domains referred to as Avolition-apathy (which includes avolition, anhedonia and asociality) and Expressive deficit (which includes alogia and blunted affect) (Kimhy et al., 2006; Galderisi et al., 2013a; Kirkpatrick, 2014a, Kirkpatrick, 2014b). These two domains have been shown to have different behavioral and neurobiological correlates and they may also be targeted by different therapeutic options (Galderisi et al., 2013a; Kirkpatrick, 2014b; Galderisi et al., 2015; Mucci et al., 2015; Kaiser et al., 2016; Kirschner et al., 2017; Marder and Galderisi, 2017; Mucci et al., 2017).
Avolition-apathy has been associated with a dysfunction of brain circuits involved in motivation (Gard et al., 2007; Heerey and Gold, 2007; Heerey et al., 2007; Waltz et al., 2007; Kring and Moran, 2008; Barch and Dowd, 2010; Cohen and Minor, 2010; Dowd and Barch, 2010; Foussias and Remington, 2010; Pizzagalli, 2010; Simpson et al., 2012; Mann et al., 2013; Strauss, 2013; Morris et al., 2015; Mucci et al., 2015), in particular to those subtending the ability to anticipate pleasure and learn from rewards (Waltz et al., 2007; Gold et al., 2012; Strauss, 2013) or to integrate reward value with performed actions (Morris et al., 2015; Mucci et al., 2015; Waltz and Gold, 2016). These functions are subtended by the circuit corresponding to the NIMH Research Domain Criteria (https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml) “positive valence system” (Bromberg-Martin et al., 2010; Miller et al., 2014; Bissonette and Roesch, 2016; O'Doherty, 2016). This system involves dopamine neurons located in the ventro-tegmental area (VTA) and in the ventro-medial substantia nigra pars compacta (VMSNpc) with projections to orbito-frontal cortex (OFC), ventro-medial prefrontal cortex (VMPFC), nucleus accumbens (NA), dorsal striatum (DS), amygdala and insular cortex (Bromberg-Martin et al., 2010; Galderisi et al., 2018).
Open issues in the field include whether Avolition-apathy and all component symptoms share the same neurobiological underpinnings, in particular for what concerns the anticipatory (failure to anticipate reward or pleasurable experiences) and consummatory aspects of anhedonia (in the moment experience of pleasure during pleasurable situations).
It is highly controversial whether consummatory anhedonia can be found in non-depressed individuals with schizophrenia (Andreasen, 1982; Horan et al., 2006; Tremeau, 2006; Kring and Moran, 2008; Tremeau et al., 2009; Cohen and Minor, 2010), as several studies documented an intact ability to experience pleasure in the moment in these subjects (Messinger et al., 2011; Kirkpatrick et al., 2017; Marder and Galderisi, 2017). In contrast, an impairment in pleasure anticipation, due to a dysfunction of the motivation circuits, was consistently reported in individuals with schizophrenia, both at first-episode and during the chronic phase of the disease (Gard et al., 2007; Heerey and Gold, 2007; Heerey et al., 2007; Waltz et al., 2007; Kring and Moran, 2008; Barch and Dowd, 2010; Cohen and Minor, 2010; Foussias and Remington, 2010; Pizzagalli, 2010; Dowd and Barch, 2012; Simpson et al., 2012; Mann et al., 2013; Strauss, 2013; Morris et al., 2015). Thus, only anticipatory anhedonia might share the same neurobiological correlates of the Avolition-apathy domain (Barch et al., 2016). This issue could not be tested adequately in the past. In fact, most of the old assessment instruments either did not evaluate anhedonia or did not distinguish between consummatory and anticipatory anhedonia (Knutson et al., 2001; Horan et al., 2006; Berridge, 2007; Gard et al., 2007; Blanchard et al., 2011). Indeed, both the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987) and the Scale for the Assessment of Negative Symptoms (SANS) (Andreasen, 1989) had several limitations. The PANSS did not evaluate avolition and anhedonia, while the SANS rated together anhedonia and asociality, and did not distinguish between the two aspects of anhedonia. These limitations have been overcome by the introduction of second generation clinician-rated scales, such as the Brief Negative Symptoms Scale (BNSS) (Kirkpatrick et al., 2011), in which the anhedonia subscale has separate items for the assessment of the intensity and frequency of pleasure during activities (consummatory pleasure) in the last week and the intensity of expected pleasure from future activities (anticipatory pleasure).
Some electrophysiological studies, investigating abnormalities of reward anticipation and evaluation in individuals with schizophrenia, found that anhedonia was associated with abnormal reward processing, evaluated using the Stimulus Preceding Negativity (SPN) and the P300 event-related potential (ERP) (Wynn et al., 2010; Vignapiano et al., 2016). In the study conducted by Wynn et al. (Wynn et al., 2010), the SPN, a negative ERP component involved in the anticipation of feedback, correlated with trait anhedonia and with the total negative symptoms score, assessed using the Scale for the Assessment of Negative Symptoms (SANS). Vignapiano et al. (2016) investigated cue P300 ERP components during the anticipation of reward and loss (using the Monetary Incentive Delay task). They found that in healthy controls and in individuals with schizophrenia, early P300 amplitude for large reward and large loss was inversely related to social anhedonia, assessed using the Chapman Social Anhedonia Scale (Chapman et al., 1976). The same study did not find any correlation of the P300 amplitude with the Avolition-apathy or Expressive deficit domain.
The results of task-related ERP investigations could be confounded by neurocognitive deficits that are prominent in individuals with schizophrenia (Heinrichs and Zakzanis, 1998; Green et al., 2000; Galderisi et al., 2002; Bora et al., 2009; Galderisi et al., 2009; Bora et al., 2010) and suffer from an a priori selection of the component of interest.
For these reasons, ERP results might be integrated by complementary analyses, using, for instance, indices of resting state activity within distributed neural networks. One such method is the so-called microstates analysis (Lehmann et al., 1987; Pascual-Marqui et al., 1995). This kind of EEG analysis defines the global functional state of the brain by its momentary scalp electric field configuration (Koenig et al., 2002). Cluster analytical approaches showed that there is a small set of prototypical microstate configurations, which constitutes a basic repertoire of brain functional states. The sequence of microstates and the rules governing these sequences may represent the subsecond switching between various types of such integrative states (Khanna et al., 2015). The microstates can be considered “atoms of thought”, since they are associated with different classes of mentation and reflect the coordinated activity of distributed neural networks (Lehmann et al., 1998). As a matter of fact, the different microstate (MS) classes were associated with distinct resting state neural networks (RSNs) in different studies, using different methodologies (Britz et al., 2010; Yuan et al., 2012; Pascual-Marqui et al., 2014; Custo et al., 2017), with only few exceptions in which only one MS demonstrated a significant association with two RSNs (Musso et al., 2010).
Two of the studies reporting a significant association between MSs and RSNs, used simultaneous EEG-fMRI recording (Britz et al., 2010; Yuan et al., 2012). In the study conducted by Britz et al. (2010), the authors extracted 4 MSs (MS-A/-B/-C/−D) and they reported that these MSs were associated with BOLD signal within brain areas subtending phonological, visual, salience and attention networks. Yuan et al. (2012), using a temporal independent component analysis, extracted 13 MS and found that six of them were associated with one or two RSNs, while the others to more than two networks.
The other studies investigated the relationships between MSs and RSNs, applying source localization methods to multichannel data (Pascual-Marqui et al., 2014; Custo et al., 2017; Milz et al., 2017). The four MS classes were found to be associated with sources in the anterior and posterior cingulate cortices and bilateral parietal-occipital regions, areas which belong to the default mode network (Pascual-Marqui et al., 2014). A recent study (Custo et al., 2017), using a source localization method closer to the approach used by Britz et al. (2010), extracted seven maps and demonstrated that maps labelled as A, B, F and D (corresponding to maps A-B-C-D in Britz et al., 2010) were linked to similar RSNs described in Britz et al. (2010).
In addition, other studies attempted to alter the temporal characteristics of the four MSs through behavioral manipulation (Milz et al., 2016; Seitzman et al., 2017) and reported that temporal parameters of MS-B were increased during visualization (Seitzman et al., 2017) or verbalization (Milz et al., 2016), and those of MS-A were increased during visualization (Milz et al., 2016).
Taking into account that MSs A and B have been linked to fronto-temporal and occipital regions, areas belonging to the phonological and visual networks and that MSs C and D have been linked to cingulate cortex, right superior and middle frontal gyri, the right superior and inferior parietal lobules, regions involved in the default mode, salience and attention networks (Britz et al., 2010; Custo et al., 2017), it is possible to extrapolate that MSs A and B reflect the extrinsic system of distributed resting state connectivity (as demonstrated also by the task-specific alteration of the temporal parameters of these two MSs), while the other two classes are the expression of the intrinsic system (for definition and characteristics of the two systems, see Doucet et al., 2011; Orliac et al., 2017). Indeed, the extrinsic system is constituted by two modules, which are located in the primary somatosensory areas (M2a) and visual areas (M2b), while the intrinsic system includes three modules that are involved in generation of spontaneous thoughts (M1a), inner maintenance and manipulation of information (M1b), cognitive control and switching activity (M1c).
Although the comparison of aforementioned studies is made difficult due to the methodological differences, taken together they suggested a relationship between MSs and RSNs. Therefore, EEG microstate analysis can be an advantageous, inexpensive, and non-invasive method to assess global functional states of the brain and to correlate these states with clinically relevant measures.
In individuals with schizophrenia, several studies have suggested changes in MS topography and/or temporal parameters (Kinoshita et al., 1998; Koenig et al., 1999; Strelets et al., 2003; Lehmann et al., 2005; Kikuchi et al., 2007; Nishida et al., 2013; Tomescu et al., 2015).
All the aforementioned studies have suggested a reduced presence of MSs B and D and increased presence of C and A (although the latter alteration is far less consistent within studies) in individuals with schizophrenia versus healthy controls.
A recent meta-analysis demonstrated that the reduced contribution and duration of MS-D, the increased contribution and occurrence of MS-C and the reduced duration of MS-B are consistent across studies (Rieger et al., 2016); on the contrary, the meta-analysis did not demonstrate any consistent effect for MS-A.
Some studies have reported that individual MS classes may be associated with specific clinical features of schizophrenia, such as hallucinations, positive symptoms, duration of illness and negative symptom total score (Stevens et al., 1997; Koenig et al., 1999; Kindler et al., 2011; Andreou et al., 2014; Tomescu et al., 2015). In particular, MS-D was found to be significantly associated to paranoid-hallucinatory symptomatology (Koenig et al., 1999) or to the experience of hallucinations (Kindler et al., 2011). An early study also has reported that, in chronic individuals with schizophrenia, the overall average duration of MSs was negatively correlated with the duration of disease and the frequency of psychotic exacerbations and was positively correlated with Brief Psychiatric Rating Scale (BPRS) total score and negative symptoms total score, assessed using the SANS (Stevens et al., 1997).
To our knowledge, no study has analyzed the correlates of the Avolition-apathy domain using the MS analysis during a resting state condition. Therefore, the current study had two primary aims: 1) to identify differences between healthy controls and clinically stable individuals with schizophrenia with respect to MS parameters and 2) to investigate the associations of these MS parameters with the Avolition-apathy domain and its component symptoms, as assessed by a second-generation rating scale, providing optimal assessment of consummatory and anticipatory anhedonia.
2. Methods
2.1. Subjects
One hundred and forty-eight outpatients with schizophrenia (SCZ) and seventy healthy controls (HC) were recruited at five research sites in Naples, Foggia, Rome “Tor Vergata”, Rome “Sapienza” and Salerno as part of the add-on EEG study of the Italian Network for Research on Psychoses (Mucci et al., 2014).
The SCZ sample included individuals consecutively seen at the outpatient units of the three mentioned Italian university psychiatric clinics. All patients had a diagnosis of schizophrenia according to DSM-IV, confirmed with the Structured Clinical Interview for DSM-IV - Patient version (SCID-I-P), and an age between 18 and 65 years. The HC group was matched with SCZ by gender and age, and recruited from the community at the same sites as the patient sample. Inclusion criteria for HC were the absence of a current or lifetime Axis I or II diagnosis.
Exclusion criteria for both groups were: (a) a history of head trauma with loss of consciousness; (b) a history of moderate to severe mental retardation or of neurological diseases; (c) a history of alcohol and/or substance abuse in the last six months; (d) current pregnancy or lactation; (e) inability to provide an informed consent. Exclusion criteria for SCZ were treatment modifications and/or hospitalization due to symptom exacerbation in the last three months.
The study has been conducted in accordance with the principles of the Declaration of Helsinki (59th World Medical Association General Assembly; October 2008) and with Uniform Requirements for manuscripts submitted to Biomedical journals and was approved by the Ethics Committee of the involved institutions. After a complete description of the study, a written informed consent was obtained from all participants.
2.2. Assessments
All subjects were evaluated for socio-demographic variables such as age, education and gender.
A semi-structured interview, the Brief Negative Symptoms Scale (Kirkpatrick et al., 2011) was used to assess negative symptoms in individuals with schizophrenia. The scale comprises 13 items, organized into six subscales (five negative symptom subscales: Anhedonia, Asociality, Avolition, Blunted Affect and Alogia, and a control subscale: Distress). All the items are rated on a 7-point (0–6) scale, thus ranging from absent (0) to moderate (3) to extremely severe (6) symptoms (except distress for which the severity rating is reversed: 0 normal distress and 6 absent). A total score was calculated by summing the 13 individual items (possible score ranging from 0 to 78); subscale scores were calculated by summing the individual items within each subscale. With regard the two domains, Avolition-apathy was computed by summing the scores on the subscales Anhedonia (consummatory and anticipatory anhedonia), Avolition, Asociality, with higher scores indicating greater Avolition-apathy (Kirkpatrick et al., 2011). Expressive deficit was computed by summing the scores on the subscales Blunted Affect and Alogia (Kirkpatrick et al., 2011).
Patients were also administered the Positive and Negative Syndrome Scale (Kay et al., 1987). According to the five-factor model proposed by Wallwork et al. (2012), we computed the positive dimension by summing the scores on the items “Delusions” (P1), “Hallucinatory behavior” (P3), “Grandiosity” (P5) and “Unusual thought content” (G9); the disorganization dimension was made up of three PANSS items: “Conceptual disorganization” (P2), “Difficulty in abstract thinking” (N5), and “Poor attention” (G11) and, finally, the negative dimension was assessed by summing the scores on the items “Blunted Affect” (N1), “Emotional Withdrawal” (N2), “Poor Rapport” (N3), “Passive/Apathetic Social Withdrawal” (N4) and “Lack of Spontaneity” (N6). All items are rated on a 7-point scale from 1 to 7, ranging from absent (1) to moderate (4) to extremely severe (7).
We also assessed depressive symptoms using the Calgary Depression Scale for Schizophrenia (CDSS) (Addington et al., 2014) and extrapyramidal symptoms using the St. Hans Rating Scale (SHRS) for Extrapyramidal Syndromes (Gerlach et al., 1993).
2.3. Recording procedure
EEGs were recorded using two highly comparable EEG recording systems: EASYS2 (Brainscope, Prague) and Galileo MIZAR-sirius (EBNeuro, Florence). Before starting the study, a harmonization of the amplifier settings and recording procedure was carried out to ensure the same settings in all centers.
All EEGs were recorded using a cap electrode system with 29 unipolar leads (Fpz, Fz, Cz, Pz, Oz, F3, F4, C3, C4, FC5, FC6, P3, P4, O1, O2, Fp1, Fp2, F7, F8, T3, T4, T5, T6, AF3, AF4, PO7, PO8, RM, LM), placed following the 10–20 system (American Electroencephalographic Society Guidelines in Electroencephalography, 1994). All leads were referenced to linked earlobes (a resistor of 10 kΩ was interposed between the earlobe leads). A ground electrode was placed on the forehead.
A horizontal electro-oculogram (HEOG) was recorded from the epicanthus of each eye, and a vertical EOG (VEOG) from the leads beneath and above the right eye for artifact monitoring. All impedances of the leads were kept below 5 kΩ.
The EEG data were filtered with a band-pass of 0.15–70 Hz and recorded with a sampling rate of 512 Hz. A 50 Hz notch filter was used. A calibration was performed for all channels, using a 50 μV sine wave, before each recording session.
For each recording, subjects were seated in a reclining chair, in a sound attenuated room and were asked to relax and sit comfortably while upright with eyes closed, minimizing eye movement or muscle tension. EEG was continuously recorded for 5 min while the subjects were at rest with closed eyes.
Participants were instructed not to drink coffee, tea and abstain from smoking cigarettes in the 2 hours before the beginning of the recording session and not to take the psychotropic drugs during the morning. Information on the quality of sleep during the night prior to the recording was collected and the EEG session was postponed if the subject reported a non-restoring sleep.
2.4. Data pre-processing
Off-line analysis was performed by a single expert (G. M. G.) from the coordinating center (Naples) using Brain Vision Analyzer software (Brain Products, Munich, Germany). Data with high line noise were excluded from further analysis. Eye movements and eye blinks were corrected using independent component analysis (Delorme et al., 2007). We did not use the interpolation for bad or artefactual channels. Body movements, muscle activity and technical artifacts were discarded through visual inspection during centralized artefacting. The data were parsed into epochs of 2-s duration, digitally filtered from 2 to 20 Hz and recomputed to the average reference.
2.5. Microstates analysis
The microstate analysis was performed using an in-house plugin for the Brain Vision Analyzer.
We decided to extract the individual MS parameters using an existing set of grand mean MS map templates (Fig. 1), which was extracted from a very large sample of subjects (Koenig et al., 2002). This methodological approach is justified by the fact that a recent review of the literature on MS analysis has revealed general consistencies among studies for MS classes A, B and D, but some discrepancy in class C (Michel and Koenig, 2017). The main discrepancy was in the occipital or parietal focus of the posterior pole. One study (Custo et al., 2017), which did not impose the 4-MS limit of previous studies, reported two separate MSs, one with the occipital pole (MS-C) and the other with the parietal pole (MS-F). The study also reported a very high spatial correlation between the two MSs (r = 0.70). Thus, acknowledging that in studies imposing the 4 MS limit, the two spatially-correlated MSs (C and F in their study) are combined in a single MS-C (which might retain either the parietal or the occipital focus of the posterior pole). Furthermore, Custo's et al. (2017) MS-F showed an association with the same neural network reported for MS-C by Britz et al. (2010) and MS-C of Custo et al. (2017) showed an association with a network belonging to the intrinsic system, i.e., the same functional system of the network associated with MS-C in the studies conducted by Britz et al., 2010 and Milz et al., 2016. In studies on schizophrenia, the two topographic variants were equally represented in different studies, independently of the number of channels used, variance explained or sample size of the individual studies (Michel and Koenig, 2017). However, all these studies had limited sample sizes and used quite heterogeneous MS analyses.
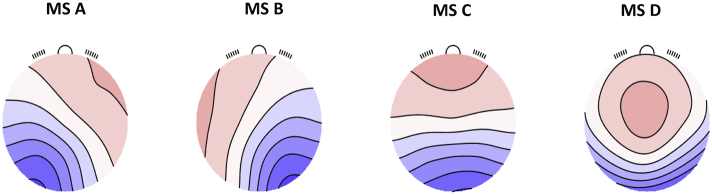
Fig. 1.
Microstate normative maps.
MS-A, microstate A; MS-B, microstate B; MS-C, microstate C; MS-D, microstate D.
Therefore, in the light of this heterogeneity, we decided to use the MS map templates from the normative study (Koenig et al., 2002), which for MS-C might represent a more reliable combination of the two topographies, as it was extracted in the largest sample of subjects (N = 496).
Individual EEG data were spatially down-sampled to 19 channels and all maps at momentary GFP peaks were assigned to the microstate template maps using a best-fit criterion. Finally, the assignment of the time-points between GFP peaks was obtained using a nearest neighbor interpolation.
MSs were then quantified for each class in terms of their relative time contribution (percentage of time covered by a given MS class), duration (average continuous time period in ms covered by a given MS class), and occurrence (number of distinct microstates of a given MS class occurring within a 1 s window).
To check for eventual influence of used template maps on main results, we also performed control analyses, in which the data-driven approach was adopted to identify the 4 MS template maps, using either the whole sample or the SCZ data. The methodology and results of the control analyses are reported in the Supplemental Materials (paragraph 1, Fig. S2 and S3, Tables S5-S7; paragraph 2, Table S8).
2.6. Statistical analyses
Statistics were computed using SPSS Version 22.0 (IBM Corporation, 2014). Analysis of variance (ANOVA) was used to test group differences on continuous variables. Group differences in sex distribution were assessed by the Pearson's χ2 test.
With regard to MS parameters, we focused only on contribution and duration as the occurrence is calculated as the ratio between the latter two parameters. Therefore, to test group differences between SCZ and HC on MS parameters, we performed a repeated measure multivariate analysis of variance (MANOVA), with MS parameters (contribution and duration) and MS classes (MS-A, MS-B, MS-C, MS-D) as within-subject factors and diagnosis (SCZ and HC) as between subjects factor. The Huynh-Feldt correction for multiple comparisons was applied. Follow-up univariate ANOVAs for investigation of simple effects were carried out only when significant group main effects or interactions were found in the MANOVA.
Associations of negative symptoms with MS parameters were assessed using Pearson's correlation coefficients. Only when a significant correlation of BNSS total score with MS measures (contribution and duration) was observed, correlations of the same parameters with the two negative symptom domains (Avolition-apathy and Expressive deficit) and their component symptoms were further assessed.
Since positive symptoms, depression and extrapyramidal symptoms might influence the relationship between MS measures and negative symptoms, correlations between these variables were also examined using partial correlation analyses, controlling for PANSS positive score, CDSS total scores and Parkinsonism global score derived from SHRS.
3. Results
3.1. Subject characteristics
One hundred and forty-eight SCZ and seventy HC were originally enrolled in the study. However, six patients and six healthy controls were removed from the final study sample as outliers for MS measures as identified by using the SPSS outlier detection program: they showed MS parameter values with deviation (positive) from the third quartile or (negative) from the first quartile >3 times the interquartile range.
Therefore, one hundred and forty-two SCZ and sixty-four HC were included in the group-level analysis.
Table 1 summarizes demographic, psychometric and clinical characteristics of HC and SCZ. There was no significant group difference in sex (χ2 = 3.20; p = .07) and age (F = 0.36; p = .55), but patients had significantly lower education level than controls (F = 7.97; p = .005). SCZ were characterized by absent to mild severity of both positive and disorganization dimensions (PANSS mean dimension score < 9 for both) and mild to moderate severity of the negative symptoms (PANSS negative dimension mean score of 15.69 and BNSS total score of 33.05). They had a low mean level of depression (CDSS total score < 4) and of parkinsonism (SHRS Parkinsonism score < 1).
Table 1.
Demographic and clinical characteristics of the study sample.
| HC (N = 64) |
SCZ (N = 142) |
F/χ2 |
p |
|
|---|---|---|---|---|
| Demographic and clinical information | ||||
| Gender (M/F) | 34/30 | 94/48 | 3.20 | 0.07 |
| Age (years, mean ± SD) | 35.73 ± 12.95 | 36.71 ± 9.6 | 0.36 | 0.55 |
| Education (years, mean ± SD) | 13.67 ± 3.79 | 12.27 ± 3.03 | 7.97 | 0.005 |
| BNSS total (mean ± SD) | 33.05 ± 14.1 | |||
| Expressive deficit (mean ± SD) | 11.84 ± 7.51 | |||
| Blunted Affect (mean ± SD) | 7.68 ± 4.92 | |||
| Alogia (mean ± SD) | 4.16 ± 3.20 | |||
| Avolition-apathy (mean ± SD) | 18.75 ± 8.56 | |||
| Anticipatory Anhedonia (mean ± SD) | 3.03 ± 1.52 | |||
| Consummatory Anhedonia (mean ± SD) | 2.96 ± 1.51 | |||
| Avolition(mean ± SD) | 6.16 ± 2.90 | |||
| Asociality (mean ± SD) | 6.6 ± 2.63 | |||
| PANSS Positive (mean ± SD) | 8.38 ± 4.56 | |||
| PANSS Negative (mean ± SD) | 15.69 ± 6.22 | |||
| PANSS Disorganization (mean ± SD) | 8.56 ± 3.52 | |||
| CDSS total (mean ± SD) | 3.9 ± 4.36 | |||
| SHRS Global Parkinsonism (mean ± SD) | 0.91 ± 1.17 | |||
HC, healthy controls; SCZ, individuals with schizophrenia; BNSS, Brief Negative Symptom Scale; PANSS, Positive and Negative Syndrome Scale; CDSS, Calgary Depression Scale for Schizophrenia; SHRS, St. Hans Rating Scale.
Additional clinical information concerning SCZ sample are reported in the Supplementary Materials (Table S1).
3.2. Group comparison on EEG parameters
3.2.1. Data quality
There was no difference in the number of 2-second epochs included in the analysis after exclusion of those with artifacts (F = 0.009; p = .93) between HC and SCZ (means±SD, 132.75 ± 29.46 and 133.18 ± 30.66, respectively). In addition, there was no recording site effect on the number of included 2-second epochs for both HC (F = 0.147; p = .864) and SCZ (F = 1.133; p = .325).
3.2.2. Group differences on microstate parameters
The four MS classes explained 66% of the variance in the whole sample.
Repeated measure MANOVA showed an interaction group x MS parameters x MS class (F[1204] = 3.44, p < .03), due to differences in the contribution (F[1204] = 6.91; p < .009; effect size = 0.4) and duration (F[1204] = 5.88; p < .016; effect size = 0.37) of MS-C between HC and SCZ (Fig. 2). Complete results of the MANOVA are reported in the supplementary materials (Table S2). Group means and standard deviations for all considered parameters and MS classes are reported in Table S3, together with post-hoc univariate test results.
Fig. 2.
Group comparison on the microstate C parameters.
This figure illustrates that the procedure results from fitting 4 template maps to the data. The between group comparison indicates which parameters of the four maps (2 A, contribution; 2 B, duration) are significantly different between SCZ and HC. This is particularly relevant for the contribution and duration of MS-C.
*A: SCZ, in comparison to HC, showed increased contribution of MS-C (p < .009).
*B: SCZ, in comparison to HC, showed increased duration of MS-C (p < .016).
HC, healthy controls; SCZ, individuals with schizophrenia.
Error bars represent standard error.
In addition, as there are more males in the patient group, we carried out a control analysis, using sex as covariate and we included the results in the supplementary materials (Table S4).
3.3. Relationship between microstate measures and negative symptoms
BNSS total score was significantly correlated with contribution of MS A (Table 2).
Table 2.
Correlations between microstate A contribution and negative symptoms in individuals with schizophrenia.
| Brief Negative Symptom Scale | Pearson's r | p-value |
|---|---|---|
| BNSS total score | 0.19 | 0.03 |
| Avolition-apathy | 0.22 | 0.01 |
| Consummatory Anhedonia | 0.13 | 0.13 |
| Anticipatory Anhedonia | 0.20 | 0.02 |
| Avolition | 0.20 | 0.02 |
| Asociality | 0.25 | 0.003 |
| Expressive Deficit | 0.12 | 0.15 |
BNSS, Brief Negative Symptom Scale.
The contribution of microstate A was positively correlated with BNSS total score, Avolition-apathy domain, anticipatory anhedonia, avolition and asociality.
These correlations remained significant after controlling for the effects of PANSS positive, CDSS total score and SHRS Parkinsonism Global score (BNSS total score: r = 0.17, p = .05; Avolition-apathy: r = 0.2, p = .02; anticipatory anhedonia: r = 0.18, p = .04; avolition: r = 0.18, p = .035; asociality: r = 0.24, p = .006).
Bold p-values are those statistically significant.
The two negative symptom domains showed a different pattern: while the Avolition-apathy domain correlated positively with the contribution of MS-A, no correlation was found for the Expressive deficit domain (Table 2; Fig. S1).
Since there was a significant correlation between the Avolition-apathy domain and the MS-A, we computed separate correlations between component symptoms of Avolition-apathy (consummatory and anticipatory anhedonia, avolition and asociality) and the contribution of MS-A. We found that within the Avolition-apathy domain, anticipatory anhedonia, avolition and asociality, but not consummatory anhedonia, were positively correlated with contribution of MS-A (Table 2). These correlations remained significant after controlling for the effects of PANSS positive, CDSS total score and SHRS Parkinsonism Global score (BNSS total score: r = 0.17, p = .05; Avolition-apathy: r = 0.2, p = .02; anticipatory anhedonia: r = 0.18, p = .04; avolition: r = 0.18, p = .035; asociality: r = 0.24, p = .006).
4. Discussion
The present study had two primary aims: 1) to identify differences between healthy controls and clinically stable individuals with schizophrenia in brain electrical MS parameters and 2) to investigate the associations of the MS parameters with the Avolition-apathy domain and its component symptoms, as assessed by a second-generation rating scale, providing optimal assessment of consummatory and anticipatory anhedonia.
The main results of our study included: 1) an increased contribution and duration of MS-C in individuals with schizophrenia, compared to healthy controls, 2) a relationship between negative symptoms, assessed by BNSS scale, with contribution of MS A, 3) a positive correlation between Avolition-apathy, but not expressive deficit, with contribution of MS-A, and 4) a positive correlation between anticipatory anhedonia, avolition and asociality, but not consummatory anhedonia with the contribution of MS-A.
Our results, in line with earlier studies (Koenig et al., 1999; Lehmann et al., 2005; Kikuchi et al., 2007; Nishida et al., 2013), demonstrated that MS of class C covers more of the total time in individuals with schizophrenia. In addition, Tomescu et al. (2014) reported that adolescents with 22q11.2 deletion syndrome, with higher risk to develop schizophrenia, showed an increased contribution, occurrence and duration of MS-C, as compared to healthy controls. The increased duration of MS-C was associated to prodromal psychotic symptoms and was proposed as a potential vulnerability marker to develop schizophrenia. This hypothesis was confirmed by a subsequent study in which the authors found the same abnormalities of MS-C in adolescents with 22q11.2 deletion syndrome and in individuals with schizophrenia (Tomescu et al., 2015).
Furthermore, Koenig et al. (2002), in their across-age study, demonstrated that MS-C was more present during late adolescence, the typical age of onset of schizophrenia symptoms, while decreasing during adulthood, suggesting that its increase in schizophrenia might be related to neurodevelopmental aberrations. MS-C increase has been reported when connections to contextual information are loosened: during reduced attention states (Brandeis and Lehmann, 1989), psychosis (Rieger et al., 2016), sleep (Brodbeck et al., 2012), reduced bodily perception states (Pipinis et al., 2017) and hypnosis (Katayama et al., 2007), conditions that might be associated with an altered experience of salience. Furthermore, a recent study using simultaneous EEG and fMRI recording has suggested that MS-C reflects the cerebral activity in the posterior part of the anterior cingulate cortex, bilateral inferior frontal gyri, the right anterior insula and the left claustrum (Britz et al., 2010), regions that have been found to be part of the salience network (Fox et al., 2006; Mantini et al., 2007; Seeley et al., 2007). Salience has been linked to the capacity in selecting internal or external stimuli deserving our attention, and aberrant salience attribution has been reported in individuals with schizophrenia (Menon and Uddin, 2010; Palaniyappan and Liddle, 2012).
In our study we did not find any consistent effect in MS of class A, B and D.
Contrary to the literature, we did not find a reduced duration of MS-D in SCZ as compared to HC (Rieger et al., 2016). The reduced duration of MS-D has been previously found to correlate with paranoid-hallucinatory symptomatology (Koenig et al., 1999), and with the acute experience of hallucinations (Kindler et al., 2011), in drug-free individuals with schizophrenia. Differences between our results and previous studies with regard to MS-D characteristics in individuals with schizophrenia might be explained by the inclusion in our study of a sample of chronic, clinically stable, medicated individuals with schizophrenia, in which more than half of the subjects had remitted hallucinatory-paranoid and disorganized symptomatology. Our results suggest that the heterogeneity of the clinical characteristics of included samples might explain discrepant findings in the literature. Discrepant findings in our study concerning the MS-D could also result from the different methodological approaches, as previous studies that reported increased MS-C and decreased MS-D used data-driven template maps, rather than the normative templates. However, the confirmation of the increased duration and contribution of MS-C argues against a role of the MS analysis method in explaining the inconsistencies. Furthermore, in our control analysis in which we compared the two groups using the data-driven approach, we could not replicate the findings concerning MS-D. These considerations argue against the hypothesis that methodological differences might account for the discrepancies concerning MS-D. However, only investigations including both subjects with acute psychotic decompensation and those clinically-stable, as well as the two methods of MS analysis might further clarify the issue.
For the overall view on microstate studies, it is also important to note that the topography of microstate classes C and D has shown a substantial amount of variation across studies, that may at one point have to be systematically scrutinized across the range of existing microstate investigations in schizophrenia.
Our results concerning MS-B and MS-A were expected. Indeed, the small effect size for reduced duration of MS-B reported in a recent meta-analysis, did not survive Bonferroni correction (Rieger et al., 2016), suggesting heterogeneity across studies. The same meta-analysis did not find a consistent group effect for MS-A (Rieger et al., 2016). For this MS, discrepant findings were reported: MS-A was found to occur more frequently (Lehmann et al., 2005; Nishida et al., 2013), to cover more percent of time (Lehmann et al., 2005) and to have a reduced duration (Strelets et al., 2003; Nishida et al., 2013) in individuals with schizophrenia as compared to healthy controls. In our study, no difference on MS-A parameters between individuals with schizophrenia and healthy controls was observed, although we found a correlation between MS-A contribution and Avolition-apathy. Our results might help understanding the discrepancies in the literature findings concerning this microstate. In fact, negative symptoms, even those which have the highest frequency, such as Avolition-apathy, are generally present at a clinically-significant level in about one half of the patient population. When comparing the whole group of patients with healthy controls, the deviance may not be statistically detectable due to the heterogeneity of the patient sample with respect to negative symptoms. Therefore, the discrepancy in the different studies concerning this MS might be due to differences in the frequency and severity of negative symptoms in included samples.
Our study demonstrated a relationship of MS-A contribution with the negative symptom domain of Avolition-apathy which, as documented by partial correlation analysis, was not mediated by positive symptoms, extrapyramidal symptoms or depression, frequently causing secondary negative symptoms. No correlation was found between the Expressive deficit domain and MS-A. These results support the view that, in individuals with schizophrenia, Avolition-apathy and Expressive deficit have different neurobiological underpinnings (Galderisi et al., 2013a; Kirkpatrick, 2014b; Mucci et al., 2015; Kaiser et al., 2016; Kirkpatrick et al., 2017; Mucci et al., 2017). The findings were not biased by the type of microstate analysis used in our study, as they were replicated in control analyses using two further methods, i.e., fitting to the patient data the template maps derived by combining data of our healthy control and patient groups or using data from the patient group alone.
With regard to component symptoms, within Avolition-apathy, we found that only avolition, asociality and anticipatory anhedonia, but not consummatory anhedonia correlated with MS-A. These findings lend support to the hypothesis that only anticipatory anhedonia might be linked to the Avolition-apathy domain of negative symptoms. Anticipatory pleasure is important to formulate, maintain and complete goals (Barch and Dowd, 2010; Simpson et al., 2012). Research has shown the so called “emotion paradox” in individuals with schizophrenia (Pizzagalli, 2010; Strauss, 2013). In fact, they have apparently intact affective responses to stimuli that produce hedonistic experiences in the present but they are impaired in generating representations of hedonic values for past (e.g., report of experienced consummatory pleasure) or future experiences (anticipatory pleasure) and in integrating information on value with the selection of an action (Gard et al., 2007; Heerey and Gold, 2007; Heerey et al., 2007; Waltz et al., 2007; Kring and Moran, 2008; Barch and Dowd, 2010; Cohen and Minor, 2010; Foussias and Remington, 2010; Pizzagalli, 2010; Dowd and Barch, 2012; Simpson et al., 2012; Mann et al., 2013; Strauss, 2013; Morris et al., 2015; Mucci et al., 2015). In our sample, individuals with schizophrenia had comparable levels of anticipatory and consummatory anhedonia in terms of means and standard deviations, and percentages of moderate to severe scores (67% for anticipatory and 61% for consummatory anhedonia). However, only anticipatory anhedonia was found to correlate with the same electrophysiological marker as avolition-apathy. The lack of correlation of consummatory anhedonia with MS-A contribution does not seem to be due to reduced frequency or severity of this symptom in our sample. Future replications of these results might shed further light on the apparent “emotion paradox” in schizophrenia. In fact, the clinical assessment of both consummatory and anticipatory anhedonia is dependent on the ability of patients to generate hedonic representations (for the past and future experiences, respectively) which might be influenced by cognitive dysfunctions of the subjects (Strauss, 2013). The different pattern of correlation with MS-A temporal parameters strongly suggests a substantial proportion of non-shared variance between the two types of anhedonia.
The occurrence of both consummatory and anticipatory anhedonia might be due to the presence of depression. However, our sample did not show clinically-significant levels of depression (as documented by the low CDSS mean score) which might confound the correlational results and the assessment of negative symptoms. Furthermore, partialling out the effect of depression, the correlation between increased MS-A contribution and anticipatory anhedonia remained significant. Previous findings on MS temporal parameters in individuals with depression reported decreased overall MS duration (Strik et al., 1995), but the authors did not investigate the symptom correlates of the abnormality. In future analyses it could be of interest to carry out a study on the electrophysiological correlates of anhedonia in subjects with depression.
The clinical implications of the relationship between MS-A and Avolition-apathy are still speculative, as the functional role of this MS is not fully understood. However, two studies (Britz et al., 2010; Milz et al., 2016) contributed to characterize this MS as the one related to the RSN devoted to extrinsic processing, i.e., to the M2 system according to Doucet et al. (2011). This network is involved in processing of signals from the external world (Doucet et al., 2011; Orliac et al., 2017). In fact, the study by Britz et al. (2010) reported that MS of class A correlated with cerebral activity in the bilateral superior and middle temporal gyri, regions of the phonological network, as well as in primary visual areas (Damoiseaux et al., 2006; Mantini et al., 2007), which are core regions of M2 (Doucet et al., 2011). The more recent study by Milz et al. (2016) reported an increase of MS-A parameters during visualization, establishing a possible link to core connectivity patterns included in the M2b. For the other MS classes, Britz et al. (2010) found associations with networks belonging to the M1 or intrinsic processing module (as reported above, MS-C was associated with core regions of the salience circuit and MS-D with those of the attentional network).
The association of Avolition-apathy with MS-A might indicate a relationship of this domain of negative symptoms with sensory processing abnormalities. These abnormalities have been described in individuals with schizophrenia and are related to functional outcome (Green et al., 1994; Rassovsky et al., 2004; Green et al., 2012). Importantly, this relationship was mediated by social perception (Rassovsky et al., 2004) and by negative symptoms (Rassovsky et al., 2011; Green et al., 2012). Within negative symptoms, anhedonia, asociality and avolition, rather than blunted affect and alogia, significantly predicted the functional outcome and explained the relationship between early visual processing and functioning (Rassovsky et al., 2011). In the light of these observations, our results seem to confirm the relationship between sensory processing deficits and the Avolition-apathy domain.
The main strengths of the present study included the use of the resting state MS and the assessment of negative symptom domains using the BNSS scale.
Certain limitations of this study should be taken into account. Contrary to other studies, our analyses were based on previously published normative MS templates rather than on templates obtained from the dataset itself, to overcome the problem of the topographic heterogeneity of the MS-C template across studies in individuals with SCZ. The use of different templates might influence the results and all the studies were limited by small sample sizes. We explained in the methods section why we decided to use the template maps. However, the new method is not uncontroversial and points to a larger problem in the EEG MS literature in general. Since the MS-C template maps vary considerably across studies, implying that the overlap of their functional correlates may only be partial, future studies on EEG MS should provide the employed template maps, so that other studies can test and compare the functional conclusions that they yield. However, we can exclude an influence of the template maps used on our results as they were confirmed in a control analysis on both MS parameters differences between groups and in their clinical correlates.
In addition, age and pharmacological treatment might have an impact on MS measures. In our study, we used a sample in which subjects were matched for age: therefore, we could exclude the effect of age on the differences between healthy controls and individuals with schizophrenia.
With regard to medication, the typical pattern of MS abnormalities in individuals with schizophrenia has been reported both before and after treatment with antipsychotics (Kikuchi et al., 2007). Therefore, our results concerning the differences between individuals with schizophrenia and healthy controls on MS-C do not seem to be related to pharmacological treatment. Moreover, we excluded the confounding effect of medication on correlation between MS-A contribution and Avolition-apathy, using partial correlation analysis in which we controlled for extrapyramidal symptoms that might cause secondary negative symptoms.
5. Conclusions
In conclusion, in line with previous studies, our results suggested that chronic individuals with schizophrenia have an increased contribution and duration of MS of class C.
In addition, for the first time, we report a correlation of MS-A contribution with the Avolition-apathy but not with the Expressive deficit domain of negative symptoms. These results are in line with those reporting the existence of different neurophysiological correlates of the two negative symptom domains. Within the Avolition-apathy domain only the anticipatory component of anhedonia, shares the same neurophysiological correlates of this domain.
Given the association of MS-A with the extrinsic resting state networks, the correlation between this MS and the Avolition-apathy domain suggests that motivational deficits in schizophrenia might be subtended by sensory processing abnormalities, in line with other literature findings (Green et al., 2012). Further studies, using simultaneous EEG and functional magnetic resonance imaging, might confirm the relationship between MS-A and extrinsic resting state networks.
If our findings will be replicated, the detection of MS-A abnormalities could help in stratifying patients to reduce clinical heterogeneity for treatment development.
Role of funding source
The study was funded by the Italian Ministry of Education (grant number: 2010XP2XR4), the Italian Society of Psychopathology (SOPSI), the Italian Society of Biological Psychiatry (SIPB), Roche, Lilly, AstraZeneca, Lundbeck and Bristol-Myers Squibb. These entities had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the paper for publication.
Conflict of interest
All authors declared no conflict of interest.
Acknowledgments
Members of the add-on EEG study of the Italian Network for Research on Psychoses participating in this study include: Giuseppe Piegari, Eleonora Merlotti, Giuseppe Maria Plescia, Valentina Montefusco, Olimpia Gallo, Paola Romano (University of Campania “Luigi Vanvitelli”); Raffaella Carnevale, Girolamo Francavilla, Stefania Malerba, Daniele Marasco (University of Foggia); Fabiola Ferrentino, Andrea Daverio, Cinzia Niolu, Michele Ribolsi (University of Rome “Tor Vergata”); Anna Comparelli (University of Rome “Sapienza”); Giulio Corrivetti (Department of Mental Health, Salerno).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.08.031.
Appendix A. Supplementary data
Supplementary material
References
- Addington J., Shah H., Liu L., Addington D. Reliability and validity of the Calgary Depression Scale for Schizophrenia (CDSS) in youth at clinical high risk for psychosis. Schizophr. Res. 2014;153(1–3):64–67. doi: 10.1016/j.schres.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Electroencephalographic Society Guidelines in Electroencephalography, E.P.a.P J. Clin. Neurophysiol. 1994;11:1–147. [PubMed] [Google Scholar]
- Andreasen N.C. Negative symptoms in schizophrenia. Definition and reliability. Arch. Gen. Psychiatry. 1982;39(7):784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Andreasen N.C. The scale for the assessment of negative symptoms (SANS): conceptual and theoretical foundations. Br. J. Psychiatry Suppl. 1989;(7):49–58. [PubMed] [Google Scholar]
- Andreou C., Faber P.L., Leicht G., Schoettle D., Polomac N., Hanganu-Opatz I.L., Lehmann D., Mulert C. Resting-state connectivity in the prodromal phase of schizophrenia: insights from EEG microstates. Schizophr. Res. 2014;152(2–3):513–520. doi: 10.1016/j.schres.2013.12.008. [DOI] [PubMed] [Google Scholar]
- Barch D.M., Dowd E.C. Goal representations and motivational drive in schizophrenia: the role of prefrontal-striatal interactions. Schizophr. Bull. 2010;36(5):919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch D.M., Pagliaccio D., Luking K. Mechanisms underlying motivational deficits in psychopathology: similarities and differences in depression and schizophrenia. Curr. Top. Behav. Neurosci. 2016;27:411–449. doi: 10.1007/7854_2015_376. [DOI] [PubMed] [Google Scholar]
- Berridge K.C. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology. 2007;191(3):391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Bissonette G.B., Roesch M.R. Neurophysiology of reward-guided behavior: correlates related to predictions, value, motivation, errors, attention, and action. Curr. Top. Behav. Neurosci. 2016;27:199–230. doi: 10.1007/7854_2015_382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard J.J., Kring A.M., Horan W.P., Gur R. Toward the next generation of negative symptom assessments: the collaboration to advance negative symptom assessment in schizophrenia. Schizophr. Bull. 2011;37(2):291–299. doi: 10.1093/schbul/sbq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E. International Universities Press; 1959. Dementia Praecox or the Group of Schizophrenias. [Google Scholar]
- Bora E., Yucel M., Pantelis C. Cognitive functioning in schizophrenia, schizoaffective disorder and affective psychoses: meta-analytic study. Br. J. Psychiatry. 2009;195(6):475–482. doi: 10.1192/bjp.bp.108.055731. [DOI] [PubMed] [Google Scholar]
- Bora E., Yucel M., Pantelis C. Cognitive impairment in schizophrenia and affective psychoses: implications for DSM-V criteria and beyond. Schizophr. Bull. 2010;36(1):36–42. doi: 10.1093/schbul/sbp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandeis D., Lehmann D. Segments of event-related potential map series reveal landscape changes with visual attention and subjective contours. Electroencephalogr. Clin. Neurophysiol. 1989;73(6):507–519. doi: 10.1016/0013-4694(89)90260-5. [DOI] [PubMed] [Google Scholar]
- Britz J., Van De Ville D., Michel C.M. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. NeuroImage. 2010;52(4):1162–1170. doi: 10.1016/j.neuroimage.2010.02.052. [DOI] [PubMed] [Google Scholar]
- Brodbeck V., Kuhn A., von Wegner F., Morzelewski A., Tagliazucchi E., Borisov S., Michel C.M., Laufs H. EEG microstates of wakefulness and NREM sleep. NeuroImage. 2012;62(3):2129–2139. doi: 10.1016/j.neuroimage.2012.05.060. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin E.S., Matsumoto M., Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–834. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman L.J., Chapman J.P., Raulin M.L. Scales for physical and social anhedonia. J. Abnorm. Psychol. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Cohen A.S., Minor K.S. Emotional experience in patients with schizophrenia revisited: meta-analysis of laboratory studies. Schizophr. Bull. 2010;36(1):143–150. doi: 10.1093/schbul/sbn061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custo A., Van De Ville D., Wells W.M., Tomescu M.I., Brunet D., Michel C.M. Electroencephalographic Resting-State Networks: Source Localization of Microstates. Brain Connect. 2017;7(10):671–682. doi: 10.1089/brain.2016.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Sejnowski T., Makeig S. Enhanced detection of artifacts in EEG data using higher-order statistics and independent component analysis. NeuroImage. 2007;34(4):1443–1449. doi: 10.1016/j.neuroimage.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet G., Naveau M., Petit L., Delcroix N., Zago L., Crivello F., Jobard G., Tzourio-Mazoyer N., Mazoyer B., Mellet E., Joliot M. Brain activity at rest: a multiscale hierarchical functional organization. J. Neurophysiol. 2011;105(6):2753–2763. doi: 10.1152/jn.00895.2010. [DOI] [PubMed] [Google Scholar]
- Dowd E.C., Barch D.M. Anhedonia and emotional experience in schizophrenia: neural and behavioral indicators. Biol. Psychiatry. 2010;67(10):902–911. doi: 10.1016/j.biopsych.2009.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd E.C., Barch D.M. Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS One. 2012;7(5) doi: 10.1371/journal.pone.0035622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foussias G., Remington G. Negative symptoms in schizophrenia: avolition and Occam's razor. Schizophr. Bull. 2010;36(2):359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M.D., Corbetta M., Snyder A.Z., Vincent J.L., Raichle M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 2006;103(26):10046–10051. doi: 10.1073/pnas.0604187103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi S., Maj M., Mucci A., Cassano G.B., Invernizzi G., Rossi A., Vita A., Dell'Osso L., Daneluzzo E., Pini S. Historical, psychopathological, neurological, and neuropsychological aspects of deficit schizophrenia: a multicenter study. Am. J. Psychiatry. 2002;159(6):983–990. doi: 10.1176/appi.ajp.159.6.983. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Davidson M., Kahn R.S., Mucci A., Boter H., Gheorghe M.D., Rybakowski J.K., Libiger J., Dollfus S., Lopez-Ibor J.J., Peuskens J., Hranov L.G., Fleischhacker W.W., group, E Correlates of cognitive impairment in first episode schizophrenia: the EUFEST study. Schizophr. Res. 2009;115(2–3):104–114. doi: 10.1016/j.schres.2009.09.022. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Bucci P., Mucci A., Kirkpatrick B., Pini S., Rossi A., Vita A., Maj M. Categorical and dimensional approaches to negative symptoms of schizophrenia: focus on long-term stability and functional outcome. Schizophr. Res. 2013;147(1):157–162. doi: 10.1016/j.schres.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Mucci A., Bitter I., Libiger J., Bucci P., Fleischhacker W.W., Kahn R.S., Eufest Study G. Persistent negative symptoms in first episode patients with schizophrenia: results from the European first Episode Schizophrenia Trial. Eur. Neuropsychopharmacol. 2013;23(3):196–204. doi: 10.1016/j.euroneuro.2012.04.019. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Merlotti E., Mucci A. Neurobiological background of negative symptoms. Eur. Arch. Psychiatry Clin. Neurosci. 2015;265(7):543–558. doi: 10.1007/s00406-015-0590-4. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Farden A., Kaiser S. Dissecting negative symptoms of schizophrenia: history, assessment, pathophysiological mechanisms and treatment. Schizophr. Res. 2016;186:1–2. doi: 10.1016/j.schres.2016.04.046. [DOI] [PubMed] [Google Scholar]
- Galderisi S., Mucci A., Buchanan R.W., Arango C. Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry. 2018;5(8):P664–P677. doi: 10.1016/S2215-0366(18)30050-6. [DOI] [PubMed] [Google Scholar]
- Gard D.E., Kring A.M., Gard M.G., Horan W.P., Green M.F. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 2007;93(1–3):253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach J., Korsgaard S., Clemmesen P., Lauersen A.M., Magelund G., Noring U., Povlsen U.J., Bech P., Casey D.E. The St. Hans Rating Scale for extrapyramidal syndromes: reliability and validity. Acta Psychiatr. Scand. 1993;87(4):244–252. doi: 10.1111/j.1600-0447.1993.tb03366.x. [DOI] [PubMed] [Google Scholar]
- Gold J.M., Waltz J.A., Matveeva T.M., Kasanova Z., Strauss G.P., Herbener E.S., Collins A.G., Frank M.J. Negative symptoms and the failure to represent the expected reward value of actions: behavioral and computational modeling evidence. Arch. Gen. Psychiatry. 2012;69(2):129–138. doi: 10.1001/archgenpsychiatry.2011.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M.F., Nuechterlein K.H., Mintz J. Backward masking in schizophrenia and mania. II. Specifying the visual channels. Arch. Gen. Psychiatry. 1994;51(12):945–951. doi: 10.1001/archpsyc.1994.03950120017004. [DOI] [PubMed] [Google Scholar]
- Green M.F., Kern R.S., Braff D.L., Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr. Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Green M.F., Hellemann G., Horan W.P., Lee J., Wynn J.K. From perception to functional outcome in schizophrenia: modeling the role of ability and motivation. Arch. Gen. Psychiatry. 2012;69(12):1216–1224. doi: 10.1001/archgenpsychiatry.2012.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey P.D., Heaton R.K., Carpenter W.T., Jr., Green M.F., Gold J.M., Schoenbaum M. Functional impairment in people with schizophrenia: focus on employability and eligibility for disability compensation. Schizophr. Res. 2012;140(1–3):1–8. doi: 10.1016/j.schres.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heerey E.A., Gold J.M. Patients with schizophrenia demonstrate dissociation between affective experience and motivated behavior. J. Abnorm. Psychol. 2007;116(2):268–278. doi: 10.1037/0021-843X.116.2.268. [DOI] [PubMed] [Google Scholar]
- Heerey E.A., Robinson B.M., McMahon R.P., Gold J.M. Delay discounting in schizophrenia. Cogn. Neuropsychiatry. 2007;12(3):213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs R.W., Zakzanis K.K. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Kring A.M., Blanchard J.J. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr. Bull. 2006;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser S., Lyne J., Agartz I., Clarke M., Morch-Johnsen L., Faerden A. Individual negative symptoms and domains - Relevance for assessment, pathomechanisms and treatment. Schizophr. Res. 2017, Aug;186:39–45. doi: 10.1016/j.schres.2016.07.013. [DOI] [PubMed] [Google Scholar]
- Katayama H., Gianotti L.R., Isotani T., Faber P.L., Sasada K., Kinoshita T., Lehmann D. Classes of multichannel EEG microstates in light and deep hypnotic conditions. Brain Topogr. 2007;20(1):7–14. doi: 10.1007/s10548-007-0024-3. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Khanna A., Pascual-Leone A., Michel C.M., Farzan F. Microstates in resting-state EEG: current status and future directions. Neurosci. Biobehav. Rev. 2015;49:105–113. doi: 10.1016/j.neubiorev.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi M., Koenig T., Fau - Wada Y., Wada Y., Fau - Higashima M., Higashima M., Fau - Koshino Y., Koshino Y., Fau - Strik W., Strik W Fau - Dierks T., Dierks T. Native EEG and treatment effects in neuroleptic-naive schizophrenic patients: time and frequency domain approaches. Schizophr. Res. 2007;97(1-3):163–172. doi: 10.1016/j.schres.2007.07.012. (0920–9964 (Print)) [DOI] [PubMed] [Google Scholar]
- Kimhy D., Yale S., Goetz R.R., McFarr L.M., Malaspina D. The factorial structure of the schedule for the deficit syndrome in schizophrenia. Schizophr. Bull. 2006;32(2):274–278. doi: 10.1093/schbul/sbi064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler J., Hubl D Fau - Strik W.K., Strik Wk Fau - Dierks T., Dierks T., Fau - Koenig T., Koenig T. Resting-state EEG in schizophrenia: auditory verbal hallucinations are related to shortening of specific microstates. Electronic. 2011:1872–8952. doi: 10.1016/j.clinph.2010.10.042. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Kuginuki T., Yagyu T., Saito N., Hirota T., Saito M. Spatial EEG field configuration in schizophrenics. Psychiatry Res. Neuroimaging. 1998;83(58) [Google Scholar]
- Kirkpatrick B. Developing concepts in negative symptoms: primary vs secondary and apathy vs expression. J. Clin. Psychiatry. 2014;75(Suppl. 1):3–7. doi: 10.4088/JCP.13049su1c.01. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B. Progress in the study of negative symptoms. Schizophr. Bull. 2014;40(Suppl. 2):S101–S106. doi: 10.1093/schbul/sbt158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B., Fischer B. Subdomains within the negative symptoms of schizophrenia: commentary. Schizophr. Bull. 2006;32(2):246–249. doi: 10.1093/schbul/sbj054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B., Strauss G.P., Nguyen L., Fischer B.A., Daniel D.G., Cienfuegos A., Marder S.R. The brief negative symptom scale: psychometric properties. Schizophr. Bull. 2011;37(2):300–305. doi: 10.1093/schbul/sbq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick B., Mucci A., Galderisi S. Primary, enduring negative symptoms: an update on research. Schizophr. Bull. 2017;43(4):730–736. doi: 10.1093/schbul/sbx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M., Aleman A., Kaiser S. Secondary negative symptoms - a review of mechanisms, assessment and treatment. Schizophr. Res. 2017;186:29–38. doi: 10.1016/j.schres.2016.05.003. [DOI] [PubMed] [Google Scholar]
- Knutson B., Fong G.W., Adams C.M., Varner J.L., Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Koenig T., Lehmann D., Merlo M.C., Kochi K., Hell D., Koukkou M. A deviant EEG brain microstate in acute, neuroleptic-naive schizophrenics at rest. Eur. Arch. Psychiatry Clin. Neurosci. 1999;249(4):205–211. doi: 10.1007/s004060050088. [DOI] [PubMed] [Google Scholar]
- Koenig T., Prichep L., Lehmann D., Sosa P.V., Braeker E., Kleinlogel H., Isenhart R., John E.R. Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. NeuroImage. 2002;16(1):41–48. doi: 10.1006/nimg.2002.1070. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. In: Dementia Praecox and Paraphrenia. Robertson G.M., editor. E & S Livingstone; Edinburgh: 1919. Transcribed by Barclay RM. [Google Scholar]
- Kring A.M., Moran E.K. Emotional response deficits in schizophrenia: insights from affective science. Schizophr. Bull. 2008;34(5):819–834. doi: 10.1093/schbul/sbn071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann D., Ozaki H., Pal I. EEG alpha map series: brain micro-states by space-oriented adaptive segmentation. Electroencephalogr. Clin. Neurophysiol. 1987;67(3):271–288. doi: 10.1016/0013-4694(87)90025-3. [DOI] [PubMed] [Google Scholar]
- Lehmann D., Strik W.K., Henggeler B., Koenig T., Koukkou M. Brain electric microstates and momentary conscious mind states as building blocks of spontaneous thinking: I. Visual imagery and abstract thoughts. Int. J. Psychophysiol. 1998;29(1):1–11. doi: 10.1016/s0167-8760(97)00098-6. [DOI] [PubMed] [Google Scholar]
- Lehmann D., Faber P.L., Galderisi S., Herrmann W.M., Kinoshita T., Koukkou M., Mucci A., Pascual-Marqui R.D., Saito N., Wackermann J., Winterer G., Koenig T. EEG microstate duration and syntax in acute, medication-naive, first-episode schizophrenia: a multi-center study. Psychiatry Res. 2005;138(2):141–156. doi: 10.1016/j.pscychresns.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lysaker P.H., Davis L.W. Social function in schizophrenia and schizoaffective disorder: associations with personality, symptoms and neurocognition. Health Qual. Life Outcomes. 2004;2:15. doi: 10.1186/1477-7525-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann C.L., Footer O., Chung Y.S., Driscoll L.L., Barch D.M. Spared and impaired aspects of motivated cognitive control in schizophrenia. J. Abnorm. Psychol. 2013;122(3):745–755. doi: 10.1037/a0033069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D., Perrucci M.G., Del Gratta C., Romani G.L., Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2007;104(32):13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marder S.R., Galderisi S. The current conceptualization of negative symptoms in schizophrenia. World Psychiatry. 2017;16(1):14–24. doi: 10.1002/wps.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messinger J.W., Tremeau F., Antonius D., Mendelsohn E., Prudent V., Stanford A.D., Malaspina D. Avolition and expressive deficits capture negative symptom phenomenology: implications for DSM-5 and schizophrenia research. Clin. Psychol. Rev. 2011;31(1):161–168. doi: 10.1016/j.cpr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel C.M., Koenig T. EEG microstates as a tool for studying the temporal dynamics of whole-brain neuronal networks: a review. NeuroImage. 2017 doi: 10.1016/j.neuroimage.2017.11.062. [DOI] [PubMed] [Google Scholar]
- Miller E.M., Shankar M.U., Knutson B., McClure S.M. Dissociating motivation from reward in human striatal activity. J. Cogn. Neurosci. 2014;26(5):1075–1084. doi: 10.1162/jocn_a_00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milz P., Faber P.L., Lehmann D., Koenig T., Kochi K., Pascual-Marqui R.D. The functional significance of EEG microstates—associations with modalities of thinking. NeuroImage. 2016;125:643–656. doi: 10.1016/j.neuroimage.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Milz P., Pascual-Marqui R.D., Achermann P., Kochi K., Faber P.L. The EEG microstate topography is predominantly determined by intracortical sources in the alpha band. NeuroImage. 2017;162:353–361. doi: 10.1016/j.neuroimage.2017.08.058. [DOI] [PubMed] [Google Scholar]
- Morris R.W., Quail S., Griffiths K.R., Green M.J., Balleine B.W. Corticostriatal control of goal-directed action is impaired in schizophrenia. Biol. Psychiatry. 2015;77(2):187–195. doi: 10.1016/j.biopsych.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Mucci A., Rucci P., Rocca P., Bucci P., Gibertoni D., Merlotti E., Galderisi S., Maj M., Italian Network for Research on, P The specific Level of Functioning Scale: construct validity, internal consistency and factor structure in a large Italian sample of people with schizophrenia living in the community. Schizophr. Res. 2014;159(1):144–150. doi: 10.1016/j.schres.2014.07.044. [DOI] [PubMed] [Google Scholar]
- Mucci A., Dima D., Soricelli A., Volpe U., Bucci P., Frangou S., Prinster A., Salvatore M., Galderisi S., Maj M. Is avolition in schizophrenia associated with a deficit of dorsal caudate activity? A functional magnetic resonance imaging study during reward anticipation and feedback. Psychol. Med. 2015;45(8):1765–1778. doi: 10.1017/S0033291714002943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci A., Merlotti E., Ucok A., Aleman A., Galderisi S. Primary and persistent negative symptoms: Concepts, assessments and neurobiological bases. Schizophr. Res. 2017;186:19–28. doi: 10.1016/j.schres.2016.05.014. [DOI] [PubMed] [Google Scholar]
- Musso F., Brinkmeyer J., Mobascher T., Warbrick A., Winterer G. Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. Neuroimage. 2010, Oct 1;52(4):1149–1161. doi: 10.1016/j.neuroimage.2010.01.093. (Epub 2010 Feb 6) [DOI] [PubMed] [Google Scholar]
- Nishida K., Morishima Y., Yoshimura M., Isotani T., Irisawa S., Jann K., Dierks T., Strik W., Kinoshita T., Koenig T. EEG microstates associated with salience and frontoparietal networks in frontotemporal dementia, schizophrenia and Alzheimer's disease. Clin. Neurophysiol. 2013;124(6):1106–1114. doi: 10.1016/j.clinph.2013.01.005. [DOI] [PubMed] [Google Scholar]
- O'Doherty J.P. Multiple systems for the motivational control of behavior and associated neural substrates in humans. Curr. Top. Behav. Neurosci. 2016;27:291–312. doi: 10.1007/7854_2015_386. [DOI] [PubMed] [Google Scholar]
- Orliac F., Delamillieure P., Delcroix N., Naveau M., Brazo P., Razafimandimby A., Dollfus S., Joliot M. Network modeling of resting state connectivity points towards the bottom up theories of schizophrenia. Psychiatry Res. 2017;266:19–26. doi: 10.1016/j.pscychresns.2017.04.003. [DOI] [PubMed] [Google Scholar]
- Orsel S., Akdemir A., Dag I. The sensitivity of quality-of-life scale WHOQOL-100 to psychopathological measures in schizophrenia. Compr. Psychiatry. 2004;45(1):57–61. doi: 10.1016/j.comppsych.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Palaniyappan L., Liddle P.F. Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. J. Psychiatry Neurosci. 2012;37(1):17–27. doi: 10.1503/jpn.100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual-Marqui R.D., Michel C.M., Lehmann D. Segmentation of brain electrical activity into microstates: model estimation and validation. IEEE Trans. Biomed. Eng. 1995;42(7):658–665. doi: 10.1109/10.391164. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui R.D., Lehmann D., Faber P., Milz P., Kochi K., Yoshimura M., Nishida K., Isotani T., Kinoshita T. The Resting Microstate Networks (RMN): Cortical Distributions, Dynamics, and Frequency Specific Information Flow. 2014. arXiv:1411.1949 (eprint)
- Pipinis E., Melynyte S., Koenig T., Jarutyte L., Linkenkaer-Hansen K., Ruksenas O., Griskova-Bulanova I. Association between resting-state microstates and ratings on the amsterdam resting-state questionnaire. Brain Topogr. 2017;30(2):245–248. doi: 10.1007/s10548-016-0522-2. [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A. The "anhedonia paradox" in schizophrenia: insights from affective neuroscience. Biol. Psychiatry. 2010;67(10):899–901. doi: 10.1016/j.biopsych.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassovsky Y., Green M.F., Nuechterlein K.H., Breitmeyer B., Mintz J. Paracontrast and metacontrast in schizophrenia: clarifying the mechanism for visual masking deficits. Schizophr. Res. 2004;71(2–3):485–492. doi: 10.1016/j.schres.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Rassovsky Y., Horan W.P., Lee J., Sergi M.J., Green M.F. Pathways between early visual processing and functional outcome in schizophrenia. Psychol. Med. 2011;41(3):487–497. doi: 10.1017/S0033291710001054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger K., Diaz Hernandez L., Baenninger A., Koenig T. 15 years of microstate research in schizophrenia – where are we? A meta-analysis. Front. Psychiatry. 2016;7:22. doi: 10.3389/fpsyt.2016.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitzman B.A., Abell M., Bartley S.C., Erickson M.A., Bolbecker A.R., Hetrick W.P. Cognitive manipulation of brain electrical microstates. NeuroImage. 2017;146:533–543. doi: 10.1016/j.neuroimage.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E.H., Waltz J.A., Kellendonk C., Balsam P.D. Schizophrenia in translation: dissecting motivation in schizophrenia and rodents. Schizophr. Bull. 2012;38(6):1111–1117. doi: 10.1093/schbul/sbs114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens A., Lutzenberger W., Fau - Bartels D.M., Bartels Dm Fau - Strik W., Strik W Fau - Lindner K., Lindner K. 1997. Increased Duration and Altered Topography of EEG Microstates during Cognitive Tasks in Chronic Schizophrenia. (0165–1781 Print) [DOI] [PubMed] [Google Scholar]
- Strauss G.P. The emotion paradox of anhedonia in schizophrenia: or is it? Schizophr. Bull. 2013;39(2):247–250. doi: 10.1093/schbul/sbs192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss J.S., Carpenter W.T., Jr., Bartko J.J. The diagnosis and understanding of schizophrenia. Summary and conclusions. Schizophr. Bull. 1974;11:70–80. [PubMed] [Google Scholar]
- Strelets V., Faber P.L., Golikova J., Novototsky-Vlasov V., Koenig T., Gianotti L.R., Gruzelier J.H., Lehmann D. Chronic schizophrenics with positive symptomatology have shortened EEG microstate durations. Clin. Neurophysiol. 2003;114(11):2043–2051. doi: 10.1016/s1388-2457(03)00211-6. [DOI] [PubMed] [Google Scholar]
- Strik W.K., Dierks T., Becker T., Lehmann D. Larger topographical variance and decreased duration of brain electric microstates in depression. J. Neural Transm. Gen. Sect. 1995;99(1–3):213–222. doi: 10.1007/BF01271480. [DOI] [PubMed] [Google Scholar]
- Tomescu M.I., Rihs T.A., Becker R., Britz J., Custo A., Grouiller F., Schneider M., Debbane M., Eliez S., Michel C.M. Deviant dynamics of EEG resting state pattern in 22q11.2 deletion syndrome adolescents: a vulnerability marker of schizophrenia? Schizophr. Res. 2014:1573–2509. doi: 10.1016/j.schres.2014.05.036. (Electronic) [DOI] [PubMed] [Google Scholar]
- Tomescu M.I., Rihs T.A., Roinishvili M., Karahanoglu F.I., Schneider M., Menghetti S., Van De Ville D., Brand A., Chkonia E., Eliez S., Herzog M.H., Michel C.M., Cappe C. Schizophrenia patients and 22q11.2 deletion syndrome adolescents at risk express the same deviant patterns of resting state EEG microstates: a candidate endophenotype of schizophrenia. Schizophr. Res. 2015;2(3):159–165. doi: 10.1016/j.scog.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin. Neurosci. 2006;8(1):59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremeau F., Antonius D., Cacioppo J.T., Ziwich R., Jalbrzikowski M., Saccente E., Silipo G., Butler P., Javitt D. In support of Bleuler: objective evidence for increased affective ambivalence in schizophrenia based upon evocative testing. Schizophr. Res. 2009;107(2–3):223–231. doi: 10.1016/j.schres.2008.09.020. [DOI] [PubMed] [Google Scholar]
- Vignapiano A., Mucci A., Ford J., Montefusco V., Plescia G.M., Bucci P., Galderisi S. Reward anticipation and trait anhedonia: an electrophysiological investigation in subjects with schizophrenia. Clin. Neurophysiol. 2016;127(4):2149–2160. doi: 10.1016/j.clinph.2016.01.006. [DOI] [PubMed] [Google Scholar]
- Wallwork R.S., Fortgang R., Hashimoto R., Weinberger D.R., Dickinson D. Searching for a consensus five-factor model of the positive and negative Syndrome Scale for schizophrenia. Schizophr. Res. 2012;137(1–3):246–250. doi: 10.1016/j.schres.2012.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz J.A., Gold J.M. Motivational deficits in schizophrenia and the representation of expected value. Curr. Top. Behav. Neurosci. 2016;27:375–410. doi: 10.1007/7854_2015_385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz J.A., Frank M.J., Robinson B.M., Gold J.M. Selective reinforcement learning deficits in schizophrenia support predictions from computational models of striatal-cortical dysfunction. Biol. Psychiatry. 2007;62(7):756–764. doi: 10.1016/j.biopsych.2006.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegener S., Redoblado-Hodge M.A., Lucas S., Fitzgerald D., Harris A., Brennan J. Relative contributions of psychiatric symptoms and neuropsychological functioning to quality of life in first-episode psychosis. Aust. N. Z. J. Psychiatry. 2005;39(6):487–492. doi: 10.1080/j.1440-1614.2005.01608.x. [DOI] [PubMed] [Google Scholar]
- Wynn J.K., Horan W.P., Kring A.M., Simons R.F., Green M.F. Impaired anticipatory event-related potentials in schizophrenia. Int. J. Psychophysiol. 2010;77(2):141–149. doi: 10.1016/j.ijpsycho.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Zotev V., Phillips R., Drevets W.C., Bodurka J. Spatiotemporal dynamics of the brain at rest—exploring EEG microstates as electrophysiological signatures of BOLD resting state networks. NeuroImage. 2012;60(4):2062–2072. doi: 10.1016/j.neuroimage.2012.02.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material