Abstract
Transcranial magnetic stimulation (TMS) is a non-invasive method that produces neural excitation in the cortex by means of brief, time-varying magnetic field pulses. The initiation of cortical activation or its modulation depends on the background activation of the neurons of the cortical region activated, the characteristics of the coil, its position and its orientation with respect to the head. TMS combined with simultaneous electrocephalography (EEG) and neuronavigation (nTMS-EEG) allows for the assessment of cortico-cortical excitability and connectivity in almost all cortical areas in a reproducible manner. This advance makes nTMS-EEG a powerful tool that can accurately assess brain dynamics and neurophysiology in test-retest paradigms that are required for clinical trials. Limitations of this method include artifacts that cover the initial brain reactivity to stimulation. Thus, the process of removing artifacts may also extract valuable information. Moreover, the optimal parameters for dorsolateral prefrontal (DLPFC) stimulation are not fully known and current protocols utilize variations from the motor cortex (M1) stimulation paradigms. However, evolving nTMS-EEG designs hope to address these issues. The protocol presented here introduces some standard practices for assessing neurophysiological functioning from stimulation to the DLPFC that can be applied in patients with treatment resistant psychiatric disorders that receive treatment such as transcranial direct current stimulation (tDCS), repetitive transcranial magnetic stimulation (rTMS), magnetic seizure therapy (MST) or electroconvulsive therapy (ECT).
Keywords: Neuroscience, Issue 138, Combined transcranial magnetic stimulation and electroencephalography, short intracortical inhibition, long intracortical inhibition, intracortical facilitation, repetitive transcranial magnetic stimulation, magnetic seizure therapy, depression
Introduction
Transcranial magnetic stimulation (TMS) is a neurophysiological tool that allows for the non-invasive assessment of cortical neuronal activity through the use of rapid, time-varying magnetic field pulses1. These magnetic field pulses induce a weak current in the superficial cortex beneath the coil which results in membrane depolarization. The ensuing cortical activation or modulation is directly related to the characteristics of the coil, its angle and orientation to the skull2. The waveform of the pulse discharged from the coil and the underlying state of the neurons also influence the resultant cortical activation3.
TMS enables the assessment of cortical functions by evoking behavioral or motor responses or through the interruption of task-related processing. The excitability of cortico-spinal processes can be evaluated through recording electromyographic (EMG) responses elicited from single TMS pulses over the motor cortex, whereas intracortical excitatory (intracortical facilitation; ICF) and inhibitory mechanisms (short and long intracortical inhibition; SICI and LICI) can be probed with paired-pulse TMS. Repetitive TMS can disturb various cognitive processes, but is primarily used as a therapeutic tool for a variety of neuropsychiatric disorders. Furthermore, the combination of TMS with simultaneous electroencephalography (TMS-EEG) can be used to assess cortico-cortical excitability and connectivity4. Finally, if the administration of TMS is delivered with neuronavigation (nTMS), it will allow for precise test-retest paradigms since the exact site of the stimulation can be recorded. Most of the cortical mantle can be targeted and stimulated (including those areas that do not produce measurable physical or behavioral responses) thus the cortex can be functionally mapped.
The EEG signal evoked from single or paired pulse TMS can facilitate the assessment of cortico-cortical connectivity5 and the current state of the brain. The TMS-induced electric current results in action potentials that can activate synapses. The distribution of the postsynaptic currents can be recorded through EEG6. The EEG signal can be used to quantify and locate synaptic current distributions through dipole modelling7 or minimum-norm estimation8, when multichannel EEG is employed, and with the conductivity structure of the head accounted for. Combined TMS-EEG can be employed to study cortical inhibitory processes9, oscillations10, cortico-cortical11 and interhemispheric interactions12, and cortical plasticity13. Most importantly, TMS-EEG can probe excitability changes during cognitive or motor tasks with good test-retest reliability14,15. Importantly, TMS-EEG has the potential to determine neurophysiological signals that may serve as the predictors of response to therapeutic interventions (rTMS or pharmacological effects) in test-retest designs16,17.
The principles of neuronavigation for TMS is based on the principles of frameless stereotaxy. The systems use an optical tracking system18 that employs a light-emitting camera that communicates with light-reflecting optical elements attached to both the head (via a reference tracker) and the TMS coil. Neuronavigation allows for coil localization on the 3-D MRI model with the aid of a digitizing reference tool or pen. The use of neuronavigation facilitates the capture of the coil orientation, location and alignment to the subject's head as well as the digitization of the EEG electrode positions. These features are essential for test-retest design experiments and for accurate stimulation of a specified location within dorsolateral prefrontal cortex.
In order to utilize a TMS-EEG protocol in a test-retest experiment, there needs to be accurate targeting and consistent stimulation of the cortical region to obtain reliable signals. TMS-EEG recording can be vulnerable to different artifacts. The TMS induced artifact on the EEG electrodes can be filtered with amplifiers that can recover after a delay19,20 or with amplifiers that cannot be saturated21. However, other types of artifact generated by eye movements or blinks, cranial muscle activation in proximity to the EEG electrodes, random electrode movement and their polarization, and by the coil click or somatic sensation must be taken into consideration. Careful subject preparation that ensures electrode impedances below 5 kΩ, immobilization of the coil over the electrodes and a foam between coil and electrodes to reduce vibration (or a spacer to eliminate low frequency artifacts22), earplugs and even auditory masking should be used to minimize these artifacts23. The protocol presented here introduces a standard process for assessing neurophysiological functioning when the stimulation is applied over the dorsolateral prefrontal (DLPFC). The focus is on common paired-pulse paradigms that have been validated in the studies of M19,15,16.
Protocol
All the experimental procedures presented here have been approved by our Local Ethical Committee following guidelines of the Declaration of Helsinki.
1. Head Registration for Neuronavigated TMS — EEG
Obtain a high resolution whole head T1-weighted structural MRI for each participant. Scan according to the neuronavigation manufacturer guidelines.
Upload the images on the navigation system. Check if MRIs are correctly scanned. Choose the cardinal points (pre-auricular points, the nasion and the tip of the nose). Insert the stimulation targets (based on anatomy or based on the head coordinates, MNI, or Talairach coordinates).
Place the head tracker in such a way so that it will not move during the stimulation session and allow free moving of the TMS coil. Have the participant insert the earplugs before the registration starts.
Align the participant's head to the 3-D MRI model. Touch on the participant's head with the digitizing pen at the cardinal points that were selected on the images of the MRI stack. Select and mark additional points over the parietal, temporal and occipital areas of the head to reduce the registration error over those areas.
Validate the registration. Place the digitizing pen on the participant's head. Check its representation on the computer. If it is not on the corresponding point in the MR, repeat step 1.4.
- Calibrate the TMS coil in use (in some systems this step is not needed).
- Attach the trackers to the coil.
- Place the coil on the calibration block so all trackers are visible from the camera.
- Press the calibration button on the computer screen and keep the coil in the calibration position for 5 s.
2. TMS-EEG Experiment
- Place the EEG cap on the head and prepare the electrodes
- Choose a cap that fits the head well. Ensure that all electrodes are tightly touching the scalp and are functional. If more than 2 electrodes do not work, then use another cap of the same or smaller size.
- Place the Cz electrode at vertex, half way between the line connecting the nasion and inion and the Iz electrode over the inion. NOTE: Place the vertical (above and under the eye contralateral to the stimulation eye) and/or horizontal electrodes (left from the left eye and right from the right, a little above each zygomatic bone) for electrooculography (EOG).
- Adjust the blunt tip of the syringe and fill it with electroconductive gel. Place the tip inside the hole of the electrode, and then lightly press the plunger flange until there is some paste on the skin. Scrub the scalp lightly using cross-like moves with the blunt tip. Ensure that the paste is not spilling out over the top to avoid bridging (short-circuit between the electrodes).
Place the EMG electrodes. Place two disposable disc electrodes (diameter of about 30 mm) over the right abductor pollicis brevis muscle (APB) for a belly tendon montage. Place the ground according to the manufacturer's guidelines.
Start the head registration. Follow steps 1.3–1.6. Use the MNI or Talairach coordinates of the DLPFC.
- Hot spot and motor threshold.
- Add a sponge (artificial fiber made from polyutherane) under the coil in order to minimize the coil vibration over the electrodes during the TMS pulses. Note that the foam should be about 10 mm thick.
- Instruct the participant to be at rest — comfortable and with relaxed hands, legs and spinal column.
- Find the hot spot. Target the motor knob24 as the initial landmark of cortical representation of APB in M1 and move the coil until there is corresponding APB movement. Use TMS intensities evoking MEPs of around 500 µV over APB. Optimize the coil orientation by changing its angle and tilt to evoke the biggest response over the hot spot.
- Save the coil positioning in the neuronavigator software and reduce the output intensity in steps of 2–3%. Give 10 pulses and if more than 5 out of 10 MEP responses over 50 µV are obtained, then continue reducing the intensity.
- When less than 5 out of 10 responses are evoked, increase the intensity by steps of 1–2%. MT is represented as the intensity that produces MEPs larger than 50 µV 5 out of 10 times25. The inter-stimulus interval (ISI) for MT should be longer than 1 s, usually set at 3, 4 or 5 s.
- Adjust the intensity using the following steps:
- Start at 120% of MT intensity to produce MEPs over M1 from 500 to 1,500 µV. Record 10 pulses with this stimulator's output so the average response is 1 mV. Increase or decrease the intensity in steps of 1–2% until reaching an average of 1 mV.
- For stimulation intensity, choose the intensity as a percentage of stimulator's output, e.g., 110%, 120%, etc.
- Find the corresponding induced field in V/m (if the system allows). Place the coil over DLPFC; adjust the stimulator's output until the calculation of the induced field becomes the same as the one over M1 for the same cortical depth.
Digitize the EEG electrodes, so that their position is registered to the brain anatomy. NOTE: This is a very important step for locating the distribution of neuronal activation and for accurate repositioning of the electrodes in the follow-up session.
- Record the TMS-EEG
- Replace the earplugs with the earplugs with air tubes to connect to audio masking (e.g., white noise) if available and add headphones over them. Play the audio masking only during TMS pulse delivery. NOTE: This step can be applied to step 2.4.2 without playing the audio masking and with care so the head trackers are not moved.
- Mount the coil on the coil holder and make sure that the coil does not move or press the electrodes under it. Make sure that the sponge is between the electrodes and the coil.
- Remove all active screens out of the sight of the participant. Give instructions to the participant to stare on a fixed point, not to change his/her head position during TMS delivery and not to blink between the TMS pulses.
- Switch off any fluorescent lights. Run single pulse TMS, SICI, ICF and LICI in a random order for each participant. Give 100 single and paired pulses. Use various ISI's of 3–4 s (±20%) or a constant of 3–5 s (see Note). Give a break of 3–5 min between each condition so the participant can relax and stretch. NOTE: SICI and ICF involve a paired-pulse TMS paradigm with a subthreshold conditioning stimulus (CS) and a suprathreshold test stimulus (TS). The CS used in this protocol is 80% of MT and the TS at the intensity evoking a 1 mV MEP peak-to-peak26. The inter-pulse interval used for optimal SICI is at 2 ms and for ICF at 12–1327. The LICI paradigm involves the pairing of a supra-threshold CS at the intensity evoking the 1 mV MEP peak-to-peak followed by another suprathreshold TS again using the intensity that evoked a 1 mV MEP peak-to-peak and at an inter-pulse interval of 100 ms. The ISI for both single and paired pulse paradigms is determined by the stimulator's charging time (our system can allow paired pulses every 4 s), the amount of sessions (longer experiments would require smaller ISI to not overburden the participants) and the analysis that is going to take place. In this study, we used a constant ISI of 5 s due to our stimulator's restrictions and also because we would need several cycles of low frequency band (theta rhythm) for the time-frequency and power spectrum analysis.
Representative Results
Figure 1A illustrates the TMSevoked potentials after DLPFC stimulation over the F3 electrode after averaging 100 epochs from each session for one healthy volunteer. In this illustration, we highlight the effect of the CS on the TS in comparison to the single pulse condition when TS is applied alone. The CS modulates the N100 deflection in a clear manner even in one subject. In the SICI and LICI sessions, N100 is usually increased and in ICF decreases in absolute values when compared to the SP condition16. In Figure 1B, the topographical distribution of N100 component of SP, SICI and ICF paradigm has been localized bilaterally as it has been shown in many previous studies16,17,28,29.
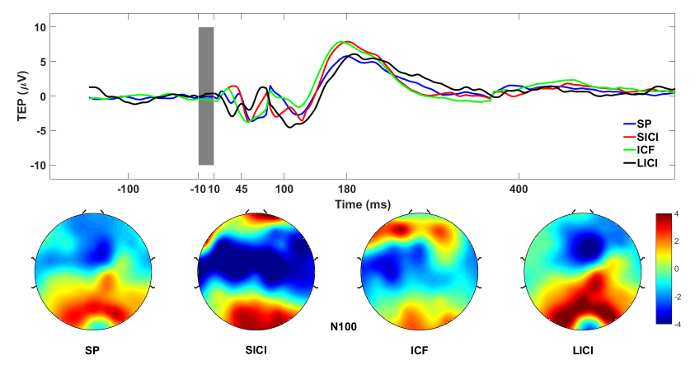
Figure 1: TMS-EEG measures of cortical excitability. (A) Grand average of TMS-evoked EEG responses from DLPFC ROI electrodes after DLPFC stimulation. (B) N100 values plotted topographically across all electrodes for each session. Please click here to view a larger version of this figure.
Discussion
TMS-EEG enables the direct and noninvasive stimulation of most cortical areas and the acquisition of the resulting neuronal activity with very good spatio-temporal resolution30, especially when neuronavigation is utilized. The benefit of this methodological advance is based on the fact that TMS-evoked EEG signals originate from the electrical neural activity and it is an index of cortico-cortical excitability. This has tremendous potential in neuropsychiatric patient populations where TMS-EEG can be used as a biomarker of current and future therapeutic interventions.
The most critical step of the protocol is the preparation of the electrodes and the determination of the stimulation intensity. This is because the TMS-EEG signal is susceptible to the TMS artifact, irrespective of the kind of amplifiers used31. The electrodes should be very carefully prepared, so they do not bridge with each other and their impedance is kept below 5 kΩ, and the signal-to-noise ratio is high. In addition, a sponge made from artificial polyutherane fiber of 5-10 mm adjusted under the coil can further reduce the mechanical pressure and the artifact of the coil click sound through bone conduction.
The MT determines the TMS-intensity; therefore, it should be measured precisely as higher intensities will lead to bigger artifacts and less focal stimulation, while smaller intensities may result in very weak signals. Thus, the motor hot spot should be found with the assistance of neuronavigation and the MT is estimated with EMG recordings (noise below 50 µV and muscles completely relaxed). However, one should not forget that the focality and the accuracy of each stimulation derives from the shape and duration of the TMS pulses32.
The lack of measures for a DLPFC threshold also suggests that the intensity should be adjusted according to the amplitude of the estimated induced electrical field23 and not based on the stimulator's intensity output as the conventional method. This requires that the MT intensity needs to be estimated in V/m for a specific cortical depth and then the same depth and V/m to be used to recalculate the stimulator's output intensity for DLPFC stimulation. This is a particularly important issue for future investigation of paired pulse protocols like the ones presented here, where the TS is always at suprathreshold intensities. However, there is a need to define the DLPFC intensity from the recorded TEP33 or oscillations34 during DLPFC stimulation as it has been suggested in recent studies for M1 by means of cortical and non-corticospinal measures.
Importantly, the DLPFC stimulation site should be chosen based on the MNI or Talairach coordinates and inserted on the MRIs of the neuronavigation. The MNI coordinates for the left DLPFC (-35, 45, 38) are drawn from a study identifying this site as optimal, based on clinical outcomes and resting-state functional connectivity35. The positioning of the coil with respect to orientation and tilt is another important variable. There are two ways to approach coil orientation and tilt: a) 45 degrees to the midline with the handle pointing to the lateral parts of the hemisphere9 and b) perpendicular to the middle frontal sulcus with lateral to medial current direction14. The first is usually applied when no navigation exists, while the second one requires real MRI and navigation and it induces the maximum of the field. Before starting the recordings, fine tuning of the coil so it evokes minimum muscle artifacts5 without affecting the stimulation physiological responses needs to be performed (small changes of 1-2 mm of the center of the coil, as well as tilt and orientation subtle changes).
Comparison of the different orientations needs to be done since there are no known studies that have examined the effect of different coil positioning over DLPFC. Even more importantly, there is a need of a method to define the DLPFC hot spot based on EEG measures in a similar manner that the M1 hot spot is defined by the EMG. Finally, a very important aspect here is the positioning of the electrodes and their digitization of their location. In test-retest designs, as soon as the cap is placed for the follow up experiments, the electrodes should be digitized. Then both digitizations (of the first and the consequents experiment) should be visualized over the 3D MRI model or the MRI template (which can be a good reliable solution when individual MRIs cannot be obtained). Then the cap should be moved if necessary so the positioning over the skull of the electrodes in the follow up experiment matches the positioning of the first measurement. This will ensure that the data will be derived from the exact same locations of the electrodes that were stimulated with the exact same magnetic field.
Before starting the stimulation, the chosen cortical site should be checked for cranial nerves passing under the coil. Therefore, a few TMS-EEG epochs should be recorded, and the artifacts evaluated. Thus, signal needs to be checked for amplitudes larger than 70 µV and non-synchronized high frequency-low amplitude oscillations (muscle and cranial nerves artifacts). Eliminating such artifacts can be done by fine and subtle repositioning of the coil or its orientation only, as it has been proposed in previous studies36. Finally, during the TMS-EEG sessions, the TMS coil should be monitored by real time neuronavigation and kept immobilized. The best way is to mount it on a tripod or on a mechanical arm. This solution also prevents from pressing the coil with the hands against the electrodes, adding mechanical pressure artifacts on them. Any changes should be immediately corrected and the respective epochs marked as bad and excluded from the data analysis, due to the fact that EEG responses to TMS are very sensitive to the perturbation of these parameters37. All these detailed suggestions can ensure test-retest reliability of TMS-EEG in single14 and paired pulse paradigms15 over the DLPFC. The attention to these important details will ensure that the data have the highest chance of reflecting changes related to the therapeutic interventions.
TMS-EEG like every other experimental method has its own specific limitations. The major issue is the various kinds of artifacts and the fact that TMS-compatible EEG amplifiers cannot eliminate the remaining artifacts. Artifacts from cranial muscles, particularly when frontal and lateral sites over the skull are stimulated, can obscure and modulate the EEG signal. These artifacts can be larger than the TMS-EEG signal and usually last longer, thus they may obscure the TEPs. Similarly, but only in areas such as the DLPFC, TMS can evoke large eye blink artifacts. Additionally, many other artifacts such as electrode movement, skin sensation and auditory activations due to TMS coil click can make the EEG analysis even more difficult (for details, see previous publications31,38). Much work in the field has been directed towards rejecting a variety of artifacts, resulting in more reliable spatio-temporal localization of the sources of the brain responses38,39,40,41,42. However, one should not forget that the careful preparation of the participants, the choice of the equipment and accurate performance of the measurement determine the quality of the raw TMS-EEG data.
TMS-EEG is a powerful tool to assess intracortical inhibition and excitation mechanisms related to the stimulation of the DLPFC. By just changing a few parameters, it allows for the study of circuits mediated by GABAAR (SICI), GABABR (LICI), and NMDAR (ICF). Modulation of different TEP components through pharmacological or electromagnetic therapeutic interventions can serve as a marker to identify inhibitory and excitatory neurotransmission, cortical plasticity and many more brain state changes and conditions43. In addition to TEP's, TMS-evoked oscillatory activity through time frequency and spectral analysis can assess the natural or the intrinsic frequency of the above circuitry10. Electrical brain indices such as current source density4 applicable for any cortical area may help to unravel the mechanisms of plasticity in damaged brain circuits in DLPFC44.
Further pharmacological validation studies of these paradigms in the DLPFC are necessary. However, there is tremendous potential for TMS-EEG to be used to study the mechanisms of various therapeutic interventions, such as neuromodulation therapies (e.g., rTMS, ECT, MST) or pharmacological ones in healthy volunteers or in various psychiatric disorders9,15,16,17,45,46, but also alternative interventions or combinations of them43. Most importantly, TMS-EEG can reliably assess the brain dynamics prior to and after an intervention and therefore potentially serve as biomarker.
Disclosures
Pantelis Lioumis has been a paid consultant for Nexstim Plc. (Helsinki, Finland) outside the submitted work (i.e., for the motor and speech mapping rTMS applications before 2017). Reza Zomorrodi is a member of the advisory board of Vielight Inc. (Toronto, Canada). Zafiris J. Daskalakis receives research support from the Canadian Institutes of Health Research (CIHR), National Institutes of Health - US (NIH), Weston Brain Institute, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for this investigator-initiated study. Daniel M. Blumberger receives research support from the Canadian Institutes of Health Research (CIHR), National Institutes of Health - US (NIH), Weston Brain Institute, Brain Canada and the Temerty Family through the CAMH Foundation and the Campbell Research Institute. He received research support and in-kind equipment support for an investigator-initiated study from Brainsway Ltd. and he is the site principal investigator for three sponsor-initiated studies for Brainsway Ltd. He received in-kind equipment support from Magventure for this investigator-initiated study. He received medication supplies for an investigator-initiated trial from Indivior. He has participated in an advisory board for Janssen.
Acknowledgments
This work was funded in part by NIMH R01 MH112815. This work was also supported by the Temerty Family Foundation, Grant Family Foundation and Campbell Family Mental Health Research Institute at the Centre for Addiction and Mental Health.
References
- Barker AT, Jalinous R, Freeston IL. Lancet. 8437. Vol. 1. London, England: 1985. Non-invasive magnetic stimulation of human motor cortex; pp. 1106–1107. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Ruohonen J, Karhu J. Transcranial magnetic stimulation--a new tool for functional imaging of the brain. Critical Reviews in Biomedical Engineering. 1999;27(3-5):241–284. [PubMed] [Google Scholar]
- Matthews PB. The effect of firing on the excitability of a model motoneurone and its implications for cortical stimulation. The Journal of Physiology. 1999;518:867–882. doi: 10.1111/j.1469-7793.1999.0867p.x. Pt 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casali AG, Casarotto S, Rosanova M, Mariotti M, Massimini M. General indices to characterize the electrical response of the cerebral cortex to TMS. NeuroImage. 2010;49(2):1459–1468. doi: 10.1016/j.neuroimage.2009.09.026. [DOI] [PubMed] [Google Scholar]
- Massimini M, Ferrarelli F, Huber R, Esser SK, Singh H, Tononi G. Science. 5744. Vol. 309. New York, N.Y: 2005. Breakdown of cortical effective connectivity during sleep; pp. 2228–2232. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport. 1997;8(16):3537–3540. doi: 10.1097/00001756-199711100-00024. [DOI] [PubMed] [Google Scholar]
- Scherg M, Ebersole JS. Models of brain sources. Brain Topography. 1993;5(4):419–423. doi: 10.1007/BF01128700. [DOI] [PubMed] [Google Scholar]
- Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Medical & Biological Engineering & Computing. 1994;32(1):35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Farzan F, Barr MS, Maller JJ, Chen R, Fitzgerald PB. Long-interval cortical inhibition from the dorsolateral prefrontal cortex: a TMS-EEG study. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2008;33(12):2860–2869. doi: 10.1038/npp.2008.22. [DOI] [PubMed] [Google Scholar]
- Rosanova M, Casali A, Bellina V, Resta F, Mariotti M, Massimini M. Natural frequencies of human corticothalamic circuits. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2009;29(24):7679–7685. doi: 10.1523/JNEUROSCI.0445-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppa S, Muthuraman M, Otto B, Deuschl G, Siebner HR, Raethjen J. Subcortical substrates of TMS induced modulation of the cortico-cortical connectivity. Brain Stimulation. 2013;6(2):138–146. doi: 10.1016/j.brs.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Borich MR, Wheaton LA, Brodie SM, Lakhani B, Boyd LA. Evaluating interhemispheric cortical responses to transcranial magnetic stimulation in chronic stroke: A TMS-EEG investigation. Neuroscience Letters. 2016;618:25–30. doi: 10.1016/j.neulet.2016.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SW, et al. Demonstration of short-term plasticity in the dorsolateral prefrontal cortex with theta burst stimulation: A TMS-EEG study. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2017;128(7):1117–1126. doi: 10.1016/j.clinph.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Lioumis P, Kicić D, Savolainen P, Mäkelä JP, Kähkönen S. Reproducibility of TMS-Evoked EEG responses. Human Brain Mapping. 2009;30(4):1387–1396. doi: 10.1002/hbm.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzan F, et al. Reliability of long-interval cortical inhibition in healthy human subjects: a TMS-EEG study. Journal of Neurophysiology. 2010;104(3):1339–1346. doi: 10.1152/jn.00279.2010. [DOI] [PubMed] [Google Scholar]
- Cash RFH, et al. Characterization of Glutamatergic and GABAA-Mediated Neurotransmission in Motor and Dorsolateral Prefrontal Cortex Using Paired-Pulse TMS-EEG. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2017;42(2):502–511. doi: 10.1038/npp.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premoli I, et al. TMS-EEG signatures of GABAergic neurotransmission in the human cortex. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2014;34(16):5603–5612. doi: 10.1523/JNEUROSCI.5089-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiles AD, Thompson DG, Frantz DD. Accuracy assessment and interpretation for optical tracking systems. SPIE. 2004;5367:421–433. [Google Scholar]
- Iramina K, Maeno T, Nonaka Y, Ueno S. Measurement of evoked electroencephalography induced by transcranial magnetic stimulation. Journal of Applied Physics. 2003;93(10):6718–6720. [Google Scholar]
- Virtanen J, Ruohonen J, Näätänen R, Ilmoniemi RJ. Instrumentation for the measurement of electric brain responses to transcranial magnetic stimulation. Medical & Biological Engineering & Computing. 1999;37(3):322–326. doi: 10.1007/BF02513307. [DOI] [PubMed] [Google Scholar]
- Ives JR, Rotenberg A, Poma R, Thut G, Pascual-Leone A. Electroencephalographic recording during transcranial magnetic stimulation in humans and animals. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2006;117(8):1870–1875. doi: 10.1016/j.clinph.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Ruddy KL, Woolley DG, Mantini D, Balsters JH, Enz N, Wenderoth N. Improving the quality of combined EEG-TMS neural recordings: Introducing the coil spacer. Journal of Neuroscience Methods. 2017;294:34–39. doi: 10.1016/j.jneumeth.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Massimini M, et al. Cortical reactivity and effective connectivity during REM sleep in humans. Cognitive Neuroscience. 2010;1(3):176–183. doi: 10.1080/17588921003731578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousry TA, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain: A Journal of Neurology. 1997;120:141–157. doi: 10.1093/brain/120.1.141. Pt 1. [DOI] [PubMed] [Google Scholar]
- Rossini PM, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2015;126(6):1071–1107. doi: 10.1016/j.clinph.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, et al. Intracortical inhibition and facilitation in different representations of the human motor cortex. Journal of Neurophysiology. 1998;80(6):2870–2881. doi: 10.1152/jn.1998.80.6.2870. [DOI] [PubMed] [Google Scholar]
- Saisane L, et al. Short- and intermediate-interval cortical inhibition and facilitation assessed by navigated transcranial magnetic stimulation. Journal of Neuroscience Methods. 2011;195(2):241–248. doi: 10.1016/j.jneumeth.2010.11.022. [DOI] [PubMed] [Google Scholar]
- Ferreri F, et al. Human brain connectivity during single and paired pulse transcranial magnetic stimulation. NeuroImage. 2011;54(1):90–102. doi: 10.1016/j.neuroimage.2010.07.056. [DOI] [PubMed] [Google Scholar]
- Premoli I, et al. Characterization of GABAB-receptor mediated neurotransmission in the human cortex by paired-pulse TMS-EEG. NeuroImage. 2014;103:152–162. doi: 10.1016/j.neuroimage.2014.09.028. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, Fitzgerald PB. Assessing cortical network properties using TMS-EEG. Human Brain Mapping. 2013;34(7):1652–1669. doi: 10.1002/hbm.22016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilmoniemi RJ, Kicić D. Methodology for combined TMS and EEG. Brain Topography. 2010;22(4):233–248. doi: 10.1007/s10548-009-0123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterchev AV, D'Ostilio K, Rothwell JC, Murphy DL. Controllable pulse parameter transcranial magnetic stimulator with enhanced circuit topology and pulse shaping. Journal of Neural Engineering. 2014;11(5):056023. doi: 10.1088/1741-2560/11/5/056023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fecchio M, et al. The spectral features of EEG responses to transcranial magnetic stimulation of the primary motor cortex depend on the amplitude of the motor evoked potentials. PLOS ONE. 2017;12(9):0184910. doi: 10.1371/journal.pone.0184910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saari J, Kallioniemi E, Tarvainen M, Julkunen P. Oscillatory TMS-EEG-Responses as a Measure of the Cortical Excitability Threshold. IEEE transactions on neural systems and rehabilitation engineering: a publication of the IEEE Engineering in Medicine and Biology Society. 2018;26(2):383–391. doi: 10.1109/TNSRE.2017.2779135. [DOI] [PubMed] [Google Scholar]
- Fox MD, Liu H, Pascual-Leone A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. NeuroImage. 2013;66:151–160. doi: 10.1016/j.neuroimage.2012.10.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casarotto S, et al. Transcranial magnetic stimulation-evoked EEG/cortical potentials in physiological and pathological aging. Neuroreport. 2011;22(12):592–597. doi: 10.1097/WNR.0b013e328349433a. [DOI] [PubMed] [Google Scholar]
- Casarotto S, et al. EEG responses to TMS are sensitive to changes in the perturbation parameters and repeatable over time. PloS One. 2010;5(4):10281. doi: 10.1371/journal.pone.0010281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, et al. ARTIST: A fully automated artifact rejection algorithm for single-pulse TMS-EEG data. Human Brain Mapping. 2018. [DOI] [PMC free article] [PubMed]
- Mutanen TP, Metsomaa J, Liljander S, Ilmoniemi RJ. Automatic and robust noise suppression in EEG and MEG: The SOUND algorithm. NeuroImage. 2018;166:135–151. doi: 10.1016/j.neuroimage.2017.10.021. [DOI] [PubMed] [Google Scholar]
- Ilmoniemi RJ, et al. Dealing with artifacts in TMS-evoked EEG. Conference proceedings: ...Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Annual Conference. 2015;2015:230–233. doi: 10.1109/EMBC.2015.7318342. [DOI] [PubMed] [Google Scholar]
- Rogasch NC, et al. Removing artefacts from TMS-EEG recordings using independent component analysis: importance for assessing prefrontal and motor cortex network properties. NeuroImage. 2014;101:425–439. doi: 10.1016/j.neuroimage.2014.07.037. [DOI] [PubMed] [Google Scholar]
- Mutanen TP, Kukkonen M, Nieminen JO, Stenroos M, Sarvas J, Ilmoniemi RJ. Recovering TMS-evoked EEG responses masked by muscle artifacts. NeuroImage. 2016;139:157–166. doi: 10.1016/j.neuroimage.2016.05.028. [DOI] [PubMed] [Google Scholar]
- Farzan F, Vernet M, Shafi MMD, Rotenberg A, Daskalakis ZJ, Pascual-Leone A. Characterizing and Modulating Brain Circuitry through Transcranial Magnetic Stimulation Combined with Electroencephalography. Frontiers in Neural Circuits. 2016;10:73. doi: 10.3389/fncir.2016.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casula EP, Pellicciari MC, Picazio S, Caltagirone C, Koch G. Spike-timing-dependent plasticity in the human dorso-lateral prefrontal cortex. NeuroImage. 2016;143:204–213. doi: 10.1016/j.neuroimage.2016.08.060. [DOI] [PubMed] [Google Scholar]
- Noda Y, et al. Characterization of the influence of age on GABAA and glutamatergic mediated functions in the dorsolateral prefrontal cortex using paired-pulse TMS-EEG. Aging. 2017;9(2):556–572. doi: 10.18632/aging.101178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PB, Maller JJ, Hoy K, Farzan F, Daskalakis ZJ. GABA and cortical inhibition in motor and non-motor regions using combined TMS-EEG: a time analysis. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology. 2009;120(9):1706–1710. doi: 10.1016/j.clinph.2009.06.019. [DOI] [PubMed] [Google Scholar]


