Abstract
Background:
The aim of the study was to evaluate the anti-inflammatory effect of chorion as a barrier membrane in periodontal pocket therapy by assessing interleukin-11 (IL-11) level in gingival crevicular fluid (GCF).
Materials and Methods:
The study was a randomized, controlled, single-blind clinical trial with split-mouth design. In each patient, two sites in two quadrants were selected and randomly allocated in Group 1 (flap surgery) and Group 2 (flap surgery + chorion membrane placement). After Phase 1 therapy, clinical and biochemical parameters were recorded at baseline and at 4 weeks. Plaque index (PI), sulcus bleeding index (SBI), probing pocket depth (PPD), clinical attachment level (CAL), and IL-11 in GCF were assessed. GCF was collected using microcapillary tubes. IL-11 was assayed in GCF with ELISA kit (Genxbio®).
Results:
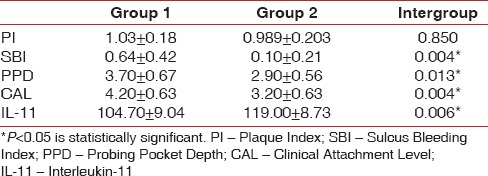
At baseline, the clinical and the biochemical parameters were similar in both the groups. Intragroup comparison revealed statistically significant decrease in all clinical and biochemical parameters in Group 1 (P < 0.001) and Group 2 (P < 0.001). Intergroup comparison showed statistically significant decrease in SBI (P = 0.004), PPD (P = 0.004), CAL (P = 0.013), and IL-11 (P = 0.006) in Group 2 compared to Group 1 at 4 weeks. However, PI did not show any statistically significant difference on intergroup comparison.
Conclusion:
Adjunctive use of chorion membrane in flap surgery provides an additive anti-inflammatory effect along with improvement in clinical outcomes enhancing the long-term prognosis.
Keywords: Anti-inflammatory, chorion membrane, gingival crevicular fluid, interleukin-11, periodontal pocket therapy
INTRODUCTION
Amniotic membranes have been used for various purposes in the field of medicine since 100 years. Amniotic membrane comprises amnion and chorion tissues where amnion is thin and chorion is 4 times thicker containing various cytokines and growth factors. Thickness of the chorion is responsible for its higher content of growth factors and cytokine than amnion. According to studies, fresh and dehydrated chorion has greater growth factor and cytokine load compared to fresh and dehydrated amnion.[1] Thus, chorion membrane may aid in better reduction of inflammation.
Chorion membrane is a well-tolerated, biodegradable membrane. It is widely used since they are bestowed with property of being immune privileged. Its antimicrobial and antibacterial properties are well known. In periodontics, chorion membrane has been used in the treatment of furcation defects, infrabony defects, and gingival soft-tissue coverage.[2,3] However, to the best of our knowledge, there have been no studies assessing the anti-inflammatory effect of chorion membrane in periodontal therapy.
Thus, the study was aimed to evaluate the anti-inflammatory effect of chorion as a barrier membrane in periodontal pocket therapy by assessing interleukin-11 (IL-11) in gingival crevicular fluid (GCF). IL-11 is an immunoregulatory, pleiotropic cytokine produced by various stromal cells, including fibroblasts, epithelial cells, and osteoblasts which can be detected in GCF.[4] IL-11 has the potential to downregulate several pro-inflammatory mediators and thereby reducing inflammation. Thus, it is a promising marker of inflammation.[5] IL-11 can be assessed in GCF which is regarded as the window for analysis of markers of inflammation.
MATERIALS AND METHODS
Patient selection
A total of 10 patients diagnosed as having severe chronic periodontitis were selected for the study.[6] The inclusion and exclusion criteria for the study were as follows:
Inclusion criteria
Patients with chronic periodontitis within the age group of 25–45 years
Patients with at least two teeth on each side of the quadrant probing pocket depth (PPD) of ≥6 mm
Radiographic evidence of bone loss.
Exclusion criteria
Patients who had undergone any kind of periodontal treatment during the past 6 months
Patients on antibiotics or immunosuppressant medication 6 months before the study
Chronic smokers, alcoholics, and smokeless tobacco users
Patients with systemic disease that could influence periodontal outcome
Pregnant and lactating women.
The study was a randomized controlled, single-blind clinical trial with split-mouth design, wherein from each patient, two sites were selected in two quadrants and the sites were randomly divided into test and control groups by flipping a coin.
Group 1 (control) – Modified flap operation
Group 2 (test) – Modified flap operation in conjunction with chorion membrane placement.
An informed consent was taken from patients after explaining the procedure in their own language. The ethical clearance was obtained from the Institutional Ethical Committee. The surgical procedure was performed by the single examiner. The chorion membrane was obtained from Tissue Bank of Tata Memorial Hospital, Mumbai, India.
Presurgical procedure
All the 10 patients received the initial therapy consisting of scaling and root planing. After completion of Phase 1 therapy, the following clinical and biochemical parameters were recorded at baseline and at 4 weeks:
Collection of gingival crevicular fluid
The participants were seated comfortably in an upright position in the dental chair. Absorbent cotton rolls and a saliva ejector were used to maintain isolation during the GCF collection. The calibrated microcapillary tube (0–5 μL) was placed extrasulcular, and a standardized volume of 2–3 μL was collected [Figure 1]. The microcapillary tubes which were contaminated with blood or inadequate volume of fluid were discarded. After collection of GCF from both the sites, GCF was transferred in sterilized microcentrifuge tubes (Eppendorf tubes) containing 100 μl of phosphate-buffered saline. The samples were stored at −80°C until they were assayed for IL-11. The samples were transported in ice packs to the Department of Clinical Biochemistry, K.L.E. ‘s Dr. Prabhakar Kore Hospital and Medical Research Centre, Belagavi, where they were assayed for IL-11with ELISA kit. (Genxbio®) [Figure 2].
Figure 1.
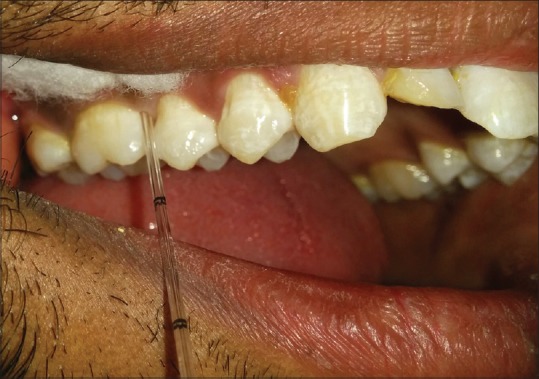
Collection of gingival crevicular fluid with a microcapillary tube
Figure 2.
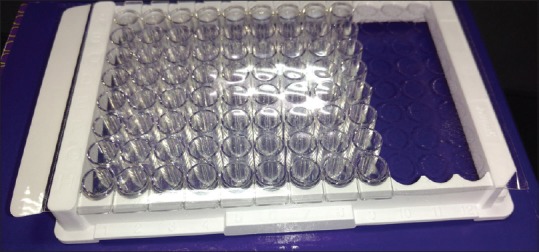
ELISA microplate for interleukin-11
Periodontal pocket therapy
The periodontal pocket therapy was performed with the administration of local anesthesia. Following the incisions, a mucoperiosteal flap was reflected [Figures 3 and 4a]. Thorough root planing and degranulation was done using appropriate site-specific curettes. The chorion membrane [Figure 4b] was trimmed to appropriate size and adapted over the alveolar bone extending from the base of the flap reflection to the alveolar crest on buccal and lingual aspects [Figure 4c]. Flap was repositioned and sutured by interrupted suture technique [Figure 4d]. The periodontal dressing was placed. In the Group 1, all surgical procedures were identical except no membrane was placed [Figure 5a and b]. All patients received postoperative instructions and were prescribed antibiotics and analgesics. The periodontal dressing and sutures were removed 7 days postsurgery. The patients were recalled at 4 weeks to assess clinical and biochemical parameters.[9]
Figure 3.
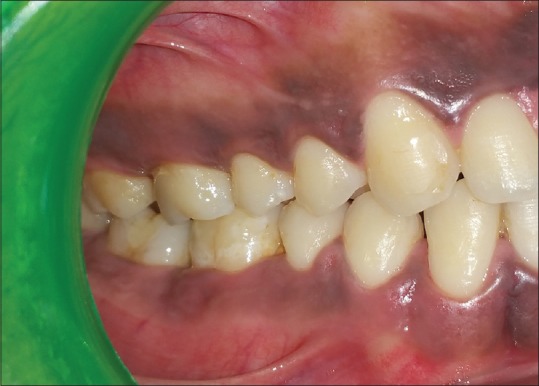
Preoperative split-mouth: Group 1 – 46 and 47; Group 2 – 16 and 17
Figure 4.
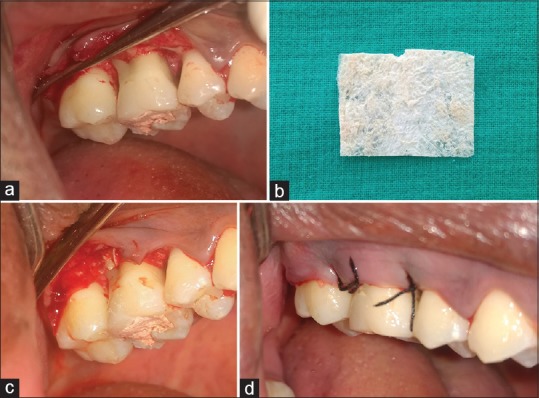
(a) Full-thickness flap reflected at 16 and 17 in Group 2; (b) chorion membrane; (c) chorion membrane placed at 16 and 17 in Group 2; (d) sutures placed at 16 and 17 in Group 2
Figure 5.
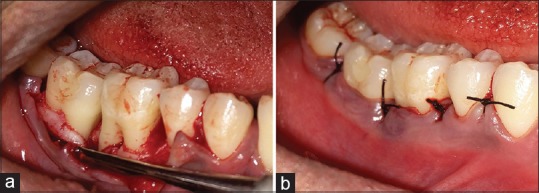
(a) Full-thickness flap reflected at 46 and 47 in Group 1; (b) sutures placed at 46 and 47 in Group 1
Statistical analysis
Wilcoxon signed-rank test was used to assess the significance within each group. Mann–Whitney U-test was applied to analyze the intergroup significance.
RESULTS
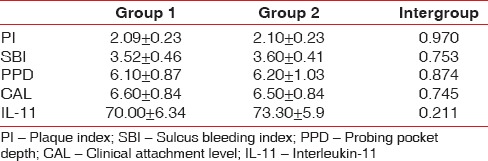
The baseline clinical parameters in Group 1 and Group 2 are depicted Tables 1 and 2. Baseline values were similar in both the groups as depicted in Table 3. Intragroup comparison revealed statistically significant decrease in all clinical parameters in Group 1 (P < 0.001) and Group 2 (P < 0.001) at 4 weeks [Tables 1 and 2].
Table 1.
Intragroup comparison of clinical and biochemical parameters in Group 1
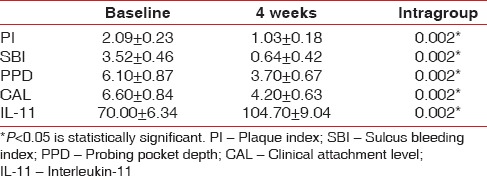
Table 2.
Intragroup comparison of clinical and biochemical parameters in Group 2
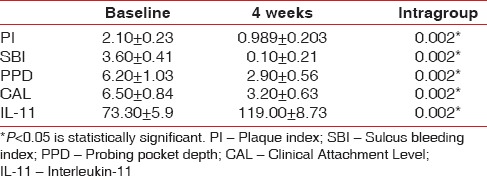
Table 3.
Intergroup comparison of clinical and biochemical parameters at baseline
Intergroup comparison showed statistically significant decrease in SBI (P = 0.004), PPD (P = 0.004), and CAL (P = 0.013) in Group 2 compared to Group 1 at 4 weeks [Table 4]. However, PI did not show statistically significant difference on intergroup comparison at 4 weeks (P = 0.850).
Table 4.
Intergroup comparison of clinical and biochemical parameters at 4 weeks
Biochemical parameter
The baseline biochemical parameter in Group 1 and Group 2 is depicted Tables 1 and 2. Baseline values were similar in both the groups as depicted in Table 3. Intragroup comparison showed statistically significant increase in levels of IL-11 in Group 1 (P < 0.001) and Group 2 (P < 0.001) at 4 weeks [Tables 1 and 2].
Intergroup comparison at baseline showed that IL-11 values were similar for both the groups as shown in Table 3. Intergroup comparison at 4 weeks showed statistically significant increase (P = 0.006) in IL-11 levels in the Group 2 compared to Group 1 as depicted in Table 4.
DISCUSSION
The main goal of periodontal treatment is to eliminate inflammatory processes in order to arrest the progression of the disease and keep the dentition in a state of the health. To the best of our knowledge, among various treatment modalities in periodontal treatment, this is the first clinical study assessing the anti-inflammatory effects of chorion membrane on periodontal tissues by assessing IL-11 levels in GCF.
There are studies which have investigated levels of IL-11 in GCF or gingival tissues of the periodontitis patients where it was observed that its levels were decreased in patients with chronic periodontitis.[4] Yücel et al. also reported decreased levels of IL-11 in chronic periodontitis compared to gingivitis or healthy patients.[10] IL-11 acts as a key mediator in preventing progression of inflammation and induces the osteoblastic differentiation in vitro and in vivo. This shows that there is a predictive role of IL-11 in periodontitis.
In the present study, after Phase I therapy, in Group 1, the baseline IL-11 value (70.00 ± 6.34) increased to 104.70 ± 9.04 at 4 weeks after flap surgery, suggesting that IL-11 increased with decrease in inflammation [Table 1]. In Group 2, the baseline value (73.30 ± 5.9) increased to 119.00 ± 8.73 after 4 weeks [Table 2]. Addition of chorion membrane as an adjunct to flap surgery significantly raised the IL-11 levels in Group 2 compared to Group 1 (P = 0.006).
Chorion membrane decreases influx of inflammatory cells to the wound area and consequently reduces inflammatory mediators by serving as a barrier. The chorion tissue contains tissue inhibitors of metalloproteinases which suppress matrix metalloproteinases and transforming growth factor-beta. Two very potent pro-inflammatory mediators, IL-1α and IL-1β, are suppressed by matrix of stroma of chorion tissue.[11] The chemokines and cytokines can directly affect the T-cells, B-cells, and natural killer cells and thus may modulate inflammation.[12] Hence, these proteins present in the chorion membrane lead to suppression of inflammation and collagenous degradation.[12] IL-10 and IL-1 receptor antagonists expressed in chorion tissue suppress the inflammatory process by their own mechanism.[13]
These mechanisms explain the statistically significant increase in IL-11 and decrease in SBI scores when chorion membrane was used as an adjunct to flap surgery even though the plaque scores in both the groups at 4 weeks did not show statistical significant difference [Table 4].
The chorion membrane histologically consists of the following layers: reticular, basement membrane, and trophoblasts. This reticular layer of chorion membrane contains collagen Types I, III, IV, V, VI, and VII along with proteoglycans. In basement membrane, collagen Type IV, fibronectins, and laminins are present. Immunohistochemical staining analysis of chorion membranes showed intense concentrations of laminin and laminin-5 throughout the barrier which is of particular importance due to its high affinity for binding gingival epithelial cells for better adaptation to the root surface.[14,15] These characteristics may have contributed in better PPD reduction and CAL gain in the Group 2 [Table 4]. Johnson et al. reported the decrease in the IL-11 in the periodontal pockets that were deeper than 6 mm and higher in pocket with <3 mm and healthy tissues.[4] In the present study, increase in the IL-11 along with PD reduction is in accordance with the study by Johnson et al.[4]
The chorion membrane has numerous advantages owing to its structure and composition. Moreover, the membrane when placed is dry and quickly hydrates with blood, becomes very pliable, and closely adapts to the contours of the underlying alveolar bone. It is self-adherent in nature and does not need to be fixed into place using sutures. It is resorbable, avoiding secondary surgical intervention for its removal.
CONCLUSION
Thus, within the limits of our study, it is suggested that adjunctive use of chorion membrane during periodontal pocket therapy provided an additive anti-inflammatory effect along with improvement in clinical outcomes enhancing the long-term prognosis. However, further long-term studies with larger sample size for varied anti-inflammatory markers need to be evaluated.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
We would like to thank Vaishali Dodamani, Biochemist, Department of Clinical Biochemistry, Dr. Prabhakar Kore Hospital and Medical Research Centre, Belagavi.
REFERENCES
- 1.McQuilling JP, Vines JB, Kimmerling KA, Mowry KC. Proteomic comparison of amnion and chorion and evaluation of the effects of processing on placental membranes. Wounds. 2017;29:E38–42. [PMC free article] [PubMed] [Google Scholar]
- 2.Kothiwale SV, Anuroopa P, Gajiwala AL. A clinical and radiological evaluation of DFDBA with amniotic membrane versus bovine derived xenograft with amniotic membrane in human periodontal grade II furcation defects. Cell Tissue Bank. 2009;10:317–26. doi: 10.1007/s10561-009-9126-3. [DOI] [PubMed] [Google Scholar]
- 3.Kothiwale SV. The evaluation of chorionic membrane in guided tissue regeneration for periodontal pocket therapy: A clinical and radiographic study. Cell Tissue Bank. 2014;15:145–52. doi: 10.1007/s10561-013-9386-9. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RB, Wood N, Serio FG. Interleukin-11 and IL-17 and the pathogenesis of periodontal disease. J Periodontol. 2004;75:37–43. doi: 10.1902/jop.2004.75.1.37. [DOI] [PubMed] [Google Scholar]
- 5.Prasad R, Suchetha A, Lakshmi P, Darshan MB, Apoorva SM, Ashit GB, et al. Interleukin-11 – Its role in the vicious cycle of inflammation, periodontitis and diabetes: A clinicobiochemical cross-sectional study. J Indian Soc Periodontol. 2015;19:159–63. doi: 10.4103/0972-124X.152108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Silness J, Loe H. Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 8.Ciancio SG. Current status of indices of gingivitis. J Clin Periodontol. 1986;13:375. doi: 10.1111/j.1600-051x.1986.tb01476.x. [DOI] [PubMed] [Google Scholar]
- 9.Newman MG, Takei H, Klokkevold PR, Carranza FA. Carranza's Clinical Periodontology. 10th ed. St. louis: Saunders; 2006. p. 936. [Google Scholar]
- 10.Yücel OO, Berker E, Gariboğlu S, Otlu H. Interleukin-11, interleukin-1beta, interleukin-12 and the pathogenesis of inflammatory periodontal diseases. J Clin Periodontol. 2008;35:365–70. doi: 10.1111/j.1600-051X.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 11.Solomon A, Rosenblatt M, Monroy D, Ji Z, Pflugfelder SC, Tseng SC, et al. Suppression of interleukin 1alpha and interleukin 1beta in human limbal epithelial cells cultured on the amniotic membrane stromal matrix. Br J Ophthalmol. 2001;85:444–9. doi: 10.1136/bjo.85.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J Biomed Mater Res B Appl Biomater. 2014;102:1353–62. doi: 10.1002/jbm.b.33141. [DOI] [PubMed] [Google Scholar]
- 13.Hao Y, Ma DH, Hwang DG, Kim WS, Zhang F. Identification of antiangiogenic and antiinflammatory proteins in human amniotic membrane. Cornea. 2000;19:348–52. doi: 10.1097/00003226-200005000-00018. [DOI] [PubMed] [Google Scholar]
- 14.Bryant-Greenwood GD. The extracellular matrix of the human fetal membranes: Structure and function. Placenta. 1998;19:1–1. doi: 10.1016/s0143-4004(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 15.Pakkala T, Virtanen I, Oksanen J, Jones JC, Hormia M. Function of laminins and laminin-binding integrins in gingival epithelial cell adhesion. J Periodontol. 2002;73:709–19. doi: 10.1902/jop.2002.73.7.709. [DOI] [PubMed] [Google Scholar]


