Abstract
Background:
Low-level laser therapy (LLLT) is based on the principle of biostimulation or biomodulation effect. LLLT after gingivectomy has resulted in better wound healing because of its action on collagen synthesis, angiogenesis, and growth factor release.
Aim:
The aim of this split-mouth controlled clinical trial was to assess the effect of LLLT, using diode laser (InGaAsP), on wound healing and patients' response after scalpel gingivectomy.
Materials and Methods:
Forty patients with gingival enlargement in the maxillary and mandibular anterior region (bilaterally symmetrical) were included in the study. After gingivectomy, a diode laser (InGaAsP) was randomly applied to one side of the surgical area on the 1st, 3rd, and 7th day postoperatively. The surgical areas were disclosed by a solution (Alpha Plac®) to visualize the areas where the epithelium was absent. Comparison of the surface areas on the LLLT-applied sites and controls was made clinically by visualizing the stained area by two examiners.
Results:
LLLT-applied sites had significantly lower stained areas signifying improved wound healing compared with the controls on the postoperative 7th and 30th day.
Conclusion:
Within the limitations of this study, the results indicated that LLLT might improve wound healing after gingivectomy.
Keywords: Biostimulation, diode laser, gingivectomy, healing, low-level laser therapy
INTRODUCTION
The periodontal surgical procedures are primarily aimed at reestablishment of anatomical and physiological conditions, resulting in long-term health and proper functioning of the periodontium. Gingival enlargement is the overgrowth of the gingiva characterized by an expansion and accumulation of the connective tissue with occasional presence of increased number of cells.[1] The gingival enlargement or overgrowth is related to various etiologic factors such as dental plaque, mouth breathing, hormonal imbalance, and medications.[2] Gingival enlargement may hamper patients' esthetics if present in the anterior areas and may also lead to further plaque accumulation due to altered gingival contours, thus causing further destruction. Thus, gingival enlargement frequently needs to be treated with surgical intervention. Gingivectomy is the most commonly performed procedure for the treatment of gingival enlargements.[3]
Gingivectomy refers to the removal of diseased gingiva, which can be traced back to the Romans. Gingivectomy involves the removal of the pocket wall and provides visibility and accessibility for complete removal of calculus and thorough smoothening of roots. This results in a favorable environment for gingival healing and restoration of physiologic gingival contour. Gingivectomy may be indicated in elimination of suprabony pockets regardless of their depth with fibrous and firm pocket wall, elimination of gingival enlargements, and elimination of suprabony periodontal abscesses.[4,5] Apart from these indications, it can also be performed for prosthetic and esthetic reasons to provide normal gingival architecture.
Gingivectomy can be performed with the use of scalpel, lasers, electrosurgical unit, and chemicals such as 5% paraformaldehyde or potassium hydroxide. The scalpel gingivectomy method can be performed either using gingivectomy knives (e.g., Kirkland knife and Orbans knife) or using surgical blades. Improved understanding of healing processes and the development of more sophisticated flap methods have transferred gingivectomy to a lesser role in the current repertoire of available techniques. However, gingivectomy still remains an effective form of treatment when indicated.[5]
Healing of gingivectomy wound is known to take by secondary intention and it takes about 4 weeks for complete epithelialization and about 7 weeks for connective tissue maturation.[6] This finding confirms the fact that wound-healing process after scalpel gingivectomy is a slow phenomenon.[6] Thus, to accelerate the wound-healing process, several studies have been conducted evaluating the effect of various topical medicaments, antibiotics, or amino acids.[7,8] These studies have reported improved healing of wounds after the application of various agents by secondary intention.[7,8]
In recent years, low-level lasers have also been evaluated regarding their efficacy in wound-healing process in both medical and dental fields.[9] Low-level lasers work in 1–500 mW power range and wavelengths in the red or near-infrared to visible light spectrum (400–980 nm).[10,11,12] Low-level lasers are not used for cutting or ablation of the tissues. Their basic mechanism of action is based on the principle of biostimulation or the photobiomodulation.[13] This biostimulatory action is mainly due to its nonthermal effect and helps in altering the cellular behavior.[13,14,15,16] It causes cellular changes by acting either on mitochondrial respiratory chain or on membrane calcium channels.[17,18] This further helps in promoting the cellular metabolism and proliferation.[19] The use of low-level laser therapy (LLLT) as a therapeutic agent was first investigated by Mester et al.,[19] who found improvement in wound healing in rat models. LLLT is known to not only accelerate the repair process but also possess immediate analgesic effect.[10,11] Hence, it is widely used in the pain reduction therapy nowadays.[11] However, the use of LLLT has still not been widely accepted by the medical and dental communities due to lack of sufficient number of controlled clinical trials.
LLLT is delivered using various types of light sources which include helium-neon, ruby, diode, and gallium arsenide. Diode lasers with different wavelengths are also used to perform LLLT. Diode lasers with wavelength of 588,[20] 670,[21] and 685 nm have already been used to perform LLLT.[22,23] Studies using diode lasers in the range of 940 nm for LLLT are very limited, and to the best of our knowledge, not a single study has been conducted wherein 940-nm diode laser was used to perform LLLT after gingivectomy.
Thus, the aim of this present randomized split-mouth study was to assess the effect of LLLT, using 940-nm diode laser (InGaAsP) on patients' response and wound healing after scalpel gingivectomy.
MATERIALS AND METHODS
The present clinical trial was a single-center, randomized controlled, split-mouth study, which was carried out in the Department of Periodontology for 1 year. Forty systemically healthy patients who fulfilled the following inclusion and exclusion criteria and were willing to participate in the study were recruited. All study participants were explained about the procedure after which they voluntarily signed informed consent. The study protocol was approved by the Institutional Ethical Committee.
The inclusion criteria were as follows: (1) cases indicated for gingivectomy in the maxillary and mandibular anterior region (bilaterally symmetrical); (2) inflammatory and noninflammatory gingival enlargement excluding drug-induced enlargements (e.g., plaque induced and orthodontic patients); (3) subjects free of any systemic disease and took no medication in the last 6 months; (4) nonsmokers, nontobacco chewers, subjects not using nicotine replacement therapy; and (5) patients maintaining good oral hygiene.
Patients who were medically compromised or under therapeutic regimen that could decrease the probability of optimum soft-tissue healing, pregnant ladies and nursing mothers, patients who were allergic to materials and drugs used or prescribed in the study, and uncooperative patients were excluded.
A split-mouth design was employed in each patient. Each patient acted as his/her own control, and thus, the comparison was standardized. The selection of the site (left/right) was done randomly, using a coin flip technique, into two groups.
Test group–40: Surgical gingivectomy with LLLT
Control group–40: Surgical gingivectomy without LLLT.
Surgical procedure
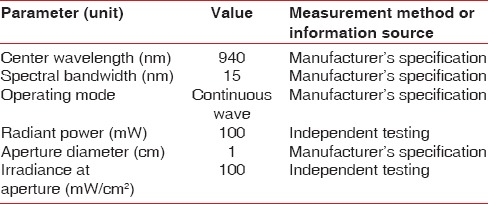
All the patients underwent presurgical preparation which consisted of thorough scaling and root planing, with oral hygiene instructions. Patients after undergoing nonsurgical therapy were recalled after 4 weeks for reevaluation. Surgical external bevel gingivectomy was performed at the same time in both test and control sides [Figure 1]. Immediately after surgery, the LLLT was performed with a 940-nm diode laser (InGaAsP) and repeated on the 3rd and 7th day postoperatively [Figure 2]. LLLT and treatment parameters are reported according to the recommendation proposed by Jenkins and Carroll[24] in Tables 1–3. To keep the patient blinded for the area receiving LLLT, the contralateral control side was exposed with laser beam with the activation button off (no energy delivered) with similar movement and time required for the test side. On the 3rd day follow-up, after LLLT, plaque-disclosing solution (methylene blue) was applied over the surgical area using a cotton pellet and kept for 2 min. After that, the patient was asked to rinse with water. The areas where epithelium was absent were stained blue with the dye. Staining of the surgical areas was repeated on the 7th day and 1 month postoperatively to visualize the surface lacking epithelium [Figures 3–5]. The surgical site was not covered with periodontal dressing.
Figure 1.
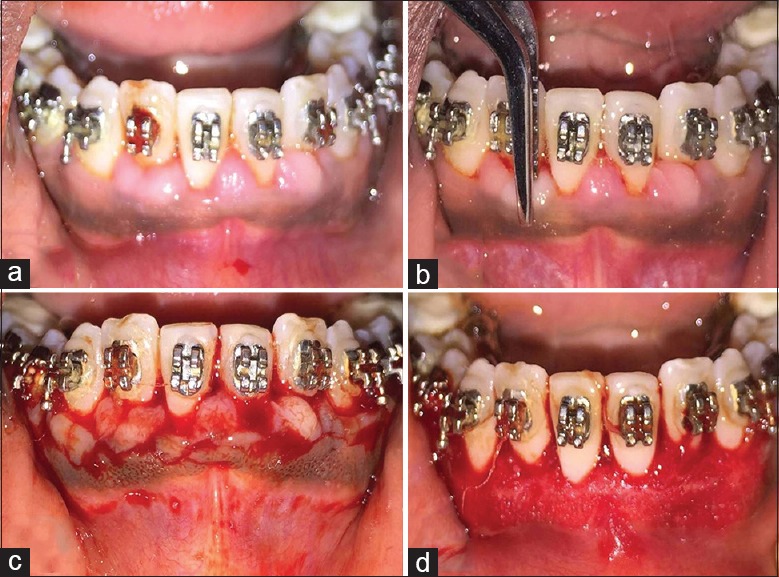
(a) Preoperative view of a patient requiring gingivectomy; (b) Bleeding points marked using pocket marker; (c) external bevel incision placed; (d) immediately after the surgery
Figure 2.
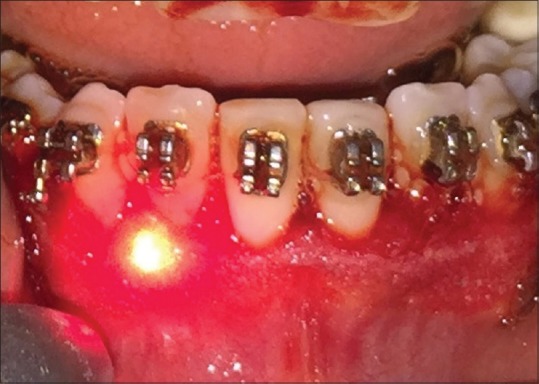
Low-level laser application
Table 1.
Device information

Table 3.
Treatment parameters

Figure 3.
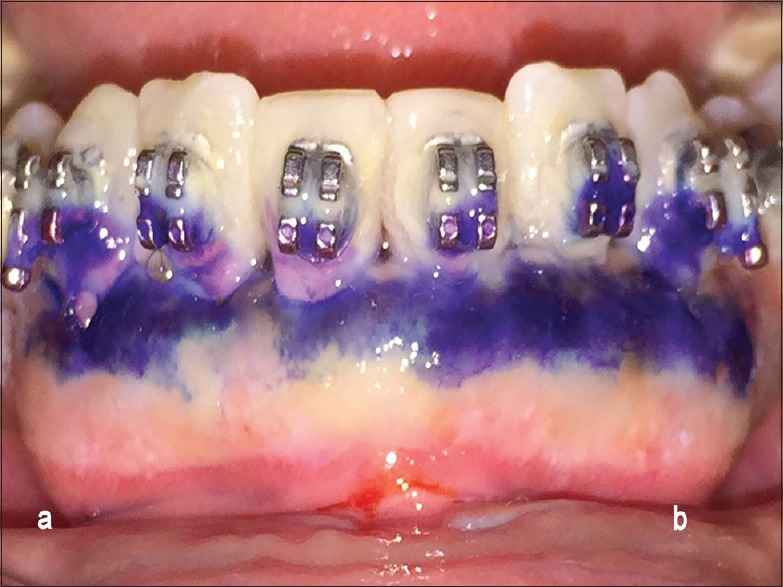
Methylene blue-stained area of test sites (a) and control sites (b) on the 3rd postoperative day
Figure 5.
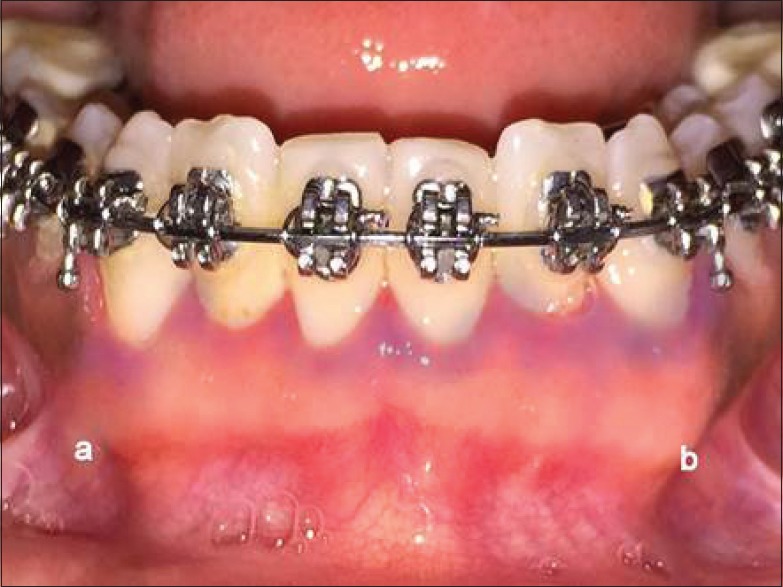
Methylene blue-stained area of test sites (a) and control sites (b) after 1 month
Table 2.
Irradiation parameters
Standard postoperative instructions were given. Patients were instructed to take pain medication if required. Patients were also instructed not to brush on the surgical site for 7 days and were asked to rinse with chlorhexidine 0.2% mouthwash 10 ml twice daily.
Patients were instructed to report any time after surgery in case of bleeding or any emergency that patients feel should be reported to the surgeon. In the absence of any concern, the patients were instructed to report back at 3rd-day, 7th-day, and 1-month postsurgery. Thus, total period required for treating a single patient was of 2 months.
At each follow-up visit, test sites received LLLT and the surgical area was stained with methylene blue to check for surface epithelialization which was rated by two observers visually according to the three-point scale. After gingivectomy, the surgical wound was stained using plaque-disclosing solution (methylene blue) at each postoperative visit. This solution has been used previously for detecting even minor areas of gingival abrasion, which would otherwise be largely undetectable, and this method has been suggested to be a sensitive tool for the identification of areas lacking epithelium.[25] A three-point scale was used to evaluate the stained surgical area.[22] Score + 1 was given when the LLLT-treated site showed less stained area, score 0 was given when both the sites showed the same amount of stained area, and score − 1 was given when control site showed less stained surface area. According to this scale, the stained surface area was visually observed by two observers for intraexaminer agreement and assessed using kappa index on 3rd, 7th, and 30th day, respectively.
Evaluation of patients' response was performed using numeric rating pain scale at every recall visit. Numeric rating scale (NRS) is a line of 10 cm in length, the extremes of which represent the limits of pain a patient might experience. Patients were asked to place a mark on the 10-cm line that indicated the intensity of their current level of sensitivity. Patients were asked to rate the pain scale on both test and control surgical sites.[26]
Wound healing was assessed clinically at every recall visits using Landry index.[27] The healing index (HI) scores healing on the basis of redness, presence of granulation tissues, bleeding, suppuration, and epithelialization. A score of 1–5 was given with score 1 for very poor healing and 5 being excellent healing of the tissues. Higher is the score, better is the healing. This index scores the surgical wound based on the clinical examination.
Statistical analysis
All the data were collected, filtered, and tabulated followed by descriptive and analytical statistics. Since the data did not follow normal distribution, Shapiro–Wilk test (nonparametric test) was used to analyze the data. The Mann–Whitney U-test and Wilcoxon signed-ranked test were used to check differences between the groups wherever appropriate. All the statistical analyses were performed using Statistical Package for the Social Sciences version 20.1 software (Chicago, IL, USA Inc.)
RESULTS
The participants (n = 40) in the study were in the age group of 14–30 years. All the patients completed the study, which was carried over 30 days [Figure 6]. The mean age of the participants was 19.15 years. Out of the total 40 participants, 21 were female with a mean age of 16.81 years and 19 were male with a mean age of 22 years [Table 4].
Figure 6.
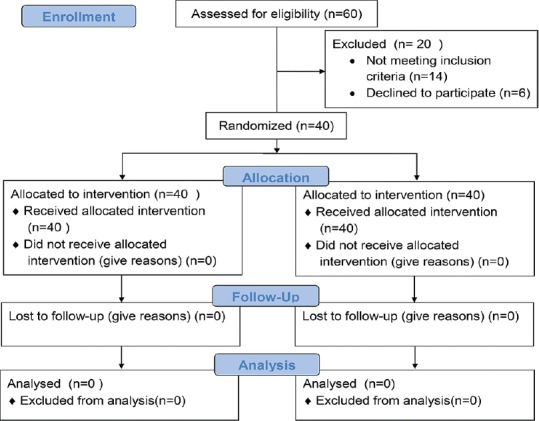
Consort flowchart
Table 4.
Study population demographics

Patient's pain response
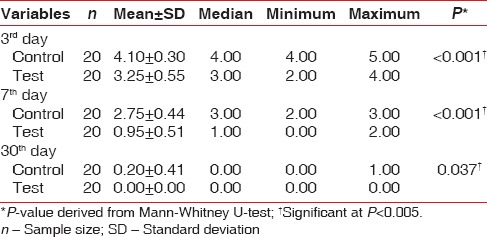
The results of this study showed that the mean NRS scores in the test group on the postoperative 3rd day was 3.25 ± 0.55 which was reduced to 0.95 ± 0.51 on the 7th day. Further reduction in the mean pain scores was observed at the end of 1 month, which was significantly lower than the NRS scores in the control group [Table 5]. In addition, the intragroup comparison of mean NRS scores in the test as well as control groups showed significant differences from 3rd day to 30th day [Table 6].
Table 5.
Intragroup comparison of numeric rating scale scores
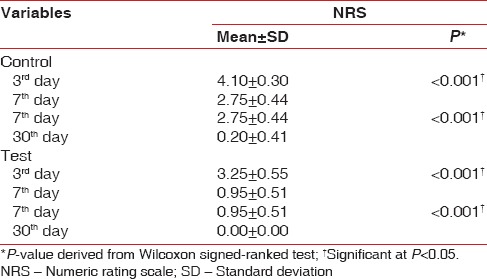
Table 6.
Intergroup comparison of numeric rating scale scores
Healing index scores
Comparison of the mean healing scores within the same group revealed statistically significant differences (P < 0.005) in both the test and control groups on the postoperative day 3, postoperative day 7, and after 1 month [Table 7]. There was statistically significant difference in the mean healing scores (P < 0.005) on the postoperative 3rd day, 7th day, and 1 month in the test as well as control groups. The mean healing score in the control group on the postoperative 3rd day was 2.60 ± 0.50, which was increased to 2.85 ± 0.36 on the 7th day. On 30th day, the mean healing scores further increased to 4.75 ± 0.44. The mean healing score in the test group on the postoperative 3rd day was 3.00 ± 0.00, which was increased to 3.35 ± 0.48 on the 7th day. On day 30th, the mean healing scores further increased to 5.00 ± 0.00 [Table 8].
Table 7.
Intragroup comparisons of mean healing index scores at 3rd day and 7th day and 7th day and 30th day of the control and test group
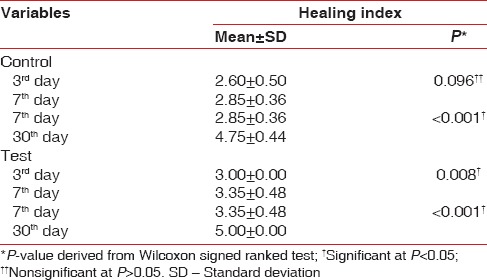
Table 8.
Intergroup comparisons of healing index scores at baseline, 3rd day, 7th day, and 30th day between the control and test group
Degree of keratinization
All the observations were tabulated, and it was seen that the LLLT-treated sides received a score + 1 at each postoperative visits in most of the patients. Scores from the two observers were tabulated, and scoring was calculated at 3rd day, 7th day, and 1 month postsurgery [Figures 2–4]. The percentage of between the observers at 3rd, 7th, and 30th day was 90%, 90%, and 80%, respectively. According to the kappa values, the level of between the two observers was strong on day 7 and almost perfect on day 3 and day 30 [Table 9].
Figure 4.
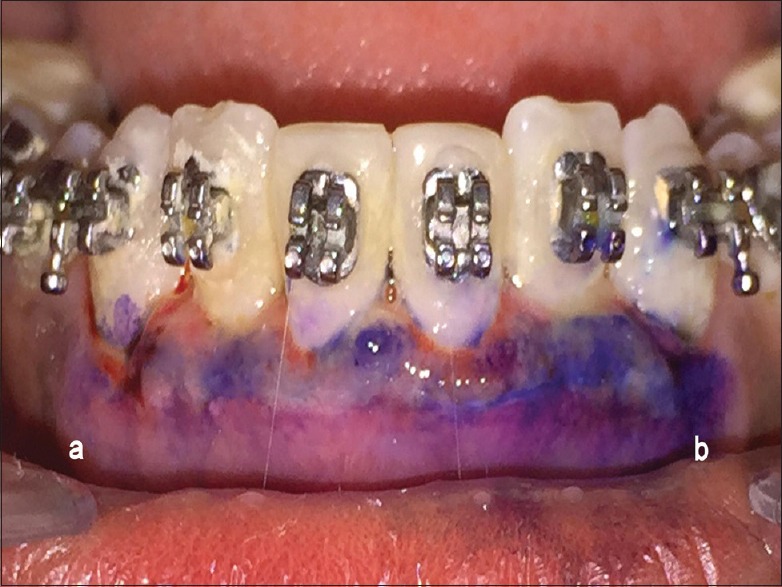
Methylene blue-stained area of test sites (a) and control sites (b) on the 7th postoperative day
Table 9.
Measurement of interexaminer variability

DISCUSSION
Numerous studies have been conducted over the years evaluating healing after periodontal surgical procedures. In the present clinical trial, effect of LLLT on the wound healing after gingivectomy was evaluated. In addition, effect of LLLT on pain reduction was evaluated to check whether the laser therapy provides any added benefit in improving patient's comfort postsurgically.
LLLT is performed with the help of low-level lasers or light-emitting diodes, which alters the cellular functions. LLLT is a noninvasive, painless process that provides biological therapeutic advantages, including analgesic effects.[11]
Various studies have shown that LLLT provides beneficial effect in pain reduction. The results of the present study showed that the mean pain score in the test group was comparatively less on the postoperative 3rd day, on the postoperative 7th day, and at the end of 1 month than the control group. These results explain the positive effect of LLLT on patient's pain response after scalpel gingivectomy. Patients in the test group that is with LLLT experienced significantly lower postoperative pain as compared to control group. Our results are in accordance with a study conducted by Sanz-Moliner et al.,[28] who concluded that less pain was reported by patients at the sites where LLLT was performed after modified Widman flap surgery. Similar results were reported in a study performed by Tomasi et al.[29] where they concluded that low-level lasers showed an analgesic effect when used during periodontal maintenance. However, these results are in contradiction to a study conducted by Masse et al.,[30] who did not find a significant analgesic effect of low-level lasers when used after the placement of free gingival grafts. These findings suggest that there is still no consensus regarding the efficacy of low-level laser in pain reduction after periodontal surgeries. This may due to the heterogeneity of studies, differences in laser parameters used, and most important the complex mechanism of LLLT. LLLT may reduce pain associated with inflammation by lowering the levels of prostaglandin E2, interleukin-1 beta, tumor necrosis factor alpha, cellular influx of neutrophils and granulocytes, oxidative stress, edema, and bleeding in a dose-dependent manner.[31] Another mechanism proposed in pain reduction is stabilization of nerve cell membrane, enhancement of cell revival system, and increased adenosine triphosphate (ATP) production.[11,32] However, it is difficult to comment regarding the mechanism by which low-level lasers caused pain reduction in the present study.
LLLT has been the focus of various in vitro and in vivo studies in oral and periodontal surgical procedures. Its growing attention is based on the patient's demands for the need of minimally invasive and less painful dental procedures. However, there is limited evidence regarding its efficacy in periodontal applications because of less number of controlled clinical trials.
LLLT apart from pain reduction is also known to help in repair process and thus subsequently accelerating the wound-healing process. Thus, in recent years, research is focused on evaluating the effect of LLLT on wound-healing processes. Low-level lasers are known to have a stimulatory effect on cells at low dosage and a suppressive effect on cells at high dosage. Mechanism by which low-level laser accelerates the healing process is by stimulating the mitochondrion to increase ATP production to increase the reactive oxygen species, which in turn influences redox signaling, affecting intercellular homeostasis of the proliferation of cells.[31] LLLT also has an effect on the microcirculation, which reduces edema by changing the capillary hydrostatic pressure.[33] The ideal dose of LLLT leads to the formation of new endothelium and blood vessels that will help in granulation tissue formation and accelerated healing.
Previous studies suggest that LLLT application may accelerate wound healing by increasing the motility of human keratinocytes and promoting early epithelialization, by increasing fibroblast proliferation and matrix synthesis, and by enhancing neovascularization. It has also been shown that the expression of fibroblast growth factors by macrophages and fibroblasts is increased after LLLT application.[34] Another effect of LLLT on wound healing is to increase the revascularization rate as it is known that successful wound healing following periodontal surgery is strongly influenced by the revascularization rate.[35]
In the present study, healing was assessed clinically using Landry HI at all postoperative visits.[27] The healing scores in the control as well as test groups increased from day 3 to day 30, indicating healing of the surgical wound. Comparison of the mean healing scores within the same group revealed statistically significant differences (P < 0.005) in both the test and control groups on postoperative day 3, day 7, and day 30. Test group showed better healing as compared to the control group. Mean scores in the test group were greater than that of the control group at all postoperative visits. Our results are in agreement with the study conducted by Amorim et al.[22] and Mârţu et al.[23] Both the studies reported that the use of LLLT showed better repair and improved healing of the damaged tissues.[22,23]
In the present study, LLLT was applied to the test sites immediately after surgery followed by day 3 and day 7. The laser used for LLLT was a diode laser with a wavelength of 940 nm and output power of 100 mW scanning an area of 1 cm2 for 40 s in noncontact mode. The resultant energy density delivered was 4 J/cm2. However, in a study conducted by Ozcelik et al.,[20] LLLT was applied using a 588-nm diode laser with an output power of 120 mW. The surgical area was irradiated for 5 min in continuous wave mode daily for 7 days and resultant energy was 4 J/cm2. The results of the present study showed better healing of the test sites at the end of 1 month which were in accordance with the study by Ozcelik et al.,[20] who reported that wound healing on the LLLT-applied sites clinically showed complete healing at around 21 days, whereas on the control sites, it was around 24 days. These results indicate that there was no difference in wound healing based on the number of application of LLLT on the test sites. In the present study, LLLT application was done immediately after surgery, on the 3rd day, and on the 7th day, and this three-time application proved to be sufficient as better wound healing was observed in the test sites.
Day 3 and day 7 were selected for LLLT application as there are formation and proliferation of newer blood vessels and fibroblasts in the initial stages of wound healing. This was done in accordance with other studies by Ozcelik et al.[20] and Ahmed et al.,[35] who also used LLLT application during the first 7 days of wound healing.
Although there are many studies reporting the positive effect of LLLT on wound healing, there are few studies with contradictory results. In one such study performed by Damante et al.,[21] it was concluded that LLLT did not accelerate the healing of oral mucosa after gingivoplasty. These authors used a 15-mW GaAlAs laser in a punctual mode over the wound surface. On the other hand, in the present study, a 100-mW diode laser was used for LLLT in continuous wave mode in a noncontact manner over the surgical area. Our results show that these parameters were adequate to elicit a better-healing process in laser-treated wounds when compared to control wounds. In another study by Ahmed,[35] where they clinically evaluated the effects of aluminum gallium arsenate laser 670 nm in wound healing after gingivectomy in 11 patients. LLLT was applied to the test sites at a 48-h interval over a period of 1 week. The best-healed sides were observed through photography of the treated areas at postsurgical periods of 7, 15, 21, 30, and 60 days. The results showed that low-intensity laser therapy did not accelerate healing after gingivectomy.
Differences in the results may be due to the laser output power and method of irradiation. Wavelength is another important parameter in the evaluation of the effects of laser radiation. Although the best wavelength for each clinical situation has not yet been determined, a diode laser with 940 nm wavelength was used for LLLT in this study.
In the present study, methylene blue was used to evaluate the wound epithelialization as compared to conventionally used hydrogen peroxide. This was because of the cytotoxic effects of hydrogen peroxide on human gingival fibroblast which could have potentially impaired the wound healing.[36] The epithelialization was assessed visually by two periodontists, and kappa values were used to assess their agreement. The results showed that LLLT-applied sites displayed better healing as compared to the control sites. There was less amount of stained surgical area (surface lacking epithelium) in the test sites. These results are in accordance with the study conducted by Ozcelik et al.,[20] where they found that the surgical area was less stained in sites treated with LLLT. However, in the present study, visual examination was performed by two examiners as compared to the previous study where image analysis was done. This was done because examination of the surgical sites clinically and visually provides help in better assessment.
The limitations of this study are as follows:
The sample size was small
Patients were followed up for a short period of time (1 month)
Healing was not assessed histologically.
CONCLUSION
Based on the results of this study, it can be concluded that the LLLT using 940-nm diode laser at an energy density of 4 J/cm2 as an adjunct to scalpel gingivectomy procedure can be used to reduce postoperative pain and discomfort and aid in better wound healing.
There is a need to establish effective protocols of laser application, allowing this novel therapy to be used in periodontology and bring more comfort for the patients. Further studies should be conducted along with various other surgical procedures to evaluate the effect of adjunctive use of LLLT on wound healing and patients response.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Agrawal AA. Gingival enlargements: Differential diagnosis and review of literature. World J Clin Cases. 2015;3:779–88. doi: 10.12998/wjcc.v3.i9.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mariotti A. Dental plaque-induced gingival diseases. Ann Periodontol. 1999;4:7–19. doi: 10.1902/annals.1999.4.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Camargo PM, Carranza FA, Pirih FQ, Takei HH. Treatment of gingival enlargement. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Carranza's Clinical Periodontology. 12th ed. Philadelphia: W.B. Saunders and Company; 2015. pp. 587–92. [Google Scholar]
- 4.Glickman I. The results obtained with the unembellished gingivectomy technique in a clinical study in humans. J Periodontol. 1956;27:247–55. [Google Scholar]
- 5.Takei HH, Carranza FA, Shin K. Gingival surgical techniques. In: Newman MG, Takei HH, Klokkevold PR, Carranza FA, editors. Carranza's Clinical Periodontology. 12th ed. Philadelphia: W.B. Saunders and Company; 2015. pp. 576–81. [Google Scholar]
- 6.Stanton G, Levy M, Stahl SS. Collagen restoration in healing human gingiva. J Dent Res. 1969;48:27–31. doi: 10.1177/00220345690480011901. [DOI] [PubMed] [Google Scholar]
- 7.Değim Z, Celebi N, Sayan H, Babül A, Erdoğan D, Take G, et al. An investigation on skin wound healing in mice with a taurine-chitosan gel formulation. Amino Acids. 2002;22:187–98. doi: 10.1007/s007260200007. [DOI] [PubMed] [Google Scholar]
- 8.Sigusch B, Beier M, Klinger G, Pfister W, Glockmann E. A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J Periodontol. 2001;72:275–83. doi: 10.1902/jop.2001.72.3.275. [DOI] [PubMed] [Google Scholar]
- 9.Liao X, Xie GH, Liu HW, Cheng B, Li SH, Xie S, et al. Helium-neon laser irradiation promotes the proliferation and migration of human epidermal stem cells in vitro: Proposed mechanism for enhanced wound re-epithelialization. Photomed Laser Surg. 2014;32:219–25. doi: 10.1089/pho.2013.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR, et al. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40:516–33. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobouti F, Khatami M, Heydari M, Barati M. The role of low-level laser in periodontal surgeries. J Lasers Med Sci. 2015;6:45–50. [PMC free article] [PubMed] [Google Scholar]
- 12.Qadri T, Miranda L, Tunér J, Gustafsson A. The short-term effects of low-level lasers as adjunct therapy in the treatment of periodontal inflammation. J Clin Periodontol. 2005;32:714–9. doi: 10.1111/j.1600-051X.2005.00749.x. [DOI] [PubMed] [Google Scholar]
- 13.Walsh LJ. The current status of low level laser therapy in dentistry. Part 1. Soft tissue applications. Aust Dent J. 1997;42:247–54. doi: 10.1111/j.1834-7819.1997.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins JT, McLoda TA, Seegmiller JG, David Baxter G. Low-level laser therapy facilitates superficial wound healing in humans: A triple-blind, sham-controlled study. J Athl Train. 2004;39:223–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Posten W, Wrone DA, Dover JS, Arndt KA, Silapunt S, Alam M, et al. Low-level laser therapy for wound healing: Mechanism and efficacy. Dermatol Surg. 2005;31:334–40. doi: 10.1111/j.1524-4725.2005.31086. [DOI] [PubMed] [Google Scholar]
- 16.Silveira PC, Streck EL, Pinho RA. Evaluation of mitochondrial respiratory chain activity in wound healing by low-level laser therapy. J Photochem Photobiol B. 2007;86:279–82. doi: 10.1016/j.jphotobiol.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Alexandratou E, Yova D, Handris P, Kletsas D, Loukas S. Human fibroblast alterations induced by low power laser irradiation at the single cell level using confocal microscopy. Photochem Photobiol Sci. 2002;1:547–52. doi: 10.1039/b110213n. [DOI] [PubMed] [Google Scholar]
- 18.Khadra M, Kasem N, Lyngstadaas SP, Haanaes HR, Mustafa K. Laser therapy accelerates initial attachment and subsequent behaviour of human oral fibroblasts cultured on titanium implant material. A scanning electron microscope and histomorphometric analysis. Clin Oral Implants Res. 2005;16:168–75. doi: 10.1111/j.1600-0501.2004.01092.x. [DOI] [PubMed] [Google Scholar]
- 19.Mester E, Spiry T, Szende B, Tota JG. Effect of laser rays on wound healing. Am J Surg. 1971;122:532–5. doi: 10.1016/0002-9610(71)90482-x. [DOI] [PubMed] [Google Scholar]
- 20.Ozcelik O, Cenk Haytac M, Kunin A, Seydaoglu G. Improved wound healing by low-level laser irradiation after gingivectomy operations: A controlled clinical pilot study. J Clin Periodontol. 2008;35:250–4. doi: 10.1111/j.1600-051X.2007.01194.x. [DOI] [PubMed] [Google Scholar]
- 21.Damante CA, Greghi SL, Sant'Ana AC, Passanezi E, Taga R. Histomorphometric study of the healing of human oral mucosa after gingivoplasty and low-level laser therapy. Lasers Surg Med. 2004;35:377–84. doi: 10.1002/lsm.20111. [DOI] [PubMed] [Google Scholar]
- 22.Amorim JC, de Sousa GR, de Barros Silveira L, Prates RA, Pinotti M, Ribeiro MS, et al. Clinical study of the gingiva healing after gingivectomy and low-level laser therapy. Photomed Laser Surg. 2006;24:588–94. doi: 10.1089/pho.2006.24.588. [DOI] [PubMed] [Google Scholar]
- 23.Mârţu S, Amălinei C, Tatarciuc M, Rotaru M, Potârnichie O, Liliac L, et al. Healing process and laser therapy in the superficial periodontium: A histological study. Rom J Morphol Embryol. 2012;53:111–6. [PubMed] [Google Scholar]
- 24.Jenkins PA, Carroll JD. How to report low-level laser therapy (LLLT)/photomedicine dose and beam parameters in clinical and laboratory studies. Photomed Laser Surg. 2011;29:785–7. doi: 10.1089/pho.2011.9895. [DOI] [PubMed] [Google Scholar]
- 25.Van der Weijden GA, Timmerman MF, Versteeg PA, Piscaer M, Van der Velden U. High and low brushing force in relation to efficacy and gingival abrasion. J Clin Periodontol. 2004;31:620–4. doi: 10.1111/j.1600-051x.2004.00529.x. [DOI] [PubMed] [Google Scholar]
- 26.Downie WW, Leatham PA, Rhind VM, Wright V, Branco JA, Anderson JA, et al. Studies with pain rating scales. Ann Rheum Dis. 1978;37:378–81. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landry RG, Turnbull RS, Howley T. Effectiveness of benzydamyne HCl in the treatment of periodontal postsurgical patients. Res Clin Forum. 1988;10:105–18. [Google Scholar]
- 28.Sanz-Moliner JD, Nart J, Cohen RE, Ciancio SG. The effect of an 810-nm diode laser on postoperative pain and tissue response after modified Widman flap surgery: A pilot study in humans. J Periodontol. 2013;84:152–8. doi: 10.1902/jop.2012.110660. [DOI] [PubMed] [Google Scholar]
- 29.Tomasi C, Schander K, Dahlén G, Wennström JL. Short-term clinical and microbiologic effects of pocket debridement with an Er: YAG laser during periodontal maintenance. J Periodontol. 2006;77:111–8. doi: 10.1902/jop.2006.77.1.111. [DOI] [PubMed] [Google Scholar]
- 30.Masse JF, Landry RG, Rochette C, Dufour L, Morency R, D'Aoust P, et al. Effectiveness of soft laser treatment in periodontal surgery. Int Dent J. 1993;43:121–7. [PubMed] [Google Scholar]
- 31.Gordon SA, Surrey K. Red and far-red action on oxidative phosphorylation. Radiat Res. 1960;12:325–39. [PubMed] [Google Scholar]
- 32.Bjordal JM, Johnson MI, Iversen V, Aimbire F, Lopes-Martins RA. Low-level laser therapy in acute pain: A systematic review of possible mechanisms of action and clinical effects in randomized placebo-controlled trials. Photomed Laser Surg. 2006;24:158–68. doi: 10.1089/pho.2006.24.158. [DOI] [PubMed] [Google Scholar]
- 33.Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A. Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg. 2005;23:3–9. doi: 10.1089/pho.2005.23.3. [DOI] [PubMed] [Google Scholar]
- 34.Tuby H, Maltz L, Oron U. Modulations of VEGF and iNOS in the rat heart by low level laser therapy are associated with cardioprotection and enhanced angiogenesis. Lasers Surg Med. 2006;38:682–8. doi: 10.1002/lsm.20377. [DOI] [PubMed] [Google Scholar]
- 35.Ahmed AH. Clinical assessment of low level laser (gaalas) on gingivectomy wound healing. Med J Babylon. 2013;10:349–53. [Google Scholar]
- 36.Furukawa M, K-Kaneyama JR, Yamada M, Senda A, Manabe A, Miyazaki A, et al. Cytotoxic effects of hydrogen peroxide on human gingival fibroblasts in vitro . Oper Dent. 2015;40:430–9. doi: 10.2341/14-059-L. [DOI] [PubMed] [Google Scholar]


