Abstract
Aim:
The aim of the present study was to assess the clinical and radiographical parameters in horizontal bone defects in patients with chronic periodontitis.
Materials and Methods:
In this randomized, controlled clinical trial study, nine individuals with 94 sites having moderate to deep periodontitis were selected and distributed to Group A – open flap debridement (OFD), Group B – open flap debridement and intra marrow penetration (OFD + IMP) and Group C – Open flap debridement + Intramarrow penetration + platelet rich fibrin matrix (OFD + IMP + PRFM). Plaque index (PI) and gingival index (GI) were evaluated at baseline, 6 months, and 9 months after surgery. Probing pocket depth (PPD) and clinical attachment level (CAL) were recorded at baseline and 9 months after surgery. Radiographic assessment was carried out to measure the periodontal defect depth and defect fill percentage (DF%) at baseline and 9 months after the surgery using radiovisiography.
Results:
The statistical evaluation obtained after 9 months showed no significant difference between PI and GI at 9 months interval. Intergroup comparison of PPD and CAL has shown significant difference in Group C as compared to Group A and B with P < 0.05. The defect depth was statistically significant at 9 months in all the groups, and DF percentage (DF%) has shown statistically significant results in Group C as compared to A and B with P = of 0.001.
Conclusion:
The addition of PRFM to horizontal type of periodontal defects has shown promising results over a 9-month follow-up period.
Keywords: Horizontal defects, intramarrow penetration, platelet-rich fibrin matrix
INTRODUCTION
Periodontitis causes inflammation and destruction of the supporting alveolar bone and periodontal ligament. The periodontal disease alters the morphologic features of bone in addition to reducing height. It leads to various patterns of bone loss of which horizontal and vertical bone loss are the most common bone destruction patterns.[1] The most common pattern of bone loss experienced is horizontal bone loss which is also called a zero wall defect as stated by Kern et al. 1984. It results in reduced height, but the bone margins remain perpendicular to the tooth surface. It was observed that vertical bone loss with a prevalence of 7.8% received 96.8% treatment options whereas horizontal bone loss, with an overwhelming prevalence of 92.2%, received only 3.2% treatment modalities.[2] The ideal goal of periodontal therapy is to promote healing through lost form, function, and esthetics and comfort.[3]
The literature has delineated several treatment modalities which have been attempted throughout the years including bone grafts, with the use of membrane and a combination of membrane and grafts materials. The biological substitute with the use of enamel matrix protein and recombinant human bone morphogenic protein has been evaluated for the treatment of horizontal defects. Various treatment outcome has been obtained and has shown an immense success rate in vertical and furcation defects.[4] In aspect of horizontal defects, open flap debridement has obtained fame in the literature.[5] With the advent of biologic approaches and other biomaterials, there was a renewed interest in the field of horizontal periodontal regeneration. Following any surgical procedure, a successful outcome mainly depends on various patient related, site-related, and surgical site-related factors. The utmost importance lies on fibrin clot adhesion and approximate flap adaptation.[6]
The year 1998 led to the innovation of platelet-rich plasma (PRP) by Marx et al which has been a boon in the field of edicine and dentistry.[7] PRP introduced required double centrifugation and addition of bovine thrombin made its preparation cumbersome.[8] Due to its drawbacks, platelet-rich fibrin matrix (PRFM) was introduced which has shown a robust release of growth factors and was known to enhance the healing process. It had shown successful results histologically, radiographically, and clinically in the treatment of periodontal defects.[9]
Thus, the present study aimed to evaluate the horizontal bony defects clinically and radiographically with and without the use of PRFM.
MATERIALS AND METHODS
Study design
The study was conducted in the Department of Periodontics. An ethical clearance was obtained from the institution. A total of nine participants with 94 sites were recruited based on the sample size calculation which showed the power of study as 95%. The conduct of the study protocol was illustrated in Figure 1.
Figure 1.
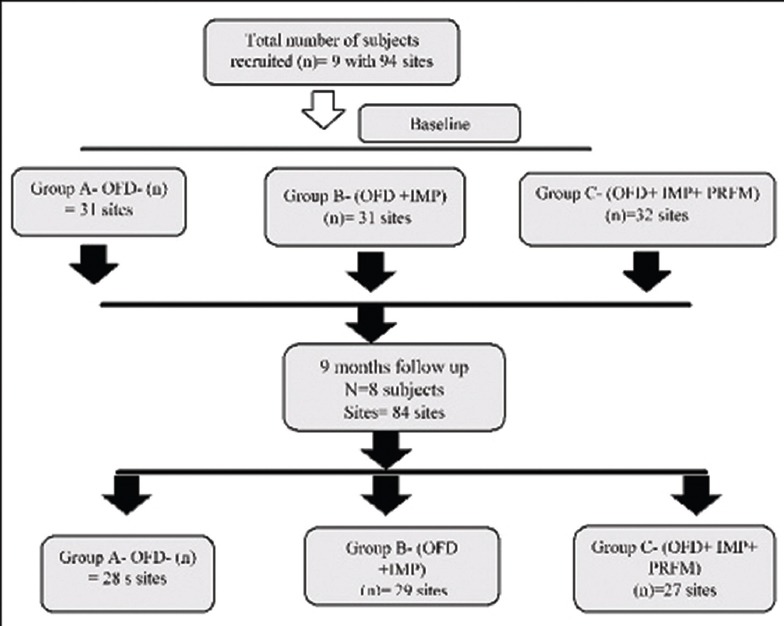
Study flow chart. n – number of subjects; OFD – Open flap debridement; IMP – Intra marrow penetration; PRFM – Platelet rich fibrin matrix
The inclusion criteria included moderate to deep chronic periodontitis with horizontal defects with pocket depths ranging from (5 to 8 mm). Horizontal bone loss was confirmed in the radiograph when the crest of the bone to the most apical area of the defect was parallel to cementoenamel junction of two adjacent sites. Patients with history of allergies to drugs and the use of antibiotics within previous 6 months were excluded from the study. Patients, with aggressive periodontitis, with known systemic illness and taking any medications known to affect the outcomes of periodontal therapy, pregnancy/lactation, and a history of smoking were also excluded.
Following the recruitment of participants based on inclusion and exclusion criteria, the participants were explained about the surgical procedure and the benefit they would achieve. Informed consent was obtained.
The 94 sites selected were randomly divided into three groups. The participants were divided accordingly into Group A in which 31 sites were treated with open flap debridement (OFD), 31 sites in Group B were treated with OFD along with intra marrow penetration (OFD + IMP), and Group C included the placement of PRFM following OFD + IMP (OFD + IMP) in the remaining 32 sites as seen in Figure 1.
Presurgical procedure included full-mouth scaling and root planing (SRP) followed by careful instructions of oral hygiene measures. At 4 weeks, reevaluation phase, periodontal examination was performed to record the suitability of the sites to be taken for the conduct of the study.
A single masked examiner performed the clinical examination at baseline, 6 months, and 9 months after the surgical procedure. At baseline, 6 months, and 9 months plaque index (PI),[10] GI[11] scores were evaluated. Periodontal probing depth (PPD), clinical attachment level (CAL), and radiovisiography (RVG) to measure the defect fill (DF) depth percentage (DF %)[12] were also recorded.
Surgical procedure
The surgical procedures followed for Group A, B, and C included anesthetization of the surgical sites with lidocaine 1: 200,000. Following anesthetization, crevicular incisions and interdental incisions were placed. A full thickness mucoperiosteal flap was raised. The periodontal surgical procedure fully exposed the horizontal defects and preserved the marginal gingiva and interdental tissue. Meticulous defect debridement and root planing were carried out to remove subgingival plaque, calculus, inflammatory granulation tissue, and pocket epithelium as observed in Figure 2. In Group B, in addition, IMP was performed with aerator handpiece and round bur with copious irrigation of water at a depth of 0.2 mm as depicted in Figure 3. In Group C following the debridment and IMP; PRFM gel was placed in the decorticated area as well as at the level of defect. In all the groups to achieve primary closure, direct interrupted sutures with 3-0 silk were performed as contemplated in Figure 4.
Figure 2.
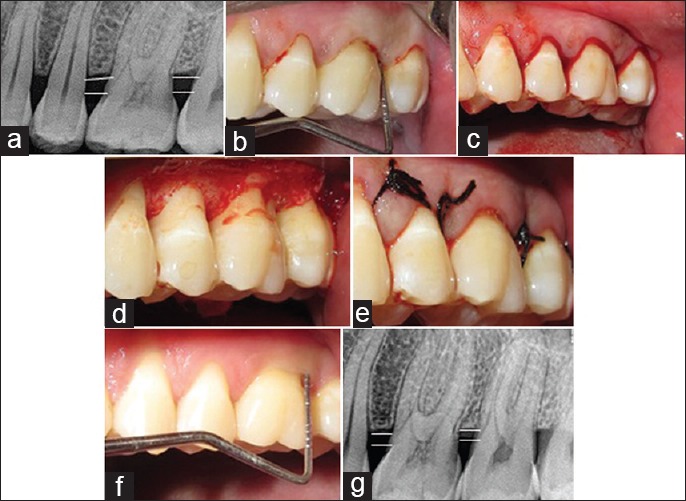
Group A OFD: (a) radiovisiography baseline-linear radiographic interpretation with AutoCAD computer software (parallel lines showed the base of the defect and crest of alveolar bone) (b) probing pocket depth, (c) incisions placed; (d) debridement done; (e) direct interrupted suture placed; (f) probing pocket depth at 9 months; (g) radiovisiography 9 months-linear radiographic interpretation with AutoCAD computer software (parallel lines showed the base of the defect and crest of alveolar bone). OFD – Open flap debridement
Figure 3.
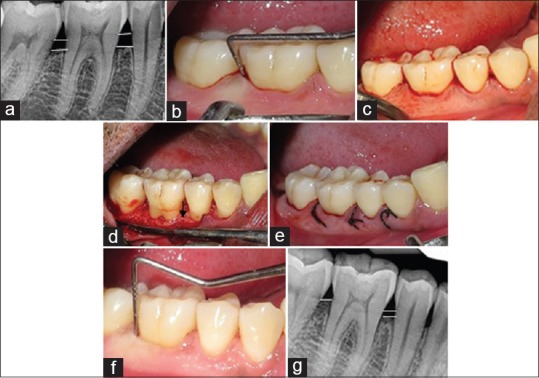
Group B (OFD + IMP) (a) radiovisiography baseline-linear radiographic interpretation with AutoCAD computer software (parallel lines showed the base of the defect and crest of alveolar bone) (b) probing pocket depth, (c) incisions placed; (d) debridement done along with intra marrow penetration; (e) direct interrupted suture placed; (f) probing pocket depth at 9 months (g) radiovisiography 9 months-linear radiographic interpretation with AutoCAD computer software (parallel lines showed the base of the defect and crest of alveolar bone). OFD + IMP – Open flap debridement with intramarrow penetration
Figure 4.
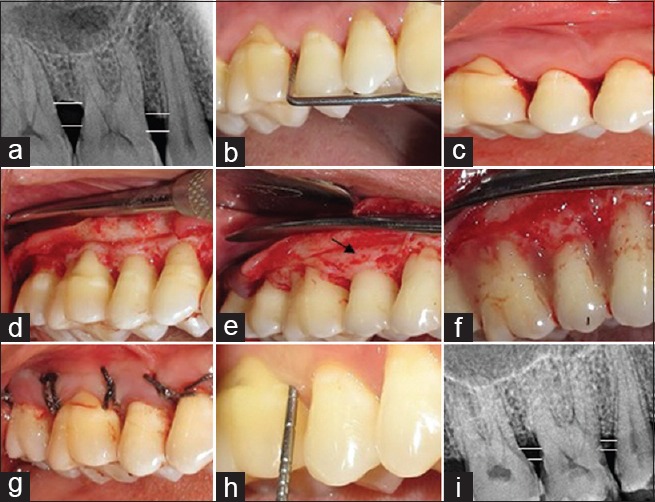
Group C (OFD + IMP + PRFM) (a) radiovisiography baseline-linear radiographic interpretation with AutoCAD computer software (parallel lines showed the base of the defect and crest of alveolar bone); (b) probing pocket depth; (c) incisions placed; (d) debridement done along with intra marrow penetration; (e) direct interrupted suture placed; (f) probing pocket depth at 9 month; (g) radiovisiography 9 months-linear radiographic interpretation with AutoCAD computer software (parallel lines showed the base of the defect and crest of alveolar bone). OFD + IMP + PRFM – Open flap debridement with intramarrow penetration and platelet-rich fibrin matrix gel
Preparation of platelet-rich fibrin matrix
Five milliliter of blood sample was collected from antecubital vein by venipuncture within 1 min from the participants recruited for Group C for the preparation of PRFM. The samples were immediately transferred within 30 s in the Meresis PRFM kit (*R-4C, REMI Laboratory Instruments, Mumbai, India) and placed in the REMI 4C centrifugation machine. It was centrifuged at rpm of 3000 for 10 min using single-spin centrifugation. The supernatant obtained at the top of gel was removed through syringe. An activator containing 0.1% gluconate was added and mixed for 9–10 times to obtain the PRFM gel as seen in Figure 5. Following centrifugation, the PRFM clot was obtained and placed in the defect area.[8]
Figure 5.
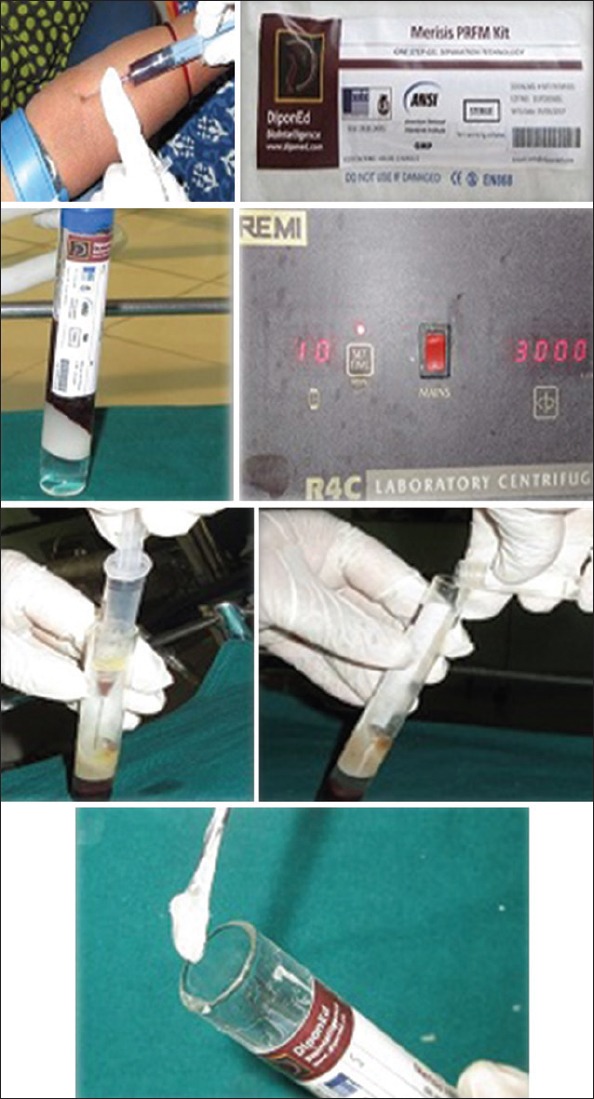
Preparation of platelet-rich fibrin matrix
Postoperative care
Postsurgical instructions were given along with 0.2% chlorhexidine digluconate rinse twice daily for one week. Sutures were removed 1-week postsurgery. Supragingival scaling was performed weekly for the first 6 weeks postsurgery, and thereafter, the patients were recalled at 3rd, 6th, and 9th month for oral hygiene reinforcement and prophylaxis. PI, GI were recorded at 3rd, 6th and 9th month visit. The clinical parameters PPD, CAL, and DF percentage were recorded at 9 months' follow-up period.
Statistical analysis
Out of nine participants recruited at initial commencement of the study; only eight participants with 84 sites had completed the entire 9-month follow-up period. Thus, the statistical evaluations were performed on 28 sites in Group A, 29 sites in Group B, and 27 sites in Group C as formulated in flowchart seen in Figure 1. The statistical analysis was done with Statistical Package for Social Sciences (SPSS) for Windows Version 22.0 Released 2013. Armonk, NY: IBM Corp. Descriptive analysis was performed for all the clinical parameters regarding mean and standard deviation. One-way ANOVA test followed by Tukey's honestly significant difference post hoc Analysis was used to compare the mean values of various study parameters at different time intervals. Student Paired t-test was used to compare the mean defect depth (in mm) between baseline and 9 months follow-up period. The level of significance was maintained at < 0.05.
RESULTS
Plaque index
The mean PI in Group A, B, and C at baseline were 1.4, 1.05, and 1.45, respectively, which were statistically nonsignificant as summarized in Table 1 and Figure 6. At 9th month follow-up period, the mean values of PI reduced to 0.66, 0.62, and 0.62 in Group A, B, and C, respectively, which showed an equal amount of improvement at follow-up periods as observed in Table 2 and Figure 7. On comparative evaluation, the intergroups also did not shown any statistical evaluation at 9 month follow-up period as tabulated in Table 3.
Table 1.
The mean values at of plaque index, gingival index, probing pocket depth, clinical attachment level, defect fill percentage at baseline in Group A, B, and C
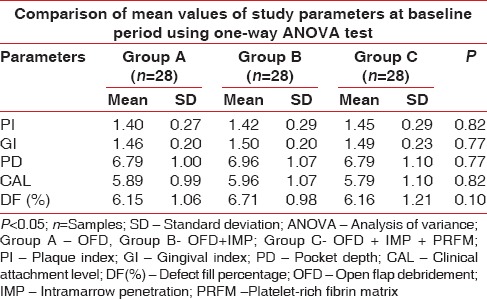
Figure 6.
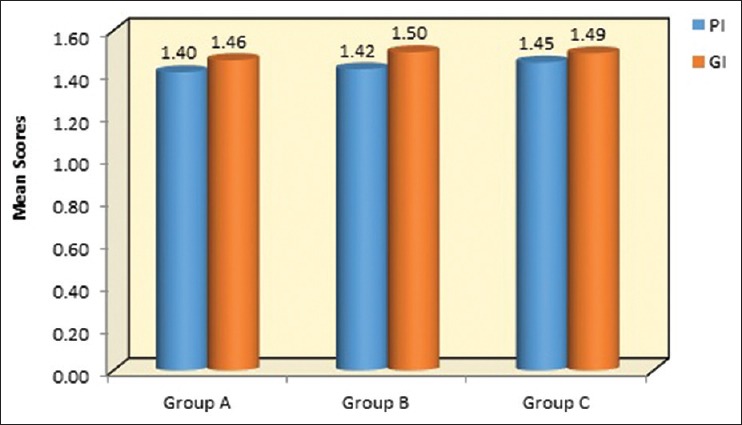
Graphical representation of mean values at of PI, GI, at baseline in group A-OFD, Group B-OFD + IMP and group C-OFD + IMP + PRFM. PI – Plaque index; GI – Gingival index; OFD – Open flap debridement; OFD + IMP – open flap debridement + intramarrow penetration; OFD + IMP + PRFM – open flap debridement + intra marrow penetration platelet-rich fibrin matrix
Table 2.
Comparison of mean values at of plaque index, Gingival index, Probing pocket depth, clinical attachment level, defect fill percentage at 9th month follow-up period in group A, B, and C using one-way analysis of variance test
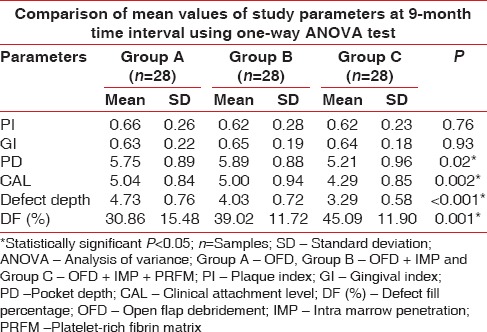
Figure 7.
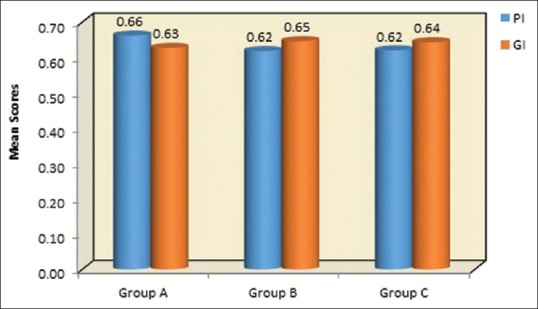
Graphical representation of mean values at of PI, GI, at 9th month follow-up period in Group A-OFD, Group B-OFD + IMP, and Group C-OFD + IMP + PRFM. PI – Plaque index; GI – Gingival index; OFD – Open flap debridement; OFD + IMP – Open flap debridement + intra marrow penetration; OFD + IMP + PRFM – Open flap debridement + intramarrow penetration Platelet-rich fibrin matrix
Table 3.
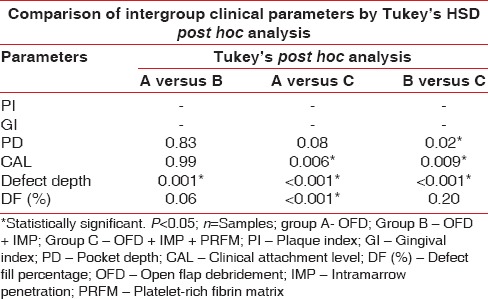
Intergroup comparison of mean values at of plaque index, gingival index probing pocket depth, clinical attachment level, Defect depth, defect fill percentage at 9th month follow-up period in group A, B and C with Turkey's post hoc analysis
Gingival index
At baseline, the mean GI in Group A, B and C were 1.46, 1.50, and 1.49, respectively, as seen in Table 1 and Figure 6. The Table 2 and Figure 7 depicts the 9th month the mean of GI reduced to 0.63, 0.65, and 0.64 in Group A, B, and C, respectively. When intergroup comparisons were made, no statistically significant difference was observed as all the groups had an equal amount of improvement during the study as contemplated in Table 3.
Probing pocket depth
Table 1 and Figure 8 shows the mean of PPD at baseline evaluated for Group A, B, and C were 6.79 mm, 6.96 mm, and 6.79 mm, respectively, which were statistically nonsignificant and all the groups had a similar mean of PPD. At 9-month interval, all the group had shown a mean reduction of PPD in Group A as 5.75 mm, Group B as 5.89 mm, and Group C as 5.21 mm which was observed in Table 2 and Figure 9. When intergroup comparison was done with post hoc analysis, Group C was statistically significant as compared to Group B with a P = 0.02. No significant difference was obtained from group in between Group A and B and Group A and C as seen in Table 2 and Figure 10.
Figure 8.
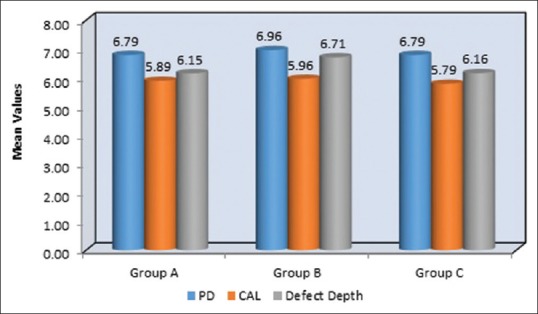
Graphical representation of mean values at of PPD, CAL, defect depth at baseline in Group A-OFD, Group B-OFD + IMP, and Group C-OFD + IMP + PRFM. PPD – Probing pocket depth; CAL – Clinical attachment level; OFD – Open flap debridement; OFD + IMP – OPEN flap debridement + intramarrow penetration; OFD + IMP + PRFM – open flap debridement + intra marrow penetration Platelet-rich fibrin matrix
Figure 9.
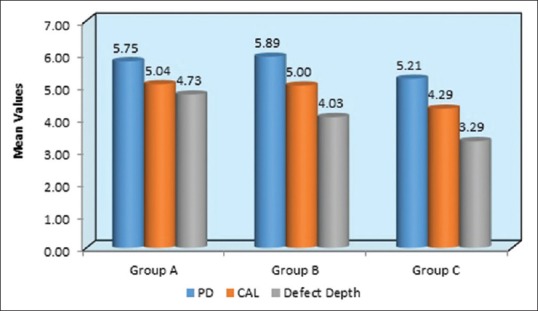
Graphical representation of mean values at PPD, CAL, defect depth at 9th month follow-up period in Group A-OFD, Group B-OFD + IMP, and Group C-OFD + IMP + PRFM. PPD – Probing pocket depth; CAL – Clinical attachment level; OFD – Open flap debridement; OFD + IMP – Open flap debridement + intra marrow penetration; OFD + IMP + PRFM – Open flap debridement + intramarrow penetration Platelet-rich fibrin matrix
Figure 10.
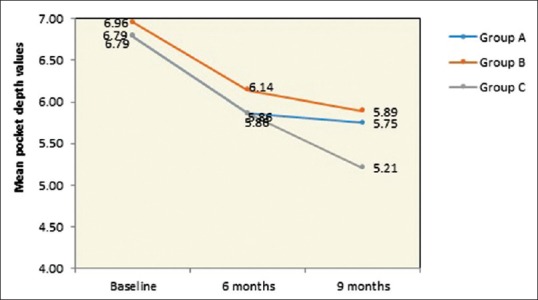
Graphical representation of intergroup comparison of PPD from baseline to 9th month follow-up period in group A-OFD, Group B-OFD + IMP and Group C-OFD + IMP + PRFM. PPD – Probing pocket depth; OFD – Open flap debridement; OFD + IMP – Open flap debridement + intra marrow penetration; OFD + IMP + PRFM – Open flap debridement + intramarrow penetration Platelet-rich fibrin matrix
Clinical attachment level
The mean of CAL at baseline showed 5.89 mm, 5.96 mm, and 5.79 mm in Group A, B, and C, respectively, which showed a similar range in all the groups at the initial conduct of the study as contemplated in Table 1 and Figure 7. At 9 months interval as observed in Table 2 and Figure 9; the CAL gain obtained was 5.04 mm, 5 mm, and 4.29 mm in Group A, B, and C, respectively. When intergroup comparison was performed, the CAL gain was significantly more in Group C as compared to Group A and B with P < 0.001 as seen in Table 3 and Figure 11.
Figure 11.
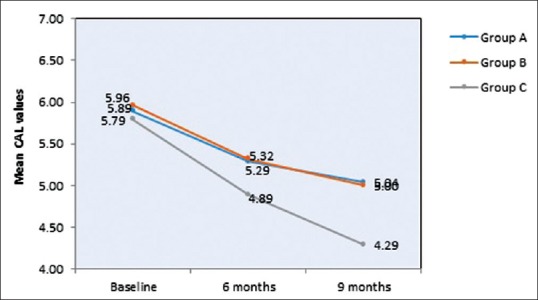
Graphical representation of intergroup comparison of CAL from baseline to 9th month follow up period in group A-OFD, Group B-OFD + IMP and Group C-OFD + IMP + PRFM. CAL – Clinical attachment level; OFD – Open flap debridement; OFD + IMP – Open flap debridement + intra marrow penetration; OFD + IMP + PRFM – Open flap debridement + intra marrow penetration Platelet-rich fibrin matrix
Defect depth
Table 1 and Figure 7 show the mean of defect depth observed through RVG at baseline for Group A, B, and C were 6.15 mm, 6.71 mm, and 6.16, respectively. At 9-month period as observed in Table 2 and Figure 9, the defect depths recorded were 4.73 mm, 4.03 mm, and 3.29 mm, respectively in Group A, B, and C. From Table 3 and Figure 12, it was contemplated that intergroup comparison has shown a statistically significant difference in Group A, B, and C.
Figure 12.
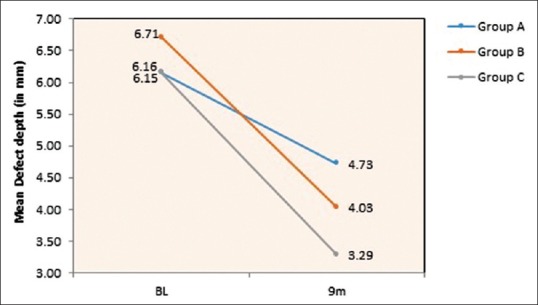
Graphical representation of intergroup comparison of defect depth from baseline to 9th month follow-up period in Group A-OFD, Group B-OFD + IMP and Group C-OFD + IMP + PRFM. OFD + IMP – Open flap debridement + intra marrow penetration; OFD + IMP + PRFM – Open flap debridement + intra marrow penetration Platelet-rich fibrin matrix
Defect fill percentage (DF %)
The DF percentage was calculated based on the linear radiographic depth of the defect obtained from RVG using the following formula:[12]

The mean DF obtained at 9 months interval was obtained in Group A as 30.86%, Group B as 39.02%, and Group C has shown a DF as measured from RVG was 45.09% as observed in Table 2. When intergroup comparison was done as observed in Table 3; Group C was statistically significant as compared to Group A with P value of 0.001 as contemplated in Figure 13. No statistically significant difference was obtained between Group A and B and Group B and C.
Figure 13.
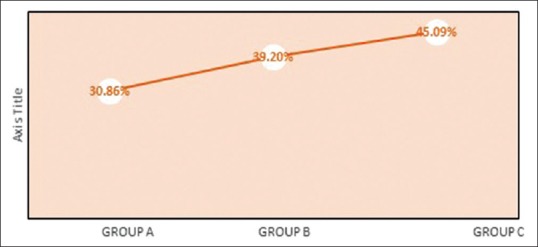
Graphical representation of intergroup comparison of defect depth percentage (DF%) from baseline to 9th month follow-up period in group A-OFD, Group B-OFD + IMP, and Group C-OFD + IMP + PRFM. OFD + IMP – Open flap debridement + intramarrow penetration; OFD + IMP + PRFM – Open flap debridement + intramarrow penetration Platelet-rich fibrin matrix
DISCUSSION
SRP is considered the gold standard in the field of periodontal therapy as it results in achieving tangible and surrogate endpoint both for the participants and operators. The residual pockets that remain following SRP due to anatomical defects or deep probing depth requires a surgical intervention.[13] Periodontal intrabony defects through surgical intervention had shown an increase in attachment level of about 2.5 mm with various bone grafts and biological mediators substitute along with stable healing of periodontium in clean, deep osseous defects with optimal osseous architecture.[14,15] The absence of bleeding on probing, presence of shallow pockets associated with periodontal regeneration, and no soft-tissue recession are considered as positive indications of a successful periodontal therapy.[16] The horizontal pattern of bone loss in the literature does not constitute a strong base in the treatment modalities except the OFD. In 2010, Jaykumar et al.[2] evaluated 3, 371 teeth radiographically where 3, 107 teeth presented the horizontal type of bone loss with only 3.2% of treatment options. Novel technologies using tissue engineering (growth factors and stem cells), miniature bone pins, or distraction osteogenesis may emerge as treatment options in the future for the most common form of bony defect of periodontal disease.[3] Thus, an attempt has been made to evaluate clinically and radiographically the effect of PRFM as a regenerative material with or without its placement in horizontal periodontal defects.
In Group A, B, and C, the PI and GI have shown an equal improvement at consecutive follow-up periods which could be attributed to disruption of bacterial plaque and other local factors in the maintenance phase that the participants has complied to. Although nonstatistical results were obtained in all groups clinically, Group C (OFD + IMP + PRFM) had shown no inflammation which could be attributed to anti-inflammatory property of PRFM and the role of IMP.[8,9] In the present study, the improvement in PI and GI were in accordance to the classical studies conducted by Lindhe et al in 1982 and 1984[17,18] and it was mainly attributed to the motivation ad re enforcement of oral hygiene.
The PPD and CAL gain evaluated in the study at baseline was equivalent in all the study groups. At 9th month, significant reduction in PPD was obtained in Group C with a mean reduction of 5.21 mm which was statistically significant as compared to Group A and C. The CAL gain reported was the maximum for Group C with a mean increase of 4.29 mm. The Groups A and B have shown almost an equal amount of improvement due to the healing pattern of any periodontal therapy which is facilitated by long junctional epithelium which does not indicate the formation of true regeneration which was in accordance with Wikesjö and Selvig in 1999.[5] Although intergroup comparison stated Group C to be statistically significant as compared to Group A and B which was attributed to the potential role of PRFM as biomaterial. The PRFM might have acted as structural support and a mechanical barrier to create space around the defects that might permit periodontal regeneration. As stated by Ozdemir and Okte et al. in 2012, the PRFM also prevented the epithelial down growth into the defect area thus preventing the formation of the long junctional epithelium.[19] The reduction in the mean PPD of 5.21 mm and CAL gain of 4.29 mm was similar to a study conducted by Joseph et al. in 2014[20] where with the use of PRF the PPD reduction of 4.43 mm and CAL gain of 4.4 mm.
Nearly 45.09% of DF was observed in Group C which was statistically significant as compared to Group A and B. This is in accordance with the study of Joseph et al. in 2015[20] where in PRF group had shown 5.25 mm of relative crest height and 50% DF in control group, whereas in PRF along with PRF, membrane had shown DF% of 52%.
The results of the present study for Group B which included OFD and IMP has shown DF% of 39.02%. This could be attributed to the decortication which was done with the help of aerator hand piece with round bur to facilitate bone regeneration. It was performed with the intention to expose cancellous bone, thereby enhancing the healing process by promoting bleeding and allowing progenitor cells and blood vessels to reach a bone-grafted site more readily.[21] Crea et al. in 2014 had evaluated OFD along with OFD and IMP and found CAL gain of >2 mm in test site which was statistically significant; however, the percentage of DF was not determined.[22]
The significant improvement observed in Group C could be attributed to the autologous biomaterial PRFM which has dense concentration of platelets as compared to normal human blood clot. The alpha granules of it contain growth factors that affect every cell and the formation of every tissue involved in the wound healing. The regeneration of soft tissue and bone has shown an immense potential role in regenerative therapy due to its robust release of growth factors which are also pivotal components of wound healing process through signaling transduction mechanism.[7] Carroll et al. in 2005 had demonstrated, in vitro, that the viable platelets in PRFM released 6 growth factors mainly platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF) in about the same concentration for the 7 days duration of their study.[23] As PRFM is from the first generation of platelet concentrate, it possesses the same properties of PRP which has shown a considerable improvement in various osseous defects by enhancing the quantity and quality of new bone formation.[24,25,26] Simon et al. in 2009[8] evaluated PRFM along with demineralized freezed dried bone graft (DFDBA) in the extraction socket where presence of nonvital DFDBA particles and the accompanying inflammatory infiltrate noted during the healing was also a significant reason for delayed healing in the sockets where that material was used for 12-week period. Sites treated with PRFM alone had a complete osseous fill of the extraction socket in 3 weeks interval. Lucarelli et al. in 2010[9] evaluated the mechanical properties which demonstrated a tear elastic modulus of 937.3 kPa, stress at a break of 1476.0 kPa, and an elongation at break of 146.3 which plays an important role as it enhances the stabilization of fibrin clot to regulate and release various cytokines required for healing process. Elevated levels of PDGF-AA, PDGF-AB, EGF, VEGF, bFGF, and TGF-beta1 were measured in the day 1-conditioned media of PRFM and growth factor levels decreased thereafter. The dense physically robust PRFM made through high-speed centrifugation of intact platelets and fibrin in the absence of exogenous thrombin yields a potential tool for accelerating tissue repair. The companionship of IMP cannot be circumvented as Majzoub et al. in 1999 stated that performing IMP in the surgical site creates a close spatial correlation between angiogenesis and bone formation. The vessel-rich medullary opening space would facilitate capillary sprouting and enhance vascular access into the surgical site. A local increase in bone morphogenetic proteins and other growth factors from the injured cortical surface, endosteal area, and wounded vessels area can enhance further new bone formation. Thus, the statistical significant difference observed in Group C was the synergistic effect of autologous PRFM and IMP.[27]
The present study as per thorough literature search was the first of its kind where first-generation platelet concentrate PRFM had been evaluated clinically and radiographically in horizontal defects.
CONCLUSION
Horizontal defect being the most prevalent form of periodontal defect demands more attention by researchers with the use of various commercially available bone grafts and the use of autologous growth factor delivery systems which when compared would give a better overview of its success rate and would also offer a new dimension in their management. The present study has shown a systematic improvement in the clinical parameters at 9-month follow-up period in all the groups with a slender improvement in Group C which was due to the beneficial effect of IMP and PRFM. Further research work is warranted in a larger sample size and its comparison with second-generation platelet concentrate (PRF) is required.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Newman MG, Takei HH, Klokkevold PR, Carranza FA. Carranza's Clinical Periodontology. 11th ed. St Louis, MO: Elsevier/Saunders; c2012. p. 1938. [Google Scholar]
- 2.Jayakumar A, Rohini S, Naveen A, Haritha A, Reddy K. Horizontal alveolar bone loss: A periodontal orphan. J Indian Soc Periodontol. 2010;14:181–5. doi: 10.4103/0972-124X.75914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nevins M, Becker M, Kornman K. Proceeding of the World Workshop in Clinical Periodontics: Princeton, New Jersey American Academy of Periodontology, Chicago III. 1989;7(Sec V) [Google Scholar]
- 4.Kao R, Nares S. Periodontal regeneration-intrabony defects: A systematic review from the AAP regeneration workshop. J Periodontol. 2015;86:S77–104. doi: 10.1902/jop.2015.130685. [DOI] [PubMed] [Google Scholar]
- 5.Wikesjö UM, Selvig KA. Periodontal wound healing and regeneration. Periodontol 2000. 1999;19:21–39. doi: 10.1111/j.1600-0757.1999.tb00145.x. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds MA, Aichelmann-Reidy ME, Branch-Mays GL. Regeneration of periodontal tissue: Bone replacement grafts. Dent Clin North Am. 2010;54:55–71. doi: 10.1016/j.cden.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR, et al. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–46. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 8.Simon BI, Zatcoff1 AL, Kong JW, O Connell S.M. Clinical and histological comparison of extraction socket healing following the use of autologous platelet-rich fibrin matrix (PRFM) to ridge preservation procedures employing demineralized freeze dried bone allograft material and membrane. Open Dent J. 2009;3:92–9. doi: 10.2174/1874210600903010092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucarelli E, Beretta R, Dozza B, Tazzari PL, O'Connel SM, Ricci F, et al. A recently developed bifacial platelet-rich fibrin matrix. Eur Cell Mater. 2010;20:13–23. doi: 10.22203/ecm.v020a02. [DOI] [PubMed] [Google Scholar]
- 10.Loe H, Silness J. Periodontal disease in pregnancy. I. Prevalence and severity. Acta Odontol Scand. 1963;21:533–51. doi: 10.3109/00016356309011240. [DOI] [PubMed] [Google Scholar]
- 11.Silness J, Loe H. Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condtion. Acta Odontol Scand. 1964;22:121–35. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 12.Singh VP, Nayak DG, Uppoor AS, Shah D. Clinical and radiographic evaluation of nano-crystalline hydroxyapatite bone graft (Sybograf) in combination with bioresorbable collagen membrane (Periocol) in periodontal intrabony defects. Dent Res J (Isfahan) 2012;9:60–7. doi: 10.4103/1735-3327.92945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palcanis KG. Surgical pocket therapy. Ann Periodontol. 1996;1:589–617. doi: 10.1902/annals.1996.1.1.589. [DOI] [PubMed] [Google Scholar]
- 14.Sculean A, Nikolidakis D, Nikou G, Ivanovic A, Chapple IL, Stavropoulos A, et al. Biomaterials for promoting periodontal regeneration in human intrabony defects: A systematic review. Periodontol 2000. 2015;68:182–216. doi: 10.1111/prd.12086. [DOI] [PubMed] [Google Scholar]
- 15.Yajamanya SR, Chatterjee A, Hussain A, Coutinho A, Das S, Subbaiah S, et al. Bioactive glass versus autologous platelet-rich fibrin for treating periodontal intrabony defects: A comparative clinical study. J Indian Soc Periodontol. 2017;21:32–6. doi: 10.4103/0972-124X.201628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Position paper: Periodontal regeneration. J Periodontol. 2005;76:1601–22. doi: 10.1902/jop.2005.76.9.1601. [DOI] [PubMed] [Google Scholar]
- 17.Lindhe J, Westfelt E, Nyman S, Socransky SS, Heijl L, Bratthall G, et al. Healing following surgical/non-surgical treatment of periodontal disease. A clinical study. J Clin Periodontol. 1982;9:115–28. doi: 10.1111/j.1600-051x.1982.tb01227.x. [DOI] [PubMed] [Google Scholar]
- 18.Lindhe J, Westfelt E, Nyman S, Socransky SS, Haffajee AD. Long-term effect of surgical/non-surgical treatment of periodontal disease. J Clin Periodontol. 1984;11:448–58. doi: 10.1111/j.1600-051x.1984.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 19.Ozdemir B, Okte E. Treatment of intrabony defects with beta-tricalciumphosphate alone and in combination with platelet-rich plasma. J Biomed Mater Res B Appl Biomater. 2012;100:976–83. doi: 10.1002/jbm.b.32660. [DOI] [PubMed] [Google Scholar]
- 20.Joseph RA. Autologous platelet rich fibrin in regenerative periodontal therapy. J Clin Diagn Res. 2014;8:ZC43–7. doi: 10.7860/JCDR/2014/9948.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Kerns DG. Mechanisms of guided bone regeneration: A review. Open Dent J. 2014;8:56–65. doi: 10.2174/1874210601408010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crea A, Deli G, Littarru C, Lajolo C, Orgeas GV, Tatakis DN, et al. Intrabony defects, open-flap debridement, and decortication: A randomized clinical trial. J Periodontol. 2014;85:34–42. doi: 10.1902/jop.2013.120753. [DOI] [PubMed] [Google Scholar]
- 23.Carroll RJ, Amoczky SP, Graham S, O'Connell SM. Edison NJ: Musculoskelatal Transplant Foundation; 2005. Characterization of autologous growth factors in Cascade platelet rich fibrin matrix (PRFM) [Google Scholar]
- 24.Danesh-Meyer MJ, Filstein MR, Shanaman R. Histologic evaliatopm of sinus augmentation using platelet-rich plasma (PRP): A case series. Int J Periodontics Restorative Dent. 2001;3:48–56. [PubMed] [Google Scholar]
- 25.Shanaman R, Filstein MR, Danesh-Meyer MJ. Localized ridge augmentation using GBR and platelet-rich plasma: Case reports. Int J Periodontics Restorative Dent. 2001;21:345–55. [PubMed] [Google Scholar]
- 26.Agarwal P, Chatterjee A, Gokhale S, Singh HP, Kandwal A. Evaluation of platelet-rich plasma alone or in combination with demineralized freeze dried bone allograft in treatment of periodontal infrabony defects: A comparative clinical trial. J Indian Soc Periodontol. 2016;20:42–7. doi: 10.4103/0972-124X.170811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majzoub Z, Berengo M, Giardino R, Aldini NN, Cordioli G. Role of intramarrow penetration in osseous repair: A pilot study in the rabbit calvaria. J Periodontol. 1999;70:1501–10. doi: 10.1902/jop.1999.70.12.1501. [DOI] [PubMed] [Google Scholar]


