Introduction
Radiotherapy has been widely used for cancer treatment either by itself or in conjunction with other treatment types such as surgery and chemotherapy (1). Despite of advanced delivery approaches, improved radiation delivery precision and aggressive fractionation schemes (2–6), tumor progression and recurrence are frequently observed (7–8). The Metastatic Breast Cancer Network showed that 20–30% of people initially diagnosed with early stage disease would develop metastatic breast cancer (9).
Recent cancer cell research and clinical data suggest that successful cancer therapy must eradicate the cancer stem cells (CSCs) (10, 14, 15). Current stem cell theory (12) supports the following highlighted characteristics of CSCs: 1) Solid tumors, such as breast, brain, prostate, lung (13) comprise a distinct heterogeneous population of CSCs; 2) CSCs are tumorigenic and markedly different than the differentiated cancer cells in their ability to proliferate and self-renew (10, 11); 3) CSCs are more persistent in tumors and cause relapse and metastasis by giving rise to new tumors; 4) CSCs can be found at any location in the tumor (16, 17, 18) and 5) are generally more resistant to ionization radiation or other cell killing agents (1). The presence of CSC challenges the limitations of current treatment method and suggests a significant opportunity to design more effective cancer treatments. CSC-targeted approaches include sensitization of CSCs to conventional drugs, promoting CSC differentiation, targeting and blocking relevant CSC signaling pathways and destroying CSC niches (21).
It is well known that individual patients respond differently when subject to the same radiotherapy regimen. The differences have been attributed to patient-specific tumor and normal tissue radiosensitivity and patient characteristics including age, performance status, staging, disease sites and metastases location, etc. Intrinsic tumor radiobiology not only differs for different types of cancer, it also varies for the same cancer in different patients. Biologically guided personalized radiotherapy based on molecular prognostic and predictive biomarkers is expected to profoundly impact radiation therapy (1). Due to the markedly different CSC radiobiology and their varying presence in tumors, radiotherapy may be individualized accordingly. The aims of this study were twofold: 1) to investigate tumor radioresistance and its correlation to the level of CSCs through in vitro experiments, developing a two-compartment mathematical model determining radiosensitivity of both BCCs and BCSCs; 2) to validate the two-compartment CSC model using pooled clinical outcome data. The potential clinical implication suggested that personalized therapy based on pre-clinical biological characteristics, e.g., the fractions of cancer stem cells, could lead to improved clinical outcomes.
Method and materials:
Surviving fraction from in vitro experiment
In vitro surviving fractions for four established breast cancer cell lines, including MCF-7, T47D (Luminal A), MDA-MB-231 (claudin-low), SUM159PT (triple-negative) molecular phenotypes, were digitized from Lagadec et al. (20). These assays were irradiated using single doses of 0, 2, 4, 6, 8 or 10 Gy under acute hypoxic conditions (2h, 0.1% O2) or normoxia (atmospheric conditions, 21% O2). In this study, we re-analyzed the survival rate for the four cell lines at atmospheric conditions. The factions of BCSCs in each cell lines were identified using two markers as described in (12,13, 20).
Dual-compartment Survival Fraction model
The linear-quadratic (LQ) model (22–24) is the most commonly used radiation-induced cell-killing model. To account for the presence of the breast CSCs in addition to the tumor cells (TCs), a more generalized dual-compartment survival fraction model was introduced:
| (1) |
where S is the total cell surviving fraction, αTC, βTC and αCSC, βCSC characterize intrinsic radiosensitivity of the tumor cells and CSCs respectively, and D is the radiation dose. The α/β ratio measures the relative importance of the linear and quadratic terms in the LQ model. The fraction of TCs to the total plated cells within a certain cell line (or a given tumor) is given by f ‘, which was determined by the aforementioned CSC assays. When f ‘=1 (no CSC), the dual-compartment model became the single-compartment tumor cell surviving model (LQ model). The least square method was used to determine the model parameters by fitting the cell surviving fractions as a function of radiation dose. The best-fit curve is defined as the parameter set that has the minimal sum of the deviations squared calculated by Eq. (2):
| (2) |
where, SCalc(Dj) is the j-th survival rate calculated from Eq. (1), SMeas(Dj) is the observed survival rate for the given dose Dj; σj2 is the error for the j-th data point.
The Function Minimization and Error Analysis package (MINUIT) from the European Laboratory for Participle Physics program library (CERNLIB, Geneva, Switzerland) was employed to fit the data (http://cernlib.web.cern.ch/cernlib).
Overall tumor radiosensitivity
The overall tumor radiosensitivity (α) was estimated by the radiosensitivities of the TCs, CSCs and their fraction by number in a heterogeneous tumor:
| (4) |
Where f ‘, αTC and αCSC were defined aforementioned.
Analysis of clinical outcome data
The generalized LQ model (22–24) is widely adopted in radiation oncology for assessing tumor control and normal tissue complications. The reported clinical local disease-free survival rate (LSR) at arbitrary time points of 5- and 8- years was assessed using a Poisson model.
| (3) |
Where K is the number of clonogenic cells, and S is the cell surviving fraction.
To avoid overfitting, the number of variables was reduced. We adopted the plausible parameter set for TCs (26), the parameters associated with BCSCs including f, αCSC, Td (CSC) were determined by the data-fitting program. As a simple approximation, we assumed βCSC=0, which was widely observed and used in many recent publications (11, 12, 20, 36, 37).
Pooled clinical data
The dual-compartment model was validated using a pooled clinical dataset of breast cancer patients (2–6). Whelan et al. (2) compared short term (42.5 Gy/16 fractions over 22 days) and long-term (50 Gy/25 fractions over 35 days) fractionation schedules based on a randomized trial of 1234 patients. Owen et al. (3) studied the effectiveness of fractionation schemes: 50 Gy (25 fractions), 39 Gy (13 fractions) and 42.9 Gy (13 fractions) for a total of 1410 women with early-stage breast cancer at long-term follow-up. Two large randomized trials in the United Kingdom Standardization of Breast Radiotherapy (UK START) trial A (2236 patients) and trial B (2215 patients) (4) and (5) were also included. START A included randomized comparisons of 41.6 Gy (13 fractions) and 39.0 Gy (13 fractions) over 5 weeks; START B compared the local–regional tumor relapse rates and late adverse effects for patients received 40 Gy (15 fractions) over 3 weeks, with a control schedule of 50 Gy (25 fractions) over 5 weeks.
Results
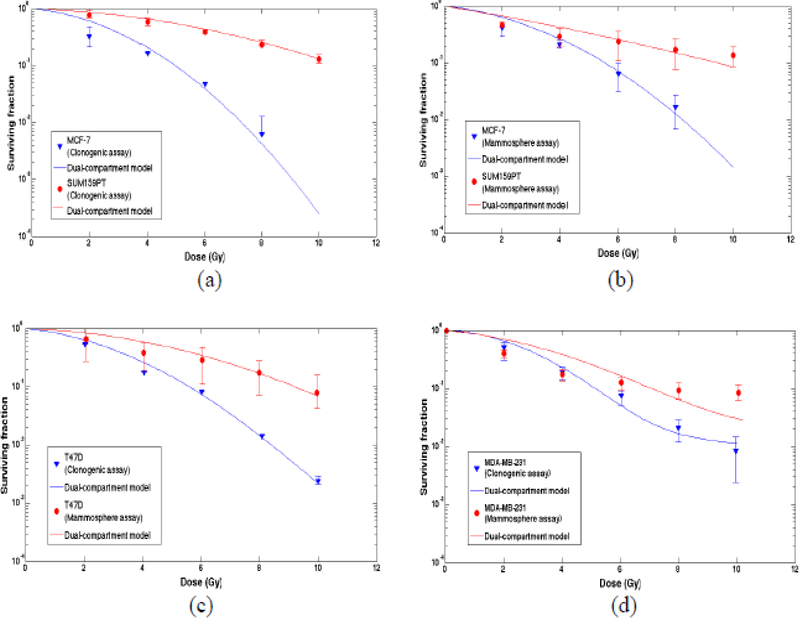
Figure 1 shows surviving fractions for breast cancer cells in clonogenic and mammosphere assays under atmospheric conditions. f is the percent of BCSCs in each cell line. The symbols (experimental data) were fitted using the dual-compartment model (curves). Figure 1 (a) surviving fractions of MCF-7 (f=0.1%) and SUM159PT (f=2.46%) cell lines in clonogenic assay; (b) surviving fractions of MCF-7 (f=0.98%) and SUM159T (f=8.68%) cell lines in mammosphere assays; (c) surviving fractions of T47D cell line in clonogenic survival assays (0.9%) versus mammosphere assays (f=1.34%) and (d) surviving fractions of MDA-MB-231 in clonogenic survival assay (f=1.18%) versus mammosphere assays (f=2.04%), respectively. In Figure 1 (d), the cell surviving curve from 0–4 Gy describe mostly cell killing while the curve from 4 Gy or higher dose range is composed of cell killing and induction of tumorigenic/spherogenic cells, which was not accounted in the two-compartment model.
Figure 1.
Surviving fraction for four types of breast cancer cells in clonogenic and mammosphere assays. The symbols (experimental data) were fitted using the dual-compartment model (curves). (a) Clonogenic assays for MCF-7 (−.1% CSC percentage of the total cells) and SUM159T (9-fold higher, 2.46%); (c) T47D in clonogenic assays (0.9%) versus mammosphere assays (1.34%) and (d) MDA-MB-231 in clonogenic assay (1.18%) versus mammosphere assays (2.04%), respectively.
Higher percentages of CSCs in the cell lines were associated more radioresistant phenotypes characterized by less curvy lines. The fractions of CSCs in mammosphere assays were greater than corresponding cell lines. Correspondingly, there was almost no shoulder found in the surviving curves (more radioresistant in mammosphere assays) with βCSC approximately zero.
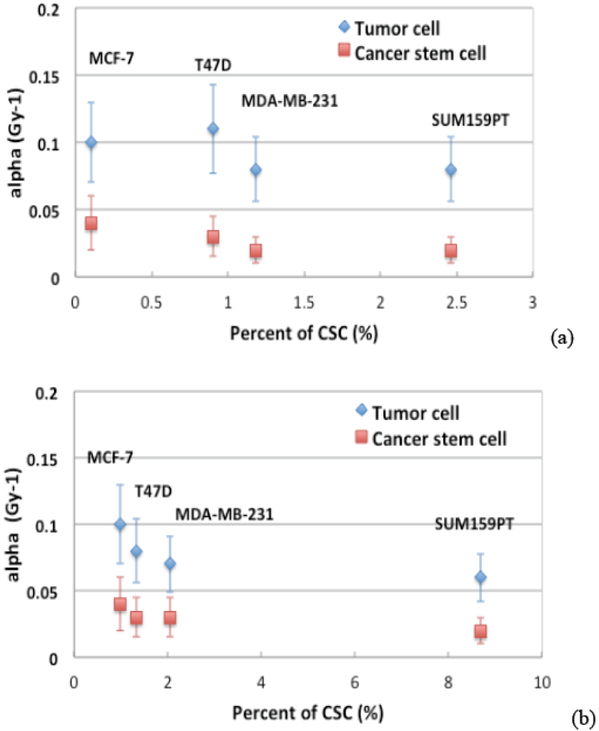
Figure 2 illustrates the derived radiosensitive parameters of αTC and αCSC as a function of percentage of CSCs in a) clonogenic assays and b) mammosphere assays, respectively. The derived αCSC was found smaller than αTC with p=0.02, p=0.003 for clonogeneic and mammosphere assays respectively, indicating that the BCSCs were less radiosensitive. Across different subtypes of the cell lines, the radiosensitivity parameters were found as not significantly different for either TCs or CSCs.
Figure 2.
The derived radiosensitive parameters of αTC and αCSC as a function of percentage of CSCs in the four cell lines in a) clonogenic survival assays and b) mammosphere assays respectively.
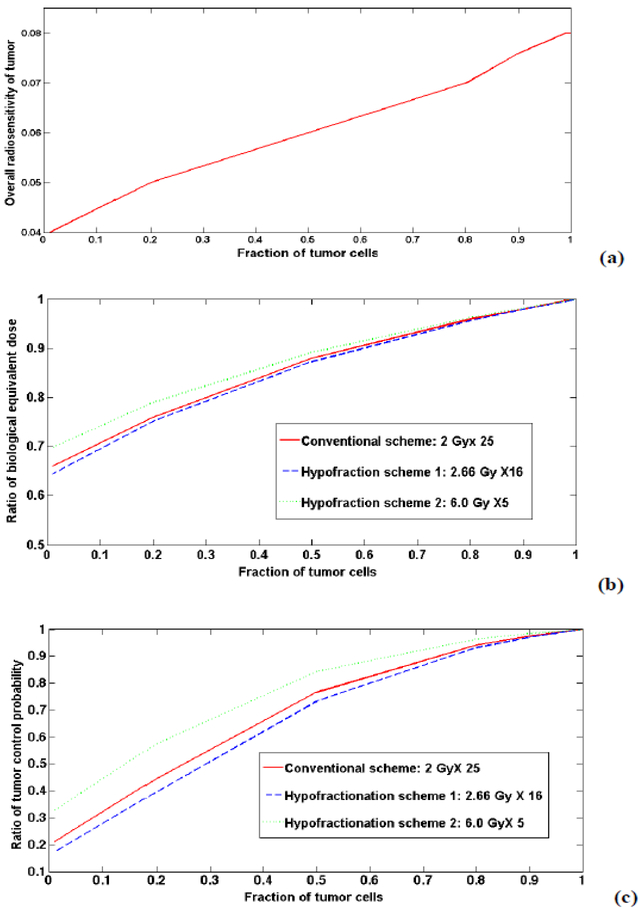
Figure 3 (a) illustrates the overall tumor radiosensitivity as a function of percent of CSCs in a tumor. Higher CSC fractions yield more radioresistant tumors (smaller α). Figure 3 (b) shows the ratio of tumor biological equivalent doses (BEDs) for a heterogeneous tumor consisting of different CSC fractions normalized to a tumor consisting of 100% TCs (hypothetically). The conventional fractionation scheme of 2 Gy×25 (solid line) and a modest hypofractionation scheme of 2.66 Gy×16 (dashed line), and hypofractionation scheme of 6.0 Gy×5 (dotted line) were displayed. The larger percentage of CSCs found from biopsy in a solid tumor, the lower BEDs would be expected. Compared to a homogenous tumor consisting of TCs only, the homogenous tumor BED may be reduced by 30–36% depending on the fraction of CSCs and different treatment schemes. Figure 3 (c) shows the ratio of calculated TCPs as a function of the fraction of TCs. Without losing any generality, the α/β ratio of 2.88 Gy (26) with an arbitrary clonogenic number K was used for the TCs in the calculation. αCSC =0.02 Gy−1, αTC=0.08 Gy −1 (derived from in vitro) for the CSC and tumor cells respectively, and the same treatment duration of 35 days was used in all TCP calculations. An increasing fraction of the CSCs resulted in lower TCPs but less so with hypofractionated approaches. To maintain the same TCPs in homogenous tumor, increased prescription doses of 2.07 Gy×25, 2.75 Gy×16 and 6.18 Gy×5 were needed assuming 10% of CSCs in a heterogeneous tumor. The hypofractoination regimens, given the presence of heterogeneous CSCs and their distinct radiosensitivity, may be slightly less affected by the intra-tumor CSC component for breast radiotherapy.
Figure 3.
The correlation of the fraction of CSCs to (a) the overall tumor radiosensitivity; (b) the ratio of biological equivalent doses for a non-uniform tumor consisting of different percentage of CSCs normalized to a uniform tumor (consisting of 100% TCs) and (c) the ratio of TCPs.
Analysis of Pooled clinical outcome data
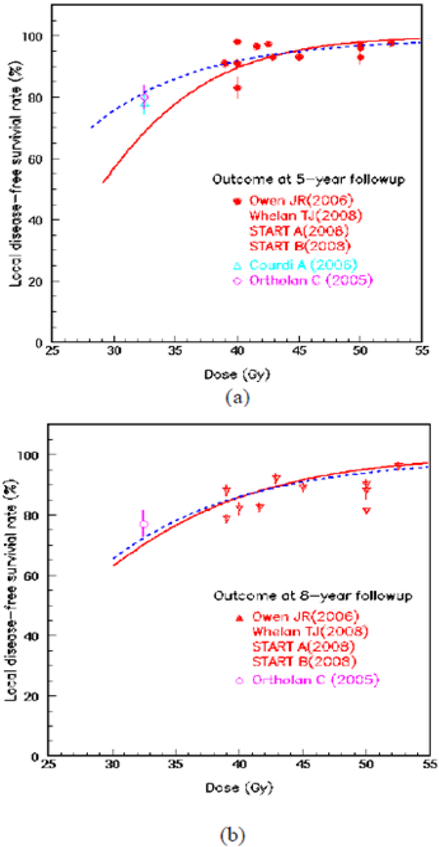
The two-compartment LQ model was then applied to fit the pooled clinical data. Figure 4 shows the LSR as a function of dose at the arbitrary follow-up time of 5 years (a) and 8 years (b). The symbols were reported clinical outcome from a series of literatures. The solid line is the best fitting results for the single-compartment model, which considered as a uniform tumor consisting of TCs; the dashed line represented the best fitting result for heterogeneous tumors consisting of both BCSCs and TCs. Figure 4 (a) illustrated the predicted LSRs at 5 years. The single-compartment model derived parameters for TCs are αTC=0.11±0.03 Gy−1, α/β (TC)=4.89±0.02 Gy, Td (TC)=10.0±4.1 days. In dual-compartment model, the plausible parameters derived from in vivo (26) were used for TCs. The derived fitting parameters for BCSCs are αBCSC=0.04±0.03 Gy−1, βBCSC = 0, Td (BCSC)=12.8±11.9 days with the fraction of BCSCs f = 0.008±0.04. Figure 4 (b) illustrated the predicted LSRs at 8 years as a function of dose. The single-compartment model yielded αTC=0.06±0.07 Gy−1, α/β (TC)=2.48±3.09 Gy, Td (TC)=17.2±5.3 days. The derived parameters for BCSCs are: αBCSC=0.06 ±0.02 Gy−1, βBCSC = 0, Td (BCSC)=14.8±12.3 days, the fraction of the BCSCs f = 0.005±0.02. The clinical results of an adjuvant once-weekly hypofractionated regimen of 32.5 Gy in 5 fractions (6.5 Gy/fx) for a group of 150 elderly patients reported by Ortholan et al (33) and Courdi et al (34) were also plotted in Figure 4. The reported 5-year and 8-year disease-free survival rates at were 80% and 71.5% respectively (33).
Figure 4.
The local disease-free survival rate (LSR) as a function of the total prescribed doses at the follow-up time of 5 years (a) and 8 years (b), respectively. Solid line: Single-compartment LQ model; Dashed line: dual-compartment LQ model.
Discussion
We derived the radiosensitivity parameters for BCSCs using four representative breast cancer cells in clonogenic and mammosphere survival assays. The BCSCs were found to be more radioresistant to ionizing radiation characterized by smaller α values compared to the differentiated tumor cells in the same cell lines. Higher radioresistance of BCSCs implied that the CSCs are more likely to survive radiotherapy, leading to a greater risk of tumor recurrence. Similar findings were also reported by others (27, 28).
Additionally, the current analysis suggested that a greater percentage of BCSCs in given cell lines is closely correlated to tumor radioresistance (20). In clonogenic assays, the fractions of BCSCs were 0.1% in MCF-7, 9-fold higher (9%) in T47D, 12-fold higher (1.18%) in MDA-MB-231, 25-fold higher (2.46%) in SUM159PT; in mammospheres assays, the concentrations of BCSCs were 1.5–10 fold higher than the corresponding cancer cells in the clonogenic assays. This study indicates the greater percentages of BCSCs were associated with greater radioresistance. Other factors such as cell proliferation may also play a role in terms of survival fraction differences between the clonogenic versus mammosphere assays for the same cell line shown in Figure 1 (c) and (d). The mammosphere assays were considered to better estimate the number of CSCs and represent the CSC repopulation in a human solid tumor than the cell line assays (20). The in vitro observation alluded to a potentially impact from the pre-treatment number of CSCs to the cell survival fractions after single dose irradiation.
The presence of CSCs and TCs can be mimicked using a dual-compartment mathematic model in vitro, we further explored the applicability of the model to access radiation response of CSCs (in addition to TCs) using pooled clinical outcome data. At different follow-up times, the dual-compartment model consistently yielded a small yet finite fraction of BCSCs, suggesting a fraction of BCSCs may exist after surgery for early stage beast cancer patients. Both single- and dual- compartment model based fitting agreed with the pooled clinical data for the prescription doses of 40 Gy or higher. The radiosensitivity parameters of αTC versus αBCSC were found not to be statistically different given the large uncertainties of the fitting parameters. The large uncertainties of the fitting parameters may due to the limited number of clinical trials using different fractionation schemes, and the available clinical prescription doses lied mostly in relatively narrow range of 40 – 50 Gy. Clinical outcome data was lacking in low dose range (i.e., < 40 Gy), which failed to constrain the fitting curves well and resulted in a large discrepancy between the single- and dual- compartment models, as well as the large uncertainties of fitting parameters. The dual-compartment model seemed to predict the reported endpoints better (33, 34) in the low dose range (Figure 4). However, it doesn’t prove that the dual-compartment model is a better fit than the single-compartment model since the elder patients (median age of 78 years old) (33, 34) might involved very different breast environment than the younger women population reported in high dose groups (3–6). Furthermore, great variability existed in patient enrollment in the selected clinical trials (including unspecified important clinical perspective such as dose escalation, patient age, tumor size, molecular subtypes of breast cancers, extent of surgery, etc) and the reported clinical outcome. Hopefully, other clinical trials such as FAST (with more similar enrolled demographics) are highly desirable to refine the derived radiosensitivity parameters in current work.
Tumor heterogeneity is well known (10, 13, 18). Radiosensitivity varies to a great extent across different tumor types and between patients bearing the same type of tumor (21, 35). We illustrated that varying fractions of CSCs in a solid tumor may result in different overall radioresistance (10, 21, 28–31), which may suggest the need for personalized radiotherapy treatments that deliver greater doses to the radioresistant areas. Integrating pretreatment biological information to optimize the radiation therapy treatment and improve outcomes is promising (29), and many on-going pre-clinical and clinical studies of cancer stem cells suggest CSC-targeted personalized treatment may dramatically improve outcome (12, 18). Adapting patient radiation treatment according to patient specific biological information, including pre-treatment number (or fraction) of CSCs derived from pre-treatment biopsy, or intrinsic radiosensitivity before the course of radiotherapy (20), may result in better cell killing and achieve better clinical outcome. The advanced treatment delivery techniques, with development of biology biomarkers and molecular/functional imaging tools allowing us to identify and track CSCs in vivo (19), enable to target those highly radioresistent CSCs with high precision dose delivery.
We acknowledge the potential shortcomings in this study, specifically the modeling analysis with the clinical outcome data. It is known that mathematical models usually rely on certain mechanisms (the model was built on), assumptions and model parameters. We assumed that the prescription dose, dose fractionation and fraction of cancer stem cells are the determining factors for LSR for breast radiotherapy. The effects from other prognostic factors, such as tumor size, tumor staging, surgery extent and molecular subtypes of breast cancers towards estrogen receptor (ER) and/or progesterone receptor (PR) subtypes etc, were not explicitly considered and simulated. Secondly, the dual-compartment model was proposed to account for the existence of CSCs and TCs, the non-linear interplay between CSCs and TCs (including the concept of cell plasticity, accounting for differentiated cells gain CSCs properties under specific conditions (38, 39), etc) in the course of radiation treatment and at later follow-up time (35–37) was not considered. It is generally believed that radiation treatment may enhance this effect by stimulating the transfer of breast TCs into the BSCS compartment. Current available clinical outcome data didn’t allow us to model the interplay mechanisms due to the lack of patient specific clinical information from the literatures. Thirdly, this is an initial study incorporating CSCs to define the radiation response of CSCs (in addition to TCs) using pooled clinical outcome data. Although inter-patient variations were not accounted for, the current study sheds new light on the understanding of underlying microscopic biological information and pooled clinical outcome, and hopefully assists in designing of personalized radiotherapy for better clinical outcome. Specifically designed in vitro experiment (or animal data) that allows the measurement of CSCs after each fraction may be highly desirable to explore the detailed cancer stem cell radiobiological mechanisms.
Conclusion
Percentage of breast cancer stem cells positively correlated to overall tumor radioresistance. This observation suggested the potential towards individualized radiotherapy to account for heterogeneous population of cancer stem cells and their distinct radiosensitivity for breast cancer.
Footnotes
Conflict of Interest: None
Reference:
- 1.Yaromina A, Krause M, Baumann M. Individualization of cancer treatment from radiotherapy perspective. Mol. Oncol 2012,6:211–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whelan TJ, Kim DH, Sussman J. Clinical experience using hypofractionated radiation schedules in breast cancer. Semin Radiat Oncol, 2008;18:57–64. [DOI] [PubMed] [Google Scholar]
- 3.Owen JR, Ashton A, Bliss JM, et al. Effect of radiotherapy fraction size on tumor control in patients with early-stage breast cancer after local tumor excision: long-term results of a randomized trial. Lancet Oncol, 2006;7:467–471. [DOI] [PubMed] [Google Scholar]
- 4.The START Trialists’ Groups. The UK standarization of breast radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial. Lancet Oncol 2008; 9: 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The START Trialists’ Groups. The UK standarization of breast radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomized trial Lancet Oncol, 2008; 9: 1098–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shelley W, Brundage M, Hayter C, et al. A shorter fractionation schedule for post lumpectomy breast cancer patients. Int J Radiat Oncol Biol Phys, 2000;47:1219–1228. [DOI] [PubMed] [Google Scholar]
- 7.FAST Trialists group. Agrawal RK, Alhasso A, Barrett-Lee PJ, Bliss JM, Bliss P, Bloomfield D,. et al. First results of the randomised UK FAST Trial of radiotherapy hypofractionation for treatment of early breast cancer (CRUKE/04/015). Radiother Oncol 2011;100:93–100. [DOI] [PubMed] [Google Scholar]
- 8.Mast ME, Vredeveld EJ, Credoe HM, van Egmond J, Heijenbrok MW, Hug EB, Kalk P, et al. Whole breast proton irradiation for maximal reduction of heart dose in breast cancer patients. Breast Cancer Res Treat 2014;148:33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haviland JS, Owen JR, Dewar JA, Agrawal RK, Barrett J., et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. The Lancet Oncology 2013; 14: 1086 –1094. [DOI] [PubMed] [Google Scholar]
- 10.Metastatic breast cancer network. http://mbcn.org/developing-awareness/category/13-things-everyone-should-know-about-metastatic-breast-cancer.
- 11.Al-Hajj M and Clarke MF. Self-renewal and solid tumor stem cells. Oncogene, 2004; 23: 7274–7282. [DOI] [PubMed] [Google Scholar]
- 12.Bachman JW and Hillen T. Mathematical optimization of the combination of radiation and differentiation therapies for cancer. Frontiers in oncology, 2013; 3: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bomken S, Fiser K, Heidenreich O and Vormoor J. Understanding the cancer stem cell. Br J Cancer, 2010;103:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chumsri S and Shah P. Radiation Resistance of Cancer Stem Cells as an Obstacle in Cancer Therapy. Mol Cell Pharmacol 2013; 5:39–49. [Google Scholar]
- 15.Dingli D and Michor F. Successful therapy must eradicate cancer stem cells. Stem cells 2006; 24: 2603–2610. [DOI] [PubMed] [Google Scholar]
- 16.Hide T, Makino K, Nakamura H, Yano S, Snai S, et al. New treatment strategies to eradicate cancer stem cells and niches in glioblastoma. Neurologia medico-chirurgica 2013; 53: 764–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Youssefpour H, Li X, Lander AD, Lowengrub JS. Multispecies model of cell lineages and feedback control in solid tumors. Journal of theoretical biology 2012; 304: 39–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LS, Wang AX, Dong B, Pu KF, Yuan LH and Zhu YM. A new prospect in cancer therapy: targeting cancer stem cells to eradicate cancer. Chinese journal of cancer 2012; 31: 564–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vlashi E, Kim K, Dealla Donna L, Lagadec C, McDonald T, et al. In Vivo imaging, tracking, and targeting of cancer stem cells. J Natl Cancer Inst 2009;101:350–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagadec C, Dekmezian C, Lucile B, Pajonk F. Oxygen levels do not determine radiation survival of breast cancer stem cells. PloS one 2012; 7: e34545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagadec C, Vlashi E, Della Donna L, Meng Y, Dekmezian C, et al. Survival and self-renewing capacity of breast cancer initiating cells during fractionated radiation treatment. Breast Cancer Res 2010;12: R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bibault JE, Fumagalli I, Ferte C, Chargari C, Soria JC and Deutsch E. Cancer Metastasis Rev 2013;32:479–92. [DOI] [PubMed] [Google Scholar]
- 23.Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br. J Radiol 1985;58:515–28. [DOI] [PubMed] [Google Scholar]
- 24.Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J Radiol 1989;62:679–94. [DOI] [PubMed] [Google Scholar]
- 25.Dale R Use of the Linear-Quadratic Radiobiological Model for Quantifying Kidney Response in Targeted Radiotherapy. Cancer Biotherapy & Radiopharmaceuticals 2004; 19: 363–70. [DOI] [PubMed] [Google Scholar]
- 26.Qi XS, White J and Li XA. Is α/β for breast cancer really low? Radiat Oncol, 2011; 100:282–8. [DOI] [PubMed] [Google Scholar]
- 27.Baumann M, Krause M., Thames H, et al. Cancer stem cells and radiotherapy. Int. J. Biol, 2009;85:391–402. [DOI] [PubMed] [Google Scholar]
- 28.Bao S, Wu Q, McLendon RE. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006; 444:756–760. [DOI] [PubMed] [Google Scholar]
- 29.Stancanello J, Bayouth JE, Orton CG. Genomics, functional and molecular imaging will pave the road to individualized radiation therapy. Med Phys, 2008:35:4769–71. [DOI] [PubMed] [Google Scholar]
- 30.Brunner TB. Kunz-Schughart LA, Grosse-Gehling P, Baumann M. Cancer stem cells as a predictive factor in radibobiology. Semin in Rdaition Oncology 2012;22:151–74. [DOI] [PubMed] [Google Scholar]
- 31.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. [DOI] [PubMed] [Google Scholar]
- 32.Evers P, Lee PP, DeMarco J, et al. : Irradiation of the potential cancer stem cell niches in the adult brain improves progression-free survival of patients with malignant glioma. BMC Cancer 2010;10:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortholan C, Hannoun-Levi JM, Ferrero JM, Largillier R and Courdi A. Long-term results of adjunct hypofractionated radiotherapy for breast cancer in elderly patients. Int J Radiat Oncol Biol Phys 2005; 61:154–162. [DOI] [PubMed] [Google Scholar]
- 34.Courdi A, Ortholan C, Hannoun-Levi JM, et al. Long-term results of hypofractionated radiotherapy and hormonal therapy without surgery for breast cancer in elderly patients. Radiat Oncol 2006;79:156–161. [DOI] [PubMed] [Google Scholar]
- 35.Meacham CE and Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013;501:328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hillen T, Enderling H, Hahnfeldt. The tumor growth paradox and immune system-mediated selection for cancer stem cells. Bull Math Biol 2013;75:161–84. [DOI] [PubMed] [Google Scholar]
- 37.Yu VY, Nguyen D, Pajonk F, Kupelian P, et al. Incorporating cancer stem cells in radiation therapy treatment response modeling and the implication in glioblastoma multiforme treatment resistance. Int J Radiat Oncol Biol Phys 2015; 91:866–75. [DOI] [PubMed] [Google Scholar]
- 38.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Medema JP. Cancer stem cells: the challenges ahead Nat. Cell Biol Nature Publishing Group; 2013;15:338–44. [DOI] [PubMed] [Google Scholar]