SUMMARY:
Treatment options for metastatic and primary spinal tumors have expanded in recent years, in part due to the advances made in stereotactic radiosurgery. For metastatic spinal tumors, our institution utilizes the neurologic, oncologic, mechanical, and systemic (NOMS) decision framework, which provides a treatment paradigm based on the neurologic, oncologic, mechanical and systemic status of the patient. Radiosurgery as a supplement to surgical decompression has allowed for less-invasive surgical procedures carrying minimal morbidity while still providing effective local tumor control. Although wide en bloc excision has traditionally been the goal for the treatment of high-grade primary spine tumors, recent studies have shown promise for radiosurgery in providing control in tumors such as chordomas and high-grade sarcomas. Despite advances in radiosurgery, there continues to be limitations in providing effective conformational doses with minimal toxicity to critical structures. One of the ways to circumvent this and supplement external beam radiation is through the use of brachytherapy delivered by radioactive plaque or seeds.
KEYWORDS : brachytherapy, separation surgery, spine tumors, stereotactic radiosurgery
PRACTICE POINTS.
The neurologic, oncologic, mechanical, and systemic (NOMS) decision framework provides a treatment paradigm for metastatic spinal tumors by evaluating a patient’s neurologic, oncologic, mechanical and systemic status.
Stereotactic radiosurgery (SRS) has modified the treatment approach to metastatic spinal tumors by being able to provide local control to tumor histologies that are radioresistant to conventional external beam radiation therapy.
Separation surgery allows decompression of the neural elements and creation of a 2–3-mm tumor margin to allow for adjuvant SRS at tumoricidal doses that provides effective local tumor control without risking spinal cord toxicity.
Preliminary data have shown that SRS provided at a median dose of 24 Gy has been shown to be effective in providing tumor control in chordomas with low treatment-related morbidity.
32P plaque brachytherapy provides a safe alternative to previously reported methods of rigid plaque brachytherapy in order to supplement postoperative external beam radiation therapy through improved dosimetry.
Early data indicate that high-dose-rate interstitial 192Ir brachytherapy can be applied either intraoperatively or percutaneously for effective tumor and pain control in patients without external beam radiation options.
Management of primary and metastatic spinal tumors has evolved to include a combination of surgical resection and radiation. Developments in stereotactic radiosurgery (SRS) have allowed greater flexibility in the treatment of spinal tumors. Metastatic tumors that demonstrated poor responses to conventional external beam radiation therapy (cEBRT) are now being recognized as radiosensitive to SRS [1]. SRS has also expanded radiation strategies against primary spinal tumors. The emerging roles of SRS in spinal tumors have allowed the modification in the surgical approaches to these tumors. For metastatic spinal tumors, aggressive cytoreductive tumor resection may no longer be necessary. Surgical goals have been modified to maintain stability and neural function, and decompression of the neural elements in order to allow safe and effective delivery of radiosurgery in order to achieve local control. In primary spinal tumors, radiosurgery is emerging as a treatment in providing tumor control and may possibly be used as a neoadjuvant therapy to optimize surgical treatment or as the definitive therapy. In addition to cEBRT and SRS, brachytherapy has also been employed and has shown promise in helping to control tumor growth by circumventing the limitations of external beam radiation.
Metastatic tumors
Twenty percent of cancer patients will develop metastases to the spine [2,3]. The treatment for spinal metastases remains palliative with goals directed at pain management, preservation of neural function, spinal stabilization and local durable tumor control. The evolution of treatment for metastatic spinal disease has evolved from radiation therapy alone to include surgical decompression and stabilization [4]. Advances in SRS have further expanded the treatment options for radiation therapy. At our institution, we utilize the neurologic, oncologic, mechanical and systemic (NOMS) decision framework as a decision-making guide for the treatment of metastatic spinal disease.
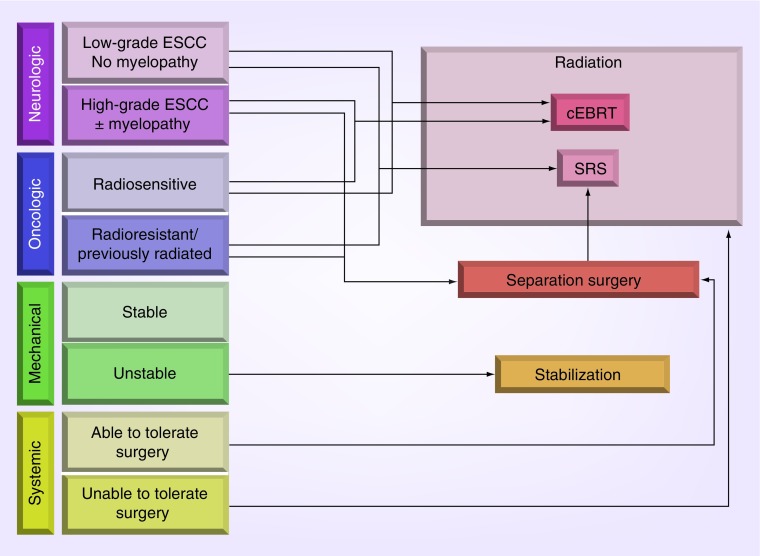
The NOMS framework is a treatment paradigm that takes into account four key elements in a patient’s clinical status to arrive at the optimal treatment approach (Figure 1). NOMS assesses the patient’s neurologic, oncologic, mechanical and systemic status [5]. The neurologic component takes into account the patient’s degree of epidural spinal cord compression (ESCC) and the clinical manifestations including myelopathy or functional radiculopathy. The oncologic component takes into account the tumor histology, which considers the tumor responsiveness to systemic or radiation therapy. The mechanical component addresses the stability of the spine at the level of disease, which dictates the type stabilization that may be needed. The systemic component takes into consideration the disease burden and associated medical comorbidities that contribute to the expected survival, which may influence the patient’s tolerance of possible treatments.
Figure 1. . Neurologic, oncologic, mechanical, and systemic (NOMS) decision framework.
cEBRT: Conventional external beam radiation therapy; ESCC: Epidural spinal cord compression; SRS: Stereotactic radiosurgery.
Reproduced with permission from [5].
One of the key decision points in the NOMS framework is tumor histology, which dictates the radiosensitivity of the tumor. Metastatic spine tumors are considered to be radiosensitive or radioresistant based on their responsiveness to cEBRT. cEBRT is typically delivered in one or two radiation beams without precise conformal techniques. The typical field of radiation encompasses the postoperative bed and includes the vertebral bodies above and below, which results in a large area of normal tissue included in the field. The inclusion of the spinal cord and other organs at risk in the radiation field limits the fraction dose that can be delivered using cEBRT. Recent advances have led to the development of image-guided radiation therapy (IGRT), which is a method of radiation treatment that provides conformal radiation doses with high spatial precision. This allows for the delivery of high doses of radiation to the desired treatment regions while minimizing the radiation dose of critical adjacent structures.
A review of the literature shows that the effectiveness of cEBRT greatly depends on the histology of the tumor [1]. Lymphoma, seminoma and myeloma are the most radiosensitive histologies. Among the solid tumors, breast, prostate, ovarian and certain neuroendocrine tumors are radiosensitive. Treatment of radiosensitive spinal metastases with cEBRT has been fairly consistently shown to result in durable local control. Treatment of 131 patients with spinal breast metastases resulted in 86% 2year local control rate [6]. The median duration of improvement in motor capacity in patients with spinal metastases from myeloma, breast and prostate cancers were 16, 12 and 10 months, respectively [7]. Renal, thyroid, hepatocellular, colon and non-small-cell lung carcinomas, sarcoma, as well as melanoma, represent radioresistant tumors in which cEBRT often fails to reliably provide durable local control. The 2year local control rates for lung and gastrointestinal metastases after cEBRT were 69 and 30%, respectively [6]. The median duration of motor improvement after cEBRT in patients with kidney, lung and bladder metastases were 5, 4 and 1 months, respectively [7]. For those histologies that were deemed radioresistant, SRS delivered via IGRT has been shown to achieve durable tumor control.
SRS has been shown to be effective in controlling tumor growth in multiple tumor histologies, including renal cell, melanoma and lung metastases [8,9]. Gerszten et al. reported an aggregate of 500 spinal metastasis patients, of which the most commonly treated histologies included renal cell, breast and lung cancer, and melanoma, with median follow-up of 21 months and tumor volume range between 0.2 and 264 cm3 [10]. They reported 86% overall pain improvement and 88% radiographic control. Yamada et al. reported similar results in a prospective study of 362 patients with 413 treated spinal metastases [11]. They reported a 2.4% risk of recurrence at 3 years after treatment when death was accounted as a competing risk to recurrence. Excellent tumor control was achieved in all histologies, with 3year local control rates of 89% for renal cell, 90% for melanoma, 92% for thyroid, 96% for sarcoma and 98% for breast, lung, prostate and colorectal cancer. Multiple oncology centers have reported similar results demonstrating the utility of radiosurgery to control tumor progression with low rates of toxicity and excellent rates of pain resolution [12–17].
The majority of studies indicate that local control with radiosurgery is independent of tumor size or histology, but there does appear to be an inverse relationship between radiation dose and local recurrence risk. Patients receiving 24 Gy single-fraction SRS exhibited significantly lower recurrence risk than patients with lower-dose treatments [18,19]. Furthermore, analysis of recurrence risk factors indicated that spinal metastases that received at least 15 Gy to the entire tumor volume exhibited no recurrence regardless of primary histology. This emphasizes the importance of optimizing dosimetry to the entire tumor volume [18]. This can only be achieved when separation between the tumor and the spinal cord exists, since a 15 Gy dose to the spinal cord has not been shown to be safe, and most physicians use lower spinal cord tolerance levels when planning spinal SRS. A systematic review of the literature performed by Gerszten et al. suggests that SRS provides durable symptomatic and radiographic tumor control independent of histology or prior fractionated radiotherapy, and that while the available quality of evidence is low, a strong recommendation based on a Cochran review of the literature is that radiosurgery should be used in the treatment of oligometastatic or radioresistant solid metastases to the spine [1].
Publications indicate a favorable toxicity profile after spinal SRS. The majority of reported complications consist of low-grade and well-tolerated toxicities. However, significant toxicities have also been reported and include vertebral compression fractures (VCF), myelopathy, neuropathy and plexopathy, and esophageal injuries. The rate of radiographic VCF has been reported to range between 11 and 39% [20–22]. Unfortunately, none of the studies have addressed the rate of symptomatic VCF after SRS, limiting the reporting to radiographic findings. Our preliminary data indicate that the rate of symptomatic VCF after 24Gy single-fraction SRS requiring cement or instrumented stabilization is approximately 10%. Majority of symptomatic post-SRS fractures can be stabilized with verterbo- or kypho-plasty or percutaneous screw stabilization with complete resolution of instability pain. Therefore, the 10% rate of symptomatic fracture after SRS appears to be quite acceptable considering the superior local control provided by 24 Gy. Increased awareness of this phenomenon has allowed us to proactively determine which patients are at high-risk of post-SRS VCF and to address their symptoms early and with minimal morbidity. Cox et al. reported a 6.8% risk of significant (grade ≥3) esophageal toxicity [23]. Patients with history of iotragenic esophageal manipulation and chemotherapy associated with radiation recall were at the highest risk of severe toxicity. Finally, the risk of spinal cord myelopathy and plexopathy after SRS appears to be quite low with only a few case reports of these events available [24].
For all radiosensitive tumors regardless of the ESCC grade [25], the NOMS framework directs treatment toward cEBRT. Radioresistant tumors without high-grade ESCC can be treated with radiosurgery, since the full tumor volume can receive the full dose of radiation without risking spinal cord toxicity. For radioresistant tumors with high-grade ESCC, surgical decompression and stabilization are required before SRS can be delivered. The primary goals of surgery are maintenance of mechanical stability and preservation of neural function through decompression of the neural elements. Prior to the integration of SRS, the goal of surgery was to maximize tumor resection in order to lessen the probability of recurrence due to the poor responses, using cEBRT as a postoperative adjuvant. Aggressive tumor resection may put the patients at increased risk of surgical morbidity and in the metastatic tumor population postoperative complications prohibit or delay patients from returning to meaningful systemic therapy. Historically, the data regarding postoperative tumor control are sparse but Klekamp and Samii reported that despite aggressive tumor resection, only 30% control rate at 1year was achieved using cEBRT in the postoperative setting [26]. With the integration of SRS, the need for aggressive tumor resection may significantly decrease as SRS markedly improves control similar to its use in the upfront setting. The integration of SRS led to the development of the concept of ‘separation surgery’ in patients operated for high-grade spinal cord compression from radioresistant histologies (Figure 2). Separation surgery is accomplished via a posterolateral laminectomy, unilateral or bilateral facetectomy and pedicle resection, followed by long posterior segmental fixation [27]. Instead of attempting a gross total resection of the tumor, the goal of separation surgery is to decompress the neural elements by creating a 2–3-mm separation between the tumor and the dural margin to allow for a tumoricidal dose of radiation to be applied to the entire tumor volume with sufficient room for dose fall-off to prevent toxicity to the spinal cord. Radiosurgery as an adjunct to surgical decompression has been shown to be effective in providing local tumor control. Rock et al. administered single-fraction SRS to 18 patients as postoperative treatment with a median follow-up of 7 months. Ninety-two percent of the patients remained neurologically stable or improved, while one deteriorated due to rapid tumor progression [28]. Moulding et al. evaluated 21 patients who were treated with single-fraction SRS, which resulted in a 1-year recurrence risk of 9.5%. Patients who received 24 Gy had significantly better local control with a 1-year recurrence risk of 6.3% [19]. This patient group was included in the larger analysis of 186 patients who underwent surgery followed by single-fraction or hypofractionated postoperative radiation [29]. The overall 1-year recurrence risk was 16.4%, with patients receiving high-dose hypofractionated radiation having a 1year recurrence risk of 4.1%, which was significantly lower than after low-dose hypofractionated radiation. The 1year recurrence rate after single-fraction SRS was 9.0%. A smaller series of patients confirmed our finding that high-dose per-fraction postoperative SRS provides superior local control compared with low-dose SRS and also noted that patients with complete spinal cord decompression had better post-SRS local tumor control compared with patients with persistent postoperative spinal cord compression [30]. Furthermore, it has been shown that hypofractionated SRS may also be safely employed in previously irradiated patients [31,32].
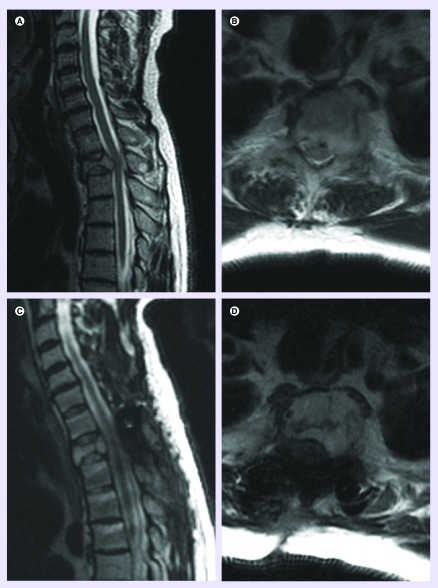
Figure 2. . Separation surgery followed by stereotactic radiosurgery for metastatic spinal tumor.
(A & B) A 63-year-old male with a history of metastatic thyroid carcinoma and metastasis to T2. (C & D) Two months following posterior decompression and stabilization followed by radiation of 2700 Gy in three fractions.
In patients with spinal instability who do not require surgical decompression, vertebral cement augmentation has been shown to be effective alone or with percutaneous instrumentation. Percutaneous cement augmentation has been demonstrated to provide effective pain control and stabilization in pathologic compression fractures. Cancer Patient Fracture Evaluation (CAFE) fractures that extend into the posterior elements including the pedicle or facet joints cannot be stabilized using cement augmentation alone [33]. In a study of 18 patients with metastatic spinal tumors with evidence of radiographic and symptomatic instability, percutaneous pedicle screw fixation and cement kyphoplasty were shown to consistently provide pain reduction and spinal stabilization. This strategy provides a minimally invasive approach to spinal stability in this particular patient population [34].
Primary tumors
Treatment goals for primary spinal tumors range from cure to palliation, depending on the histology and stage of the tumor. When amenable, en bloc excision to provide wide margins may provide a definitive cure. Depending on the tumor grade, chemotherapy or radiation may be needed in addition to surgery.
The surgical approach for primary spinal tumors was adopted from the Enneking system of staging musculoskeletal neoplasms, which recommends intracapsular or marginal excision of benign tumors and wide excision of malignant tumors [35]. The Weinstein, Boriani, Biagini (WBB) system was created to apply the concepts of the Enneking staging system to the spine [36]. A review of the literature shows that when wide margins can be achieved through en bloc resection of chordomas and chondrosarcomas, local recurrence and disease-free survival is significantly improved when compared with intralesional resections [37]. The Spine Oncology Study Group (SOSG) has concluded that surgery represents the primary treatment modality for local control of osteoblastoma, aneurysmal bone cyst, giant cell tumors, chordoma, chondrosarcoma, Ewing’s sarcoma and osteosarcoma [37–39].
Total excision of primary spinal tumors can be difficult to achieve due tumors that extend into the epidural space, large paraspinal masses or circumferential bone disease. Surgical resection to achieve wide margins in the sacral spine carries a high morbidity. Thus, while surgery may represent the best chance for cure in select patients, alternative therapies that are able supplement surgical resections are needed.
Chemotherapy has long played a major role in the treatment of osteogenic sarcoma and is being explored in the treatment of chordomas. A Phase II clinical study reported that imatinib, a tyrosine kinase inhibitor that targets PDGFB/PDGFRB-positive chordomas, has shown promise in providing local tumor control [40].
Chordomas, chondrosarcomas and most osteosarcomas have proven to be resistant to low-dose fractionated photon therapy [37]. On the other hand, heavy particle irradiation with protons and carbon ions has yielded promising results. Combination of photon irradiation with surgery has yielded 5-year local control rates ranging from 62 to 100% [41–43]. The results of fractionated carbon ion irradiation of large sacral chordomas have been even more promising, with two publications reporting local control rates of 94% in 34 patients with mean 43-month follow-up and 100% in seven patients with mean 58month follow-up [44,45].
Long-term response of spinal chordomas to SRS has yet to be determined. Several studies have shown the efficicacy of SRS in the treatment of cranial chordomas [46–50]. Based on the cranial results and convincing evidence that SRS is able to provide durable tumor control in significantly radioresistant metastases, SRS has been used as a therapy in inoperable and recurrent tumors and as planned postoperative adjuvant treatment. Henderson et al. treated three sacral, eight mobile spine and seven clival chordomas with CyberKnife in four or five fractions as a postoperative adjuvant treatment [51]. The 5-year actuarial control rate was 59% for the entire series. None of the patients with gross total resection of spinal chordomas who received at least 37.5 Gy to the tumor margin had a recurrence. Gerszten et al. reported the use of single-fraction SRS in the treatment of two spinal chordomas [52]. Wu et al. described the results of a pathologic examination of an L3 chordoma that was treated with 2400cGy single-fraction SRS [53]. The tumor was excised when 14 weeks after SRS the patient developed mechanical instability. The pathology showed the tumor was almost entirely necrotic with only a small focus of viable tissue. This result may lead to the use of radiation therapy as neoadjuvant therapy to assist in surgical resection.
We recently reported on 24 spinal chordomas that were treated at Memorial Sloan–Kettering Cancer Center with single-fraction SRS, with a median dose of 24 Gy [54]. This series includes primary, recurrent and metastatic chordomas of the mobile spine and the sacrum, with a median follow-up of 24 months. Only one instance of local progression has been diagnosed, resulting in an actuarial local control rate of 95%. Initially, SRS was only used as a salvage strategy in patients with recurrent tumors or patients who were excluded from surgery due to medical comorbidities or the extensive nature of their tumors. The encouraging results with these patients led to the use of SRS as a neoadjuvant treatment in an attempt to reduce the risk of postoperative recurrence. Six patients underwent surgery after SRS, with three of them having greater than 90% tumor necrosis at the time of surgery and one with 50% necrosis. The timing of surgery after SRS was variable; therefore, the range in the extent of tumor necrosis may not be reflective of the efficacy of SRS but may rather reflect the various time points in the cellular response to high-dose radiation. Six patients underwent SRS as an intended neoadjuvant treatment, but based on the lack of tumor progression refused to have surgery and are being followed radiographically. Most of the patients only experienced grade I or II skin reactions or odynophagia. One patient developed a sciatic radiculopathy and one developed partial vocal cord paralysis requiring vocal cord augmentation.
Brachytherapy
Brachytherapy represents an alternative form of radiation therapy that can circumvent some of the limitations of external beam radiation. Despite the advances achieved through SRS in enabling highly conformal radiotherapy for the delivery of cytotoxic tumoral doses assisted with separation surgery, concern about toxicity to the spinal cord remains a dose-limiting problem, particularly in instances when there is circumferential disease with tumor around the posterior aspect of the spinal dura. In order to supplement postoperative radiation therapy in these cases following separation surgery, we have employed the use of 32P plaques as single-dose intraoperative brachytherapy.
Rigid plaque brachytherapy systems have been reported in the past using 192Ir- and 90Y-based sources with a polycarbonate backing. Drawbacks of these systems include long fabrication time, short half-life and inflexibility of the plaque, which compromised its clinical utility on the dural surface [55]. Our plaque is developed from 32P bound chemically to a flexible and transparent polymer layer coated with silicone. 32P does not require special shielding and has a relatively long shelf life, retaining sufficient activity for 1–2 weeks, which allows for flexibility in the timing of the surgical intervention. The malleability of the plaque itself allows for contouring when applied to the dural surface for maximal contact. The source is available at maximal capacity of 4 mCi/cm2, and the manufacture-specific dose rate is approximately 38 cGy/min/mCi·cm2 at 1 mm from the foil surface. With the spinal cord at an estimated 3 mm from the dural surface, a standard subscription dose of 1000 cGy at 1 mm will result in a maximum cord dose of approximately 160 cGy. Plaques were applied to the surface of the dura after being wrapped in sterile plastic film (3M™ Ioban™ surgical film) to minimize errant exposure (Figure 3).
Figure 3. . 32P plaque brachytherapy for metastatic spinal tumors.
(A) 32P plaque sealed in Ioban™ surgical film. (B) Plaque placed on the dorsal dura following laminectomy.
Reproduced with permission from Department of Neurosurgery, Memorial Sloan–Kettering Cancer Center.
We applied 32P plaques in a study of 25 patients with recurrent primary or metastatic spinal tumors to assess local disease control. In our treatment group, 24 (96%) patients had failed at least one course of external beam radiation, consisting of cEBRT, SRS or proton therapy, prior to surgical resection and 12 (48%) patients had failed two or more courses of radiation. Patients were selected due to a history of prior radiation that would make effective re-radiation exceed spinal cord tolerance. Separation surgery was performed in these patients at which time the 32P plaque was applied to the posterior dural surface. Median exposure time was 15.1 min. All patients received postoperative EBRT. Dosing was calculated by integrating the dosing from the 32P plaque into the treatment plan, which accounted for the radiation to the posterior aspect of the dura. Coverage from the plaque extended the overall radiation coverage allowing simplification of the external beam treatment plan by reducing coverage burden, which may have been otherwise difficult or impossible given the need to meet dose fall off to the spinal cord. Mean local control rate of spinal sarcomas was 62.5% with a mean follow-up of 10.2 months and a mean time to failure of 7.3 months. Local control rate of metastatic disease was 82.4% with a mean follow-up of 7.3 months and a time to failure of 3.2 months. Local progression-free survival and overall survival rates for all spine tumor patients was 83.3% [56].
We have also developed the use of intraoperative and percutaneously placed 192Ir brachytherapy seeds [57]. High-dose-rate (HDR) interstitial brachytherapy techniques have been developed to use image-guided techniques to deliver tumoricidal doses of radiation directly to tumor in the vertebral bodies and paraspinal tissues with less risk to adjacent critical structures. We recently reported our study of five patients with metastatic spinal tumors treated with intraoperative or percutaneous 192Ir HDR spine brachytherapy. Patients in the study were selected because of symptomatic progressive disease at previously irradiated sites in the spine and determined that further cEBRT or SRS would exceed departmental constraint guidelines to the adjacent critical structures. Two patients in the study had recurrent tumors with epidural involvement that was previously treated with separation surgery followed by radiation. These patients were taken to the operating room for repeat decompression followed by intraoperative placement of HDR brachytherapy. Three patients with recurrent tumors treated with radiation therapy only received percutaneous HDR brachytherapy. The median dose delivered was 14 Gy (range: 12–18 Gy). At a median follow-up of 9 months, there was no local progression of disease. Four patients had either a reduction or complete resolution of pain. No evidence of brachytherapy-related complications were recorded and there was no evidence of toxicity to the adjacent structures. A prospective clinical trial at our institution is currently underway to validate the use of HDR 192Ir brachytherapy [58].
Limitations of current evidence
A wealth of data have been published discussing the results of spinal radiation in the treatment of metastatic and primary tumors. Unfortunately, the variability of radiation fractionation and dosing, reported outcome measures and stratification variables complicate consistent interpretation and synthesis of these into high-quality evidence and strong recommendations. Currently we have to rely on the best possible interpretation and comparison of the data and expert opinion. Several years ago the Spine Oncology Study Group, a multidisciplinary panel of experts who specialize in the treatment of spine tumors, performed the best possible analysis of the available data and made several recommendations. They concluded that the data support a strong recommendation that oligometastatic and radioresistant spinal metastases should be treated with SRS and delineated the histologies that have been shown to be resistant to cEBRT. To date, there is no prospective randomized trial comparing single-fraction or hypofractionated SRS to long-duration cEBRT and we are relying on historical data to make the comparison between spinal SRS and cEBRT.
Conclusion
The treatment of metastatic and primary spinal tumors is comprised from a combination of surgical resection and reconstruction, radiation therapy and chemotherapy. The ultimate goal is to achieve local tumor control while minimizing morbidity. Advances in radiation therapy have allowed more effective methods of treating spinal tumors to supplement less-invasive surgical interventions. Future developments will be directed toward advancements in radiation therapy that can provide more effective local tumor control without escalating risks to critical adjacent structures.
Future perspective
The goals of treatment in patients with spinal tumors include reliable and durable local control, minimizing the morbidity of disease and treatment, and optimizing the quality of life. Furthermore, we must achieve these goals in a financially responsible manner. Our understanding of tumor biology continues to improve with increasing emphasis on targeting alterations that drive tumor cell proliferation. Such targeted therapies will continue to develop and to gain prominence in the treatment of primary and metastatic spinal tumors. While the radiation techniques continue to improve, certain tumors recur even after high-dose SRS. We are working to optimize the dosimetry protocols in order to maximize tumor coverage without risking toxicity to surrounding tissues in order to further minimize the risk of recurrence. Finally, while surgery continues to play a prominent role in the treatment of spinal tumors, surgical goals will continue to evolve in order to adapt to the advances in systemic and radiation therapies. Minimally invasive surgical techniques are already decreasing or eliminating the need to interrupt systemic or radiation therapy and the prominence of such techniques continues to increase.
Future research is likely to be directed toward determining the optimal dosing of single-fraction and hypofractionated SRS and toward decreasing the risk of recurrence even further using combination of surgery, systemic therapy, radiosensitizers and improved radiation dosimetry. Furthermore, improvement in our understanding of toxicity risks should make this treatment safer. The significance of preservation of ambulation in the cancer population cannot be underestimated, but the cost–effectiveness of these treatments will have to be determined. Multicenter prospective protocols will facilitate collection of uniform information that will be broadly applicable across various patient demographic profiles.
Footnotes
Financial & competing interests disclosure
MH Bilsky serves on the advisory board for Varian Medical Systems. I Laufer is a consultant of SpineWave and DePuy/Synthes. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine. 2009;34(22 Suppl.):S78–92. doi: 10.1097/BRS.0b013e3181b8b6f5. [DOI] [PubMed] [Google Scholar]; •• Systematic literature review from the Spinal Oncology Study Group assessing the effectiveness of conventional external beam radiation therapy and stereotactic radiosurgery for metastatic spine disease, delineating between radiosensitive and radioresistant tumor histologies.
- 2.Cobb CA, 3rd, Leavens ME, Eckles N. Indications for nonoperative treatment of spinal cord compression due to breast cancer. J. Neurosurg. 1977;47(5):653–658. doi: 10.3171/jns.1977.47.5.0653. [DOI] [PubMed] [Google Scholar]
- 3.Walsh GL, Gokaslan ZL, McCutcheon IE. Anterior approaches to the thoracic spine in patients with cancer: indications and results. Ann. Thorac. Surg. 1997;64(6):1611–1618. doi: 10.1016/s0003-4975(97)01034-5. [DOI] [PubMed] [Google Scholar]
- 4.Patchell RA, Tibbs PA, Regine WF. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]; •• Landmark study for the support of surgical decompression in the treatment of metastatic spinal disease.
- 5.Laufer I, Rubin DG, Lis E. The NOMS framework: approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(6):744–751. doi: 10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Describes the neurologic, oncologic, mechanical, and systemic (NOMS) multidisciplinary approach to spinal metastases.
- 6.Mizumoto M, Harada H, Asakura H. Radiotherapy for patients with metastases to the spinal column: a review of 603 patients at Shizuoka Cancer Center Hospital. Int. J. Radiat. Oncol. Biol. Phys. 2011;79(1):208–213. doi: 10.1016/j.ijrobp.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 7.Maranzano E, Bellavita R, Rossi R. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a Phase III, randomized, multicenter trial. J. Clin. Oncol. 2005;23(15):3358–3365. doi: 10.1200/JCO.2005.08.193. [DOI] [PubMed] [Google Scholar]
- 8.Gerszten PC, Burton SA, Belani CP. Radiosurgery for the treatment of spinal lung metastases. Cancer. 2006;107(11):2653–2661. doi: 10.1002/cncr.22299. [DOI] [PubMed] [Google Scholar]
- 9.Gerszten PC, Burton SA, Ozhasoglu C. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J. Neurosurg. Spine. 2005;3(4):288–295. doi: 10.3171/spi.2005.3.4.0288. [DOI] [PubMed] [Google Scholar]
- 10.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 11.Yamada Y, Cox BW, Zelefsky MJ. An analysis of prognostic factors for local control of malignant spine tumors treated with spine radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(2):S132–S133. [Google Scholar]
- 12.Chang EL, Shiu AS, Mendel E. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J. Neurosurg. Spine. 2007;7(2):151–160. doi: 10.3171/SPI-07/08/151. [DOI] [PubMed] [Google Scholar]
- 13.Gerszten PC, Ozhasoglu C, Burton SA. CyberKnife frameless single-fraction stereotactic radiosurgery for tumors of the sacrum. Neurosurg. Focus. 2003;15(2):E7. doi: 10.3171/foc.2003.15.2.7. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs IC, Chang SD. Radiosurgery and radiotherapy for sacral tumors. Neurosurg. Focus. 2003;15(2):E8. doi: 10.3171/foc.2003.15.2.8. [DOI] [PubMed] [Google Scholar]
- 15.Gibbs IC, Kamnerdsupaphon P, Ryu MR. Image-guided robotic radiosurgery for spinal metastases. Radiother. Oncol. 2007;82(2):185–190. doi: 10.1016/j.radonc.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Ryu S, Fang Yin F, Rock J. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97(8):2013–2018. doi: 10.1002/cncr.11296. [DOI] [PubMed] [Google Scholar]
- 17.Ryu S, Jin R, Jin JY. Pain control by image-guided radiosurgery for solitary spinal metastasis. J. Pain Symptom Manage. 2008;35(3):292–298. doi: 10.1016/j.jpainsymman.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Lovelock DM, Zhang Z, Jackson A. Correlation of local failure with measures of dose insufficiency in the high-dose single-fraction treatment of bony metastases. Int. J. Radiat. Oncol. Biol. Phys. 2010;77(4):1282–1287. doi: 10.1016/j.ijrobp.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moulding HD, Elder JB, Lis E. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J. Neurosurg. Spine. 2010;13(1):87–93. doi: 10.3171/2010.3.SPINE09639. [DOI] [PubMed] [Google Scholar]
- 20.Boehling NS, Grosshans DR, Allen PK. Vertebral compression fracture risk after stereotactic body radiotherapy for spinal metastases. J. Neurosurg. Spine. 2012;16(4):379–386. doi: 10.3171/2011.11.SPINE116. [DOI] [PubMed] [Google Scholar]
- 21.Cunha MV, Al-Omair A, Atenafu EG. Vertebral compression fracture (VCF) after spine stereotactic body radiation therapy (SBRT): analysis of predictive factors. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(3):e343–349. doi: 10.1016/j.ijrobp.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 22.Rose PS, Laufer I, Boland PJ. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J. Clin. Oncol. 2009;27(30):5075–5079. doi: 10.1200/JCO.2008.19.3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox BW, Jackson A, Hunt M, Bilsky M, Yamada Y. Esophageal toxicity from high-dose, single-fraction paraspinal stereotactic radiosurgery. Int. J. Radiat. Oncol. Biol. Phys. 2012;83(5):e661–667. doi: 10.1016/j.ijrobp.2012.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahgal A, Whyne CM, Ma L, Larson DA, Fehlings MG. Vertebral compression fracture after stereotactic body radiotherapy for spinal metastases. Lancet Oncol. 2013;14(8):e310–320. doi: 10.1016/S1470-2045(13)70101-3. [DOI] [PubMed] [Google Scholar]
- 25.Bilsky MH, Laufer I, Fourney DR. Reliability analysis of the epidural spinal cord compression scale. J. Neurosurg. Spine. 2010;13(3):324–328. doi: 10.3171/2010.3.SPINE09459. [DOI] [PubMed] [Google Scholar]
- 26.Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neuro. 1998;140(9):957–967. doi: 10.1007/s007010050199. [DOI] [PubMed] [Google Scholar]
- 27.Wang JC, Boland P, Mitra N. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J. Neurosurg. Spine. 2004;1(3):287–298. doi: 10.3171/spi.2004.1.3.0287. [DOI] [PubMed] [Google Scholar]
- 28.Rock JP, Ryu S, Shukairy MS. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58(5):891–898. doi: 10.1227/01.NEU.0000209913.72761.4F. [DOI] [PubMed] [Google Scholar]
- 29.Laufer I, Iorgulescu JB, Chapman T. Local disease control for spinal metastases following ‘separation surgery’ and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: outcome analysis in 186 patients. J. Neurosurg. Spine. 2013;18(3):207–214. doi: 10.3171/2012.11.SPINE12111. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Reports the results of using separation surgery followed by stereotactic radiosurgery for metastatic spinal disease for local tumor control.
- 30.Al-Omair A, Masucci L, Masson-Cote L. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15(10):1413–1419. doi: 10.1093/neuonc/not101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damast S, Wright J, Bilsky M. Impact of dose on local failure rates after image-guided reirradiation of recurrent paraspinal metastases. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(3):819–826. doi: 10.1016/j.ijrobp.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 32.Katsoulakis E, Riaz N, Cox B. Delivering a third course of radiation to spine metastases using image-guided, intensity-modulated radiation therapy. J. Neurosurg. Spine. 2013;18(1):63–68. doi: 10.3171/2012.9.SPINE12433. [DOI] [PubMed] [Google Scholar]
- 33.Aghayev K, Papanastassiou ID, Vrionis F. Role of vertebral augmentation procedures in the management of vertebral compression fractures in cancer patients. Curr. Opin. Support. Palliat. Care. 2011;5(3):222–226. doi: 10.1097/SPC.0b013e328349652d. [DOI] [PubMed] [Google Scholar]
- 34.Schwab JH, Gasbarrini A, Cappuccio M, et al. Minimally invasive posterior stabilization improved ambulation and pain scores in patients with plasmacytomas and/or metastases of the spine. Int. J. Surg. Oncol. 2011;2011:239230. doi: 10.1155/2011/239230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enneking WF. A system of staging musculoskeletal neoplasms. Clin. Orthop. Relat. Res. 1986;204:9–24. [PubMed] [Google Scholar]; • Provides the basis for total surgical excision of primary bone tumors that has been applied to current thinking in primary spinal tumors.
- 36.Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22(9):1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 37.Boriani S, Saravanja D, Yamada Y, Varga PP, Biagini R, Fisher CG. Challenges of local recurrence and cure in low grade malignant tumors of the spine. Spine. 2009;34(22 Suppl.):S48–S57. doi: 10.1097/BRS.0b013e3181b969ac. [DOI] [PubMed] [Google Scholar]
- 38.Harrop JS, Schmidt MH, Boriani S, Shaffrey CI. Aggressive ‘benign’ primary spine neoplasms: osteoblastoma, aneurysmal bone cyst, and giant cell tumor. Spine. 2009;34(22 Suppl.):S39–S47. doi: 10.1097/BRS.0b013e3181ba0024. [DOI] [PubMed] [Google Scholar]
- 39.Sciubba DM, Okuno SH, Dekutoski MB, Gokaslan ZL. Ewing and osteogenic sarcoma: evidence for multidisciplinary management. Spine. 2009;34(22 Suppl.):S58–S68. doi: 10.1097/BRS.0b013e3181ba6436. [DOI] [PubMed] [Google Scholar]
- 40.Stacchiotti S, Longhi A, Ferraresi V. Phase II study of imatinib in advanced chordoma. J. Clin. Oncol. 2012;30(9):914–920. doi: 10.1200/JCO.2011.35.3656. [DOI] [PubMed] [Google Scholar]
- 41.Delaney TF, Liebsch NJ, Pedlow FX. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2009;74(3):732–739. doi: 10.1016/j.ijrobp.2008.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Staab A, Rutz HP, Ares C. Spot-scanning-based proton therapy for extracranial chordoma. Int. J. Radiat. Oncol. Biol. Phys. 2011;81(4):e489–e496. doi: 10.1016/j.ijrobp.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 43.Wagner TD, Kobayashi W, Dean S. Combination short-course preoperative irradiation, surgical resection, and reduced-field high-dose postoperative irradiation in the treatment of tumors involving the bone. Int. J. Radiat. Oncol. Biol. Phys. 2009;73(1):259–266. doi: 10.1016/j.ijrobp.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 44.Imai R, Kamada T, Tsuji H. Carbon ion radiotherapy for unresectable sacral chordomas. Clin. Cancer Res. 2004;10(17):5741–5746. doi: 10.1158/1078-0432.CCR-04-0301. [DOI] [PubMed] [Google Scholar]
- 45.Serizawa I, Imai R, Kamada T. Changes in tumor volume of sacral chordoma after carbon ion radiotherapy. J. Comput. Assist. Tomogr. 2009;33(5):795–798. doi: 10.1097/RCT.0b013e31818f0d49. [DOI] [PubMed] [Google Scholar]
- 46.Bridges M, Diamond DL. The financial impact of teaching surgical residents in the operating room. Am. J. Surg. 1999;177(1):28–32. doi: 10.1016/s0002-9610(98)00289-x. [DOI] [PubMed] [Google Scholar]
- 47.Kano H, Iqbal FO, Sheehan J. Stereotactic radiosurgery for chordoma: a report from the North American Gamma Knife Consortium. Neurosurgery. 2011;68(2):379–389. doi: 10.1227/NEU.0b013e3181ffa12c. [DOI] [PubMed] [Google Scholar]
- 48.Kato TA, Tsuda A, Uesaka M. In vitro characterization of cells derived from chordoma cell line U-CH1 following treatment with x-rays, heavy ions and chemotherapeutic drugs. Radiat. Oncol. 2011;6:116. doi: 10.1186/1748-717X-6-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krishnan S, Foote RL, Brown PD, Pollock BE, Link MJ, Garces YI. Radiosurgery for cranial base chordomas and chondrosarcomas. Neurosurgery. 2005;56(4):777–784. doi: 10.1227/01.neu.0000156789.10394.f5. [DOI] [PubMed] [Google Scholar]
- 50.Martin JJ, Niranjan A, Kondziolka D, Flickinger JC, Lozanne KA, Lunsford LD. Radiosurgery for chordomas and chondrosarcomas of the skull base. J. Neurosurg. 2007;107(4):758–764. doi: 10.3171/JNS-07/10/0758. [DOI] [PubMed] [Google Scholar]
- 51.Henderson FC, McCool K, Seigle J, Jean W, Harter W, Gagnon GJ. Treatment of chordomas with CyberKnife: georgetown university experience and treatment recommendations. Neurosurgery. 2009;64(2 Suppl.):A44–A53. doi: 10.1227/01.NEU.0000341166.09107.47. [DOI] [PubMed] [Google Scholar]
- 52.Gerszten PC, Ozhasoglu C, Burton SA. CyberKnife frameless single-fraction stereotactic radiosurgery for benign tumors of the spine. Neurosurg. Focus. 2003;14(5):e16. doi: 10.3171/foc.2003.14.5.17. [DOI] [PubMed] [Google Scholar]
- 53.Wu AJ, Bilsky MH, Edgar MA, Yamada Y. Near-complete pathological response of chordoma to high-dose single-fraction radiotherapy: case report. Neurosurgery. 2009;64(2):E389–E390. doi: 10.1227/01.NEU.0000338073.49649.1A. [DOI] [PubMed] [Google Scholar]
- 54.Yamada Y, Laufer I, Cox BW. Preliminary results of high-dose single-fraction radiotherapy for the management of chordomas of the spine and sacrum. Neurosurgery. 2013;73(4):673–680. doi: 10.1227/NEU.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 55.Delaney TF, Chen GT, Mauceri TC. Intraoperative dural irradiation by customized 192iridium and 90yttrium brachytherapy plaques. Int. J. Radiat. Oncol. Biol. Phys. 2003;57(1):239–245. doi: 10.1016/s0360-3016(03)00505-4. [DOI] [PubMed] [Google Scholar]
- 56.Folkert MR, Bilsky MH, Cohen GN. Intraoperative 32P high-dose rate brachytherapy of the dura for recurrent primary and metastatic intracranial and spinal tumors. Neurosurgery. 2012;71(5):1003–1010. doi: 10.1227/NEU.0b013e31826d5ac1. [DOI] [PubMed] [Google Scholar]; • States the first experience using intraoperative 32P plaque brachytherapy to help supplement postoperative radiation.
- 57.Folkert MR, Bilsky MH, Cohen GN. Intraoperative and percutaneous iridium-192 high-dose-rate brachytherapy for previously irradiated lesions of the spine. Brachytherapy. 2013;12(5):449–456. doi: 10.1016/j.brachy.2013.01.162. [DOI] [PubMed] [Google Scholar]
- 58.http://www.clinicaltrials.gov/show/NCT01757717 Image-Guided Navigation for High Dose Rate Temporary Interstitial Brachytherapy in the Palliative Management of Previously Treated Tumors of the Spine.