Abstract
Francisella tularensis is the causative agent of the life-threatening disease tularemia. However, the molecular tools to study Francisella are limited. Especially, expression plasmids are sparse and difficult to use, as they are unstable and prone to spontaneous loss. Most Francisella expression plasmids lack inducible promoters making it difficult to control gene expression levels. In addition, available expression plasmids are mainly designed for F. tularensis, however, genetic differences including restriction-modification systems impede the use of these plasmids in F. novicida, which is often used as a model organism to study Francisella pathogenesis. Here we report construction and characterization of two mobilizable plasmids (pFNMB1 and pFNMB2) designed for regulated gene expression in F. novicida. pFNMB plasmids contain a tetracycline inducible promoter to control gene expression levels and oriT for RP4 mediated mobilization. We show that both plasmids are stably maintained in bacteria for more than 40 generations over 4 days of culturing in the absence of selection against plasmid loss. Expression levels are dependent on anhydrotetracycline concentration and homogeneous in a bacterial population. pFNMB1 and pFNMB2 plasmids differ in the sequence between promoter and translation start site and thus allow to reach different maximum levels of protein expression. We used pFNMB1 and pFNMB2 for complementation of Francisella Pathogenicity Island mutants ΔiglF, ΔiglI, and ΔiglC in-vitro and pFNMB1 to complement ΔiglI mutant in bone marrow derived macrophages.
Keywords: Francisella novicida, expression plasmid, conjugation, ATc inducible, complementation, type VI secretion system, bacterial mutagenesis, tularemia
Introduction
Francisella tularensis is the causative agent of tularemia and can cause life-threatening disease in animals and humans. Essential for Francisella virulence is the Francisella pathogenicity island (FPI), which encodes a dynamic type VI secretion system (T6SS) (Bröms et al., 2010; Chong and Celli, 2010; Clemens et al., 2015; Brodmann et al., 2017). The most virulent subspecies F. tularensis subspecies tularensis, classified as a Tier 1 agent (Oyston et al., 2004), and subspecies holarctica (hereafter F. tularensis) contain two FPIs. The related subspecies F. tularensis subspecies novicida (hereafter F. novicida) possesses only one FPI and is highly virulent in mice but rarely infects humans. These features make F. novicida an ideal model organism for investigating tularemia and Francisella T6SS (Kingry and Petersen, 2014).
Molecular tools to make chromosomal in-frame deletion mutations in Francisella are available and commonly used to study the role of a certain gene of interest on a particular phenotype (Anthony et al., 1991; Golovliov et al., 2003; Frank and Zahrt, 2007). However, gene deletion and insertions may alter the expression of neighboring genes and cause so called “polar effects”. If expression of the gene of interest in trans from an inducible plasmid reverses the mutant phenotype, a possible polar effect can be ruled out. Unfortunately, only few expression plasmids are available for Francisella. Therefore, many recent studies lack in trans complementation of in-frame deletion mutations (Nano and Schmerk, 2007; Santic et al., 2011; Eshraghi et al., 2016; Brodmann et al., 2017) or use chromosomal complementation in cis (de Bruin et al., 2007; Weiss et al., 2007; Lindgren et al., 2013).
All available expression plasmids for Francisella are derived from the pFNL10 plasmid except for pCUG18, which is derived from pC194 and pUC18 (Rasko et al., 2007). pFNL10 was isolated from the F. novicida-like strain F6168 (Pavlov et al., 1996). The function of pFNL10 is unclear; however, the five encoding regions on the plasmid were identified. ORF1—ORF3 are required for plasmid replication and encode replication initiation protein RepA (ORF1), an ATP-dependent RNA helicase/endonuclease (ORF2), and an integrase/recombinase (ORF3). ORF4 and ORF5 encode a putative toxin-antitoxin system together with a possible regulatory feature ORFm (Pomerantsev et al., 2001a) (Figure 1A). Over the last 20 years, pFNL10 was modified to meet the needs of the Francisella research community. First, tetracycline and chloramphenicol resistance cassettes were introduced for selection resulting in pFNL200 (Pavlov et al., 1996). Since pFNL200 was restricted to replicate in Francisella, the p15A origin of replication of Escherichia coli was added thus obtaining a shuttle vector pKK202 (Norqvist et al., 1996). Later, the constitutively active groESL promoter was successfully used to express gfp and other genes (pKK214, pKK289Km, Figure 1B) in-vitro and in eukaryotic cells (Abd et al., 2003; Bönquist et al., 2008). Other pFNL10 derivatives are pFNLTP, which includes a version that only replicates at 32°C but not at 42°C due to a mutation in repA (Maier et al., 2004) and pMP, which includes a version of a bla promoter that is not recognized in E. coli to allow cloning of toxic genes in E. coli (LoVullo et al., 2006, 2009). So far, only two controllable Francisella promoter systems exist; a glucose repressible system (pTCD3) (Horzempa et al., 2008) and a tetracycline inducible or repressible version of the groESL promoter (pEDL) (LoVullo et al., 2012). The tetracycline inducible promoter system is a preferred choice for many bacterial model organisms because it allows tight and concentration dependent regulation of expression levels. It is also applicable for infection models such as cell cultures or animals since tetracycline passively penetrates most mammalian membranes (Bertram and Hillen, 2008). The tetracycline inducible promoter systems consists of constitutively expressed TetR, which binds to the tetO sequence and thereby transcriptionally represses the tetA promoter. Tetracycline or anhydrotetracycline (ATc), which is less toxic but has even higher affinity to TetR, binds TetR, and derepresses the tetA promoter (Gossen and Bujard, 1992). In the case of the tetracycline repressible promoter system, TetR binds tetO only if tetracycline or ATc is present, therefore, transcription is repressed upon addition of ATc (Scholz et al., 2004).
Figure 1.
Plasmid maps. Promoters, genes, terminators, and some relevant restriction sites are shown. Maps were designed with SnapGene Version 4.0.3. (A) Plasmid map of pFNL10 according to Pomerantsev et al. (2001a). (B) Plasmid map of pKK289Km gfp according to Bönquist et al. (2008). (C) Plasmid map of pFNMB msfgfp with variable RBS sequences. pFNMB1 contains the RBS sequence of iglC and pFNMB2 of pKK289Km. The underline highlights the RBS, the arrows mark the MluI restriction site and the start codon is represented in bold.
Despite the efforts in recent years, complementation from plasmid remains difficult in Francisella. Non-native expression levels (Santic et al., 2007; Zogaj and Klose, 2010) and spontaneous deletions in pFNL200 (Pomerantsev et al., 2001b) and pFNLTP (Maier et al., 2004) were reported. Another problem is the relatively low electroporation efficiency in Francisella and especially in F. novicida for plasmids isolated from E. coli. This is thought to be due to active restriction-modification systems in Francisella (Maier et al., 2004; LoVullo et al., 2006). In F. novicida, 4 restriction-modification systems were identified to restrict unmodified plasmid DNA, while in F. tularensis most restriction-modification system were annotated as pseudogenes (Gallagher et al., 2008). Expression plasmids were mainly tested in F. tularensis (Norqvist et al., 1996; Abd et al., 2003; LoVullo et al., 2006, 2009, 2012; Rasko et al., 2007) and consequently, in-frame deletions were more often complemented from plasmid in F. tularensis (Lai et al., 2004; Gil et al., 2006; Maier et al., 2006; Bönquist et al., 2008; Ark and Mann, 2011; Lindemann et al., 2011; Schmidt et al., 2013). On the other hand, suitable expression plasmids are mostly lacking in F. novicida and therefore only few studies include complementation experiments (Tempel et al., 2006; de Bruin et al., 2011).
Here we report construction of expression plasmids derived from pKK289Km specially designed for F. novicida. pFNMB1 and pFNMB2 plasmids can be mobilized by conjugation to overcome the need for electroporation. In addition, ATc induction allows homogeneous gene expression and the plasmids are stably maintained in a population for 4 days without selection pressure. As a proof of concept, we successfully complemented in-frame deletion of FPI genes iglF, iglI, and iglC in-vitro and iglI in bone marrow derived macrophages.
Materials and methods
Bacterial strains and growth conditions
Francisella tularensis subsp. novicida strain U112 and the derivative strains were grown in brain heart infusion (BHI) broth supplemented with 0.2% L-cysteine (Sigma). Ampicillin (100 μg/ml, AppliChem) or kanamycin (15 μg/ml, AppliChem) were added if not stated otherwise. Liquid cultures were grown aerobically at 37°C. Gene expression from plasmid was induced by adding the indicated concentration of anhydrotetracycline (ATc, IBA) to the liquid culture at OD600 of 0.02 for 3 h. Escherichia coli DH5α λpir and derivative strains were grown aerobically in Luria broth (LB) or on LB agar plates both supplemented with 50 μg/ml kanamycin at 37°C. All strains used are listed in Supplementary Table 1.
Construction of plasmids
All plasmids and corresponding primers are listed in Supplementary Table 2. Expression plasmids pFNMB1 and pFNMB2 were constructed by using the backbone of pKK289Km gfp (Bönquist et al., 2008) and inserting the RP4 mobilization site of pDMK3 (Lindgren et al., 2007) at EcoRI and SbfI restriction sites, thereby removing the truncated chloramphenicol resistance cassette, a leftover of pKK214CAT (Abd et al., 2003). Then the ATc inducible promoter cassette of pEDL17 (tetR with rpsL promoter and groESL promoter with tetO; LoVullo et al., 2012), the multiple cloning site of pDMK3 and the E. coli rrnB T1 and T2 terminators of pBAD24 (Guzman et al., 1995) were combined by overlap-extension PCR. The PCR product was placed into pKK289Km gfp at SpeI and EcoRI restriction sites to remove the original groESL promoter and gfp gene. For pFNMB1, the iglC RBS was inserted together with msfgfp at XhoI and XmaI restriction sites by amplification of msfgfp with primers containing the sequence of iglC RBS and an additional MluI restriction site in front of the msfgfp start codon (AGAGGAGAACGCGT). For pFNMB2, the iglC RBS was exchanged for the RBS of pKK289Km by combining the ATc inducible promoter cassette of pEDL17 and msfgfp by overlap-extension PCR using primers containing the RBS of pKK289Km with a MluI restriction site. The PCR product was placed into pFNMB1 at SpeI and XmaI restriction sites. All cloning products were sequenced. Plasmid maps were generated with SnapGene Version 4.0.3. pFNMB1 msfgfp (Addgene ID: 113191) and pFNMB2 msfgfp (Addgene ID: 113192) were deposited to Addgene.
Bacterial mutagenesis
Suicide vector pDMK3 was used for generating in-frame deletions as reported previously (Lindgren et al., 2007; Brodmann et al., 2017), except that an optimized conjugation procedure was used (described below). Various genes were cloned into pKK289Km using NdeI and EcoRI or SacI restriction sites and into pFNMB1 and pFNMB2 using MluI and SacI restriction sites. Plasmids, remaining peptides of in-frame deletions and primers are listed in Supplementary Table 3. Cloning products were sequenced and the site of homologous recombination was verified by PCR with primers located outside of the replaced regions.
pKK289Km and derivatives were transformed by electroporation as reported previously (Maier et al., 2004). Up to 1 μg of plasmid was used for electroporation. pFNMB1, pFNMB2 and derivatives were mobilized by conjugation as described below.
Conjugation
F. novicida was grown on BHI agar plates supplemented with 0.2 % L-cysteine and 100 μg/ml ampicillin and the donor E. coli strain (kind gift of A. Harms and C. Dehio, Harms et al., 2017) harboring the plasmid of interest was grown on Luria-Bertani (LB) agar plates supplemented with 300 μM 2,6-Diaminopimelic acid (Sigma) and 50 μg/ml kanamycin. Both plates were incubated over night at 37°C. The following day, about 100 μl of F. novicida and E. coli dense bacterial cultures were transferred to a fresh LB plate supplemented with 300 μM 2,6-Diaminopimelic acid and mixed thoroughly. After 2 h incubation at 37°C, about 50 μl of the mixture was resuspended in 100 μl Mueller–Hinton (MH) broth and plated on MH agar plates supplemented with 0.1% L-cysteine, 0.1% D-glucose (Millipore), 0.1% FCS (BioConcept), 100 μg/ml ampicillin, and 15 μg/ml kanamycin and incubated for 2 days at 37°C. Single F. novicida colonies were purified by passaging on selective plates.
To assess conjugation efficiency, the donor and recipient strains were first concentrated to an OD600 of 10 and then mixed in a 1 to 1 ratio (each 50 μl). Five microliters of the mixture was spotted on a LB agar plate supplemented with 300 μM 2,6-Diaminopimelic acid in two technical replicates. After 2 h, the spots were cut out and resuspended in 100 μl of MH broth. The resuspended bacteria were plated on MH agar plates supplemented with 0.1% L-cysteine, 0.1% D-glucose, 0.1% FCS, 100 μg/ml ampicillin, and 15 μg/ml kanamycin. The CFU per ml and the conjugation efficiency were calculated in the following manner:
The assay was performed in three biological replicates.
Plasmid stability assay
On day 0, F. novicida harboring pFNMB1 msfgfp or pFNMB2 msfgfp were diluted to an OD600 of 0.02 and grown in liquid overnight (ON) cultures supplemented with 15 μg/ml kanamycin and 500 ng/ml ATc to induce gene expression. On days 1–4, the old ON cultures were diluted to an OD600 of 0.02 and supplemented with 100 μg/ml ampicillin and 500 ng/ml ATc. For every ON culture, OD600 was measured and aliquots were taken for imaging, serial dilutions and inoculation of new ON cultures. Serial dilutions were plated on MH agar plates supplemented with 0.1% L-cysteine, 0.1% D-glucose, 0.1% FCS, and 100 μg/ml ampicillin and on MH agar plates supplemented with 0.1% L-cysteine, 0.1% D-glucose, 0.1% FCS, and 100 μg/ml ampicillin and 15 μg/ml kanamycin. Colony forming units (CFU) were counted and the concentrations of CFU/ml were calculated. Number of generations were calculated with following formula:
The experiment was carried out in three biological replicates.
Plasmid recovery
pFNMB1 msfgfp and pFNMB2 msfgfp were recovered from F. novicida with a Zyppy Plasmid Miniprep Kit (Zymo Research) after passaging the cultures for 4 days in liquid BHI supplemented with 100 μg/ml ampicillin and 500 ng/ml ATc as described above. About 250 ng of each plasmid DNA was then transformed into chemo-competent E. coli DH5α λpir. The transformed E. coli were plated on LB agar plates supplemented with 50 μg/ml kanamycin. Three independent experiments were carried out. The next day, colonies were grown in liquid LB supplemented with 50 μg/ml kanamycin, plasmid DNA was isolated and 250 ng of each plasmid was digested with SacI-HF and SpeI restriction enzymes (New England BioLabs) for 1 h. As control, both plasmids were additionally isolated from E. coli directly, without passaging in F. novicida, and digested identically. After heat inactivation of the enzymes (80°C for 20 min), the digested plasmids were loaded on a 1% agarose gel (BioConcept) together with a 1 kb ladder (New England BioLabs). DNA was stained with RedSafe (iNtRON Biotechnology) and a Red imaging system (Alpha Innotech) was used for imaging.
Fluorescence microscopy
Microscope set up was described previously (Kudryashev et al., 2015; Vettiger and Basler, 2016; Brodmann et al., 2017). F. novicida strains were prepared as described in Brodmann et al. (2017). For assessment of plasmid stability, 1.5 μl ON culture was spotted on a pad of 1% agarose in phosphate buffered saline (PBS) and imaged immediately. For measuring the GFP signal intensities after induction with ATc, the spotted bacteria were imaged immediately. For assessing T6SS function of complemented in-frame deletion mutants, the bacteria were incubated on a pad at 37°C for 1 h before imaging. All imaging experiments were performed in three independent experiments.
Image analysis
Image analysis and manipulations were performed with Fiji software (Schindelin et al., 2012) as described previously (Basler et al., 2013; Vettiger and Basler, 2016). For calculation of the GFP signal intensities after ATc induction, the background intensity was subtracted with the plugin “BackgroundSubstracter.” Then the plugin “Time Series Analyzer V3.0” was used to quantify the total GFP signal intensity of the whole field of view. The total GFP signal intensity was divided by the number of bacteria in the field of view. Number of bacteria was calculated with the “Find Maxima” function from phase contrast images. Contrast on compared images was adjusted equally. For the Supplementary Movies, the contrast used for F. novicida U112 ΔiglC pFNMB2 iglC induced with 500 ng/ml was set to match the other strains.
Cell culture and infection assay
The day before infection experiment, bone marrow derived macrophages (BMDMs) were seeded into 96-well plates (Eppendorf) at a density of 5*104 cells/well in DMEM (Thermo Fisher) with 20% M-CSF (supernatant of L929 mouse fibroblasts, BioConcept), 10% FCS (BioConcept), 10 mM HEPES (BioConcept), and non-essential amino acids (Thermo Fisher). The BMDMs were primed with 100 ng/ml LPS from E. coli O111:B4 (InvivoGen). F. novicida strains were grown aerobically in liquid BHI culture supplemented with the corresponding antibiotics and with 0 or 500 ng/ml ATc at 37°C ON. The next day, the medium of the BMDMs was replaced with fresh medium supplemented with 0 or 1,000 ng/ml ATc and the bacteria were added to the BMDMs at a multiplicity of infection (MOI) of 100. The 96-well plates were centrifuged at 300 g for 5 min to synchronize the infection process and afterwards incubated at 37°C. After 2 h, the medium was replaced with fresh medium supplemented with 0 or 1,000 ng/ml ATC and with 10 μg/ml gentamycin (BioConcept). Then the 96-well plates were incubated for 10 h at 37°C. Afterwards, a lactate dehydrogenase (LDH) release assay was carried out with an LDH Cytotoxicity Detection Kit (Takara). The percentage of LDH release was calculated with the following formula:
Infection experiments were carried out in biological triplicates. The unpaired two-tailed t-test with Welch's correction was used to identify significant differences. P-values are given in the figure legend.
Results
The need for expression plasmids for F. novicida, motivated us to construct the mobilizable and inducible expression plasmids pFNMB1 and pFNMB2 (Figure 1C). We constructed pFNMB1 and pFNMB2 by using the backbone of pKK289Km, which is transformed by electroporation and contains a constitutively active promoter groESL (Bönquist et al., 2008). As electroporation can be difficult in F. novicida (Maier et al., 2004; LoVullo et al., 2006), the need for electroporation was circumvented by inserting the RP4 mobilization site of pDMK3 (Lindgren et al., 2013) encoding traI (relaxase), traX (regulation of traI and traJ), traJ, and traK (oriT binding proteins) and origin of transfer (oriT) (Haase et al., 1995) at the site of the truncated chloramphenicol resistance cassette. The constitutively active groESL promoter was exchanged for the tetracycline inducible grotet promoter (LoVullo et al., 2012). Two different RBS were inserted to achieve a wider range of expression levels. pFNMB1 was designed for lower expression and contains the ribosomal binding site (RBS) of iglC in front of a MluI restriction site. Higher expression levels in pFNMB2 were reached by inserting the RBS of pKK289Km in front of a MluI restriction site. In addition, the well characterized E. coli rrnB T1 and T2 terminators from pBAD (Guzman et al., 1995) were inserted after a multiple cloning site.
First, we tested the conjugation efficiency of pFNMB1 msfgfp from an E. coli strain harboring a chromosomally encoded RP4 machinery (Harms et al., 2017) to F. novicida. Both strains were mixed in a 1:1 ratio and spotted on an agar plate. After 2 h incubation at 37°C, the bacteria were resuspended and plated on agar plates containing both ampicillin and kanamycin to select for F. novicida harboring the plasmid. On average, about 5.1*10−7 ± 2.5*10−7 bacterial cells were transformed per donor cell.
As plasmid instability is reported for certain Francisella plasmids (Pomerantsev et al., 2001b; Maier et al., 2004), we tested the stability of pFNMB1 and pFNMB2 with msfgfp in F. novicida over 4 days by inducing expression with 500 ng/ml ATc but without addition of kanamycin to select for plasmid maintenance (Figure 2). To assess plasmid stability, we monitored msfGFP expression by fluorescence microscopy (Figures 2A,B) and counted the kanamycin resistant colonies (Figure 2C). Over 4 days and during ~40 generations, the plasmids were stable in the bacterial population. Importantly both, the msfgfp and the kanamycin resistance cassette, which are located at different sites on the plasmid (Figure 1C), stayed fully functional. To exclude that the plasmids integrated into the chromosome, pFNMB1 msgfp and pFNMB2 msfgfp were recovered from F. novicida after passaging the bacteria for 4 days as described above. Then the isolated plasmid DNA was transformed into E. coli. The plasmids were recovered again, digested with SacI and SpeI restriction enzymes and loaded on an agarose gel to analyze the size of the DNA fragments. Two bands of the correct size (about 6,000 base pairs and 1,700 base pairs) were observed for pFNMB1 msfgfp and pFNMB2 msfgfp similarly to the controls pFNMB1 msfgfp and pFNMB2 msfgfp, which were not passaged in F. novicida (Figures 2D,E). These results strongly suggest that the plasmids are maintained extra-chromosomally in F. novicida without any rearrangements.
Figure 2.
Plasmid stability. GFP expression was induced with 500 ng/ml of ATc. ON culture of day 0 was supplemented with 15 μg/ml kanamycin, while ON cultures of days 1–4 were supplemented with 50 μg/ml ampicillin. (A) GFP expression in F. novicida U112 pFNMB1 msfgfp ON cultures of day 0 and day 4. (B) GFP expression in F. novicida U112 pFNMB2 msfgfp ON cultures of day 0 and day 4. (A,B) Images are a merge of phase contrast and GFP channel. 26 × 39 μm fields of view are shown. Scale bar represent 5 μm. Representative replicates are shown. Three independent experiments were performed. (C) Survival assay performed with ON cultures of F. novicida U112 pFNMB1 msfgfp and F. novicida U112 pFNMB2 msfgfp plated on ampicillin and ampicillin/kanamycin plates. Three independent experiments were performed. (D) Digestion of pFNMB1 msfgfp with SacI and SpeI restriction enzymes. (E) Digestion of pFNMB2 msfgfp with SacI and SpeI restriction enzymes. (D,E) Plasmids were passaged in F. novicida for 4 days before being transformed into E. coli. Controls were isolated directly from E. coli. Representative replicates are shown. Three independent experiments were performed.
To test if the grotet promoters of pFNMB1 and pFNMB2 respond to ATc in F. novicida, we used different ATc concentrations to induce expression of msfGFP (Figure 3). Indeed, msfGFP intensity increased in a concentration dependent manner for both plasmids. However, the level of induction differed; GFP expression from pFNMB1 was in general lower than from pFNMB2 (Figure 3D) indicating that the pKK289Km RBS starts translation more efficiently than the iglC RBS. Furthermore, we compared GFP expression from pFNMB1, pFNMB2 and pKK289Km by fluorescence microscopy (Figures 3A–C). Interestingly, bacteria harboring pKK289Km gfp showed a heterogeneous expression of GFP (Figure 3C), while all bacteria harboring pFNMB1 msfgfp or pFNMB2 msfgfp expressed similar levels of GFP after induction with 500 ng/ml ATc. Without ATc, no GFP fluorescence was observed indicating that expression is well repressed by the TetR in the absence of ATc (Figures 3A,B). However, GFP expression was higher in some bacteria containing pKK289Km plasmid than in those with pFNMB1 and pFNMB2 (Figures 3A–C).
Figure 3.
GFP intensities of pFNMB1 msfgfp, pFNMB2 msfgfp, and pKK289Km gfp. (A) Homogeneous GFP expression in F. novicida U112 pFNMB1 msfgfp upon induction with 500 ng/ml ATc. (B) Homogeneous GFP expression in F. novicida U112 pFNMB2 msfgfp upon induction with 500 ng/ml ATc. (C) Heterogeneous GFP expression in F. novicida U112 pKK289Km gfp. (A–C) GFP expression was induced with 0 ng/ml ATc and 500 ng/ml of ATc. GFP channels are shown. 39 × 26 μm fields of view. Scale bars represent 5 μm. (D) GFP expression in F. novicida U112 pFNMB1 msfgfp and F. novicida U112 pFNMB2 msfgfp was induced with 0, 25, 100, 250, 500, and 1,000 ng/ml of ATc. GFP intensity per bacterium was calculated as described in the section Materials and Methods. Three independent experiments were performed. Standard deviations are shown.
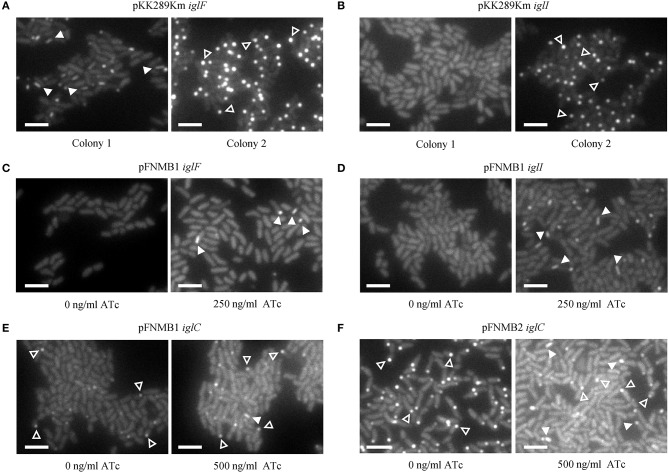
In our previous study, we constructed several in-frame deletion mutants in F. novicida and assessed T6SS function using fluorescence microscopy (Brodmann et al., 2017). For two mutants (ΔiglF and ΔiglI) with abolished T6SS function, we were unable to exclude polar effects as the deletion of the downstream genes (iglG and iglJ) resulted in similar phenotypes. Here, we generated F. novicida mutants carrying the respective complementation plasmids and successfully restored T6SS sheath assembly in ΔiglF and ΔiglI mutants by expression of IglF or IglI from pFNMB1 after induction with 250 ng/ml ATc (Figures 4C,D, Supplementary Movies 1, 2). Importantly, independently isolated colonies exhibited the same phenotypes. This was in contrast with several problems we experienced when using pKK289Km plasmid. First, electroporation of pKK289Km was very inefficient, as we routinely obtained only 1–10 transformed colonies even when using 1 μg of the plasmid DNA and 3*1010 F. novicida cells. In addition, independently isolated colonies exhibited different phenotypes such as no complementation, partial complementation or we only detected IglA-GFP aggregates in cells (Figures 4A,B, Supplementary Movies 1, 2) suggesting spontaneous deletions or variable expression levels. As previously characterized (Brodmann et al., 2017), T6SS dynamics in F. novicida consists of assembly, contraction and disassembly of a long cytosolic sheath at the bacterial poles and thus non-dynamic GFP aggregates likely represent non-functional T6SS (Supplementary Movies 1, 2). We also tested ATc inducible plasmid pEDL17 (LoVullo et al., 2012) for complementation, however, we failed to obtain any F. novicida colonies containing the plasmid.
Figure 4.
Complementation of in-frame deletion mutants on agar pads. The first frame of the GFP channel of one of the examples in the Supplementary Movie 2 is shown. T6SS assemblies were distinguished from GFP aggregates by analyzing dynamics in the movies. A maximum of 4 T6SS assemblies (arrow heads) and/or GFP aggregates (empty arrow heads) are highlighted. 13 × 9.75 μm fields of view. Scale bars represent 2 μm. (A) T6SS sheath assemblies (arrow heads) in F. novicida U112 iglA-sfgfp ΔiglF pKK289Km iglF colony 1 and GFP aggregates (empty arrow heads) in colony 2 (B) Failed T6SS sheath assembly in F. novicida U112 iglA-sfgfp ΔiglI pKK289Km iglI colony 1 and GFP aggregates (empty arrow heads) in colony 2. (C) T6SS sheath assemblies (arrow heads) in F. novicida U112 iglA-sfgfp ΔiglF pFNMB1 iglF, IglF expression induced with 250 ng/ml of ATc. (D) T6SS sheath assemblies (arrow heads) in F. novicida U112 iglA-sfgfp ΔiglI pFNMB1 iglI, IglI expression induced with 250 ng/ml of ATc. (E) One T6SS sheath assembly (arrow head) and GFP aggregates (empty arrow heads) in F. novicida U112 iglA-sfgfp ΔiglC pFNMB1 iglC, IglC expression induced with 500 ng/ml of ATc. (F) T6SS sheath assemblies (arrow heads) and GFP aggregates (empty arrow heads) in F. novicida U112 iglA-sfgfp ΔiglC pFNMB2 iglC, IglC expression induced with 500 ng/ml of ATc.
To further test pFNMB1 and pFNMB2 plasmids, we attempted to restore T6SS function in a ΔiglC mutant. The IglC protein is likely forming the T6SS inner tube, which was shown to be required in a large copy number in canonical T6SS, e.g., up to ~1,000 molecules for a single Vibrio cholerae T6SS sheath-tube complex (Wang et al., 2017). As shown on Figure 4E and Supplementary Movies 1, 2, T6SS sheath dynamics was only partially restored when inducing IglC expression from pFNMB1 with 500 ng/ml of ATc. However, T6SS sheath dynamics was restored to the levels similar to the parental strain when using pFNMB2 for IglC expression (Figure 4F, Supplementary Movies 1, 2). Overall, this suggests that pFNMB plasmids are superior to the previously used plasmids for complementation in F. novicida and that pFNMB2 plasmid can be used to achieve high levels of protein expression.
Intracellular F. novicida require a functional T6SS to escape from the phagosome in order to reach the replicative niche in the cytosol (Chong and Celli, 2010). Cytosolic F. novicida bacteria activate the absent in melanoma 2 (AIM2) inflammasome among other defense mechanisms, which leads to pyroptotic cell death and pro-inflammatory cytokine release (Fernandes-Alnemri et al., 2010; Jones et al., 2010). To test whether pFNMB1 can be used for complementation in bone marrow derived macrophages (BMDMs), we analyzed in-frame deletion mutant ΔiglI and the respective complemented strain for induction of pyroptosis in infected cells as a measure for phagosomal escape and thus T6SS function. We pre-induced expression of IglI from pFNMB1 with 0 and 500 ng/ml ATc overnight and then infected BMDMs, which were supplemented with 0 and 1,000 ng/ml ATc. After 10 h of infection, we observed significantly higher cell death for the complemented strain than for the in-frame deletion mutant without induced gene expression or for the in-frame deletion mutant without the plasmid (Figure 5). This result indicates that pFNMB1 can be used to restore T6SS activity in F. novicida mutant in BMDMs.
Figure 5.
Complementation of ΔiglI mutant in BMDMs. Release of LDH from primed BMDMs supplemented with 0 and 1,000 ng/ml ATc 10 h after infection with F. novicida U112 iglA-sfgfp ΔiglI and F. novicida U112 iglA-sfgfp ΔiglI pFNMB1 iglI. Bacterial cultures were supplemented with 0 and 500 ng/ml ATc overnight. Black columns represent conditions without induction by ATc and gray columns represent conditions with ATc. Means and standard deviations of nine measurements originating from three biological replicates are shown. The two-tailed unpaired t-test with Welch's correction was used to test significant differences between two groups. ***P < 0.001 and ****P < 0.0001.
Discussion
We generated stable mobilizable expression plasmids pFNMB1 and pFNMB2 for F. novicida. There are two major advantages using these plasmids. First, they can be easily mobilized from E. coli to F. novicida; second, they allow for inducible and homogeneous expression of inserted genes in-vitro and inside eukaryotic cells. We modified pKK289Km by insertion of the RP4 mobilization site as we experienced great difficulties transforming F. novicida by electroporation similarly to what was reported previously (Maier et al., 2004; LoVullo et al., 2006). The low electroporation efficiency in F. novicida is probably caused by the capsule and restriction-modification systems (Maier et al., 2004; LoVullo et al., 2006; Frank and Zahrt, 2007; Gallagher et al., 2008). Gallagher et al. (2008) suggested to first transform plasmid DNA into a F. novicida strain with all restriction-modification systems deleted and then use this isolated plasmid DNA to transform wild-type F. novicida. Importantly, the high efficiency of mobilization of the pFNMB plasmids can be reached without this step and therefore may allow for generation of large libraries of mutants and thus facilitate future screens and selections.
To express genes in a controlled manner, pFNMB1 and pFNMB2 contain a tetracycline inducible promoter system, which was used for F. tularensis (LoVullo et al., 2012). We could show that expression levels were dependent on ATc concentration in F. novicida (Figure 3D). In contrast to F. tularensis (LoVullo et al., 2012), we observed no growth defects of F. novicida in the presence of 1,000 ng/ml ATc. However, we noticed that the expression levels achieved from pKK289Km were higher than those from our constructs. One possible explanation for the lower induction levels of pFNMB1 compared to pKK289Km are the different RBS. However, pFNMB2 has a similar RBS as pKK289Km (except for the MluI restriction site); therefore, it is also possible that pFNMB2 is still partially repressed even at 1,000 ng/ml of ATc. This may suggest that cytosolic concentration of ATc reaches lower level in F. novicida than in F. tularensis. Indeed, differences in resistance levels toward tetracycline antibiotics and number of transporters were reported (Kingry and Petersen, 2014; Sutera et al., 2014). Additionally, in contrast to pKK289Km, the expression from pFNMB1 and pFNMB2 is homogenous throughout the bacterial population (Figures 3A–C). The reason for the heterogeneous gene expression from pKK289Km in F. novicida is unknown; however, spontaneous deletions or differential activation of the groESL promoter could be responsible.
Other suitable inducible promoter systems are difficult to use in Francisella. The araBAD promoter requires the uptake of L-arabinose for induction (Guzman et al., 1995); similarly the lac promoter requires lactose or isopropyl-β-D-thiogalactopyranosid (Polisky et al., 1976). Since Francisella lacks the L-arabinose and lactose degradation pathway (NCBI, RefSeq NC_008601.1, Larsson et al., 2005), it is questionable if these inducers are taken up. In addition, Francisella has a unique RNA polymerase composition with two different α subunits, which may interfere with promoter recognition of these commonly used inducible promoter systems subunits (Charity et al., 2007). A glucose repressible promoter system was described for F. tularensis (Horzempa et al., 2008), however, since glucose is a common carbon source, the use of such repressor could be problematic. In addition, a temperature dependent promoter was constructed for F. tularensis (Maier et al., 2004). However, since Francisella is an intracellular pathogen (Chong and Celli, 2010), many cell culture infections or in vivo experiments are performed at defined temperature and temperature shifting is impossible. Overall, the tetracycline inducible promoter system is likely the best option for F. novicida despite the apparent suboptimal level of derepression by ATc. Importantly, the possibility of inducing gene expression in cell culture or in vivo is a crucial advantage for testing the role of expressed genes during the pathogenesis of F. novicida.
In summary, we show that pFNMB1 and pFNMB2 are easy to mobilize into F. novicida and are stably maintained in the population. The tetracycline inducible promoter system is functional in F. novicida and can be used to tune gene expression levels. pFNMB1 and pFNMB2 exhibit homogeneous expression patterns in a population and can be used to complement chromosomal in-frame deletions. Overall, pFNMB1 and pFNMB2 may serve as useful tools for future studies of F. novicida.
Author contributions
MBr and MBa designed experiments, analyzed, and interpreted the results. MBr generated strains and acquired all data except for the BMDM infection experiments. PB and RH designed, analyzed, and interpreted the BMDM infection experiments. RH acquired the data for the BMDM infection experiments. MBr and MBa wrote the manuscript. All authors read and approved the manuscript.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank M. A. Horwitz (UCLA) for providing the strain F. novicida U112 iglA-sfGFP, D. M. Monack (Stanford University) for the conjugation plasmid pDMK3, T. H. Kawula (University of North Carolina at Chapel Hill) for the inducible and repressible plasmids pEDL17 and pEDL47, A. Sjöstedt (Umeå University) for the expression plasmid pKK289Km gfp and A. Harms and C. Dehio (Biozentrum, University of Basel) for the E. coli conjugation strain. The work was supported by Swiss National Science Foundation (SNSF) grants 31003A_159525 and PP00P3_165893/1 and the University of Basel. MBr was supported by the Biozentrum Basel International Ph.D. Program Fellowships for Excellence.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2018.00284/full#supplementary-material
References
- Abd H., Johansson T., Golovliov I., Sandström G., Forsman M. (2003). Survival and growth of Francisella tularensis in Acanthamoeba castellanii. Appl. Environ. Microbiol. 69, 600–606. 10.1128/AEM.69.1.600-606.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony L. S., Gu M. Z., Cowley S. C., Leung W. W., Nano F. E. (1991). Transformation and allelic replacement in Francisella spp. J. Gen. Microbiol. 137, 2697–2703. 10.1099/00221287-137-12-2697 [DOI] [PubMed] [Google Scholar]
- Ark N. M., Mann B. J. (2011). Impact of Francisella tularensis pilin homologs on pilus formation and virulence. Microb. Pathog. 51, 110–120. 10.1016/j.micpath.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler M., Ho B. T., Mekalanos J. J. (2013). Tit-for-tat: type VI secretion system counterattack during bacterial cell-cell interactions. Cell 152, 884–894. 10.1016/j.cell.2013.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram R., Hillen W. (2008). The application of Tet repressor in prokaryotic gene regulation and expression. Microb. Biotechnol. 1, 2–16. 10.1111/j.1751-7915.2007.00001.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bönquist L., Lindgren H., Golovliov I., Guina T., Sjöstedt A. (2008). MglA and Igl proteins contribute to the modulation of Francisella tularensis live vaccine strain-containing phagosomes in murine macrophages. Infect. Immun. 76, 3502–3510. 10.1128/IAI.00226-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann M., Dreier R. F., Broz P., Basler M. (2017). Francisella requires dynamic type VI secretion system and ClpB to deliver effectors for phagosomal escape. Nat. Commun. 8:15853. 10.1038/ncomms15853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröms J. E., Sjöstedt A., Lavander M. (2010). The role of the Francisella tularensis pathogenicity island in type VI secretion, intracellular survival, and modulation of host cell signaling. Front. Microbiol. 1:136. 10.3389/fmicb.2010.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charity J. C., Costante-Hamm M. M., Balon E. L., Boyd D. H., Rubin E. J., Dove S. L. (2007). Twin RNA polymerase-associated proteins control virulence gene expression in Francisella tularensis. PLoS Pathog. 3:e84. 10.1371/journal.ppat.0030084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong A., Celli J. (2010). The francisella intracellular life cycle: toward molecular mechanisms of intracellular survival and proliferation. Front. Microbiol. 1:138. 10.3389/fmicb.2010.00138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D. L., Ge P., Lee B. Y., Horwitz M. A., Zhou Z. H. (2015). Atomic structure of T6SS reveals interlaced array essential to function. Cell 160, 940–951. 10.1016/j.cell.2015.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin O. M., Duplantis B. N., Ludu J. S., Hare R. F., Nix E. B., Schmerk C. L, et al. (2011). The biochemical properties of the Francisella pathogenicity island (FPI)-encoded proteins IglA, IglB, IglC, PdpB and DotU suggest roles in type VI secretion. Microbiology 157, 3483–3491. 10.1099/mic.0.052308-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruin O. M., Ludu J. S., Nano F. E. (2007). The Francisella pathogenicity island protein IglA localizes to the bacterial cytoplasm and is needed for intracellular growth. BMC Microbiol. 7:1. 10.1186/1471-2180-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshraghi A., Kim J., Walls A. C., Ledvina H. E., Miller C. N., Ramsey K. M., et al. (2016). Secreted effectors encoded within and outside of the Francisella pathogenicity island promote intramacrophage growth. Cell Host Microbe 20, 573–583. 10.1016/j.chom.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Alnemri T., Yu J. W., Juliana C., Solorzano L., Kang S., Wu J., et al. (2010). The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393. 10.1038/ni.1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank D. W., Zahrt T. C. (2007). Genetics and genetic manipulation in Francisella tularensis. Ann. N.Y. Acad. Sci. 1105, 67–97. 10.1196/annals.1409.008 [DOI] [PubMed] [Google Scholar]
- Gallagher L. A., McKevitt M., Ramage E. R., Manoil C. (2008). Genetic dissection of the Francisella novicida restriction barrier. J. Bacteriol. 190, 7830–7837. 10.1128/JB.01188-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil H., Platz G. J., Forestal C. A., Monfett M., Bakshi C. S., Sellati T. J., et al. (2006). Deletion of TolC orthologs in Francisella tularensis identifies roles in multidrug resistance and virulence. Proc. Natl. Acad. Sci. U.S.A. 103, 12897–12902. 10.1073/pnas.0602582103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovliov I., Sjöstedt A., Mokrievich A., Pavlov V. (2003). A method for allelic replacement in Francisella tularensis. FEMS Microbiol. Lett. 222, 273–280. 10.1016/S0378-1097(03)00313-6 [DOI] [PubMed] [Google Scholar]
- Gossen M., Bujard H. (1992). Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. U.S.A. 89, 5547–5551. 10.1073/pnas.89.12.5547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L. M., Belin D., Carson M. J., Beckwith J. (1995). Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130. 10.1128/jb.177.14.4121-4130.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase J., Lurz R., Grahn A. M., Bamford D. H., Lanka E. (1995). Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J. Bacteriol. 177, 4779–4791. 10.1128/jb.177.16.4779-4791.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms A., Segers F. H., Quebatte M., Mistl C., Manfredi P., Körner J., et al. (2017). Evolutionary dynamics of pathoadaptation revealed by three independent acquisitions of the VirB/D4 type IV secretion system in Bartonella. Genome Biol. Evol. 9, 761–776. 10.1093/gbe/evx042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horzempa J., Tarwacki D. M., Carlson P. E., Robinson C. M., Nau G. J. (2008). Characterization and application of a glucose-repressible promoter in Francisella tularensis. Appl. Environ. Microbiol. 74, 2161–2170. 10.1128/AEM.02360-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. W., Kayagaki N., Broz P., Henry T., Newton K., O'Rourke K., et al. (2010). Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. U.S.A. 107, 9771–9776. 10.1073/pnas.1003738107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingry L. C., Petersen J. M. (2014). Comparative review of Francisella tularensis and Francisella novicida. Front. Cell. Infect. Microbiol. 4:35. 10.3389/fcimb.2014.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryashev M., Wang R. Y. R., Brackmann M., Scherer S., Maier T., Baker D., et al. (2015). Structure of the type VI secretion system contractile sheath. Cell 160, 952–962. 10.1016/j.cell.2015.01.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai X. H., Golovliov I., Sjöstedt A. (2004). Expression of IglC is necessary for intracellular growth and induction of apoptosis in murine macrophages by Francisella tularensis. Microb. Pathog. 37, 225–230. 10.1016/j.micpath.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Larsson P., Oyston P. C. F., Chain P., Chu M. C., Duffield M., Fuxelius H. H., et al. (2005). The complete genome sequence of Francisella tularensis, the causative agent of tularemia. Nat. Genet. 37, 153–159. 10.1038/ng1499 [DOI] [PubMed] [Google Scholar]
- Lindemann S. R., Peng K., Long M. E., Hunt J. R., Apicella M. A., Monack D. M., et al. (2011). Francisella tularensis Schu S4 O-antigen and capsule biosynthesis gene mutants induce early cell death in human macrophages. Infect. Immun. 79, 581–594. 10.1128/IAI.00863-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren H., Shen H., Zingmark C., Golovliov I., Conlan W., Sjöstedt A. (2007). Resistance of Francisella tularensis strains against reactive nitrogen and oxygen species with special reference to the role of KatG. Infect. Immun. 75, 1303–1309. 10.1128/IAI.01717-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren M., Bröms J. E., Meyer L., Golovliov I., Sjöstedt A. (2013). The Francisella tularensis LVS ΔpdpC mutant exhibits a unique phenotype during intracellular infection. BMC Microbiol. 13:20. 10.1186/1471-2180-13-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVullo E. D., Miller C. N., Pavelka M. S., Kawula T. H. (2012). TetR-based gene regulation systems for Francisella tularensis. Appl. Environ. Microbiol. 78, 6883–6889. 10.1128/AEM.01679-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVullo E. D., Sherrill L. A., Pavelka M. S. (2009). Improved shuttle vectors for Francisella tularensis genetics. FEMS Microbiol. Lett. 291, 95–102. 10.1111/j.1574-6968.2008.01440.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoVullo E. D., Sherrill L. A., Perez L. L., Pavelka M. S. (2006). Genetic tools for highly pathogenic Francisella tularensis subsp. tularensis. Microbiology 152, 3425–3435. 10.1099/mic.0.29121-0 [DOI] [PubMed] [Google Scholar]
- Maier T. M., Havig A., Casey M., Nano F. E., Frank D. W., Zahrt T. C. (2004). Construction and characterization of a highly efficient Francisella shuttle plasmid. Appl. Environ. Microbiol. 70, 7511–7519. 10.1128/AEM.70.12.7511-7519.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier T. M., Pechous R., Casey M., Zahrt T. C., Frank D. W. (2006). In vivo Himar1-based transposon mutagenesis of Francisella tularensis. Appl. Environ. Microbiol. 72, 1878–1885. 10.1128/AEM.72.3.1878-1885.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nano F. E., Schmerk C. (2007). The Francisella pathogenicity island. Ann. N.Y. Acad. Sci. 1105, 122–137. 10.1196/annals.1409.000 [DOI] [PubMed] [Google Scholar]
- Norqvist A., Kuoppa K., Sandström G. (1996). Construction of a shuttle vector for use in Francisella tularensis. FEMS Immunol. Med. Microbiol. 13, 257–260. 10.1111/j.1574-695X.1996.tb00248.x [DOI] [PubMed] [Google Scholar]
- Oyston P. C. F., Sjostedt A., Titball R. W. (2004). Tularaemia: bioterrorism defence renews interest in Francisella tularensis. Nat. Rev. Microbiol. 2, 967–978. 10.1038/nrmicro1045 [DOI] [PubMed] [Google Scholar]
- Pavlov V. M., Mokrievich A. N., Volkovoy K. (1996). Cryptic plasmid pFNL10 from Francisella novicida-like F6168: the base of plasmid vectors for Francisella tularensis. FEMS Immunol. Med. Microbiol. 13, 253–256. 10.1111/j.1574-695X.1996.tb00247.x [DOI] [PubMed] [Google Scholar]
- Polisky B., Bishop R. J., Gelfand D. H. (1976). A plasmid cloning vehicle allowing regulated expression of eukaryotic DNA in bacteria. Proc. Natl. Acad. Sci. U.S.A. 73, 3900–3904. 10.1073/pnas.73.11.3900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomerantsev A. P., Golovliov I. R., Ohara Y., Mokrievich A. N., Obuchi M., Norqvist A., et al. (2001a). Genetic organization of the Francisella plasmid pFNL10. Plasmid 46, 210–222. 10.1006/plas.2001.1548 [DOI] [PubMed] [Google Scholar]
- Pomerantsev A. P., Obuchi M., Ohara Y. (2001b). Nucleotide sequence, structural organization, and functional characterization of the small recombinant plasmid pOM1 that is specific for Francisella tularensis. Plasmid 46, 86–94. 10.1006/plas.2001.1538 [DOI] [PubMed] [Google Scholar]
- Rasko D. A., Esteban C. D., Sperandio V. (2007). Development of novel plasmid vectors and a promoter trap system in Francisella tularensis compatible with the pFLN10 based plasmids. Plasmid 58, 159–166. 10.1016/j.plasmid.2007.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santic M., Molmeret M., Barker J. R., Klose K. E., Dekanic A., Doric M., et al. (2007). A Francisella tularensis pathogenicity island protein essential for bacterial proliferation within the host cell cytosol. Cell. Microbiol. 9, 2391–2403. 10.1111/j.1462-5822.2007.00968.x [DOI] [PubMed] [Google Scholar]
- Santic M., Ozanic M., Semic V., Pavokovic G., Mrvcic V., Kwaik Y. A. (2011). Intra-vacuolar proliferation of F. Novicida within H. Vermiformis. Front. Microbiol. 2:78. 10.3389/fmicb.2011.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., et al. (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. 10.1038/nmeth.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M., Klimentova J., Rehulka P., Straskova A., Spidlova P., Szotakova B., et al. (2013). Francisella tularensis subsp. holarctica DsbA homologue: a thioredoxin-like protein with chaperone function. Microbiol. Read. Engl. 159, 2364–2374. 10.1099/mic.0.070516-0 [DOI] [PubMed] [Google Scholar]
- Scholz O., Henssler E. M., Bail J., Schubert P., Bogdanska-Urbaniak J., Sopp S., et al. (2004). Activity reversal of Tet repressor caused by single amino acid exchanges. Mol. Microbiol. 53, 777–789. 10.1111/j.1365-2958.2004.04159.x [DOI] [PubMed] [Google Scholar]
- Sutera V., Levert M., Burmeister W. P., Schneider D., Maurin M. (2014). Evolution toward high-level fluoroquinolone resistance in Francisella species. J. Antimicrob. Chemother. 69, 101–110. 10.1093/jac/dkt321 [DOI] [PubMed] [Google Scholar]
- Tempel R., Lai X. H., Crosa L., Kozlowicz B., Heffron F. (2006). Attenuated Francisella novicida transposon mutants protect mice against wild-type challenge. Infect. Immun. 74, 5095–5105. 10.1128/IAI.00598-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vettiger A., Basler M. (2016). Type VI secretion system substrates are transferred and reused among sister cells. Cell 167, 99–110.e12. 10.1016/j.cell.2016.08.023 [DOI] [PubMed] [Google Scholar]
- Wang J., Brackmann M., Castaño-Díez D., Kudryashev M., Goldie K. N., Maier T., et al. (2017). Cryo-EM structure of the extended type VI secretion system sheath-tube complex. Nat. Microbiol. 2, 1507–1512. 10.1038/s41564-017-0020-7 [DOI] [PubMed] [Google Scholar]
- Weiss D. S., Brotcke A., Henry T., Margolis J. J., Chan K., Monack D. M. (2007). In vivo negative selection screen identifies genes required for Francisella virulence. Proc. Natl. Acad. Sci. U.S.A. 104, 6037–6042. 10.1073/pnas.0609675104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zogaj X., Klose K. E. (2010). Genetic manipulation of francisella tularensis. Front. Microbiol. 1:142. 10.3389/fmicb.2010.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.