Abstract
Background
We describe a case of pan-resistant Pseudomonas aeruginosa postsurgical meningitis associated with the presence of an external ventricular device. We changed therapy twice; finally, by using amikacin and a continuous infusion of cefepime, we obtained clinical improvement.
Case presentation
A female patient, who underwent surgery for a cavernous angioma, presented with meningitis. Cerebrospinal fluid culture revealed a multidrug-resistant Pseudomonas aeruginosa, initially sensitive only to colistin. We successfully used intrathecal amikacin and intravenous cefepime continuous infusion plus intravenous amikacin after two previous ineffective therapeutic approaches.
Conclusion
The evaluation of the antibiotic concentration and the bactericidal activity in cerebrospinal fluid may contribute to the choice of the drug in cases of multidrug-resistant meningitis.
Keywords: pan-resistant meningitis, Pseudomonas aeruginosa, amikacin
Introduction
Literature data on the management of neurosurgical infections associated with neurosurgical devices are still scanty, and currently no standardized treatment procedures are available.1 The use of intraventricular (IVT) colistin has been associated with positive clinical outcomes,2,3 and its daily doses and regimens vary considerably and are often empirically chosen.4
Imberti et al described, for the first time, the pharmacokinetics of colistin after IVT administration.5 They found that IVT of colistimethate at a dose of ≥5.22 mg/day was pharmacokinetically (PK)/pharmacodynamically appropriate, but since external cerebrospinal fluid (CSF) efflux is variable and it can influence its clearance and concentration in the CSF, the daily dose of 10 mg may be appropriate.
Although substantial publications have clearly highlighted the PK parameters of aminoglycosides after intravenous (IV) administration, PK models for central nervous system (CNS) drug disposition are not available, the IVT dosage regimens have not been studied in prospective, randomized trials, and empirical dosages of IVT amikacin 5–50 mg daily are recommended for meningitis and/or ventriculitis.6 Moreover, differences exist between intracranial/CSF and intravascular/blood systems, particularly regarding the blood fluid dynamics, CNS pathophysiological conditions, and external ventricular device (EVD) setting.7 For this reason, direct instillation of amikacin into the CNS via IVT administration may help bypass the physiologic barrier in order to target the drug to the site of action.
We describe a case of pan-resistant postsurgical meningitis with loss of sensitivity to colistin, while on successful treatment with a combination of IV cefepime, IV amikacin and IVT amikacin.
Case report
A 66-year-old Caucasian woman underwent surgical excision of a cavernous angioma. The surgical intervention was complicated by a cerebral ischemia that caused a low level of consciousness and a prolonged stay in the Intensive Care Unit (ICU). After 3 days, a hydrocephalus occurred, and so an EVD was placed.
On day 12, after EVD placement, the patient presented with meningitis and CSF analysis revealed white blood cell (WBC) count 1,600 cells/µL, glucose 10 mg/dL, and total protein 566 g/L; on that occasion, EVD was changed for the first time. As Gram staining showed Gram-negative rods, and due to the concurrent presence of a Pseudomonas aeruginosa–colonized patient in the ICU, an empiric therapy with IV cefepime (6 g/day by continuous infusion after 2 g loading dose), IV colistin (4.5 million units every 12 hours after 9 million units loading dose), and IVT colistin (10 mg) was started. In fact, CSF culture revealed a multidrug-resistant (MDR) P. aeruginosa only sensitive to colistin with a minimum inhibitory concentration (MIC) value of 0.75 mg/L (Sensititre, Thermo Fisher, Scientific, Waltham, MA, USA; Etest, bioMèrieux, Craponne, France).
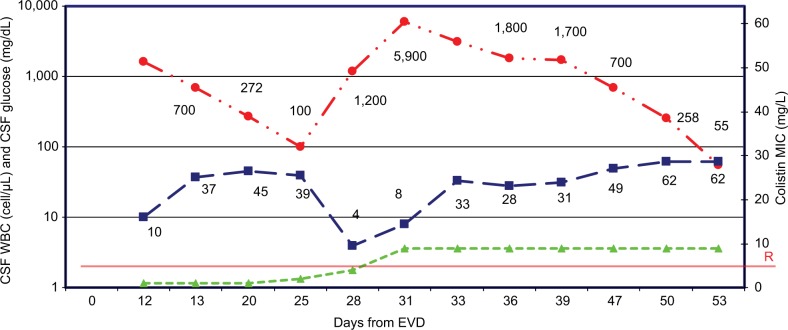
Empiric therapy was optimized by using IV colistin, meropenem (2 g every 8 hours extended infusion), and IVT colistin; but after an initial improvement of the CSF parameters, we observed an increase in the CSF WBC count and a decrease in CSF glucose. Moreover, CSF cultures continued to demonstrate growth of P. aeruginosa with colistin MIC increasing from 0.75 to 4–8 mg/L (Sensititre, Etest). Therefore, a second change occurred by setting the antimicrobial therapy as follows: IVT amikacin 30 mg/day6 and IV cefepime continuous infusion plus IV amikacin 1 g/day. We chose IVT amikacin instead of colistin because, although resistant, amikacin MIC level (32 mg/L) was the nearest to the European Committee on Antimicrobial Susceptibility Testing breakpoint (16 mg/L). Figure 1 shows the trend of CSF parameters during the changes in the therapeutic plan, with particular regard to the period when P. aeruginosa became resistant to colistin.
Figure 1.
CSF parameters according to different antibiotic treatments and colistin MIC values.
Notes: CSF WBC (cells/μL) <img>; CSF glucose (mg/dL) <img>; Colistin MIC <img>. R: colistin breakpoint=4 (EUCAST).
Abbreviations: CSF, cerebrospinal fluid; EUCAST, European Committee on Antimicrobial Susceptibility Testing; EVD, external ventricular device; MIC, minimum inhibitory concentration; WBC, white blood cell.
From day 28, P. aeruginosa could be considered pan-resistant rather than MDR.
During IVT amikacin therapy, we measured the intrathecal concentration achieved 1 hour after the end of infusion by a homogeneous Enzyme Multiplied Immuno Assay (EMIT® performed on a VIVA-E® analyzer; Siemens Healthcare Diagnostic, Newark, DE, USA), validated by the manufacturer for use in human serum/plasma matrix. The method was adapted for CSF matrix. The concentration of amikacin in the CSF sample exceeded 200 mg/L; however, this level was adequate, considering the amikacin MIC breakpoint. We did not determine IVT colistin concentration at that time. Moreover, due to the difficulty to accurately control P. aeruginosa colistin MIC level with commercial automatic systems,8–10 we observed variable colistin MIC values with the two distinct analytical methods (Sensititre, Etest).
We also verified the activity of the different therapeutic regimens by evaluating the bactericidal activity of both colistin and amikacin IVT treatments in CSF, maintained at −80°C, by using the microtiter dilution method.11 The test was performed in duplicate on both the samples taken before and after administration of antibiotics. One hundred microliters of CSF was added to the first well of a microtiter plate (Greiner-bio-One, Frickenhausen, Germany) and serially diluted in 50 µL of Mueller Hinton broth (Oxoid Ltd, Basingstoke, England). We diluted an overnight culture of P. aeruginosa 1:1,000 (5×105 colony-forming units/mL) in Mueller Hinton broth and 50 µL was added to every well of the microplate. The final volume in each well was 100 µL and the dilution range was 1:2–1:1,024. After incubation at 35°C for 18 hours, the inhibitory titer was defined as the highest dilution that prevented visual turbidity. In order to assess the bactericidal activity, 10 µL was removed from each clear well, plated onto Mueller Hinton agar, and incubated at 35°C for 18 hours.
The bactericidal activity of the CSF was defined as the highest dilution that resulted in ≥99.9% killing of the starting inoculum. In the case of IVT colistin treatment, the bactericidal activity was not optimal for trough (trough <1:2, peak=1:64), while it was more effective with amikacin (trough=1:8, peak=1:128).
The success of our strategy was objectively measured by CSF culture (that became negative after 8 days of IVT amikacin therapy) and by progressive improvement of CSF parameters, considering the complex clinical scenario of this fragile ICU patient.
Informed consent
We collected samples from the patient treated in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration. The patient signed the informed consent regarding acceptance of proposed therapies and publication of data for scientific purposes. The Ethics Committee of Humanitas Clinical and Research Center approved this publication as a retrospective study (11/18).
Data collection and processing were carried out in accordance with the Italian law for patient confidentiality and good clinical practice.
Discussion
To the best of our knowledge, no other cases have been reported, so far, describing successful treatment of meningitis in the presence of a pan-resistant P. aeruginosa that lost sensitivity even to colistin during treatment.
P. aeruginosa is defined “pan-resistant” when it is resistant to all cephalosporins, piperacillin–tazobactam, aztreonam, carbapenems, ciprofloxacin, and aminoglycosides.12
Regarding MDR P. aeruginosa, there are several definitions; nevertheless, they are usually recognized as resistant to at least three classes of antibiotics.13
Hsueh et al demonstrated synergism for cefepime–amikacin after 24 hours in two blood isolates intermediately resistant or resistant to all cephalosporins, piperacillin–tazobactam, aztreonam, carbapenems, ciprofloxacin, and aminoglycosides (pandrug-resistant Pseudomonas aeruginosa [PDRPA]).14
Ideally, monitoring of aminoglycosides concentrations in the CSF would be the correct way to individualize IVT therapy in order to achieve effectiveness and minimize risks, besides which there are doubts concerning the optimal times to withdraw CSF samples once the therapy has been initiated. The guidelines published by the Infectious Diseases Society of America in 2004 recommended an empirical dosage of IVT aminoglycosides for bacterial meningitis6 and an adjustment of therapy based on the “inhibitory quotient” to maintain antimicrobial CSF concentrations greater than 10–20 times the MIC of the isolated causative organism.
Our data respected this recommendation in terms of amikacin concentration in the CSF, 1 hour after the end of IVD infusion.
Since colistin resistance represents a challenge, especially for infections that are difficult to treat, such as meningitis, alternative options must be considered.
Conclusion
This study shows that the evaluation of antibiotic concentration and bactericidal activity in CSF may be a decisive factor in the choice of the drug and in the succesfully outcome when treating MDR meningitis. IV and IVT amikacin administration in place of colistin (P. aeruginosa became resistant during the treatment) allowed reaching therapeutic success. The optimal drug dosage, guided by both amikacin CSF concentration monitoring and antibacterial activity test, led to the negative culture in CSF, with a corresponding improvement of parameters in terms of WBC and glucose. Despite amikacin resistance (MIC: 32 mg/L, breakpoint 16 mg/L) of P. aeruginosa, the chosen therapeutic plan led to healing of the patient.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Conen A, Fux CA, Vajkoczy P, Trampuz A. Management of infections associated with neurosurgical implanted devices. Expert Rev Anti Infect Ther. 2017;15(3):241–255. doi: 10.1080/14787210.2017.1267563. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert B, Morrison C. Evaluation of intraventricular colistin utilization: a case series. J Crit Care. 2017;40:161–163. doi: 10.1016/j.jcrc.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Bargiacchi O, Rossati A, Car P, et al. Intrathecal/intraventricular colistin in external ventricular device-related infections by multi-drug resistant Gram negative bacteria: case reports and review. Infection. 2014;42(5):801–809. doi: 10.1007/s15010-014-0618-0. [DOI] [PubMed] [Google Scholar]
- 4.Imberti R, Iotti GA, Regazzi M. Intraventricular or intrathecal colistin for the treatment of central nervous system infections caused by multidrug-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther. 2014;12(4):471–478. doi: 10.1586/14787210.2014.896740. [DOI] [PubMed] [Google Scholar]
- 5.Imberti R, Cusato M, Accetta G, et al. Pharmacokinetics of colistin in cerebrospinal fluid after intraventricular administration of colistin meth-anesulfonate. Antimicrob Agents Chemother. 2012;56(8):4416–4421. doi: 10.1128/AAC.00231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis. 2004;39(9):1267–1284. doi: 10.1086/425368. [DOI] [PubMed] [Google Scholar]
- 7.Nau R, Sörgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood–brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23(4):858–883. doi: 10.1128/CMR.00007-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lat A, Clock SA, Wu F, et al. Comparison of polymyxin B, tigecycline, cefepime, and meropenem MICs for KPC-producing Klebsiella pneumoniae by broth microdilution, Vitek 2, and Etest. J Clin Microbiol. 2011;49(5):1795–1798. doi: 10.1128/JCM.02534-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bulik CC, Fauntleroy KA, Jenkins SG, et al. Comparison of meropenem MICs and susceptibilities for carbapenemase-producing Klebsiella pneumoniae isolates by various testing methods. J Clin Microbiol. 2010;48(7):2402–2406. doi: 10.1128/JCM.00267-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tan TY, Ng SY. Comparison of Etest, Vitek and agar dilution for susceptibility testing of colistin. Clin Microbiol Infect. 2007;13(5):541–544. doi: 10.1111/j.1469-0691.2007.01708.x. [DOI] [PubMed] [Google Scholar]
- 11.Reller LB, Stratton CW. Serum dilution test for bactericidal activity. II. Standardization and correlation with antimicrobial assays and susceptibility tests. J Infect Dis. 1977;136(2):196–204. doi: 10.1093/infdis/136.2.196. [DOI] [PubMed] [Google Scholar]
- 12.Wang CY, Jerng JS, Chen KY, et al. Pandrug-resistant Pseudomonas aeruginosa among hospitalised patients: clinical features, risk-factors and outcomes. Clin Microbiol Infect. 2006;12(1):63–68. doi: 10.1111/j.1469-0691.2005.01305.x. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch EB, Tam VH. Impact of multidrug-resistant Pseudomonas aeruginosa infection on patient outcomes. Expert Rev Pharmacoecon Outcomes Res. 2010;10(4):441–451. doi: 10.1586/erp.10.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsueh PR, Tseng SP, Teng LJ, Ho SW. Pan-drug-resistant Pseudomonas aeruginosa causing nosocomial infection at a university hospital in Taiwan. Clin Microbiol Infect. 2005;11(8):670–673. doi: 10.1111/j.1469-0691.2005.01196.x. [DOI] [PubMed] [Google Scholar]