Abstract
Background
Serine/arginine protein kinase 1 (SRPK1) is a protein kinase that belongs to the serine/arginine-rich domain family of splicing factors which are essential for splice-site selection, especially the modulation for RNA metabolism, localization, and translation. High expression of SRPK1 has been found in numerous human cancers, but its mechanism in colorectal cancer (CRC) is still rarely reported.
Purpose
To investigate the expression of SRPK1 in CRC tissues and cells and determine its functions and mechanism in CRC.
Methods
The expression of SRPK1 was explored in human CRC patients and cells by immunohistochemistry, real-time quantitative PCR, and Western blot; Cell Counting Kit-8, Transwell, flow cytometry, and tube formation assay were used to investigate the CRC cell viability, migration, apoptosis, and angiogenesis, respectively.
Results
SRPK1 was overexpressed in CRC tumor tissues and cells, and correlated with tumor node metastasis stage; inhibition of SRPK1 by siRNA resulted in decreased cell growth and migration, significantly increased apoptosis, and suppressed angiogenesis.
Conclusion
SRPK1 can be a prognostic indicator of CRC and may be a therapeutic target for CRC.
Keywords: serine/arginine protein kinase 1, colorectal cancer, growth, angiogenesis, apoptosis
Introduction
Colorectal cancer (CRC) is one of the most common causes of cancer-related death worldwide.1 As the etiology of CRC is associated with a variety of genetic and epigenetic abnormalities, the knowledge of genetics in the different stages of CRC is limited.2,3 It is therefore important to identify potential genetic abnormalities underlying the development of local and distant metastases in CRC patients.
Serine/arginine protein kinase 1 (SRPK1) is a serine/arginine protein kinase specific for the serine/arginine-rich domain (SR) family of splicing factors.4 SR proteins are a highly conserved family of splicing factors with 1 or 2 N terminal RNA-recognition motifs and a C-terminal domain enriched in arginine and serine dipeptides. SR proteins act as RNA-binding proteins that are essential for splice-site selection and have also been implicated as having key roles in all crucial aspects of RNA metabolism, including export, localization, translation, and nonsense-mediated decay.5
High expression of SRPK1 can induce hyperphosphorylation of SR splicing factor 1, subsequently increasing the transcription and translation of disease-related proteins.6 Vascular endothelial growth factors (VEGFs) are critical regulators of both normal and pathological angiogenesis and have been identified as antiangiogenic targets until now. A previous study showed that the IGF receptor activates SRPK1 to switch between 2 VEGF-splicing isoforms with opposite angiogenic properties, and phosphorylated SRSF1 was indicative of an angiogenic phenotype resulting in proximal splicing and production of angiogenic VEGF isoforms (VEGF-A165).7 Other studies showed that SRPK1 can bind to activated Akt to stimulate autophosphorylation and nuclear translocation of SRPK1, and subsequently phosphorylate SR and regulate splicing,8 to influence nuclear import of SRSF1 and its typical localization in speckles.9 SRPK1 can also participate in the growth factor signaling pathway to promote tumorigenesis.10
SRPK1 is involved in many biological and pathological processes, and abnormal expression of SRPK1 has been reported in several human cancers including hepatocellular carcinoma,11 melanoma,12 prostate cancer,13 breast cancer,14 ovarian cancer,15 lung cancer,16 renal cell carcinoma,17 and gastric cancer.18 In addition, SRPK1-catalyzed SRSF1 phosphorylation reportedly increases alternative splicing of tumor-related Rac1b in colorectal cells.19 However, there are only few reports regarding the expression of SRPK1 in CRC, and its mechanism remains unclear.
Overexpression of SRPK1 will be more indicative of an oncogenic function. In the present study, we aimed to explore SRPK1 expression profiles in CRC and its clinical characteristics. The expression and distribution of SRPK1 in human CRC patients was detected by immunohistochemistry (IHC), real-time quantitative PCR (RT-qPCR), and Western blot methods. Additionally, the relationship between SRPK1 expression and clinicopathological characteristics of patients with CRC was also analyzed. In CRC cells, siRNA was used to decrease SRPK1, and the viability, migration, apoptosis, and angiogenesis formation of CRC cells influenced by SRPK1 inhibition was investigated. We hypothesized that SRPK1 could be a prognostic indicator in CRC patients.
Materials and methods
Clinical specimens
A total of 85 paraffin-embedded human CRC tumor tissues and 10 normal colon tissues were collected from the Affiliated Hospital of Nantong University (Nantong, People’s Republic of China) from March 2005 to November 2011. The clinical records of all tissues were confirmed by 2 pathologists. Fifteen fresh CRC tumor tissues and matched adjacent nontumor tissues were also collected from patients and frozen at −80°C for RT-qPCR and Western blot analysis. The study protocol was approved by the Ethics Committee of the Affiliated Hospital of Nantong University, and all experiments were performed in accordance with the approved guidelines of the Affiliated Hospital of Nantong University. Written informed consent was obtained from each patient prior to study enrollment.
Cell culture and transfection
Human CRC cell lines Caco2 and HT29 and a normal human colon mucosal epithelial cell line NCM460 were obtained from the Cell Bank of Chinese Academy of Science (Shanghai, People’s Republic of China). Caco2, HT29, and NCM460 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA, USA) at 37°C in a humidified incubator with 5% CO2. siRNA was designed for targeting SRPK1 (siSRPK1), and an siSRPK1 sequence-scrambled RNA oligo used as the siRNA negative control (siNC). Overexpression of SRPK1 plasmid (pSRPK1) (FulenGen, Guangzhou, People’s Republic of China) was used to increase endogenous SRPK1 expression. The SRPK1 open reading frame sequence was obtained from NCBI Genbank Accession no NM_003137.4; the vector with no SRPK1 ORF sequence was used as the negative control. siRNAs and plasmid were transfected into Caco2 and HT29 cells using Lipofectamine® 2000 transfection reagent (Thermo Fisher Scientific) according to the manufacturer’s instructions. siRNA and siNC were obtained from Biomics Biotechnologies Co., Ltd (Nantong, People’s Republic of China), and the sequences were as follows:
For siSRPK1: sense, 5′-GGCAAUGAAAGUAGUUAAAdTdT-3′ antisense, 5′-UUUAACUACUUUCAUUGCCdTdT-3′;
For siNC: sense, 5′-GAAUUACGUGAAUAGAAGAdTdT-3′ antisense, 5′-UCUUCUAUUCACGUAAUUCdTdT-3′.
IHC staining and evaluation
The paraffin-embedded tissue samples were cut into 4 µm sections and deparaffinized in xylene and rehydrated in graded alcohol, finishing with distilled water. The endogenous peroxidase activity was blocked in 0.3% hydrogen peroxide and absolute methanol. Antigen retrieval was performed by pressure cooking in sodium citrate buffer (10 mM sodium citrate monohydrate, pH 6.0). Subsequently, the sections were blocked in goat serum for 20 minutes at room temperature, then incubated with rabbit anti-human SRPK1 antibody (dilution 1:200) (Abcam, Cambridge, MA, USA) overnight at 4°C. After washing in PBS, the slides were incubated with the HRP-conjugated goat anti-rabbit secondary antibody for 30 minutes, and then with diaminobenzidine (Dako, Troy, MI, USA) for 5 minutes, followed by hematoxylin counterstaining. The percentages of SRPK1-positive cells were scored in 5 categories according to staining as follows: 0 for ≤5%, 1 for 6%–25%, 2 for 26%–50%, 3 for 51%–75%, and 4 for >75%. The sum of the percentages and intensity scores was used as the final staining score as follows: 0 (no staining), 1–3 for + (weak staining), 4–6 for ++ (moderate staining), and 7–9 for +++ (strong staining). The expression of SRPK1 was categorized as low (score: 1–6) or high (score: 7–9) for statistical analysis.
RT-qPCR
The mRNA expression levels of SRPK1 in tissues or cells were assessed by the RT-qPCR method. The total RNA of tissues and cells was extracted using TRIzol® reagent (Thermo Fisher Scientific) according to the manufacturers’ instructions. β-actin was used as an internal control. RT-qPCR reactions were carried out using SYBR Green One-Step RT-qPCR kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. The relative mRNA expression levels were evaluated by the 2−ΔΔCt method.20 The primer sequences were as follows:
For SRPK1: forward, 5′-GGTGTGCCAGTCTTCCTCAAC-3′ reverse: 5′-GGTCCGTTATGTTCTTGCTCTTG-3′;
For β-actin: forward, 5′-GCGTGACATTAAGGAGAAG-3′ reverse: 5′-GAAGGAAGGCTGGAAGAG-3′.
Western blot analysis
The protein expression levels of SRPK1 in tissues or cells were detected by Western blot. Cells were plated into 6-well plates and grown for 24 hours, and posttreatment took 48 hours, as described earlier. The cells were then collected and lysed in RIPA lysis and extraction buffer (Thermo Fisher Scientific) on ice and centrifuged for collecting proteins. The proteins were separated by 10% SDS-PAGE and transferred onto a PVDF membrane (Millipore, Burlington, MA, USA). Subsequently, the membrane was incubated with rabbit anti-human SRPK1 antibody (1:1,000 dilution) or mouse anti-human β-actin antibody (1:5,000 dilution) (all from Abcam) as an internal control. After washing with TBST, the membrane was incubated with HRP-conjugated secondary antibody (1:5,000 dilution) (Abcam) for 1.5 hour at room temperature, then washed in TBST. The specific proteins were detected with ECL substrate (Thermo Fisher Scientific).
Cell viability assay
Cell Counting Kit-8 (CCK-8) (Sigma-Aldrich, St Louis, MO, USA) was used to determine the cell viability. Briefly, 5×103 Caco2 and HT29 cells/well were seeded onto 96-well plates before treatment with siSRPK1 or siNC and grown to about 75% confluence over 24 hours at 37°C. Posttreatment for 0, 24, 48, 72, and 96 hours, cells were washed thrice with PBS and 100 µL/well of PBS was readded to each well. Next, 10 µL of the FCCK working solution was added to each well. After incubation at 37°C for 30 minutes, the fluorescence intensity of each well was recorded at 535 nm using a fluorescence microplate reader (BioTek, Winooski, VT, USA).
Cell migration assay
Transwell assay was performed to detect cell migration abilities. Briefly, 2×105 Caco2 and HT29 cells/well were seeded onto 24-well plates and grown for 24 hours. After treatment with siSRPK1 or siNC for 48 hours, cells were suspended in DMEM at a density of 1×106 cells/mL. Transwell chambers (Corning, Corning, NY, USA) were incubated with DMEM for 1 hour before treatments; the upper and lower chamber was separated by an 8 µm pore polycarbonate membrane. About 100 µL cell suspension was added to each upper chamber, while 600 µL DMEM containing 10% FBS and conditioned medium (1:1 dilution) was added to the lower chamber; the conditioned medium was obtained from the cultures of Caco2 or HT29 cells, which were transfected with siSRPK1 or siNC for 48 hours. The cells on the top surface of the membrane were carefully removed using cotton swabs posttreatment for 24 hours. Cells on the transwell chambers were fixed in 10% formaldehyde for 30 seconds, and then stained with 0.5% crystal violet solution. After washing in PBS, the cells on the top surface of the membrane were carefully removed again, and the cells on the bottom surface of the membrane were observed and counted under the microscope.
Cell apoptosis assay
Flow cytometry (FCM) with Annexin V-FITC/PI double staining was performed to detect cell apoptosis. Briefly, after Caco2 or HT29 cells were treated with siSRPK1 or siNC for 48 hours, 1×105 cells/well were harvested and washed using PBS in a 6-well plate, then resuspended in annexin-binding buffer, and incubated with Annexin V-FITC conjugate and PI for 15 minutes at room temperature. Then, the cells were measured by FCM and the results were analyzed by BD CELLQuest software (BD Biosciences, Franklin Lakes, NJ, USA).
Tube formation assay
Human umbilical vein endothelial cells (HUVECs) were used as an in vitro angiogenesis model to evaluate the effects of downregulated SRPK1 on angiogenesis. Briefly, 24-well plates were coated with 100 µL/well Matrigel (BD Biosciences) and incubated at 37°C for 30 minutes. About 1×105 HUVECs/well were resuspended in conditioned medium, which was obtained from the cell cultures of Caco2 and HT-29 cells treated with siSRPK1 or siNC for 48 hours, then seeded onto Matrigel-coated 24-well plates and cultured in 37°C/5% CO2 incubator for 24 hours. The numbers of nodes of the tubular structures were counted and quantified under a microscope.
Statistical analysis
All experiments were performed independently at least 3 times. Data are presented as the means ± SD. All data were analyzed using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). χ2 test was used to explore the association between SRPK1 expression and clinicopathological variables. Kaplan–Meier curves were constructed and the log-rank test was used for analysis of survival data. Multivariate analysis was performed using the Cox proportional hazards model, and the HR as well as its 95% CI was presented. For cellular experiments, all data were expressed as mean ± SD of 3 independent experiments performed in triplicate. Statistical significance was assessed with the Student’s t-test or one-way analysis of variance. P<0.05 was considered statistically significant.
Results
Overexpression of SRPK1 in CRC patients and correlation with clinicopathological parameters
To investigate whether SRPK1 is expressed in CRC patients, a total of 85 tumor tissues from CRC patients was used for IHC staining, and 15 pairs of fresh tumor and matched adjacent nontumor tissues were used for RT-qPCR and Western blotting.
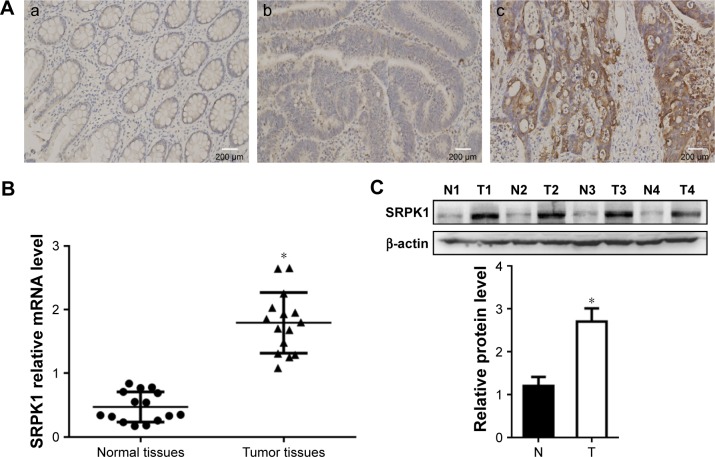
IHC confirmed high expression of SRPK1 in 51.76% (44/85) and low expression in 48.24% (41/85) of patients, and weak or no staining in normal tissues (Figure 1A). The expression level of SRPK1 was associated with clinicopathological parameters in CRC patients. SRPK1 mRNA and protein expression levels were both more highly expressed in the tumor tissues of CRC patients (P<0.05) (Figure 1B and C) than normal tissues, as detected by RT-qPCR and Western blot. The Kaplan–Meier survival curves indicated that CRC patients who presented with high SRPK1 expression had poorer overall survival (OS) rates than patients who presented with low expression (Figure 2).
Figure 1.
The expression profiles of SRPK1 expression in CRC tissues, compared with normal colon tissues.
Notes: (A) SRPK1 expression in CRC tissues, (a) normal colon tissues with no or weak SRPK1 expression; (b) low expression of SRPK1 in CRC tissues; (c) high expression of SRPK1 in CRC tissues. (B) SRPK1 mRNA levels in CRC tissues, *P<0.05 vs normal tissues. (C) SRPK1 protein levels in CRC tissues, *P<0.05 vs normal tissues.
Abbreviations: CRC, colorectal cancer; N, normal; SRPK1, serine/arginine protein kinase 1; T, tumor.
Figure 2.
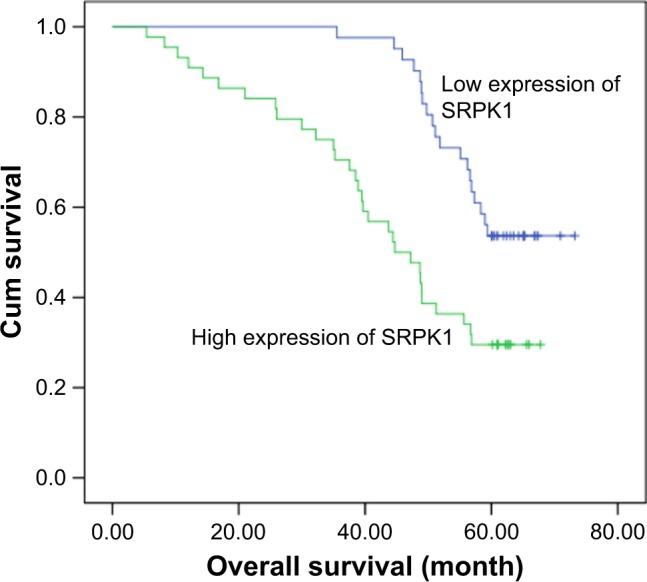
Kaplan–Meier survival curves of patients with CRC based on SRPK1 expression status.
Abbreviations: CRC, colorectal cancer; Cum, cumulative; SRPK1, serine/arginine protein kinase 1.
The clinicopathological data of 85 CRC patients is shown in Table 1. There were statistical differences between SRPK1 positive expression and the characteristics of tumor node metastasis (TNM) stage (P=0.001), T stage (P=0.030), and N stage (P<0.001).
Table 1.
Association between SRPK1 expression and clinicopathological parameters in CRC patients
| Clinicopathological parameters | Case number | SRPK1, n (%)
|
P-value | |
|---|---|---|---|---|
| Low | High | |||
| Total | 85 | 41 (48.24) | 44 (51.76) | |
| Gender | 0.562 | |||
| Male | 47 | 24 (51.06) | 23 (48.94) | |
| Female | 38 | 17 (44.74) | 21 (55.26) | |
| Age, years | 0.406 | |||
| <60 | 35 | 15 (42.86) | 20 (57.14) | |
| ≥60 | 50 | 26 (52.00) | 24 (48.00) | |
| Location | 0.724 | |||
| Colon | 39 | 18 (46.15) | 21 (53.85) | |
| Rectum | 46 | 23 (50.00) | 23 (50.00) | |
| Tumor size, cm | 0.299 | |||
| <5 | 49 | 26 (53.06) | 23 (46.94) | |
| ≥5 | 36 | 15 (41.67) | 21 (58.33) | |
| TNM stage | 0.001* | |||
| I + II | 42 | 28 (66.67) | 14 (33.33) | |
| III + IV | 43 | 13 (30.23) | 30 (69.77) | |
| Differentiation | 0.544 | |||
| Well/moderately | 66 | 33 (50.00) | 33 (50.00) | |
| Poorly | 19 | 8 (42.11) | 11 (57.89) | |
| T stage | 0.030* | |||
| T1 + T2 | 22 | 15 (68.18) | 7 (31.82) | |
| T3 + T4 | 63 | 26 (41.27) | 37 (58.73) | |
| N stage | 0.000** | |||
| N0 | 52 | 33 (63.46) | 19 (36.54) | |
| N1 + N2 | 33 | 8 (24.24) | 25 (75.76) | |
| M stage | 0.108* | |||
| M0 | 79 | 40 (50.63) | 39 (49.37) | |
| M1 | 6 | 1 (16.67) | 5 (83.33) | |
Note:
P<0.05,
P<0.001.
Abbreviations: CRC, colorectal cancer; SRPK1, serine/arginine protein kinase 1; TNM, tumor node metastasis.
Prognostic value of SRPK1 expression in CRC patients
A multivariate analysis was performed using the Cox proportional hazards model for all significant variables in the univariate analysis. In univariate survival analysis, high SRPK1 expression (HR =1.914; P=0.031), TNM stage (HR =2.984; P=0.011), and N stage (HR =2.059; P=0.062) were associated with the OS rate (Table 2). The significance of these prognostic factors in the univariate models was evaluated in a multivariate Cox regression model, and the results demonstrated that SRPK1 overexpression (HR =1.914; P=0.031), TNM stage (HR =2.984; P=0.011), and N stage (HR =2.059; P=0.062) were independent prognostic factors for poor outcomes (Table 2).
Table 2.
Univariate and multivariate analyses of various prognostic parameters in patients with CRC Cox regression analysis
| Variable | Univariate analysis
|
Multivariate analysis
|
|||
|---|---|---|---|---|---|
| OS months (mean ± SD) | P-value | HR | P-value | 95% CI | |
| Gender | 0.111 | ||||
| Male | 50.155±2.589 | ||||
| Female | 58.145±2.901 | ||||
| Age, years | 0.515 | ||||
| <60 | 51.270±4.648 | ||||
| ≥60 | 56.730±3.099 | ||||
| Location | 0.882 | ||||
| Colon | 56.830±1.036 | ||||
| Rectum | 51.270±5.251 | ||||
| Tumor size, cm | 0.983 | ||||
| <5 | 56.730±0.826 | ||||
| ≥5 | 51.270±6.930 | ||||
| TNM stage | <0.000** | ||||
| I + II | 66.571±1.492 | Reference | |||
| III + IV | 42.189±2.669 | 2.984 | 0.011* | 1.288–6.912 | |
| T stage | 0.027* | ||||
| T1, T2 | 64.946±1.981 | ||||
| T3, T4 | 48.965±2.271 | ||||
| N stage | <0.000** | ||||
| N0 | 64.107±1.556 | Reference | |||
| N1 + N2 | 38.586±3.037 | 2.059 | 0.062 | 0.965–4.394 | |
| M stage | <0.000** | ||||
| M0 | 57.126±1.835 | ||||
| M1 | 20.302±7.558 | ||||
| Differentiation | 0.323 | ||||
| Well/moderately | 57.005±2.031 | ||||
| Poorly | 44.402±5.075 | ||||
| SRPK1 expression | 0.001* | ||||
| Low | 62.248±1.788 | Reference | |||
| High | 45.101±2.901 | 1.914 | 0.031* | 1.061–3.453 | |
Note:
P<0.05,
P<0.001.
Abbreviations: CRC, colorectal cancer; OS, overall survival; SRPK1, serine/arginine protein kinase 1; TNM, tumor node metastasis.
Expression of SRPK1 in CRC cells and inhibited by siRNAs
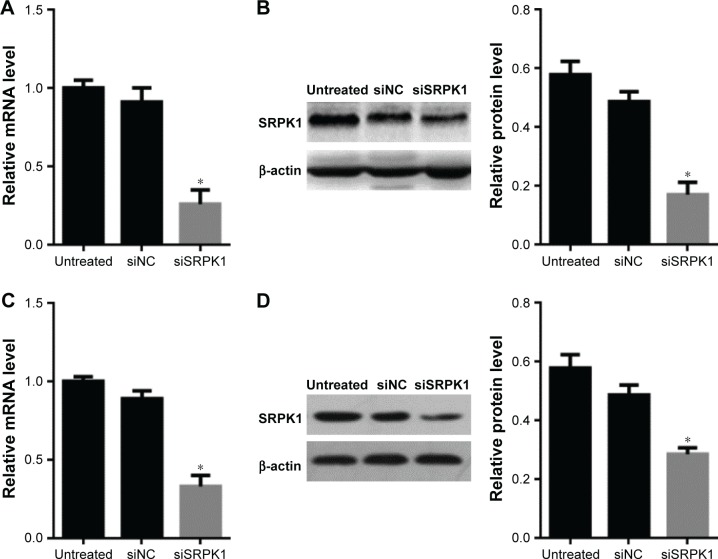
Caco2 and HT-29 CRC cells were used to investigate the SRPK1 expression and NCM460 cell was used as control. To further observe the expression of SRPK1 in CRC cells, the predesigned siRNA (siSRPK1) was used to knock down the SRPK1 levels. The results showed that SRPK1 mRNA levels were increased up to 3.89-fold and 4.17-fold in Caco2 and HT-29 cells, respectively, compared with normal human colon mucosal epithelial cell NCM460, and the protein levels were increased up to 2.42-fold and 2.95-fold (Figure 3), respectively. In addition, the mRNA and protein expression levels of SRPK1 were both inhibited significantly by siSRPK1 in Caco2 (74% and 83%, respectively) and HT-29 (67% and 71%, respectively) cells compared with untreated cells (P<0.05; Figure 4). There was no significant difference between siNC-treated and untreated cells.
Figure 3.
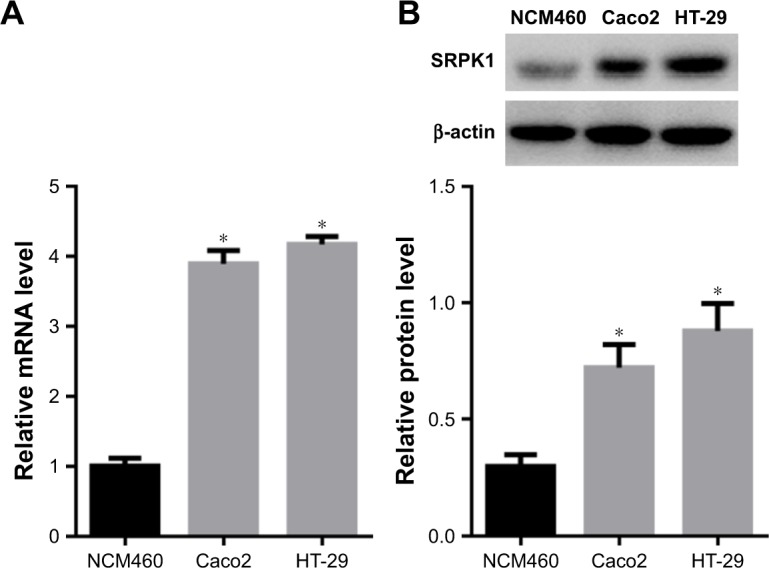
Expression levels of SRPK1 in CRC cells.
Notes: (A) SRPK1 mRNA levels in Caco2 and HT-29 CRC cells were detected by RT-qPCR, compared with that in NCM460 cells; (B) SRPK1 protein levels in Caco2 and HT-29 CRC cells were detected by Western blot. *P<0.05, compared with the normal human colon mucosal epithelial cell line NCM460.
Abbreviations: CRC, colorectal cancer; RT-qPCR, real-time quantitative PCR; SRPK1, serine/arginine protein kinase 1.
Figure 4.
Expression levels of SRPK1 were inhibited by SRPK1-targeted siRNA (siSRPK1) in Caco2 and HT-29 CRC cells.
Notes: (A) SRPK1 mRNA levels were inhibited by siSRPK1 in Caco2 cells; (B) SRPK1 protein levels were inhibited by siSRPK1 in Caco2 cells; (C) SRPK1 mRNA levels were inhibited by siSRPK1 in HT-29 cells; (D) SRPK1 protein levels were inhibited by siSRPK1 in HT-29 cells. *P<0.05, compared with siNC-treated and untreated cells.
Abbreviations: CRC, colorectal cancer; NC, negative control; RT-qPCR, real-time quantitative PCR; SRPK1, serine/arginine protein kinase 1.
The effects of SRPK1 silencing on CRC cell growth
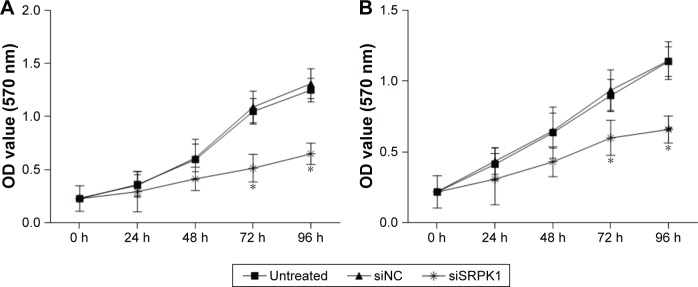
To investigate the inhibitory effect of decreased SRPK1 on CRC cell growth, a CCK-8 assay was performed. The results revealed that growth of Caco2 and HT-29 cells was inhibited by siSRPK1 at 48, 72, and 96 hours, as compared with the siNC-treated and untreated cells (P<0.05; Figure 5).
Figure 5.
Effects of SRPK1 inhibition on CRC cell growth were detected by CCK-8 assay.
Notes: (A) Growth of Caco2 cells was inhibited by siSRPK1; (B) growth of HT-29 cells was inhibited by siSRPK1. *P<0.05, compared with siNC-treated and untreated cells.
Abbreviations: CCK-8, Cell Counting Kit-8; CRC, colorectal cancer; NC, negative control; SRPK1, serine/arginine protein kinase 1.
The effects of SRPK1 silencing on CRC cell migration
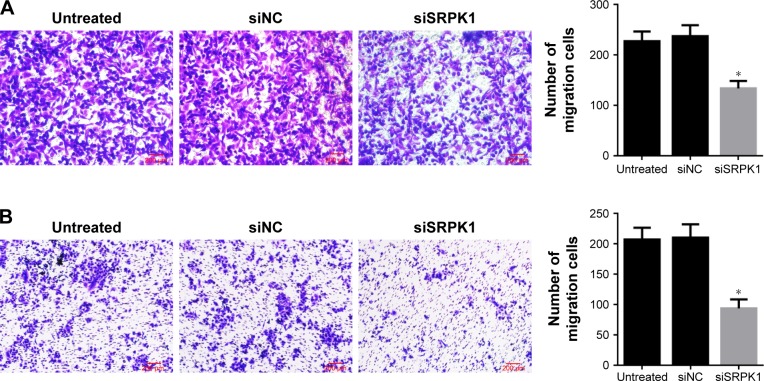
Transwell assay was used to observe the effects of siRNA- mediated SRPK1 inhibition on CRC cell migration. The results revealed that the invasion abilities of Caco2 and HT-29 cells were markedly decreased by about 41% and 55%, respectively, after siSRPK1 treatment, as compared with untreated cells (P<0.05; Figure 6). There was no significant difference between siNC-treated and untreated cells.
Figure 6.
Effects of SRPK1 inhibition on the migration of CRC cells were detected by Transwell assay.
Notes: (A) Caco2 cells migration was inhibited by siSRPK1; (B) HT-29 cells migration was inhibited by siSRPK1. *P<0.05, compared with siNC-treated and untreated cells.
Abbreviations: CRC, colorectal cancer; NC, negative control; SRPK1, serine/arginine protein kinase 1.
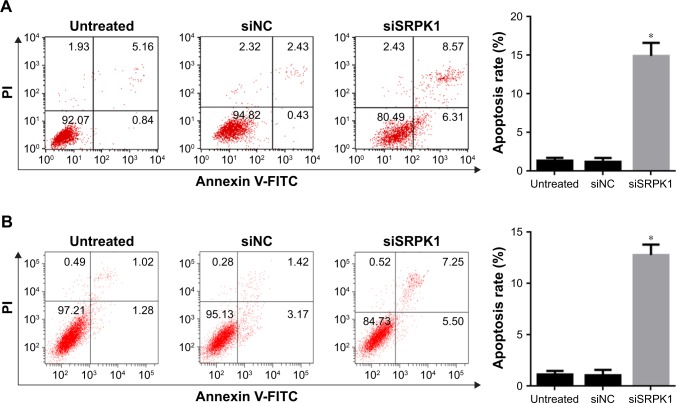
The effects of SRPK1 silencing on CRC cell apoptosis
FCM analysis after Annexin V-FITC/PI double staining was used to evaluate the effect of SRPK1 silencing on CRC cell apoptosis. The result showed that after siSRPK1 treatment, the apoptosis rate was increased by about 15% and 13% in Caco2 and HT-29 cells, respectively, compared with untreated cells (P<0.05; Figure 7). There was no significant difference between siNC-treated and untreated cells.
Figure 7.
Effects of SRPK1 inhibition on the apoptosis of CRC cells were detected by FCM analysis.
Notes: (A) Caco2 cells apoptosis was induced by siSRPK1; (B) HT-29 cells migration was induced by siSRPK1. *P<0.05, compared with siNC-treated and untreated cells.
Abbreviations: CRC, colorectal cancer; FCM, flow cytometry; NC, negative control; SRPK1, serine/arginine protein kinase 1.
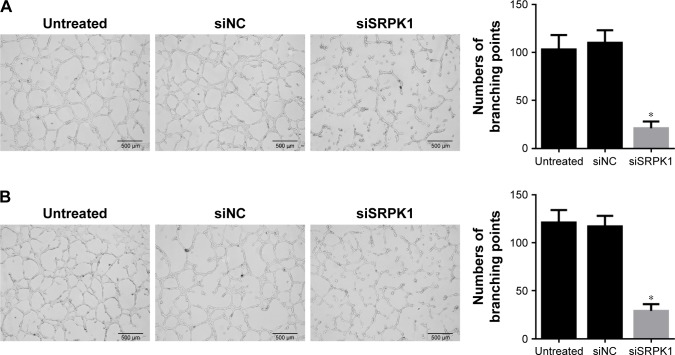
The effects of SRPK1 silencing on angiogenesis formation
In vitro tube formation assay based on HUVECs was used to observe the angiogenesis formation in CRC cells. HUVECs were stimulated by the conditioned medium, which was obtained from the cultures of Caco2 and HT-29 cells treated with siSRPK1 or siNC for 48 hours. HUVECs treated with the siSRPK1 conditioned medium were significantly inhibited from forming extensive and enclosed tube networks by about 80% (Caco2) and 76% (HT-29), compared with untreated cells (P<0.05; Figure 8). There was no significant difference between siNC-treated and untreated cells.
Figure 8.
Effects of SRPK1 inhibition on the angiogenesis formation were detected by in vitro tube formation assay.
Notes: (A) Caco2 cells inhibited by siSRPK1 on angiogenesis formation; (B) HT-29 cells inhibited by siSRPK1 on angiogenesis formation. *P<0.05, compared with siNC-treated and untreated cells.
Abbreviations: CRC, colorectal cancer; NC, negative control; SRPK1, serine/arginine protein kinase 1.
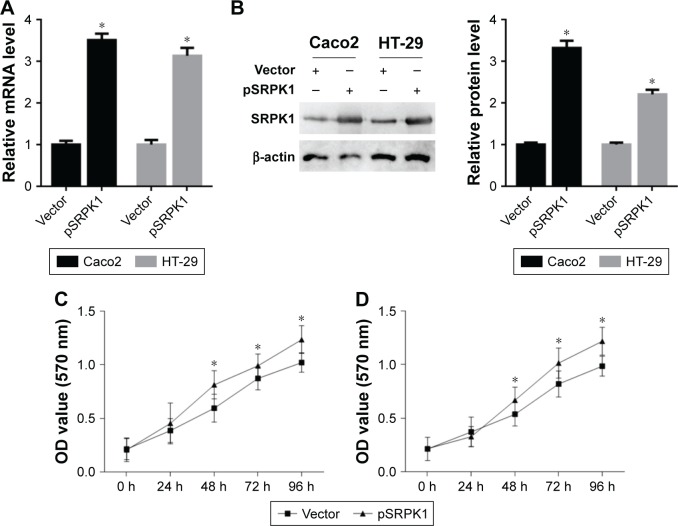
The effects of SRPK1 overexpression on CRC cell proliferation
CRC cell growth was inhibited by SRPK silencing, as shown. To further explore the effects of SRPK1 overexpression on CRC cell proliferation, the SRPK1 overexpression plasmid was used. The SRPK1 mRNA levels were increased up to 3.5-fold and 3.1-fold (Figure 9A), respectively, and the protein level was increased up to 3.3-fold and 2.2-fold (Figure 9B), respectively, in Caco2 and HT-29 cells as compared with vector-treated cells. Furthermore, the cell proliferation in those cells with SRPK1 overexpression was detected by the CCK-8 assay, which showed that Caco2 and HT-29 cell proliferation was significantly promoted by pSRPK1 (Figure 9C and D).
Figure 9.
Effects of SRPK1 overexpression on CRC cell proliferation.
Notes: (A) The SRPK1 mRNA levels increased by SRPK1 overexpression plasmid (pSRPK1) were detected by RT-qPCR; (B) the SRPK1 protein levels were increased by pSRPK1 and were detected by Western blot; (C) Caco2 cell proliferation was promoted by pSRPK1 and detected by CCK-8 assay; (D) HT-29 cell proliferation was promoted by pSRPK1 and detected by CCK-8 assay. *P<0.05, compared with vector-treated cells.
Abbreviations: CCK-8, Cell Counting Kit-8; CRC, colorectal cancer; OD, optical density; RT-qPCR, real-time quantitative PCR; SRPK1, serine/arginine protein kinase 1.
Discussion
SRPK1 is a protein kinase involved in the regulation of several mRNA processing pathways including alternative splicing.13 SRPK1 can regulate alternative splicing of VEGF-A to proangiogenic isoforms, and SRPK1 silencing can restore the balance of proangiogenic and antiangiogenic isoforms to normal physiological levels.21 SRPK1 has been found in many cancers and is known to play an oncogenic role in cancers. Several studies have shown that suppression of SRPK1 has antitumoral effects, suggesting it could serve as a new potential therapeutic target in oncology. Colorectal cancer is one of the most common malignancies,1,22 and while there are a few studies on the role of SRPK1 in CRC, its mechanism still remains largely unclear. In this study, we identified that expression of SRPK1 increased in tumor tissues of CRC patients. The result was in accordance with that of Hayes et al23 who reported that its expression increases along with tumor grade. In our study, we also found that over-expression of SRPK1 was significantly correlated with TNM stage, T stage, and N stage. Furthermore, Kaplan–Meier survival analysis indicated that CRC patients who had high SRPK1 expression had poorer OS rates than patients with low expression. Multivariate analysis also showed that SRPK1 expression might be an independent prognostic factor for poor outcomes in CRC patients.
SRPK1 has been reported to affect several processes in different cancers, eg, angiogenesis in prostate and colon cancer, apoptosis in breast and colon cancer, metastasis in breast cancer14 or renal cell carcinoma, and stimulation of a stem cell-like phenotype in non-small-cell lung cancer.24 In the study, we found that SRPK1 was highly expressed in Caco2 and HT-29 CRC cells, which was in accordance with the results to of Gonçalves et al.19 In the study of non-small-cell lung cancer, the chimeric antibody targeting SRPK1 can inhibit growth, migration, and invasion of cancer cells,25 and siRNA14,17 or micoRNA11 have also been used to achieve the same goals. We used SRPK1-targeted siRNA to explore the changes of biological behaviors of CRC cells with high SRPK1 expression. When SRPK1 was silenced by siRNA, the CRC cell viability and migration were significantly, and cell apoptosis rates of CRC cells also obviously increased; conversely, CRC cell proliferation was promoted by SRPK1 overexpression. Most human cancers have VEGF-dependent angiogenesis properties, and SRPK1 can phosphorylate VEGF-splicing factors. SRPK1 inhibitors are being investigated as new anticancer agents.26,27 Morooka et al27 screened a large-scale chemical library to identify a new inhibitor of SRPK1, and the screened inhibitor SRPIN340 was found to prevent VEGF production more effectively in a mouse model of age-related macular degeneration, suggesting that silencing of SRPK1 could prevent neovascularization; this indicated that SRPK1 was a potential target for antiangiogenic drug development. The approach of the SRPK1 inhibitor “SPHINX” was also investigated to inhibit angiogenesis for prostate cancer treatment.13 We also demonstrated that angiogenesis formation was inhibited after downregulation of SRPK1. The results showed that SRPK1 played an oncogene role in CRC cells.
Conclusion
Our study demonstrates that SRPK1 was highly expressed in CRC tissues and cells, and high SRPK1 expression was correlated with clinical characteristics in CRC patients, indicating that SRPK1 could be a prognostic indicator of CRC and may be a candidate target for CRC therapy. Silencing of SRPK1 by siRNA may be an optional strategy for CRC treatment.
Acknowledgments
This study was supported by the National Natural Science Foundation (number 81672372).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Schiff GD, Bearden T, Hunt LS, et al. Primary care collaboration to improve diagnosis and screening for colorectal cancer. Jt Comm J Qual Patient Saf. 2017;43(7):338–350. doi: 10.1016/j.jcjq.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 2.De Rosa M, Pace U, Rega D, et al. Genetics, diagnosis and management of colorectal cancer (Review) Oncol Rep. 2015;34(3):1087–1096. doi: 10.3892/or.2015.4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pancione M, Remo A, Colantuoni V. Genetic and epigenetic events generate multiple pathways in colorectal cancer progression. Patholog Res Int. 2012;2012:509348. doi: 10.1155/2012/509348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Long JC, Caceres JF. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417(1):15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- 5.Bradley T, Cook ME, Blanchette M. SR proteins control a complex network of RNA-processing events. RNA. 2015;21(1):75–92. doi: 10.1261/rna.043893.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.da Silva MR, Moreira GA, Goncalves da Silva RA. Splicing regulators and their roles in cancer biology and therapy. Biomed Res Int. 2015;2015:150514. doi: 10.1155/2015/150514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amin EM, Oltean S, Hua J, et al. WT1 mutants reveal SRPK1 to be a downstream angiogenesis target by altering VEGF splicing. Cancer Cell. 2011;20(6):768–780. doi: 10.1016/j.ccr.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Z, Qiu J, Liu W, et al. The Akt-SRPK-SR axis constitutes a major pathway in transducing EGF signaling to regulate alternative splicing in the nucleus. Mol Cell. 2012;47(3):422–433. doi: 10.1016/j.molcel.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh G, Adams JA. Phosphorylation mechanism and structure of serine-arginine protein kinases. FEBS J. 2011;278(4):587–597. doi: 10.1111/j.1742-4658.2010.07992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang P, Zhou Z, Hu A, et al. Both decreased and increased SRPK1 levels promote cancer by interfering with PHLPP-mediated dephosphorylation of Akt. Mol Cell. 2014;54(3):378–391. doi: 10.1016/j.molcel.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Liu X, Liu Z, et al. MicroRNA-1296 inhibits metastasis and epithelial-mesenchymal transition of hepatocellular carcinoma by targeting SRPK1-mediated PI3K/AKT pathway. Mol Cancer. 2017;16(1):103. doi: 10.1186/s12943-017-0675-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gammons MV, Lucas R, Dean R, Coupland SE, Oltean S, Bates DO. Targeting SRPK1 to control VEGF-mediated tumour angiogenesis in metastatic melanoma. Br J Cancer. 2014;111(3):477–485. doi: 10.1038/bjc.2014.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavrou A, Brakspear K, Hamdollah-Zadeh M, et al. Serine-arginine protein kinase 1 (SRPK1) inhibition as a potential novel targeted therapeutic strategy in prostate cancer. Oncogene. 2015;34(33):4311–4319. doi: 10.1038/onc.2014.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Roosmalen W, Le Dévédec SE, Golani O, et al. Tumor cell migration screen identifies SRPK1 as breast cancer metastasis determinant. J Clin Invest. 2015;125(4):1648–1664. doi: 10.1172/JCI74440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang F, Zhou J, Xie X, et al. Involvement of SRPK1 in cisplatin resistance related to long non-coding RNA UCA1 in human ovarian cancer cells. Neoplasma. 2015;62(3):432–438. [PubMed] [Google Scholar]
- 16.Gong L, Song J, Lin X, et al. Serine-arginine protein kinase 1 promotes a cancer stem cell-like phenotype through activation of Wnt/β-catenin signalling in NSCLC. J Pathol. 2016;240(2):184–196. doi: 10.1002/path.4767. [DOI] [PubMed] [Google Scholar]
- 17.Han X, Yang J, Jia Z, et al. Knockdown of serine-arginine protein kinase 1 inhibits the growth and migration in renal cell carcinoma cells. Oncol Res. 2017;25(3):389–395. doi: 10.3727/096504016X14743324568129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X, Wei Y, Wang S, Luo M, Zeng H. Serine-arginine protein kinase 1 (SRPK1) is elevated in gastric cancer and plays oncogenic functions. Oncotarget. 2017;8(37):61944–61957. doi: 10.18632/oncotarget.18734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonçalves V, Henriques AF, Pereira JF, et al. Phosphorylation of SRSF1 by SRPK1 regulates alternative splicing of tumor-related Rac1b in colorectal cells. RNA. 2014;20(4):474–482. doi: 10.1261/rna.041376.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Batson J, Toop HD, Redondo C, et al. Development of potent, selective SRPK1 inhibitors as potential topical therapeutics for neovascular eye disease. ACS Chem Biol. 2017;12(3):825–832. doi: 10.1021/acschembio.6b01048. [DOI] [PubMed] [Google Scholar]
- 22.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395. [DOI] [PubMed] [Google Scholar]
- 23.Hayes GM, Carrigan PE, Miller LJ. Serine-arginine protein kinase 1 overexpression is associated with tumorigenic imbalance in mitogen-activated protein kinase pathways in breast, colonic, and pancreatic carcinomas. Cancer Res. 2007;67(5):2072–2080. doi: 10.1158/0008-5472.CAN-06-2969. [DOI] [PubMed] [Google Scholar]
- 24.Bullock N, Potts J, Simpkin AJ, et al. Serine-arginine protein kinase 1 (SRPK1), a determinant of angiogenesis, is upregulated in prostate cancer and correlates with disease stage and invasion. J Clin Pathol. 2016;69(2):171–175. doi: 10.1136/jclinpath-2015-203125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu F, Li J, Du X, et al. Chimeric antibody targeting SRPK-1 in the treatment of non-small cell lung cancer by inhibiting growth, migration and invasion. Mol Med Rep. 2017;16(2):2121–2127. doi: 10.3892/mmr.2017.6833. [DOI] [PubMed] [Google Scholar]
- 26.Siqueira RP, Barros MVA, Barbosa ÉAA, et al. Trifluoromethyl aryl-amides with antileukemia effect and intracellular inhibitory activity over serine/arginine-rich protein kinases (SRPKs) Eur J Med Chem. 2017;134:97–109. doi: 10.1016/j.ejmech.2017.03.078. [DOI] [PubMed] [Google Scholar]
- 27.Morooka S, Hoshina M, Kii I, et al. Identification of a dual inhibitor of SRPK1 and CK2 that attenuates pathological angiogenesis of macular degeneration in mice. Mol Pharmacol. 2015;88(2):316–325. doi: 10.1124/mol.114.097345. [DOI] [PubMed] [Google Scholar]