Abstract
Designer self-assembling peptides are a category of emerging nanobiomaterials which have been widely investigated in the past decades. In this field, amphiphilic peptides have received special attention for their simplicity in design and versatility in application. This review focuses on recent progress in designer amphiphilic peptides, trying to give a comprehensive overview about this special type of self-assembling peptides. By exploring published studies on several typical types of amphiphilic peptides in recent years, herein we discuss in detail the basic design, self-assembling behaviors and the mechanism of amphiphilic peptides, as well as how their nanostructures are affected by the peptide characteristics or environmental parameters. The applications of these peptides as potential nanomaterials for nanomedicine and nanotechnology are also summarized.
Keywords: amphiphilic peptides, self-assembly, nanomaterials
Introduction
Molecular self-assembly is one of the most important strategies for fabricating nanomaterials. As a novel “bottom-up” strategy complementary to traditional “top-down” strategy, molecular self-assembly has received considerable attention ever since its emergence decades ago.1–3 Generally, molecular self-assembly could be briefly defined as the spontaneous aggregation of molecules into well-ordered nanostructures, which are usually driven by non-covalent bonds such as ionic bond, hydrophobic interaction, van der Waals interaction and hydrogen bond. According to this definition, it is clear that a successful design of molecular self-assembling materials requires deliberate understanding and control of the chemical and geometrical properties of the molecular building blocks. Fortunately, nature as a great master has provided us countless paradigms for designing molecular self-assembling materials: the formation of bilayer membrane by lipids, virus capsid by protein subunits, double helix by DNA strands, are all self-assembling processes. Taking these self-assembling systems created by nature as models, biomolecules such as lipids, peptides and nucleic acids have already been used as building blocks and many sophisticated self-assembling materials have been developed. Furthermore, with the recent development of biotechnology, genetic engineering, synthetic and material chemistry, de novo designer self-assembling molecules have also been developed rapidly.4–11 These novel self-assembling molecules, biomimetically derived or de novo designed, have shown great potential as nanomaterials in a wide range of fields including regenerative medicine,12–18 cancer research,19–21 three-dimensional (3-D) cell culture,22–29 drug and gene delivery,30–39 nanotechnology40–42 and so on.
Among these molecular self-assembling materials, a category of amphiphilic peptides characterized by the structure of a hydrophobic tail and a hydrophilic head have received especially rapid development. Taking hydrophobic interaction as the predominant driving force, they could undergo self-assembly in aqueous solution and form various nanostructures with certain applications. The remarkable importance of these amphiphilic peptides may come from the following advantages: 1) Since life is originated from water and all biological processes are happening in aqueous environment, hydrophobic interaction might be one of the most initial and important driving forces for self-assembly. For this reason, designing amphiphilic peptides employing hydrophobic interaction for self-assembly might be the most rational and convenient strategy. 2) The architecture consisting of only two parts, one hydrophobic and the other hydrophilic, might be the simplest to design self-assembling molecules, as well as to study the molecular mechanisms and parameters affecting the self-assembly. 3) There are many existing examples in nature, such as lipid, traditional surfactant, surfactin and siderophore, enabling us to learn to design amphiphilic peptides. 4) Amphiphilic peptides are composed of natural L-amino acids, which endow them with perfect biocompatibility for biological applications. 5) There are >20 amino acids which could be used to design self-assembling peptides. This diversity, combined with the feasibility of conjugating peptides with other biomolecules such as fatty acids and nucleic acids, brings countless possibilities for designing amphiphilic peptides.
With the rapid development in the design and application of self-assembling peptides, a promising field has already been established. This review will focus on the recent advances in the specific field of self-assembling amphiphilic peptides, from their design, self-assembling structure and mechanism to their potential applications in various fields. We note that this review will be mainly focused on amphiphilic peptides composed of typical hydrophobic moiety and hydrophilic moiety. Detailed introduction of other types of self-assembling peptides such as ionic complementary peptides could be found in other reviews.5,9
Design of amphiphilic peptides
Surfactant-like peptides
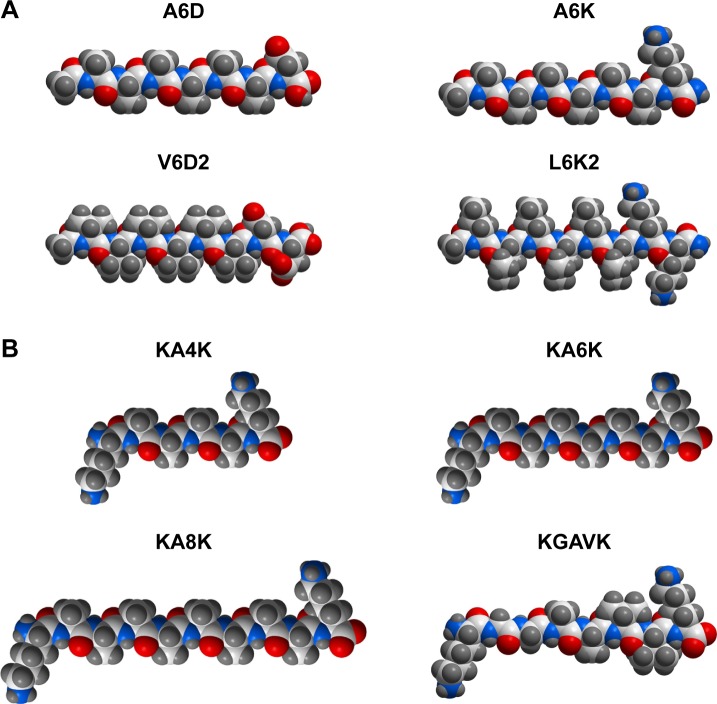
Surfactants, named for their ability to decrease the surface tension of solvent, are a category of amphiphiles composed of a hydrophobic tail and a hydrophilic head. By mimicking the structure of traditional surfactants, a large family of surfactant-like peptides has been designed.43–45 A typical surfactant-like peptide usually consists of two parts: a hydrophobic tail composed of several hydrophobic amino acids and a hydrophilic head composed of one or two hydrophilic amino acids. According to this basic rule, various surfactant-like peptides could be designed quite freely by choosing different hydrophobic or hydrophilic amino acids, which endows them with different chemical and geometrical properties. For example, the hydrophobic tail could be designed as different hydrophobic amino acids including Gly, Ala, Val, Leu and Ile with different levels of hydrophobicity, so that the overall hydrophobicity of a surfactant-like peptide could be controlled. On the other hand, the hydrophilic head could be designed as negatively charged Asp and Glu, or positively charged Lys, His and Arg, generating anionic or cationic surfactant-like peptides, respectively. Based on this simple rule, a number of surfactant-like peptides have been designed (Figure 1A).
Figure 1.
Molecular models of several typical designer amphiphilic peptides.
Notes: (A) Surfactant-like peptides composed of hydrophobic amino acids as the tail and one or two hydrophilic amino acids as the head. (B) Bolaamphiphilic peptides composed of two hydrophilic amino acids linked by a section of hydrophobic amino acids. All molecular models were generated using the ICM-Pro software package (MolSoft LLC, San Diego, CA, USA).
It should be noted that the hydrophilic head could be designed at either the C-terminal or the N-terminal, generating surfactant-like peptides with different charge distribution, but similar self-assembling behaviors.43 Furthermore, the tail length of a designer surfactant-like peptide could be controlled by changing the number of hydrophobic amino acids. Generally, the tail of a surfactant-like peptide is composed of six hydrophobic amino acids, making the whole peptide molecule 2–3 nm in length, which is similar to the length of natural phospholipids. But it has been proved that surfactant-like peptides with tails composed of 4–10 hydrophobic amino acids have similar self-assembling behavior, except for that longer tails may lead to nanostructures with more polydispersity.45 On the other hand, not only the relative position, but also the components of the head and the tail could be shifted. This means, neither the hydrophobic part should necessarily be the tail nor the hydrophilic part should necessarily be the head. In two surfactant-like peptides investigated by Capes et al, two isoleucine residues composed the hydrophobic head and six hydrophilic amino acids composed the tail.46
Peptide amphiphiles with alkyl group
Besides the surfactant-like peptides composed of only natural amino acids described earlier, peptides conjugated with other components have also been investigated. One of the most widely studied examples is peptide amphiphile using alkyl as the hydrophobic tail, which has been developed by Stupp’s group.47,48 Generally, a peptide amphiphile is composed of four sections: 1) an alkyl tail linking to the N-terminal of the peptide; this hydrophobic saturated fatty acid tail is of crucial importance for the self-assembly event of the peptide amphiphile; 2) an eight-peptide section which can form a stable β-sheet and provides hydrogen bond for self-assembly; 3) a linker region of glycine residues to provide the functional head group flexibility from the more rigid cross-linked region and 4) a hydrophilic functional section. In addition to acting as a functional group, the hydrophilic head also has the largest cross-section that makes the peptide amphiphile molecule cone shaped, which is considered as a crucial factor for self-assembly.
Generally, for a designer peptide amphiphile, the alkyl tail, β-sheet section and glycine linker are relatively conserved to ensure the self-assembling process, while the hydrophilic group could be diverse epitopes with different biological functions. Furthermore, lysine dendron can be used to form embranchment at the C-terminal of the peptide section, so that different epitopes can be attached to a same peptide amphiphile.49–51 In some cases, the hydrophobic tail could also be alternatively designed by either linking more than one alkyl tail to a peptide amphiphile52 or using a cho-lesteryl group to replace the alkyl tail.53,54
Bolaamphiphilic peptides
Since so many typical surfactant-like peptides have been successfully designed by mimicking the structure of traditional surfactants, it is expectable that more novel surfactant-like peptides could be designed by mimicking the structures of other special surfactants. Bolaamphiphilic surfactants are such a special group of surfactants. Unlike traditional surfactants with only one hydrophilic head, a bolaamphiphilic surfactant has two hydrophilic heads connected by a hydrophobic section, which is generally composed of alkyls. Bolaamphiphilic surfactants could self-assemble into various nanostructures including membrane-mimetic films,55 nanotubes56 and helical ribbons,57 which have shown promising applications such as nanotube templates for metallic nanowire,58 membrane mimetic film for bioactive functions59 and nanovesicles for drug and gene delivery.60,61
By mimicking the structure of these bolaamphiphilic surfactants, a serial of bolaamphiphilic peptides composed of natural amino acids have been designed (Figure 1B).62 Like traditional surfactant-like peptide, a bolaamphiphilic peptide usually uses Gly, Ala and Val as its hydrophobic section and charged Lys or Asp as its hydrophilic heads. By controlling the number of hydrophobic amino acids and hydrophilic amino acids, the overall hydrophobicity and length of the peptide molecule could be adjusted to generate different self-assembling structures. Furthermore, like the design of peptide amphiphiles using alkyl group as the hydrophobic tail, bolaamphiphilic peptide amphiphiles with alkyl group as their hydrophobic sections have also been designed.63 Although the research on bolaamphiphilic peptides has just been started in very recent years and only a few peptides have been designed and studied, it suggests a new strategy to design more self-assembling amphiphilic peptides by mimicking the structure of unconventional surfactants.
Self-assembling structures of amphiphilic peptides
Methods for structural characterization of amphiphilic peptides
In most cases, designer amphiphilic peptides could self-assemble into various kinds of nanostructure, and these nanostructures are their main form as useful biomedical materials. So, the first and the most important step in the study of an amphiphilic peptide is the characterization of nanostructure. Transmission electron microscopy (TEM) and atomic force microscopy (AFM) are the conventional methods for observing detailed structure of biological samples, and have been widely used for studying the nanostructures formed by self-assembling amphiphilic peptides.47,62 Furthermore, cryo-TEM and cryo-scanning electron microscopy (SEM), in which samples are pre-fixed by freezing before observation, have also been developed to study some dynamic and unstable nanostructures formed by amphiphilic peptides.64,65 On the other hand, dynamic light scattering could provide information about the average size distribution of nanoparticles in solution; therefore, it is also a conventional tool for studying the self-assembling behavior of amphiphilic peptides.44,62
Since the self-assembling behavior of amphiphilic peptides is based on their secondary structure, characterization of peptides’ secondary structure is very important to analyze the self-assembling mechanism and predict the self-assembling behavior. To date, techniques including circular dichroism (CD), Fourier transform infrared spectroscopy, solid-state nuclear magnetic resonance and X-ray scattering/diffraction have been widely used for the secondary structure characterization of amphiphilic peptides.62,66,67 Furthermore, a combination of different techniques is definitely a rational strategy to investigate the self-assembling behaviors at a high molecular detail.68 With the help of all these powerful techniques, various types of nanostructures formed by different designer amphiphilic peptides, as well as their molecular mechanisms have been extensively studied.
Nanotubes and nanovesicles
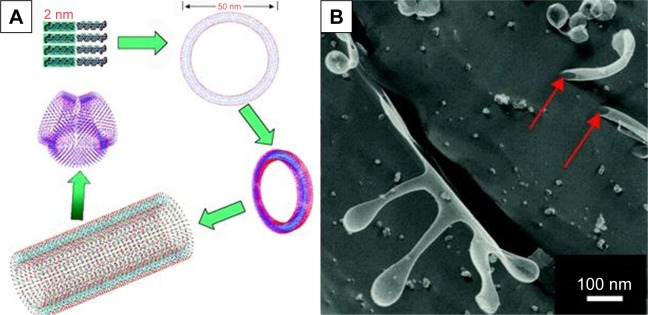
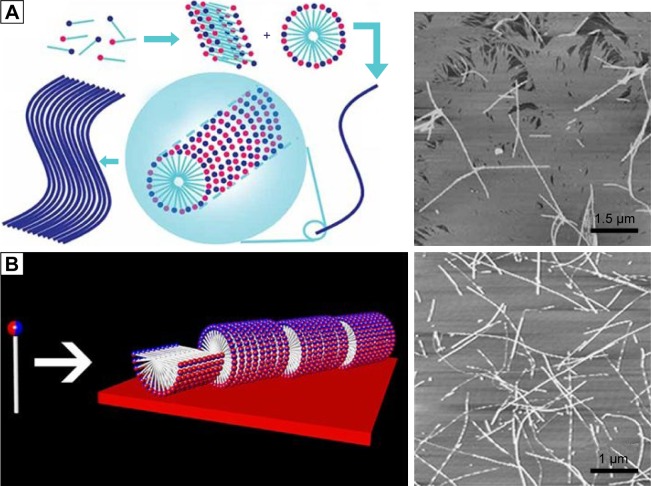
For the simplest surfactant-like peptides composed of 1–2 hydrophilic amino acids and 4–8 hydrophobic amino acids, such as A6D, V6D, V6D2, L6D2, A6K and so on, hollow nanostructures including nanotube with closed end and nanovesicle have been observed in several earliest studies. in aqueous solution.43–45 These hollow nanostructures were 30–50 nm in diameter and could further form a network with three-way junctions, which were highly unstable and dynamic. Since these typical surfactant-like peptides are structurally similar to natural lipids, it was believed that in aqueous solution, they could pack the hydrophobic part tail-to-tail to form a bilayer structure, which further forms closed nanotubes or nanovesicles (Figure 2). In this model, the peptide monomers took an irregular secondary structure as revealed by CD, which seemed to be less important for the self-assembling process, while the hydrophobic interaction between tails was regarded as the major driving force. According to this model, these nanotubes or nanovesicles were structurally similar to the widely studied liposomes, but with a much smaller size; so, they have been expected to be novel carrier materials for hydrophilic drugs and DNA/RNA.
Figure 2.
Self-assembling model and morphology of a typical surfactant-like peptide A6D forming bilayer structures.
Notes: (A) Proposed model describing the formation of bilayer nanotube and three-way junctions. (B) TEM image of nanotubes and nanovesicles formed by A6D, with red arrows indicating hollow opening at the ends. The figure has been reproduced from Vauthey S, Santoso S, Gong H, Watson N, Zhang S. Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc Natl Acad Sci U S A. 2002;99(8):5355–5360. Copyright (2002) National Academy of Sciences, USA.44
Abbreviation: TEM, transmission electron microscopy.
Although the attention of researchers was drawn upon surfactant-like peptides initially by these bilayer nanostructures, further studies have revealed other types of self-assembling structures formed by these peptides. For example, Bucak et al69 found that the model surfactant-like peptide A6K could self-assemble into ordered and presumably nematic nanotubes with a radius of 25–30 nm and very large aspect ratio. Although their size was similar to the bilayer nanostructures described earlier, these nanotubes seemed to be formed by single wall of peptide monomers. A proposed model based on in situ capillary flow X-ray diffraction indicated that A6K monomers form antiparallel β-sheets in the single wall and project the charged lysine outside. In this model, intermolecular hydrogen bond based on β-sheets secondary structure was regarded as the major driving force, and the nanotubes were stabilized by charged surfaces bearing lysine residues.70
Micellar nanostructures
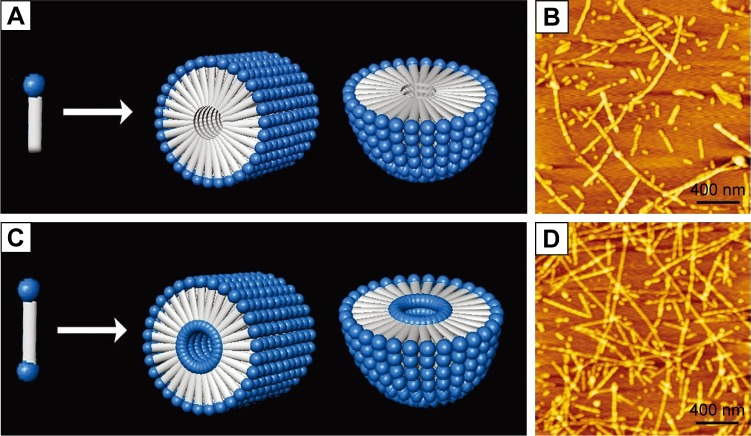
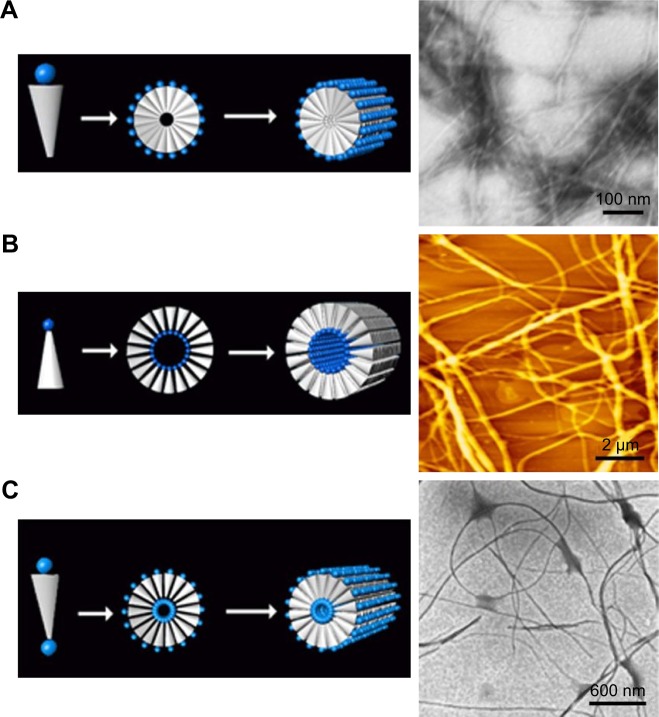
It is well known that except for tail-to-tail bilayer structures, traditional surfactants could also form micelles by packing the tails in a hydrophobic core and exposing the hydrophilic heads outside. Similar self-assembling behaviors have also been observed for surfactant-like peptides, which could form micellar nanostructures instead of hollow nanotubes or nanovesicles. As observed by AFM and TEM, A6K±, a typical surfactant-like peptide, could form nanofibers, nanorods and nanospheres with various lengths.71 These nanostructures were quite different from the nanotubes and nanovesicles described in earlier reports, considering their following features: 1) the diameter of these nanostructures was <10 nm, which was much smaller compared with those in earlier reports; 2) the nanofibers were separated from each other, rather than cross-linked by three-way junctions and 3) these nanostructures seemed to be more stable, since they could be observed by conventional AFM or TEM methods, while the nanotubes and nanovesicles were very unstable, and thus, a specialized quick-freeze/deep-etch technique was required to fix the structures for observation. All these differences indicated that these nanostructures were formed by a different self-assembling mechanism. A molecular model has been proposed to explain this alternative self-assembling behavior. As shown in Figure 3A, peptide molecules pack their hydrophobic tails inside and expose their hydrophilic heads outside, forming spherical or cylindrical micelles, for example, nanospheres or nanofibers with various lengths but similar diameter. It is not clear why and how similar surfactant-like peptides could undergo self-assembly in quite a different way, but it is no doubt that the new pathway to form micellar structures is very helpful to understand the behavior of these self-assembling surfactant-like peptides and to further exploit their potential applications.
Figure 3.
Formation of micellar structures by amphiphilic peptides.
Notes: (A) Self-assembling model of surfactant-like peptide forming cylindrical and spherical micelles. (B) AFM image of surfactant-like peptide A6K±. (C) Self-assembling model of bolaamphiphilic peptide forming cylindrical and spherical micelles. (D) AFM image of bolaamphiphilic peptide KA6K. Data from Chen et al.8
Abbreviation: AFM, atomic force microscopy.
Several de novo designer bolaamphiphilic peptides, which have a molecular structure very close to surfactant-like peptides, could also form similar micellar nanostructures, likely through a similar mechanism.62 In aqueous solution, these bolaamphiphilic peptides could also self-assemble into unbranched nanofibers or nanospheres with diameters <10 nm. Compared with micellar nanostructures formed by surfactant-like peptides, the most unique feature of these structures may be that they are hydrophilic both in the core and on the surface (Figure 3C). Due to this feature, they hold promising application as carriers for hydrophilic molecules such as DNA/RNA and some hydrophilic drugs. Furthermore, the nanofibers and nanospheres seem to be different phases of a same self-assembling process, so they could be transformed to each other. It has been found that after they are treated by ultrasound, the nanofibers could be broken into short nanorods or nanospheres, which could then undergo a slow process of reassembly to recover long nanofibers. Another feature of the nanostructures formed by bolaamphiphilic peptides is that they have considerable thermostability, making them promising materials for more applications other than being used as carriers. Although both surfactant-like peptide and bolaamphiphilic peptide take irregular secondary structure in aqueous solution as revealed by CD spectra, their hydrophobic part could be tightly packed by hydrophobic interaction. This could then induce strong hydrogen bonds among the peptide backbones, endowing the micellar nanostructures with considerable stability.
Similarly, peptide amphiphiles with alkyl group could also self-assemble into micellar structures. But unlike surfactant-like peptides and bolaamphiphilic peptides that self-assemble into a mixture of nanofibers, nanorods and nanospheres, peptide amphiphiles could preferentially self-assemble into nanofibers with a very high aspect ratio. By packing the hydrophobic alkyl tails inside and exposing the hydrophilic head groups outside, all designer peptide amphiphiles could self-assemble into extremely long cylindrical micelles. The overall cone-like shape and the hydrogen bond between the middle peptide sections of β-sheets are believed to stabilize the nanofibers, which could further form 3-D nanoscaffold with a macroscopic morphology of the hydrogel. Since all the functional head groups are exposed outside and arranged along the surface of nanofibers, these nanofiber hydrogels could be used as functionalized nanomaterials. Similarly, bolaamphiphilic peptide amphiphiles with two hydrophilic heads could also form such long nanofibers with a hydrophilic core.63
Film and lamella structures
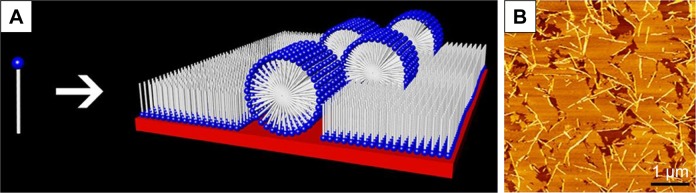
In the investigation of a classic cationic surfactant-like peptide A6K, our group has found that A6K could undergo another very surprising and interesting self-assembling behavior. When the A6K solution was spread on mica surface for AFM observation, a layer of film-like structure was observed in addition to nanofibers.71 Line profile analysis of the surface indicated that the height of the film was very close to the estimated length of a peptide monomer, indicating that the film was composed of a single layer of peptide molecules. It is proposed that the A6K monomers could attach their positively charged heads to the negatively charged mica surface and stretch their hydrophobic tails up to air, forming a layer of peptide monomers aligned shoulder-by-shoulder (Figure 4). In this manner, the peptide monolayer could cover the hydrophilic mica surface and transform it to a hydrophobic one. This interesting self-assembling behavior of A6K on the mica surface could be used as a very simple technique for surface modification with potential applications in many fields. In another work on a serial of surfactant-like peptides V6K2, V6K and V3K, similar film structures were found to be formed on silicon wafer.72 A slight difference was that the hydrophobic tails of the peptides were found to intermix to form a dense hydrophobic middle layer, leaving the hydrophilic head groups symmetrically projected outside. This difference might be caused by the difference between mica and silicon wafer used as the substrates in the two studies.
Figure 4.
Monolayer film formed by surfactant-like peptide A6K on mica surface.
Notes: (A) Self-assembling model of A6K on mica surface. (B) AFM image nanostructures formed by A6K on mica surface. Reprinted from J Colloid Interface Sci, 336(2), Qiu F, Chen Y, Zhao X. Comparative studies on the self-assembling behaviors of cationic and catanionic surfactant-like peptides, 477–484, Copyright 2009, with permission from Elsevier.71
Another type of lamella structure formed by surfactant-like peptide has been reported by Xu et al73 when studying the effect of hydrophobic tail length on self-assembling structures of AmK (number of alanine [m]=3, 6 or 9). They found that compared with A6K, A3K had a shorter hydrophobic chain; therefore, it had a higher packing parameter, which consequently led to the formation of a bilayer sheet-like structure. But these lamella structures were unstable during observation, probably because the lamellar stacks of A3K molecules were sensitive to dehydration.
Factors affecting self-assembly of amphiphilic peptides
Ionic bond in hydrophilic heads
Although it is generally believed that hydrophobic interaction and hydrogen bond are the most predominant driving forces for the self-assembly of amphiphilic peptides, it has been shown that introduction of extra ionic bond has significant effect on the self-assembling structures. In addition to traditional anionic and cationic surfactants, catanionic and zwitterionic surfactants are the other two types of surfactants. The former is a mixed system of anionic and cationic surfactants, and the latter consists of surfactants bearing both positive and negative charges in a single hydrophilic head. A common advantage of these two special types of surfactants is that compared with traditional surfactants, they could form structures with better stability, possibly because of the ionic bonds between oppositely charged heads.74–76 Similarly, ionic bond has been proved to be a very important non-covalent force driving the self-assembling process of amphiphilic peptides. In order to exploit self-assembling surfactant-like peptides with better properties, researchers have made several attempts to introduce ionic bond into the system of typical surfactant-like peptides. Khoe et al77 have studied the self-assembling behaviors of several catanionic surfactant-like peptide systems, which were mixtures of cationic A6K and anionic A6D in various ratios. They found that when A6D and A6K were mixed at a ratio of 2:1, the catanionic system could form well-ordered nanofibers (Figure 5A), indicating that in a catanionic system, the interaction between positively and negatively charged heads has significant effects on the self-assembling behavior. Similarly, Niece et al78 have combined two peptide amphiphiles with oppositely charged bioactive groups to fabricate nanofibers which bear two different functional groups on the surface.
Figure 5.
Self-assembly of amphiphilic peptides with ionic bonds introduced among hydrophilic heads.
Notes: (A) Self-assembly of a catanionic system of A6D and A6K mixed at a molar ratio of 2:1. Reproduced from Khoe U, Yang Y, Zhang S. Synergistic effect and hierarchical nanostructure formation in mixing two designer lipid-like peptide surfactants Ac-A6 D-OH and Ac-A6 K-NH2. Macromol Biosci. 2008;8(11):1060–1067.77 Copyright 2008 John Wiley and Sons. (B) Self-assembly of zwitterionic peptide A6K± with both negative and positive charges in the head groups. In each figure, the left panel shows the self-assembling model and the right panel shows the AFM image of nanofibers. Reprinted from J Colloid Interface Sci, 336(2), Qiu F, Chen Y, Zhao X. Comparative studies on the self-assembling behaviors of cationic and catanionic surfactant-like peptides, 477–484, Copyright 2009, with permission from Elsevier.71
Abbreviation: AFM, atomic force microscopy.
An alternative strategy to introduce ionic bond is to design two opposite charges in a single head, generating zwitterionic peptide. Our group has designed a zwitterionic surfactant-like peptide by simply removing the C-terminal protective amide of A6K and exposing the dissociable carboxyl.71 This new peptide, named as A6K±, could simultaneously bear a positive and a negative charge at its C-terminal. It was found that synergistically driven by the ionic bonds among hydrophilic heads and the hydrophobic interaction among tails, A6K± could form much longer nanofibers compared with traditional anionic or cationic surfactant-like peptides (Figure 5B). Furthermore, this peptide was found to have much lower critical micelle concentration value, and the self-assembled structures were also more mechanically and thermally stable than those formed by A6K. Because of the existence of an extra dissociable carboxyl group, A6K± could also respond more sensitively to the change of environmental pH and undergo complicated transformation. These results indicated that by designing zwitterionic surfactant-like peptides, novel stable and smart nanomaterials could be obtained.
Molecular geometric shape
It is well known that self-assembling behavior is not only determined by chemical complementarity, but also controlled by geometrical compatibility.79–81 For designer peptide amphiphiles, it is believed that the overall cone shape of the molecular building block is a critical property for the self-assembling process. In order to study the effect of geometrical shape on the self-assembling behavior of surfactant-like peptides, our group has designed several surfactant-like peptides with different geometrical shapes.82 As shown in Figure 6A and B, by using different amino acids in the hydrophobic tail, AVK± (Ac-AAAVVVK) has a wedge-like shape and AGK± (Ac-AAAGGGK) has the shape of an inverted wedge. The self-assembling behaviors of these peptides were compared with A6K± with a regular shape. The self-assembling behavior of A6K± was very dynamic and it tended to form a mixture of nanofibers with various lengths and nanospheres, which could transform to each other. On the contrary, the wedge-shaped AVK± underwent a more homogeneous self-assembling behavior to form much longer nanofibers. These nanofibers were relatively more stable and less prone to transform to nanospheres (Figure 6A). It is very interesting to note that AGK± with an inverted wedge-like shape could not undergo self-assembly in aqueous solution, but could form nanofibers with various lengths in a nonpolar solvent (Figure 6B). The effects of geometrical shape on self-assembling behaviors have been discussed by proposed 3-D molecular models. For AVK±, the wedge-like shape could efficiently reduce the spatial encumbrance when it formed micelles, so that it could form stable cylindrical micelles, for example, nanofibers. For AGK±, the inverted wedge-like shape facilitated it to bury the small hydrophilic heads inside and expose the large hydrophobic tails to the nonpolar solvent to form reversed micelles. Similar self-assembling behavior has also been found for negatively charged surfactant-like peptide A3V3D with a wedge-like shape, which could also form smooth, long nanofibers.83 Khoe et al84 have also reported the self-assembling behaviors of a designer cone-shaped surfactant-like peptide, Ac-GAVILRR-NH2. Through fusion or elongation of spherical micelles, this peptide could self-assemble into donut-like nanostructures.
Figure 6.
Self-assembly of amphiphilic peptides with different geometrical shapes.
Notes: (A) Wedge-shaped surfactant-like peptide and (C) bolaamphiphilic peptides formed long, cylindrical micelle nanofibers in water solution. Inverted wedge-shaped surfactant-like peptide (B) formed reverse micelle nanofibers in nonpolar environment. In each figure, the left panel shows the self-assembling model and the right panel shows the TEM images (in A and C) or AFM image (B). Data from Chen et al.82
Abbreviations: AFM, atomic force microscopy; TEM, transmission electron microscopy.
In the system of bolaamphiphilic peptides, geometrical shape of hydrophobic section has similar impact on the self-assembling behavior. A wedge-shaped bolaamphiphilic peptide KGAVK has been designed and its self-assembling behavior has been compared with regular-shaped KA6K. KA6K self-assembled into nanospheres and short nanofi-bers, which could dynamically transform to each other. On the contrary, KGAVK could homogeneously self-assemble into extremely long, flexile nanofibers (Figure 6C).82 These studies suggested that geometrical shape plays a crucial role in controlling the self-assembling behaviors of designer amphiphilic peptides, so that more rational design could be introduced for developing amphiphilic peptides with controllable self-assembling behaviors for special purpose.
Solution parameters
Since for nearly all of the designer peptides, their self-assembling processes happen in solution and most of them are expected to be applied as solution, the effects of solution parameters on the self-assembling behaviors are of high concern. Although systematic study on this issue is rare, it has been proved that solution parameters such as pH value and solvent property have critical effects on self-assembling behaviors of amphiphilic peptides.85,86 Since it has been discussed earlier that charges in hydrophilic heads are very important for self-assembly, and the net charge of a hydrophilic amino acid is determined by the environmental pH value, it is clear that the pH value of a solution will affect the self-assembling behaviors by affecting the charges. In fact, nearly all self-assembling amphiphilic peptides have been investigated in a limited range of pH, only within which the peptide molecules could undergo self-assembly as expected. The change of pH will change the charge distribution and then change the secondary structure of peptide monomers, and eventually, either change the self-assembling structure to another type or eliminate it.46,87 An example is the peptide IIEENNDD investigated by Capes et al.46 As the pH value increased from acidic to basic, the peptide gradually lost its β-sheet structure and the self-assembling structures changed from nanofibers to nanospheres.
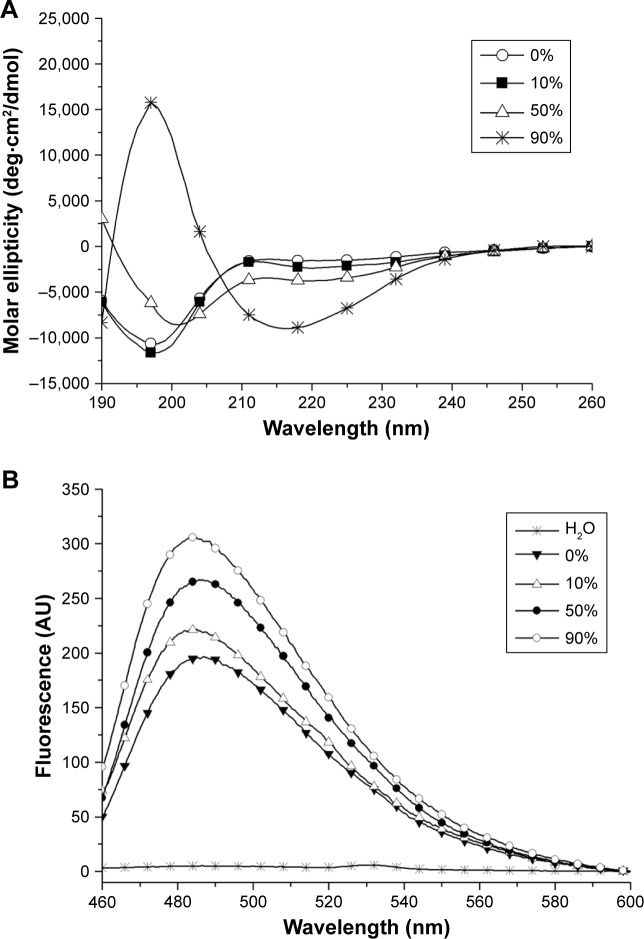
Except for water, some organic solvents have also been used in the study of peptide self-assembly. In our recent study,86 we found that ethanol as a polar aprotic solvent could induce surfactant-like peptide A6K± to take typical β-sheet secondary conformation, based on which the peptide could form long nanofibers. Most interestingly, these long nanofibers induced by ethanol showed some properties similar to well-studied amyloid fibrils (Figure 7). This phenomenon was also in accordance with the fact that ethanol could denature some normal proteins and induce them to form amyloid fibrils based on β-sheet, indicating that short surfactant-like peptides might serve as a simple model to investigate the mechanism for the formation of pathogenic amyloid fibrils.
Figure 7.
Amyloid-like aggregation of A6K± induced by ethanol.
Notes: (A) CD spectra of surfactant-like peptide A6K± in ethanol with different concentrations. Increasing band near 218 nm indicates the increase in β-sheet secondary structure induced by ethanol. (B) Thioflavin T binding fluorescent spectra of A6K± in ethanol with different concentrations. Increasing band near 490 nm indicates increasing amyloid-like fibrils. Reproduced from Chen Y, Tang C, Xing Z, Zhang J, Qiu F. Ethanol induced the formation of β-sheet and amyloid-like fibrils by surfactant-like peptide A6K. J Pept Sci. 2013;19(11):708–716.86 Copyright 2013, John Wiley & Sons, Ltd.
Abbreviations: AU, arbitrary units; CD, circular dichroism.
On the other hand, it is well known that amphiphiles forming micellar structures in polar solvent could form reversed micelle in nonpolar solvent. So, it is clear that the solvent polarity also has a critical effect on the self-assembly of amphiphilic peptides. For example, peptide AGK± dissolved in a polar water solution failed to undergo self-assembly, while it could self-assemble into nanofibers in a nonpolar mixture of tetrahydrofuran and water.82 Another example is a peptide amphiphile with two hydrophobic tails, which could self-assemble into nanofibers in both water and nonpolar solvents such as benzene and carbon tetrachloride.88
Additionally, other factors such as ionic strength, peptide concentration and even different batches of synthesized peptide could have remarkable effects on the self-assembling behaviors of designer amphiphilic peptides.89 In one word, although the basic self-assembling mechanism of these peptides is relatively simple and clear, many factors will have significant effect on the self-assembling behavior of a specialized peptide in a specialized environment. On one hand, this raises the requirement for further investigation to fully understand and deliberately control the designer peptide molecule to make it a useful nanomaterial with practical applications. On the other hand, this also makes it possible to design smart nanomaterials with sensitive responsibility to environmental factors, which would be important for some applications such as controlled drug release and molecule switch.
Applications of amphiphilic peptides
Stabilizing membrane proteins
About one-third of all cellular proteins are membrane proteins, which lie on the interface of cells and their environment and play critical roles in many important biological activities such as energy transformation, substance transport and signal transmission. For this reason, the structure and function of membrane proteins, especially G-protein coupled receptors, have become a worldwide hotspot. Unfortunately, in spite of the great importance of membrane proteins, people’s knowledge about them is still very poor. The reason for this problem is that membrane proteins in their natural active form are fully or partially embedded in a hydrophobic environment surrounded by lipids, and therefore, a purifying and crystallizing process for normal water-soluble proteins will inevitably destroy their natural structures. In the past decades, many traditional surfactants, including chemical detergents and lipids, have been used for dissolving, stabilization, purification and crystallization of membrane proteins, but the efficacy is still far from meeting the requirement for studying the structure and function of membrane proteins. Furthermore, how did these surfactants act on membrane proteins and affect their structures and functions, and how to choose the most suitable surfactant for a certain membrane protein are still unclear. For these reasons, a brand new material is badly needed to facilitate the study of membrane proteins.
Because of their structural similarity to traditional surfactants, some designer surfactant-like peptides have shown promising potential in the study of membrane proteins.90–95 The capability of surfactant-like peptides to facilitate the dissolving and stabilization of membrane proteins has already been proved by many researches, and a molecular model has been built to explain how these peptides could protect membrane proteins from denaturation.95 In this model, surfactant-like peptides bind to the hydrophobic section of a membrane protein via their tails, embedding it in a hydrophobic environment and preventing it from denaturation (Figure 8). This mechanism is similar to traditional surfactants such as lipids. Since the surfactant-like peptides are short peptides composed of natural amino acids, they can also be called as “artificial molecular chaperon”. Using these novel designer surfactant-like peptides as molecular chaperons, some membrane proteins with significant importance have been well investigated.
Figure 8.
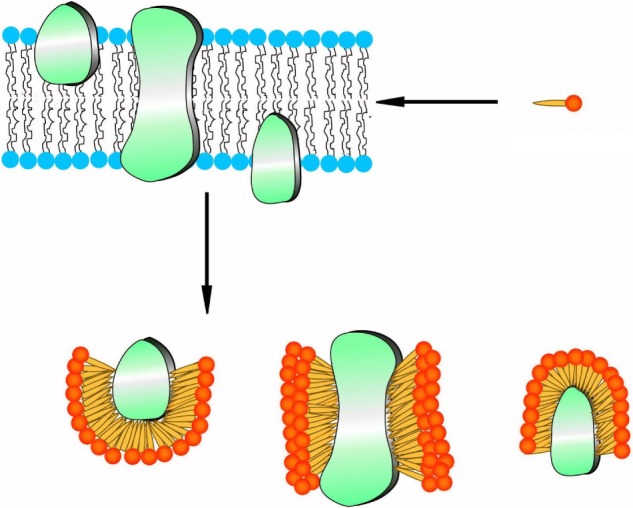
Proposed mechanism of surfactant-like peptides stabilizing membrane proteins.
Note: Peptide molecules, acting as traditional surfactants, bind their hydrophobic tails to the hydrophobic region of a membrane protein and prevent it from destabilizing.
For example, Matsumoto et al94 have designed a new class of short surfactant-like peptides and studied their ability to stabilize the functional conformation of the PSI membrane protein from T. elongatus. By measuring the light-induced electron transfer activity of PSI in aqueous media in the presence of surfactant-like peptides, they found that amphiphilicity is necessary, but not sufficient to stabilize PSI in its functional form. It was found that the amino acid sequence was crucial, and the best performing peptides for the stabilization of functional PSI were, in order of effectiveness, I6K2, A6K2, V6K2 and V6R2. These results indicated that photosynthetic complexes could be effectively stabilized and immobilized by choosing suitable surfactant-like peptides, bringing hope for the development of new energy resource based on solar bio-battery.
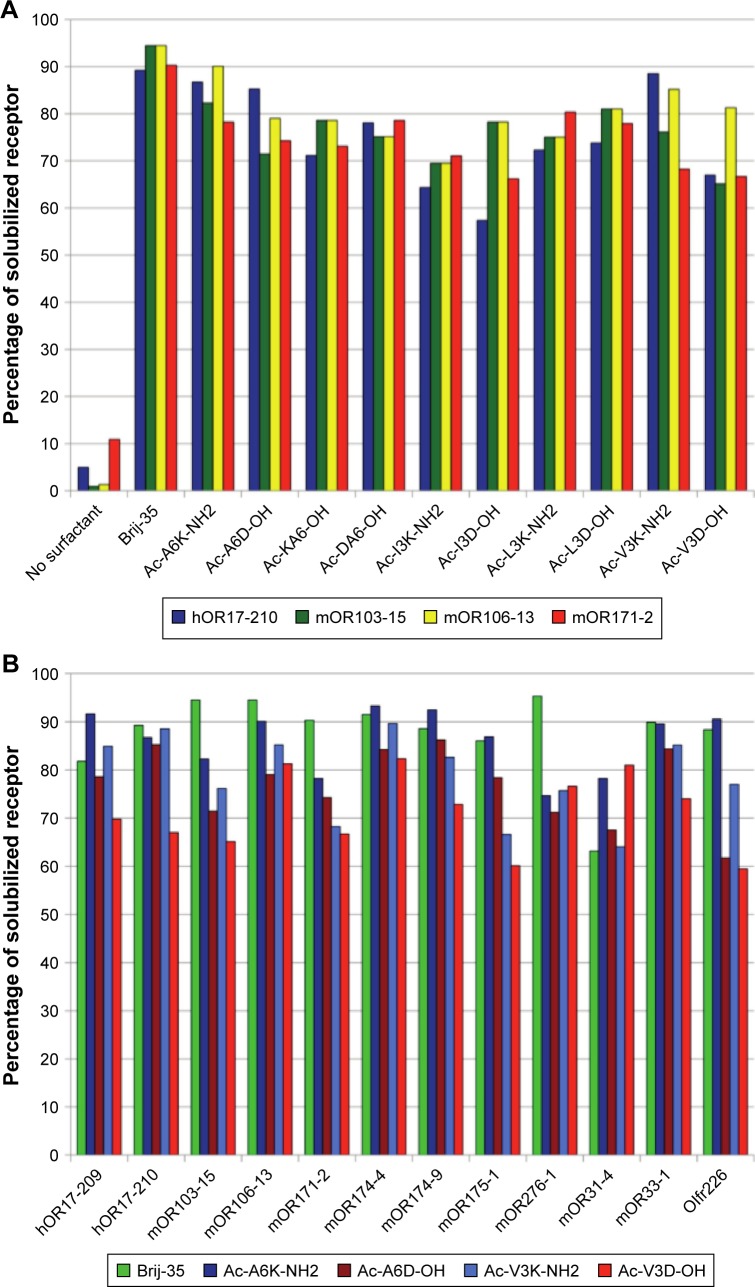
In another recent study, Corin et al96 have used surfactant-like peptides for the solubilization and stabilization of mammalian olfactory receptors, another important family of G-protein coupled receptors. It was found that a serial of surfactant-like peptides were able to solubilize 12 different olfactory receptors expressed by Escherichia coli, with an efficacy comparable to that of the detergent Brij-35, an optimal traditional detergent (Figure 9). Furthermore, olfactory receptors purified by surfactant-like peptides retained their native α-helix secondary structure and were functional to bind their odorants.
Figure 9.
Solubilization of different olfactory receptors by different surfactant-like peptides as compared with Brij-35.
Notes: (A) Using four olfactory receptors as models, ten different surfactant-like peptides showed similar ability to solubilize membrane proteins. (B) Four surfactant-like peptides were chosen to compare their potential to solubilize membrane proteins with Brij-35, and 12 different olfactory receptors could be solubilized by these peptides similarly. Reproduced from Corin K, Baaske P, Ravel DB, et al. Designer lipid-like peptides: a class of detergents for studying functional olfactory receptors using commercial cell-free systems. PLoS One. 2011;6(11):e25067.96
Antimicrobial capacity
As the resistance of microbes against conventional antibiotics keeps increasing, there is an increasing demand to develop new antimicrobial agents. To date, several hundreds of different antibacterial peptides from a wide variety of organisms have been isolated and characterized.97 On the other hand, short designer cationic amphiphilic peptides as antimicrobial agents have also been widely investigated. Although the lengths of most natural antimicrobial peptides vary from 10 to a few dozens of amino acid residues, the designed ones are normally 6–15 residues in length. Both positive charges and hydrophobicity play vital roles in the effectiveness of microbial killing. The head group is usually composed of a few Lys, Arg or His bearing positive charges at biological pH, while the hydrophobic chain can be a few hydrophobic amino acid residues. For example, the K4 peptide (KKKKPLF-GLFFGLF) was recently demonstrated to destabilize the organization of monolayer membranes or bilayer liposomes composed of anionic lipids and display good antimicrobial activities against various bacterial strains.98
Among the shortest designer amphiphilic peptides, AmK (m=3, 6 or 9) displayed a varying extent of antimicrobial activities. Unlike traditional antibiotics working as bacterial growth inhibitors, AmK killed bacteria by permeating the cell membranes, and the possible mechanism included “barrel-stave”, “carpet” and “worm-pore”.99 It has been proved that the peptides’ antimicrobial activity was determined by the length of their hydrophobic tail. Among the studied peptides, A9K exhibited the best killing capacity against both Gram-negative and Gram-positive bacteria. Furthermore, the effect of membrane permeation and bacterial clustering was determined by peptide concentration and incubating time.
Regenerative medicine
In a manner, regenerative medicine requires two complementary key ingredients. One is a biologically compatible scaffold that can be readily adopted by the body without harm, and the other is suitable cells, such as stem cells and primary cells, that could effectively replace the damaged tissues without adverse consequences. Obviously, it would be advantageous for tissue repairing if one could apply suitable biological scaffolds to stimulate cell differentiation. For this purpose, many scaffold-forming self-assembling peptides have been investigated, and one of the most widely studied families is peptide amphiphiles. These nanofiber-forming amphiphilic peptides could be designed to bear various biofunctional epitopes and act as 3-D scaffolds for cell adhesion, proliferation, differentiation and tissue reparation.100–106 To date, these functional scaffolds bearing various bioactive signals have shown potential applications for the regeneration of central nervous system,107–109 vasculature110,111 and hard tissue,112,113 as well as for the transplantation of islets.114
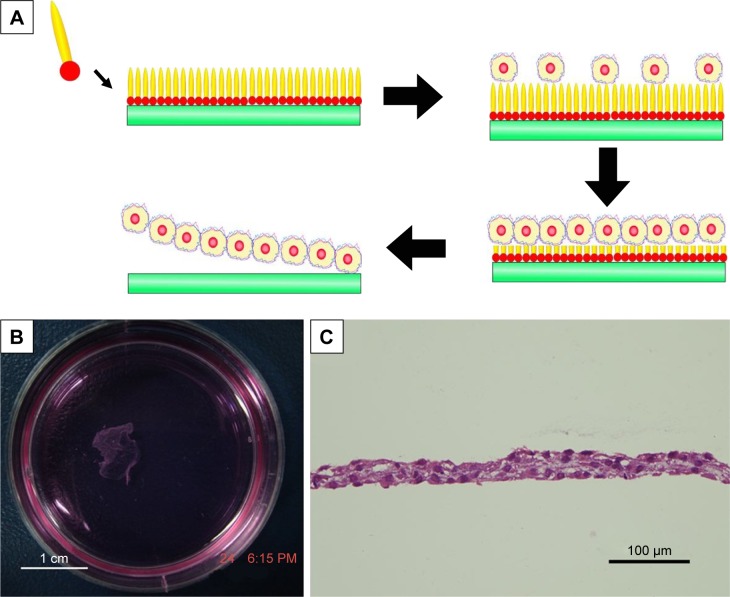
As a complementary to traditional regenerative medicine strategy requiring scaffold materials, cell sheet is an alternative technology, which means a sheet-like integrate of cells is cultured and harvested by certain methods. A cell sheet maintaining the extracellular matrix and cell-to-cell connections could be regarded as a small piece of artificial tissue, which has shown its advantages for the regeneration of soft organs including heart,115–117 liver118,119 and kidney.120 In this field, designer amphiphilic peptide has also shown its potential. In a recent work,121 it was found that A6K, a cationic surfactant-like peptide, could self-assemble on mica surface to form a monolayer. In this way, a hydrophilic mica surface could be transformed into a hydrophobic one, which was suitable for cell adhesion and growth. Along with the growth of cells, the peptides could be gradually biodegraded and the hydrophilic mica surface could be re-exposed. As the hydrophilic mica surface was negative for cell adhesion, an integrate cell sheet could be easily released from the mica surface (Figure 10). This novel technology for harvesting cell sheet based on surfactant-like peptide is very simple, effective and safe, and may become an important technique in the field of regenerative medicine.
Figure 10.
Cell sheet technology based on amphiphilic peptide A6K.
Notes: (A) Mechanism of harvesting cell sheet using A6K-modified mica surface as the substprate for cell culture. (B) Photograph shows a macroscopic piece of cell sheet harvested. (C) HE staining of cell sheet revealed intact extracellular matrix and cell-to-cell connections. Reproduced from Qiu F, Chen Y, Cheng J, Wang C, Xu H, Zhao X. A simple method for cell sheet fabrication using mica surfaces grafted with peptide detergent A(6)K. Macromol Biosci. 2010;10(8):881–886.121 Copyright 2010, John Wiley and Sons.
Drug and gene carriers
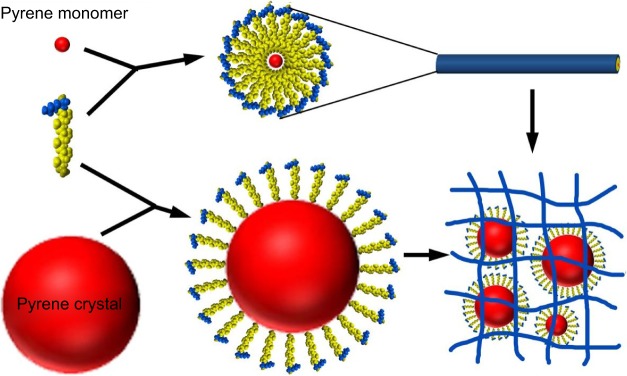
Due to their capability to form nanostructures and permeate membrane, amphiphilic peptides are excellent candidates for drug and gene delivery. In the micellar structures formed by amphiphilic peptides, the hydrophobic core could be used to embed hydrophobic drugs. This simple strategy has been recently proved in one of our studies using A6K as the carrier and pyrene as a model hydrophobic drug.122 It was found that pyrene could be embedded either as monomers in the hydrophobic core of micellar nanofibers formed by A6K or as nanocrystals surrounded by A6K monomer, just like the manner in membrane protein stabilization (Figure 11). The combination of these two mechanisms greatly enhanced the encapsulation efficiency and achieved a sustained release of pyrene. Similarly, an amphiphilic peptide with four fatty tails was also investigated as a hydrophobic drug carrier.52 The peptide could form stable micelles at a low concentration through strong hydrophobic interactions among the tails, and the micelles could encapsulate hydrophobic anticancer drug efficiently. In addition, the micelles could enter cancer cells specifically through the introduction of RGD sequence. These results indicated the great potential of amphiphilic peptides as targeted drug carriers.
Figure 11.
Two mechanisms for the encapsulation of hydrophobic pyrene by A6K.
Notes: On one hand, pyrene monomers could be embedded into the hydrophobic core of cylindrical micelles formed by A6K. On the other hand, A6K monomers could surround bigger pyrene particles with their hydrophobic tails. A combination of the two mechanisms could stabilize pyrene as nano/microparticles in suspension. Copyright ©2015. Dove Medical Press. Reproduced from Chen Y, Tang C, Zhang J, Gong M, Su B, Qiu F. Self-assembling surfactant-like peptide A6K as potential delivery system for hydrophobic drug. Int J Nanomed. 2015;10:847–858.122
Unlike amphiphilic peptides embedding drugs through nonspecific hydrophobic interactions, another strategy to design specific drug carriers was developed based on peptide amphiphiles. In a recent study, Matson and Stupp123 have designed a drug-tethered peptide amphiphile containing hydrazide in its hydrophilic head. The self-assembly and gelation ability of this peptide amphiphile was not affected by drug tethering. After gelation, the small molecule drug could be slowly degraded and released from the gel, suggesting a promising approach to employ peptide amphiphiles as drug carriers.
During the struggle of finding idea carriers for gene delivery, it was found that a number of cationic amphiphilic peptides have great potential.124–126 Contrary to drugs that were embedded in the hydrophobic core of the micelles formed by amphiphilic peptides, negatively charged DNA was expected to bind to the positively charged heads. Recently, amphiphilic cholesterol-conjugated HR15 and HR20 oligopeptides were synthesized, which were able to self-assemble into cationic micelles. The formation of the micelles increased the local density of cationic charge, leading to greater DNA binding efficiency and, thus, higher gene transfection efficiency in both HepG2 and HEK293 cell lines as compared to HR15 and HR20 without cholesterol.126
As hydrophobic drugs and genes are bound to different parts of the amphiphilic peptide carriers, it is also possible to deliver the hydrophobic drug and gene simultaneously into the same cells to achieve synergistic therapeutic effect. In a recent study, amphiphilic peptide containing three blocks of amino acids, Ac-(AF)6-H5-K15-NH2 (FA32), has been designed.127 In aqueous solution, FA32 peptide molecules could self-assemble into small micelles with a particle size of about 100 nm, which provided high capacity for loading hydrophobic DOX and delivered the drug into HepG2 cells efficiently. Meanwhile, these micelles were capable of condensing DNA and delivering it into the same cells with high efficiency. These findings suggest that FA32 micelles may be an effective carrier to deliver hydrophobic anticancer drug and gene simultaneously for improved cancer therapy.
Nanofabrication
The nanoscale organization of metallic and other inorganic materials into one-dimensional (1-D) object is a key task in nanotechnology. Biologically derived nanofibers and nanotubes have provided very useful templates for such task because of their inherent 1D organization. In this field, self-assembling biomolecules such as DNA and peptides have been investigated as intriguing building blocks for nanofabrication. Because of their ability to self-assemble into nanofibers with a large aspect ratio, amphiphilic peptides also have great potential as templates for biomineralization and nucleation, as well as for the fabrication of nanowires and nanocircuits. Recently, our group has designed a surfactant-like peptide AGD, which could undergo self-assembly to form reversed micelle nanorings in the mixture of CuCl2 solution and tetrahydrofuran. Binding with the negatively charged heads of the peptide, copper ions were sequestered in the inner core of the reversed micelle and formed nanorings composed of copper particles, which could be directly observed by TEM (Figure 12).128
Figure 12.
Copper particle nanorings induced by surfactant-like peptide.
Notes: (A) Proposed self-assembling model demonstrating the formation of reversed micelle nanotubes with copper ions (blue dots) embedded inside. (B) TEM image of nanorings. The sample was unstained; the dark rings are actually condensed copper particles with high electron density. Republished with permission of World Scientific Publishing Co., Inc from Formation of reversed micelle nanoring by a designed surfactant-like peptide. Qiu F, Chen Y, Tang C, Cheng J, Zhao X. Nano. 2012;7. Copyright 2006; permission conveyed through Copyright Clearance Center, Inc.128
Abbreviation: TEM, transmission electron microscopy.
On the other hand, peptide amphiphiles forming long unbranched nanofibers could also be used as a template for nanofabrication. For example, a lipopeptide was designed and synthesized for biomineralization by Hartgerink et al.129 Similarly, Li et al have designed a peptide amphiphile with binding functionalities, which could self-assemble into nanofibers in an aprotic environment and induce 1D assembly of gold nanoparticles.88
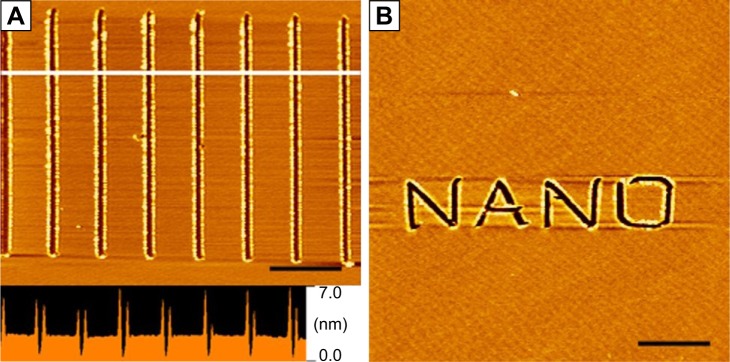
Besides serving as a template, peptide self-assembling structures could also serve as a substrate for AFM-based nanolithography, another important strategy for nanofabrication. Recently, our group has found that surfactant-like peptide A6K could be controlled to form ultra-flat monolayer on mica surface, which could be used as an ideal substrate for AFM-based nanolithography.130 Employing this method, we have created clear nanopatterns on mica surface in a well-controlled manner (Figure 13).
Figure 13.
Nanopatterns generated by AFM nanolithography on a peptide self-assembled monolayer on mica surface.
Notes: (A) Parallel linear scratches with length of 4 µm. The lower panel is the line profile analysis of the section marked by white line, indicating the depth of scratches was about 2–3 nm, the length of a peptide monomer. (B) A more complicated word pattern “NANO” is shown. Scale bars=5 µm. Republished with permission of Bentham Science Publishers, from Curr Nanosci, Xing Z, Chen Y, Tang C, Gong X, Qiu F, 10(2), Fabrication of peptide self-assembled monolayer on mica surface and its application in atomic force microscopy nanolithogrsaphy, 2014; permission conveyed through Copyright Clearance Center, Inc.130
Abbreviation: AFM, atomic force microscopy.
Conclusion and outlook
To sum up, although the design of amphiphilic peptides could be diverse, they are basically composed of a hydrophobic moiety and a hydrophilic moiety. Driven by hydrophobic interaction, these peptides could undergo self-assembly to form various nanostructures. Properties of the peptide molecule, such as overall hydrophobicity, charge distribution and geometrical shape, and the environmental conditions such as pH and solvent polarity could affect the self-assembling behaviors in different ways. Based on their diverse self-assembling nanostructures, these peptides have exhibited many applications in different fields including membrane protein stabilization, regenerative medicine, gene and drug delivery, antimicrobial agent, nanofabrication and so on, indicating their promising potential as a novel type of nanomaterials.
Amphiphilic peptides are of special importance for their simplicity, versatility and biocompatibility. From the aspect of application, these self-assembling peptides are easy to design and synthesize, ensuring their quality and purity. Also, their intrinsic biocompatibility makes them perfect materials for biological and biomedical applications. Although the application of amphiphilic peptides, especially their clinic application, still needs to be explored, it is expectable that this novel type of self-assembling peptide nanomaterials will receive more and more attention and facilitate the development of modern biomedicine and nanotechnology. On the other hand, self-assembling amphiphilic peptide is also a theoretically important system. Amphiphilic peptides consist of only two different parts, for example, the hydrophobic moiety and the hydrophilic moiety, so that their chemical structures and self-assembling mechanisms are very simple and clear. On one hand, this makes it possible to create and ameliorate more amphiphilic peptides to obtain advanced nanomaterials. On the other hand, this also provides very simple models for studying the structure and function of normal molecular machines, as well as the mechanism of some protein conformational diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 31070889, 81000658 and 31100565) and the National 985 Key Project of Sichuan University of the Education Ministry of China. Authors are grateful to Prof Shuguang Zhang of MIT for permitting the use of his original figures in this review.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Whitesides G, Mathias J, Seto C. Molecular self-assembly and nano-chemistry: a chemical strategy for the synthesis of nanostructures. Science. 1991;254(5036):1312–1319. doi: 10.1126/science.1962191. [DOI] [PubMed] [Google Scholar]
- 2.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 3.Lee I. Molecular self-assembly: smart design of surface and interface via secondary molecular interactions. Langmuir. 2013;29(8):2476–2489. doi: 10.1021/la304123b. [DOI] [PubMed] [Google Scholar]
- 4.Berger O, Gazit E. Molecular self-assembly using peptide nucleic acids. Biopolymers. 2017;108(1):e22930. doi: 10.1002/bip.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao X, Zhang S. Designer self-assembling peptide materials. Macromol Biosci. 2007;7(1):13–22. doi: 10.1002/mabi.200600230. [DOI] [PubMed] [Google Scholar]
- 6.Gelain F, Horii A, Zhang S. Designer self-assembling peptide scaffolds for 3-D tissue cell cultures and regenerative medicine. Macromol Biosci. 2007;7(5):544–551. doi: 10.1002/mabi.200700033. [DOI] [PubMed] [Google Scholar]
- 7.Acar H, Srivastava S, Chung EJ, et al. Self-assembling peptide-based building blocks in medical applications. Adv Drug Deliv Rev. 2017;(110–111):65–79. doi: 10.1016/j.addr.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao X, Pan F, Xu H, et al. Molecular self-assembly and applications of designer peptide amphiphiles. Chem Soc Rev. 2010;39(9):3480–3498. doi: 10.1039/b915923c. [DOI] [PubMed] [Google Scholar]
- 9.Zhao X, Zhang S. Molecular designer self-assembling peptides. Chem Soc Rev. 2006;35(11):1105–1110. doi: 10.1039/b511336a. [DOI] [PubMed] [Google Scholar]
- 10.Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: from molecules to nanostructures to biomaterials. Biopolymers. 2010;94(1):1–18. doi: 10.1002/bip.21328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ke Y. Designer three-dimensional DNA architectures. Curr Opin Struct Biol. 2014;27:122–128. doi: 10.1016/j.sbi.2014.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Schneider A, Garlick JA, Egles C. Self-assembling peptide nanofiber scaffolds accelerate wound healing. PLoS One. 2008;3(1):e1410. doi: 10.1371/journal.pone.0001410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meng H, Chen L, Ye Z, Wang S, Zhao X. The effect of a self-assembling peptide nanofiber scaffold (peptide) when used as a wound dressing for the treatment of deep second degree burns in rats. J Biomed Mater Res B Appl Biomater. 2009;89B(2):379–391. doi: 10.1002/jbm.b.31226. [DOI] [PubMed] [Google Scholar]
- 14.Koutsopoulos S. Self-assembling peptide nanofiber hydrogels in tissue engineering and regenerative medicine: progress, design guidelines, and applications. J Biomed Mater Res A. 2016;104(4):1002–1016. doi: 10.1002/jbm.a.35638. [DOI] [PubMed] [Google Scholar]
- 15.Wan S, Borland S, Richardson SM, Merry CLR, Saiani A, Gough JE. Self-assembling peptide hydrogel for intervertebral disc tissue engineering. Acta Biomater. 2016;46:29–40. doi: 10.1016/j.actbio.2016.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Koss KM, Unsworth LD. Neural tissue engineering: bioresponsive nano-scaffolds using engineered self-assembling peptides. Acta Biomater. 2016;44:2–15. doi: 10.1016/j.actbio.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Song H, Zhang L, Xu H, Zhao X. Self-assembly-peptide hydro-gels as tissue-engineering scaffolds for three-dimensional culture of chondrocytes in vitro. Macromol Biosci. 2010;10(10):1164–1170. doi: 10.1002/mabi.200900450. [DOI] [PubMed] [Google Scholar]
- 18.Song H, Zhang L, Zhao X. Hemostatic efficacy of biological self-assembling peptide nanofibers in a rat kidney model. Macromol Biosci. 2010;10(1):33–39. doi: 10.1002/mabi.200900129. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Li P, Jin H, Ding Q, Zou Z, Peng G. Influence of designer self-assembling nanofiber scaffolds containing anti-cancer peptide motif on hepatoma carcinoma cells. J Biomed Mater Res A. 2017;105(8):2329–2334. doi: 10.1002/jbm.a.36059. [DOI] [PubMed] [Google Scholar]
- 20.Tang C, Shao X, Sun B, Huang W, Zhao X. The effect of self-assembling peptide RADA16-I on the growth of human leukemia cells in vitro and in nude mice. Int J Mol Sci. 2009;10(5):2136–2145. doi: 10.3390/ijms10052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mi K, Wang G, Liu Z, Feng Z, Huang B, Zhao X. Influence of a self-assembling peptide, RADA16, compared with collagen I and Matrigel on the malignant phenotype of human breast-cancer cells in 3D cultures and in vivo. Macromol Biosci. 2009;9(5):437–443. doi: 10.1002/mabi.200800262. [DOI] [PubMed] [Google Scholar]
- 22.Horii A, Wang X, Gelain F, Zhang S. Biological designer self-assembling peptide nanofiber scaffolds significantly enhance osteoblast proliferation, differentiation and 3-D migration. PLoS One. 2007;2(2):e190. doi: 10.1371/journal.pone.0000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gelain F, Bottai D, Vescovi A, Zhang S. Designer self-assembling peptide nanofiber scaffolds for adult mouse neural stem cell 3-dimensional cultures. PLoS One. 2006;1(1):e119. doi: 10.1371/journal.pone.0000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modepalli VN, Rodriguez AL, Li R, et al. In vitro response to functionalized self-assembled peptide scaffolds for three-dimensional cell culture. Biopolymers. 2014;102(2):197–205. doi: 10.1002/bip.22469. [DOI] [PubMed] [Google Scholar]
- 25.Worthington P, Pochan DJ, Langhans SA. Peptide hydrogels – versatile matrices for 3D cell culture in cancer medicine. Front Oncol. 2015;5:92. doi: 10.3389/fonc.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scott CM, Forster CL, Kokkoli E. Three-dimensional cell entrapment as a function of the weight percent of peptide-amphiphile hydrogels. Langmuir. 2015;31(22):6122–6129. doi: 10.1021/acs.langmuir.5b00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hogrebe NJ, Gooch KJ. Direct influence of culture dimensionality on human mesenchymal stem cell differentiation at various matrix stiffnesses using a fibrous self-assembling peptide hydrogel. J Biomed Mater Res A. 2016;104(9):2356–2368. doi: 10.1002/jbm.a.35755. [DOI] [PubMed] [Google Scholar]
- 28.Tsukamoto J, Naruse K, Nagai Y, et al. Efficacy of a self-assembling peptide hydrogel, SPG-178-Gel, for bone regeneration and three-dimensional osteogenic induction of dental pulp stem cells. Tissue Eng Part A. 2017 doi: 10.1089/ten.TEA.2017.0025. [DOI] [PubMed] [Google Scholar]
- 29.Chen S, Zhou A, He B, Zhao W, Chen X, Jiang D. Designer D-form self-assembling peptide scaffolds promote the proliferation and migration of rat bone marrow-derived mesenchymal stem cells. Int J Mol Med. 2017;40(3):679–688. doi: 10.3892/ijmm.2017.3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fung SY, Yang H, Chen P. Formation of colloidal suspension of hydrophobic compounds with an amphiphilic self-assembling peptide. Colloids Surf B Biointerfaces. 2007;55(2):200–211. doi: 10.1016/j.colsurfb.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Wang M, Adikane HV, Duhamel J, Chen P. Protection of oligodeoxynucleotides against nuclease degradation through association with self-assembling peptides. Biomaterials. 2008;29(8):1099–1108. doi: 10.1016/j.biomaterials.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 32.Liu J, Zhang L, Yang Z, Zhao X. Controlled release of paclitaxel from a self-assembling peptide hydrogel formed in situ and antitumor study in vitro. Int J Nanomedicine. 2011;6:2143–2153. doi: 10.2147/IJN.S24038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu M, Ye Z, Liu Y, Liu B, Zhao X. Release of hydrophobic anticancer drug from a newly designed self-assembling peptide. Mol Biosyst. 2011;7(6):2040–2047. doi: 10.1039/c0mb00271b. [DOI] [PubMed] [Google Scholar]
- 34.Tang F, Zhao X. Interaction between a self-assembling peptide and hydrophobic compounds. J Biomater Sci Polym Ed. 2010;21(5):677–690. doi: 10.1163/156856209X434683. [DOI] [PubMed] [Google Scholar]
- 35.Li F, Wang J, Tang F, et al. Fluorescence studies on a designed self-assembling peptide of RAD16-II as a potential carrier for hydrophobic drug. J Nanosci Nanotechnol. 2009;9(2):1611–1614. doi: 10.1166/jnn.2009.c214. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Tang F, Li F, et al. The amphiphilic self-assembling peptide EAK16-I as a potential hydrophobic drug carrier. J Nanomater. 2008 [Google Scholar]
- 37.Fung SY, Yang H, Chen P. Sequence effect of self-assembling peptides on the complexation and in vitro delivery of the hydrophobic anticancer drug ellipticine. PLoS One. 2008;3(4):e1956. doi: 10.1371/journal.pone.0001956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang M, Law M, Duhamel J, Chen P. Interaction of a self-assembling peptide with oligonucleotides: complexation and aggregation. Biophys J. 2007;93(7):2477–2490. doi: 10.1529/biophysj.106.102624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bansal R, Kumar P. Engineered polymeric amphiphiles self-assembling into nanostructures and acting as efficient gene and drug carriers. J Biomater Appl. 2017;32(1):40–53. doi: 10.1177/0885328217710125. [DOI] [PubMed] [Google Scholar]
- 40.Zhang H, Xin X, Sun J, et al. Self-assembled chiral helical nanofibers by amphiphilic dipeptide derived from d- or l-threonine and application as a template for the synthesis of Au and Ag nanoparticles. J Colloid Interface Sci. 2016;484:97–106. doi: 10.1016/j.jcis.2016.08.052. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Guan Z, Lin J, Cai C. Strip-pattern-spheres self-assembled from polypeptide-based polymer mixtures: structure and defect features. Sci Rep. 2016;6(1):29796. doi: 10.1038/srep29796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li B, Han W, Jiang B, Lin Z. Crafting threads of diblock copolymer micelles via flow-enabled self-assembly. ACS Nano. 2014;8(3):2936–2942. doi: 10.1021/nn500193y. [DOI] [PubMed] [Google Scholar]
- 43.von Maltzahn G, Vauthey S, Santoso S, Zhang S. Positively charged surfactant-like peptides self-assemble into nanostructures. Langmuir. 2003;19(10):4332–4337. [Google Scholar]
- 44.Vauthey S, Santoso S, Gong H, Watson N, Zhang S. Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc Natl Acad Sci U S A. 2002;99(8):5355–5360. doi: 10.1073/pnas.072089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santoso S, Hwang W, Hartman H, Zhang S. Self-assembly of surfactant-like peptides with variable glycine tails to form nanotubes and nanovesicles. Nano Lett. 2002;2(7):687–691. [Google Scholar]
- 46.Capes JS, Kiley PJ, Windle AH. Investigating the effect of pH on the aggregation of two surfactant-like octapeptides. Langmuir. 2010;26(8):5637–5644. doi: 10.1021/la904528p. [DOI] [PubMed] [Google Scholar]
- 47.Cui H, Cheetham AG, Pashuck ET, Stupp SI. Amino acid sequence in constitutionally isomeric tetrapeptide amphiphiles dictates architecture of one-dimensional nanostructures. J Am Chem Soc. 2014;136(35):12461–12468. doi: 10.1021/ja507051w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Webber MJ, Berns EJ, Stupp SI. Supramolecular nanofibers of peptide amphiphiles for medicine. Isr J Chem. 2013;53(8):530–554. doi: 10.1002/ijch.201300046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harrington DA, Cheng EY, Guler MO, et al. Branched peptide-amphiphiles as self-assembling coatings for tissue engineering scaffolds. J Biomed Mater Res A. 2006;78A(1):157–167. doi: 10.1002/jbm.a.30718. [DOI] [PubMed] [Google Scholar]
- 50.Guler MO, Hsu L, Soukasene S, Harrington DA, Hulvat JF, Stupp SI. Presentation of RGDS epitopes on self-assembled nanofibers of branched peptide amphiphiles. Biomacromolecules. 2006;7(6):1855–1863. doi: 10.1021/bm060161g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guler MO, Soukasene S, Hulvat JF, Stupp SI. Presentation and recognition of biotin on nanofibers formed by branched peptide amphiphiles. Nano Lett. 2005;5(2):249–252. doi: 10.1021/nl048238z. [DOI] [PubMed] [Google Scholar]
- 52.Chen J-X, Wang H-Y, Li C, Han K, Zhang X-Z, Zhuo R-X. Construction of surfactant-like tetra-tail amphiphilic peptide with RGD ligand for encapsulation of porphyrin for photodynamic therapy. Biomaterials. 2011;32(6):1678–1684. doi: 10.1016/j.biomaterials.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 53.Hwang JJ, Iyer SN, Li L-S, Claussen R, Harrington DA, Stupp SI. Self-assembling biomaterials: liquid crystal phases of cholesteryl oligo(l-lactic acid) and their interactions with cells. Proc Natl Acad Sci U S A. 2002;99(15):9662–9667. doi: 10.1073/pnas.152667399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stendahl JC, Li L, Claussen RC, Stupp SI. Modification of fibrous poly(l-lactic acid) scaffolds with self-assembling triblock molecules. Biomaterials. 2004;25(27):5847–5856. doi: 10.1016/j.biomaterials.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 55.Sun X-L, Biswas N, Kai T, Dai Z, Dluhy RA, Chaikof EL. Membrane-mimetic films of asymmetric phosphatidylcholine lipid bolaamphiphiles. Langmuir. 2006;22(3):1201–1208. doi: 10.1021/la052125t. [DOI] [PubMed] [Google Scholar]
- 56.Zhan C, Gao P, Liu M. Self-assembled helical spherical-nanotubes from an l-glutamic acid based bolaamphiphilic low molecular mass organogelator. Chem Commun. 2005;4(4):462–464. doi: 10.1039/b413259a. [DOI] [PubMed] [Google Scholar]
- 57.Song J, Cheng Q, Stevens RC. Morphological manipulation of bolaamphiphilic polydiacetylene assemblies by controlled lipid doping. Chem Phys Lipids. 2002;114(2):203–214. doi: 10.1016/s0009-3084(02)00007-5. [DOI] [PubMed] [Google Scholar]
- 58.Gao P, Zhan C, Liu M. Controlled synthesis of double- and multiwall silver nanotubes with template organogel from a bolaamphiphile. Langmuir. 2006;22(2):775–779. doi: 10.1021/la0517787. [DOI] [PubMed] [Google Scholar]
- 59.Cameron LM, Fyles TM, Hu C-Wei, Cw H. Synthesis and membrane activity of a bis(metacyclophane)bolaamphiphile. J Org Chem. 2002;67(5):1548–1553. doi: 10.1021/jo0160930. [DOI] [PubMed] [Google Scholar]
- 60.Gaucheron J, Santaella C, Vierling P. In vitro gene transfer with a novel galactosylated spermine bolaamphiphile. Bioconjug Chem. 2001;12(4):569–575. doi: 10.1021/bc000165x. [DOI] [PubMed] [Google Scholar]
- 61.Weissig V, Torchilin V. Mitochondriotropic cationic vesicles: a strategy towards mitochondrial gene therapy. Curr Pharm Biotechnol. 2000;1(4):325–346. doi: 10.2174/1389201003378870. [DOI] [PubMed] [Google Scholar]
- 62.Qiu F, Chen Y, Tang C, et al. De novo design of a bolaamphiphilic peptide with only natural amino acids. Macromol Biosci. 2008;8(11):1053–1059. doi: 10.1002/mabi.200800180. [DOI] [PubMed] [Google Scholar]
- 63.Claussen RC, Rabatic BM, Stupp SI. Aqueous self-assembly of unsymmetric peptide bolaamphiphiles into nanofibers with hydrophilic cores and surfaces. J Am Chem Soc. 2003;125(42):12680–12681. doi: 10.1021/ja035882r. [DOI] [PubMed] [Google Scholar]
- 64.Gao J, Xie C, Zhang M, et al. RGD-modified lipid disks as drug carriers for tumor targeted drug delivery. Nanoscale. 2016;8(13):7209–7216. doi: 10.1039/c5nr05577f. [DOI] [PubMed] [Google Scholar]
- 65.Sallach RE, Cui W, Balderrama F, et al. Long-term biostability of self-assembling protein polymers in the absence of covalent crosslinking. Biomaterials. 2010;31(4):779–791. doi: 10.1016/j.biomaterials.2009.09.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pellach M, Mondal S, Harlos K, et al. A two-tailed phosphopeptide crystallizes to form a lamellar structure. Angew Chem Int Ed Engl. 2017;56(12):3252–3255. doi: 10.1002/anie.201609877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lin Y-A, Kang M, Chen W-C, et al. Isomeric control of the mechanical properties of supramolecular filament hydrogels. Biomater Sci. 2018;6(1):216–224. doi: 10.1039/c7bm00722a. [DOI] [PubMed] [Google Scholar]
- 68.Rad-Malekshahi M, Visscher KM, Rodrigues JPGLM, et al. The supramolecular organization of a peptide-based nanocarrier at high molecular detail. J Am Chem Soc. 2015;137(24):7775–7784. doi: 10.1021/jacs.5b02919. [DOI] [PubMed] [Google Scholar]
- 69.Bucak S, Cenker C, Nasir I, Olsson U, Zackrisson M. Peptide nanotube nematic phase. Langmuir. 2009;25(8):4262–4265. doi: 10.1021/la804175h. [DOI] [PubMed] [Google Scholar]
- 70.Castelletto V, Nutt DR, Hamley IW, Bucak S, Cenker Ç, Olsson U. Structure of single-wall peptide nanotubes: in situ flow aligning X-ray diffraction. Chem Commun. 2010;46(34):6270–6272. doi: 10.1039/c0cc00212g. [DOI] [PubMed] [Google Scholar]
- 71.Qiu F, Chen Y, Zhao X. Comparative studies on the self-assembling behaviors of cationic and catanionic surfactant-like peptides. J Colloid Interface Sci. 2009;336(2):477–484. doi: 10.1016/j.jcis.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 72.Pan F, Zhao X, Perumal S, Waigh TA, Lu JR, Webster JRP. Interfacial dynamic adsorption and structure of molecular layers of peptide surfactants. Langmuir. 2010;26(8):5690–5696. doi: 10.1021/la9037952. [DOI] [PubMed] [Google Scholar]
- 73.Xu H, Wang J, Han S, et al. Hydrophobic-region-induced transitions in self-assembled peptide nanostructures. Langmuir. 2009;25(7):4115–4123. doi: 10.1021/la802499n. [DOI] [PubMed] [Google Scholar]
- 74.Wang J, Song A, Jia X, Hao J, Liu W, Hoffmann H. Two routes to vesicle formation: metal−ligand complexation and ionic interactions. J Phys Chem B. 2005;109(22):11126–11134. doi: 10.1021/jp044518r. [DOI] [PubMed] [Google Scholar]
- 75.Wy Y, Yang YM, Chang CH. Cosolvent effects on the spontaneous formation of vesicles from 1:1 anionic and cationic surfactant mixtures. Langmuir. 2005;21(14):6185–6193. doi: 10.1021/la046984d. [DOI] [PubMed] [Google Scholar]
- 76.Silva BFB, Marques EF. Thermotropic behavior of asymmetric chain length catanionic surfactants: the influence of the polar head group. J Colloid Interface Sci. 2005;290(1):267–274. doi: 10.1016/j.jcis.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 77.Khoe U, Yang Y, Zhang S. Synergistic effect and hierarchical nanostructure formation in mixing two designer lipid-like peptide surfactants Ac-A6 D-OH and Ac-A6 K-NH2. Macromol Biosci. 2008;8(11):1060–1067. doi: 10.1002/mabi.200800182. [DOI] [PubMed] [Google Scholar]
- 78.Niece KL, Hartgerink JD, Donners JJJM, Stupp SI. Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. J Am Chem Soc. 2003;125(24):7146–7147. doi: 10.1021/ja028215r. [DOI] [PubMed] [Google Scholar]
- 79.Taboada P, Attwood D, Mosquera V. Influence of the structure of the hydrophobe on the ideality of mixing in micelles in binary mixtures of amphiphilic tricyclic drugs. J Colloid Interface Sci. 2002;248(1):158–162. doi: 10.1006/jcis.2001.8186. [DOI] [PubMed] [Google Scholar]
- 80.Wieslander A, Christiansson A, Rilfors L, Lindblom G. Lipid bilayer stability in membranes. Regulation of lipid composition in Acholeplasma laidlawii as governed by molecular shape. Biochemistry. 1980;19(16):3650–3655. doi: 10.1021/bi00557a002. [DOI] [PubMed] [Google Scholar]
- 81.Hinz HJ, Kuttenreich H, Meyer R, et al. Stereochemistry and size of sugar head groups determine structure and phase behavior of glycolipid membranes: densitometric, calorimetric, and X-ray studies. Biochemistry. 1991;30(21):5125–5138. doi: 10.1021/bi00235a003. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, Qiu F, Lu Y, Shi Y-K, Zhao X. Geometrical shape of hydrophobic section determines the self-assembling structures of peptide detergents and bolaamphiphilic peptides. Curr Nanosci. 2009;5(1):69–74. [Google Scholar]
- 83.Chen Y, Qiu F, Zhao X. The self-assembling structure and mechanism of a wedge-shaped peptide detergent A3V3D. Chem J Chinese Univ. 2009;30:1337–1341. [Google Scholar]
- 84.Khoe U, Yang Y, Zhang S. Self-assembly of nanodonut structure from a cone-shaped designer lipid-like peptide surfactant. Langmuir. 2009;25(7):4111–4114. doi: 10.1021/la8025232. [DOI] [PubMed] [Google Scholar]
- 85.Qiu F, Tang C, Chen Y. Amyloid-like aggregation of designer bolaamphiphilic peptides: effect of hydrophobic section and hydrophilic heads. J Pept Sci. 2018;24(2):e3062. doi: 10.1002/psc.3062. [DOI] [PubMed] [Google Scholar]
- 86.Chen Y, Tang C, Xing Z, Zhang J, Qiu F. Ethanol induced the formation of β-sheet and amyloid-like fibrils by surfactant-like peptide A6K. J Pept Sci. 2013;19(11):708–716. doi: 10.1002/psc.2553. [DOI] [PubMed] [Google Scholar]
- 87.Gurevich L, Poulsen TW, Andersen OZ, Kildeby NL, Fojan P. pH-dependent self-assembly of the short surfactant-like peptide KA6. J Nanosci Nanotechnol. 2010;10(12):7946–7950. doi: 10.1166/jnn.2010.2667. [DOI] [PubMed] [Google Scholar]
- 88.Ls L, Stupp SI. One-dimensional assembly of lipophilic inorganic nanoparticles templated by peptide-based nanofibers with binding functionalities. Angew Chem Int Ed Engl. 2005;44(12):1833–1836. doi: 10.1002/anie.200462142. [DOI] [PubMed] [Google Scholar]
- 89.Adams DJ, Holtzmann K, Schneider C, Butler MF. Self-assembly of surfactant-like peptides. Langmuir. 2007;23(25):12729–12736. doi: 10.1021/la7011183. [DOI] [PubMed] [Google Scholar]
- 90.Das R, Kiley PJ, Segal M, et al. Integration of photosynthetic protein molecular complexes in solid-state electronic devices. Nano Lett. 2004;4(6):1079–1083. [Google Scholar]
- 91.Yeh JI, Du S, Tortajada A, Paulo J, Zhang S. Peptergents: peptide detergents that improve stability and functionality of a membrane protein, glycerol-3-phosphate dehydrogenase. Biochemistry. 2005;44(51):16912–16919. doi: 10.1021/bi051357o. [DOI] [PubMed] [Google Scholar]
- 92.Kiley P, Zhao X, Vaughn M, Baldo MA, Bruce BD, Zhang S. Self-assembling peptide detergents stabilize isolated photosystem ion a dry surface for an extended time. PLoS Biol. 2005;3(7):e230. doi: 10.1371/journal.pbio.0030230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ge B, Yang F, Yu D, Liu S, Xu H. Designer amphiphilic short peptides enhance thermal stability of isolated photosystem-I. PLoS One. 2010;5(4):e10233. doi: 10.1371/journal.pone.0010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Matsumoto K, Vaughn M, Bruce BD, Koutsopoulos S, Zhang S. Designer peptide surfactants stabilize functional photosystem-I membrane complex in aqueous solution for extended time. J Phys Chem B. 2009;113(1):75–83. doi: 10.1021/jp8021425. [DOI] [PubMed] [Google Scholar]
- 95.Zhao X, Nagai Y, Reeves PJ, Kiley P, Khorana HG, Zhang S. Designer short peptide surfactants stabilize G protein-coupled receptor bovine rhodopsin. Proc Natl Acad Sci U S A. 2006;103(47):17707–17712. doi: 10.1073/pnas.0607167103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Corin K, Baaske P, Ravel DB, et al. Designer lipid-like peptides: a class of detergents for studying functional olfactory receptors using commercial cell-free systems. PLoS One. 2011;6(11):e25067. doi: 10.1371/journal.pone.0025067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heerklotz H, Seelig J. Detergent-like action of the antibiotic peptide surfactin on lipid membranes. Biophys J. 2001;81(3):1547–1554. doi: 10.1016/S0006-3495(01)75808-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Legrand B, Laurencin M, Sarkis J, et al. Structure and mechanism of action of a de novo antimicrobial detergent-like peptide. Biochim Biophys Acta. 2011;1808(1):106–116. doi: 10.1016/j.bbamem.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 99.Chen C, Pan F, Zhang S, et al. Antibacterial activities of short designer peptides: a link between propensity for nanostructuring and capacity for membrane destabilization. Biomacromolecules. 2010;11(2):402–411. doi: 10.1021/bm901130u. [DOI] [PubMed] [Google Scholar]
- 100.Webber MJ, Tongers J, Renault M-A, Roncalli JG, Losordo DW, Stupp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 2010;6(1):3–11. doi: 10.1016/j.actbio.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Webber MJ, Kessler JA, Stupp SI. Emerging peptide nanomedicine to regenerate tissues and organs. J Intern Med. 2010;267(1):71–88. doi: 10.1111/j.1365-2796.2009.02184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Standley SM, Toft DJ, Cheng H, et al. Induction of cancer cell death by self-assembling nanostructures incorporating a cytotoxic peptide. Cancer Res. 2010;70(8):3020–3026. doi: 10.1158/0008-5472.CAN-09-3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rajangam K, Arnold MS, Rocco MA, Stupp SI. Peptide amphiphile nanostructure–heparin interactions and their relationship to bioactivity. Biomaterials. 2008;29(23):3298–3305. doi: 10.1016/j.biomaterials.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tovar JD, Rabatic BM, Stupp SI. Conducting polymers confined within bioactive peptide amphiphile nanostructures. Small. 2007;3(12):2024–2028. doi: 10.1002/smll.200600645. [DOI] [PubMed] [Google Scholar]
- 105.Beniash E, Hartgerink JD, Storrie H, Stendahl JC, Stupp SI. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomater. 2005;1(4):387–397. doi: 10.1016/j.actbio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 106.Silva GA, Czeisler C, Niece KL, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303(5662):1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 107.Tysseling VM, Sahni V, Pashuck ET, et al. Self-assembling peptide amphiphile promotes plasticity of serotonergic fibers following spinal cord injury. J Neurosci Res. 2010;88(14):3161–3170. doi: 10.1002/jnr.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Angeloni NL, Bond CW, Tang Y, et al. Regeneration of the cavernous nerve by Sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials. 2011;32(4):1091–1101. doi: 10.1016/j.biomaterials.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tysseling-Mattiace VM, Sahni V, Niece KL, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28(14):3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chow LW, Bitton R, Webber MJ, et al. A bioactive self-assembled membrane to promote angiogenesis. Biomaterials. 2011;32(6):1574–1582. doi: 10.1016/j.biomaterials.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rajangam K, Behanna HA, Hui MJ, et al. Heparin binding nanostructures to promote growth of blood vessels. Nano Lett. 2006;6(9):2086–2090. doi: 10.1021/nl0613555. [DOI] [PubMed] [Google Scholar]
- 112.Shah RN, Shah NA, del Rosario Lim MM, Hsieh C, Nuber G, Stupp SI. Supramolecular design of self-assembling nanofibers for cartilage regeneration. Proc Natl Acad Sci U S A. 2010;107(8):3293–3298. doi: 10.1073/pnas.0906501107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mata A, Geng Y, Henrikson KJ, et al. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials. 2010;31(23):6004–6012. doi: 10.1016/j.biomaterials.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chow LW, Wang LJ, Kaufman DB, Stupp SI. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials. 2010;31(24):6154–6161. doi: 10.1016/j.biomaterials.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kobayashi H, Shimizu T, Yamato M, et al. Fibroblast sheets co-cultured with endothelial progenitor cells improve cardiac function of infarcted hearts. J Artif Organs. 2008;11(3):141–147. doi: 10.1007/s10047-008-0421-8. [DOI] [PubMed] [Google Scholar]
- 116.Miyahara Y, Nagaya N, Kataoka M, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12(4):459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 117.Sekiya S, Shimizu T, Yamato M, Kikuchi A, Okano T. Bioengineered cardiac cell sheet grafts have intrinsic angiogenic potential. Biochem Biophys Res Commun. 2006;341(2):573–582. doi: 10.1016/j.bbrc.2005.12.217. [DOI] [PubMed] [Google Scholar]
- 118.Ohashi K, Yokoyama T, Yamato M, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13(7):880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 119.Ito A, Jitsunobu H, Kawabe Y, Kamihira M. Construction of heterotypic cell sheets by magnetic force-based 3-D coculture of HepG2 and NIH3T3 cells. J Biosci Bioeng. 2007;104(5):371–378. doi: 10.1263/jbb.104.371. [DOI] [PubMed] [Google Scholar]
- 120.Yang J, Yamato M, Nishida K, et al. Cell delivery in regenerative medicine: the cell sheet engineering approach. J Control Release. 2006;116(2):193–203. doi: 10.1016/j.jconrel.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 121.Qiu F, Chen Y, Cheng J, Wang C, Xu H, Zhao X. A simple method for cell sheet fabrication using mica surfaces grafted with peptide detergent A(6)K. Macromol Biosci. 2010;10(8):881–886. doi: 10.1002/mabi.200900360. [DOI] [PubMed] [Google Scholar]
- 122.Chen Y, Tang C, Zhang J, Gong M, Su B, Qiu F. Self-assembling surfactant-like peptide A6K as potential delivery system for hydrophobic drug. Int J Nanomed. 2015;10:847–858. doi: 10.2147/IJN.S71696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Matson JB, Stupp SI. Drug release from hydrazone-containing peptide amphiphiles. Chem Commun. 2011;47(28):7962–7964. doi: 10.1039/c1cc12570b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seow WY, Yang Y-Y. A class of cationic triblock amphiphilic oligopeptides as efficient gene-delivery vectors. Adv Mater. 2009;21(1):86–90. [Google Scholar]
- 125.Wiradharma N, Khan M, Tong YW, Wang S, Yang Y-Y. Self-assembled cationic peptide nanoparticles capable of inducing efficient gene expression in vitro. Adv Funct Mater. 2008;18(6):943–951. [Google Scholar]
- 126.Guo XD, Tandiono F, Wiradharma N, et al. Cationic micelles self-assembled from cholesterol-conjugated oligopeptides as an efficient gene delivery vector. Biomaterials. 2008;29(36):4838–4846. doi: 10.1016/j.biomaterials.2008.07.053. [DOI] [PubMed] [Google Scholar]
- 127.Wiradharma N, Tong YW, Yang YY. Self-assembled oligopeptide nanostructures for co-delivery of drug and gene with synergistic therapeutic effect. Biomaterials. 2009;30(17):3100–3109. doi: 10.1016/j.biomaterials.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 128.Qiu F, Chen Y, Tang C, Cheng J, Zhao X. Formation of reversed micelle nanoring by a designed surfactant-like peptide. Nano. 2012;7 [Google Scholar]
- 129.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294(5547):1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 130.Xing Z, Chen Y, Tang C, Gong X, Qiu F. Fabrication of peptide self-assembled monolayer on mica surface and its application in atomic force microscopy nanolithogrsaphy. Curr Nanosci. 2014;10(2):297–301. [Google Scholar]