SYNOPSIS
GATA2 deficiency is an immunodeficiency and bone marrow failure disorder caused by pathogenic variants in GATA2 which lead to haploinsufficiency. It is inherited in an autosomal dominant pattern or can be due to de novo sporadic germline mutation. Patients commonly have B-, DC-, NK- and mono- cytopenias, and are predisposed to the development of MDS, AML, and CMML. Patients may also suffer from disseminated HPV and mycobacterial infections, pulmonary alveolar proteinosis and lymphedema. Bone marrow from GATA2 deficient patients eventually takes on a characteristic hypocellular myelodysplasia with loss of monocytes and hematogones, megakaryocytes with separated nuclear lobes, micromegakaryocytes and megakaryocytes with hypolobated nuclei.
Keywords: GATA2, monocytopenia, micromegakaryocytes, MDS
Introduction
GATA2 encodes a zinc finger transcription factor necessary for normal hematopoiesis located on chromosome 3q21.2. The protein contains two zinc fingers and a nuclear localization signal. GATA2 binds to the consensus sequence W/GATA/R (W = A or T and R = A or G) in promoter/enhancer regions of target genes including SPI1 (PU.1), LMO2, TAL1, FLI1 and RUNX1 to regulate endothelial to hematopoietic transition in the early embryo, the formation of hematopoietic stem cells (HSCs) and definitive hematopoiesis1. In the adult, GATA2 is critical for maintenance of the stem cell pool through HSC survival and self-renewal. GATA2 is also important for production of megakaryocytes, mast cells, NK cells and monocytes2.
In mice Gata2−/− leads to embryonic lethality at embryonic day 9.5 due to lack of definitive hematopoiesis, while Gata2+/− mice have decreased progenitor cell numbers and reduced transplant repopulation3,4. The level of functional GATA2 protein is critical for HSC survival and normal hematopoiesis, as shown by conditional Gata2 mouse models5.
Pathogenic germline variants in GATA2 affect exons and critical regulatory intronic regions of the gene, as well as deletions. Several groups described constellations of symptoms, each giving them different names: MonoMAC6,7; familial MDS/AML (myelodysplastic syndrome/acute myeloid leukemia)8; DCML deficiency (dendritic cell, monocyte, B, and natural killer (NK) lymphoid deficiency)9; and Emberger syndrome (MDS with lymphedema)10. In 2011, it was recognized that underlying all of these autosomal dominant disorders were heterozygous germline mutations in GATA2, hence this is best referred to as GATA2 deficiency.
Germline pathogenic variants include deletions, missense, nonsense, frameshift and splice site changes and alterations of intronic regulatory elements. Mechanistically, all variants appear to lead to haploinsufficiency11. Most pathogenic variants cluster in the two zinc fingers, leading to a non-functional protein unable to bind DNA or other transcription factor partners12,13. Whole or partial gene deletions produce haploinsufficiency by hemizygosity14. Mutations in the intron 5 enhancer lead to reduced transcription of the cis allele15. Frameshift mutations lead to nonsense mediated decay, premature stop codons or splice site alterations2.
Since gene identification in 2011, the number of cases identified has grown steadily, with associated phenotypic expansion. We will briefly review the clinical manifestations with a focus on the bone marrow failure aspects, discuss management and bone marrow transplantation.
Clinical Manifestations
Hematologic
Patients molecularly diagnosed with germline GATA2 mutations at birth, due to an affected family member, are immunologically and hematologically normal. Over time the majority will evolve cytopenias, including deficiencies in monocytes, B-lymphocytes, dendritic cells (DCs) and natural killer (NK) cells. However, there is significant variability in this presentation (Table 1). Profound monocytopenia is one of the most consistent features, but only later in the development of disease. Unlike other marrow failure syndromes, anemia and thrombocytopenia are uncommon early presentations, except in those who present with aplastic anemia (AA), MDS or AML. Lymphocyte subset evaluation may identify B and NK cytopenias. Pediatric MDS with germline GATA2 mutation can present without recognized immunodeficiency16,17.
Table 1.
Diagnostic Clue
| Medical History/Physical Exam | PeriDherai Biood |
|---|---|
| HPV- persistent warts | Monocytopenia |
| NTM- disseminated | Dendritic Cell cytopenia |
| Aspergillosis | NK Cell cytopenia |
| Lymphedema | B Cell cytopenia |
| Hearing Loss | Neutropenia |
| Panniculitis | |
| Erythema nodosum | Bone Marrow Morphology |
| Thrombosis | Loss of hematogones |
| Miscarriage/Preterm labor | Micromegakaryocytes |
| EBV Viremia | Megakaryocytes with separated nuclear lobes |
| Inverted CD4:CD8 ratio | |
| Radiological | |
| Crazy paving on chest CT | |
| Subpleural Blebs | |
| Paraseptal Emphysema | |
| Ground Glass Opacities | |
| Splenomegaly |
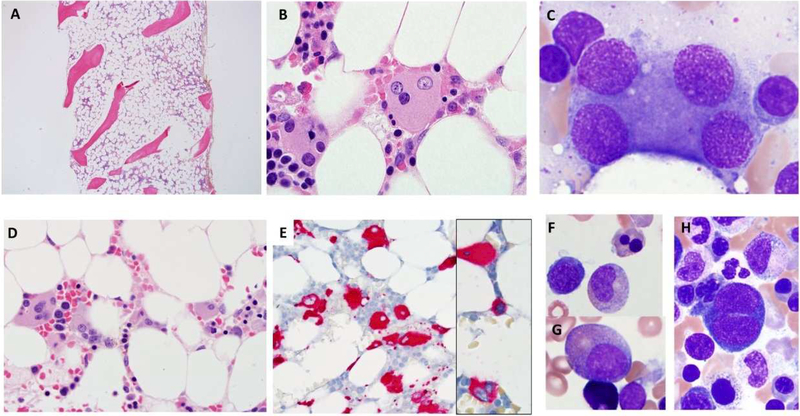
Bone marrow is typically hypocellular (Fig 1A) with characteristic features including atypical megakaryocytes, ranging from large abnormal forms with separated nuclear lobes (osteoclast-like), to smaller forms with separated nuclear lobes, micromegakaryocytes, to small hypolobated or mononuclear megakaryocytes18 (Fig 1B-E). In some cases, the marrow is very hypocellular with very few megakaryocytes. Immunohistochemistry (IHC) for CD61 performed on core biopsies may help identify megakaryocytic atypia (Fig 1E); IHC for CD34 can help identify increased blasts (Fig 1L). Patients with disseminated nontuberculous mycobacterial infections (NTM) may have granulomata in the marrow with mycobacteria. Review of aspirate smears is critical for assessing progression to MDS, which can be subtle or hindered by paucicellular specimens. GATA2 deficiency-associated hypocellular MDS may display multilineage dysplastic changes (Fig 1F-H) involving erythroid precursors (nuclear budding, binucleation, megaloblastic changes), myeloid precursors (nuclear hyposegmentation, hypogranularity, nuclear to cytoplasmic maturation asynchrony) and markedly dysplastic megakaryocytes.
Figure 1. The range of bone marrow pathologies seen in GATA2 deficiency. (A-C) Bone marrow and immunodeficiency disorder with germline GATA2 mutation.
(A) Hypocellular marrow for age (late adolescent patient with history of warts and infections) with characteristic atypical megakaryocytes with separated nuclear lobes present on (B) core biopsy and (C) aspirate. No overt morphologic dysplastic changes are present in myeloid and erythroid precursors. Cytogenetic analysis showed a normal karyotype. CBC with differential and peripheral blood flow cytometric analysis revealed severe monocytopenia, B/NK-cell lymphopenia. (D-H) Myelodysplastic syndrome with multilineage dysplasia and germline GATA2 mutation. The bone marrow is hypocellular for age (young adult in 3rd decade) with (D) increased atypical megakaryocytes with separated nuclear lobes, hypolobated and mononuclear forms. (E) Megakaryocytes are highlighted by immunohistochemistry stain for CD61 (E, inset small positive cell in center) revealing micromegakaryocytes. Aspirate smear shows (F) dyserythropoiesis with nuclear budding and (H) binucleation; myeloid maturation asynchrony with (G) nuclear hyposegmentation and (H) hypogranular myeloid cells. Cytogenetic analysis showed monosomy 7. CBC with differential and peripheral blood flow cytometric analysis revealed moderate pancytopenia with severe monocytopenia, and B/NK-cell lymphopenia. (I-J) Aplastic anemia, with unrecognized germline GATA2 mutation at diagnosis. Bone marrow is markedly hypocellular with trilineage hypoplasia. Aspirate is paucicellular with insufficient cells to evaluate for dysplastic changes. Cytogenetic analysis failed due to poor mitotic index. Bone marrow flow cytometry analysis was not performed. The patient later evolved to MDS and a GATA2 mutation was identified. (K-M) Acute myeloid leukemia with myelodysplasia related changes and germline GATA2 mutation. (K) Hypocellular marrow from a man (early 3rd decade) previously diagnosed with aplastic anemia that had evolved to MDS with monosomy 7. Marrow shows background hemosiderin laden macrophages from red cell transfusions. (L) Immunohistochemistry stain for CD34 reveals (M) numerous CD34 positive blasts which are also positive for CD117. (N) Aspirate smear showed increased blasts (35% on differential count) indicative of progression to acute myeloid leukemia. Cytogenetic analysis showed monosomy 7.
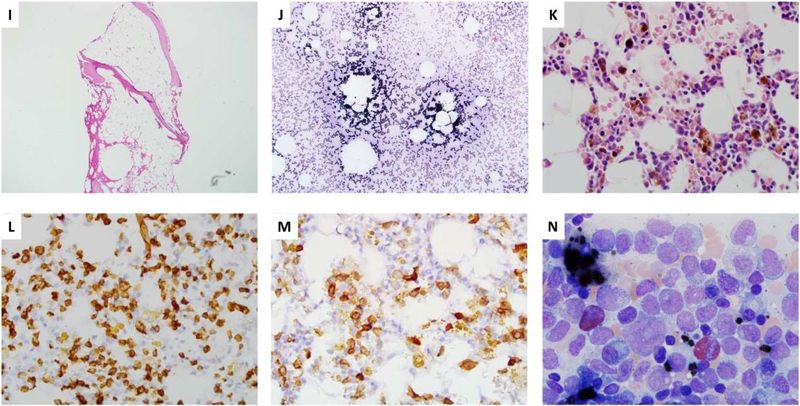
Bone marrow flow cytometry often shows hypogranular granulocytes, absent monocytes and hematogones (B cells progenitors), reduced mature B cells and NK cells and inverted CD4:CD8 ratios with relatively increased CD57+ T-cells (Fig 2). Absent hematogones, inverted CD4:CD8 ratio with mono-, NK and B cell cytopenia differentiate GATA2 deficiency from idiopathic aplastic anemia (AA)19. In pediatric MDS patients lack of B cells and B cell progenitors predicts GATA2 deficiency17. Decreased or absent intron RSS-Kde recombination excision circles are also common in pediatric MDS due to GATA2 deficiency17. Within the bone marrow compartment there is a loss of CD34+, CD38- multi-lymphoid or lymphoid-primed multipotent progenitors and depletion of CD38+ granulocytic monocytic progenitors2. Increased serum FLT3 ligand tracks with worsening of bone marrow failure and clinical disease in GATA2 patients20.
Figure 2. Flow cytometry analysis of bone marrow in idiopathic aplastic anemia (AA) and GATA2 deficiency.
(A) Monocytic cells, designated by positivity for CD14 and HLA-DR (within red box) are typically present in the bone marrow of AA and disproportionately decreased to absent in GATA2 deficiency; all nucleated cells in the marrow are gated. (B) Mature B-cells, marked by positivity for CD19 and CD20 (within red box), are typically present in AA and reduced to absent in GATA2 deficiency; lymphocytes in the marrow are gated. (C) Bcell precursors (hematogones; within red box) are often reduced but detectable in AA, but in GATA2 deficiency there is an absence of B-cell precursors. CD19+ cells in the bone marrow are gated; the cells outside of the red box are mature B-cells which are CD20+ and CD10-. (D) NK cells, positive for CD56 and negative for CD3, are present in AA and are often absent in GATA2 deficiency; the BM lymphocyte gate is displayed.
Since GATA2 deficiency can present as AA (Fig 1I-J), it is critical to differentiate it from idiopathic AA21. One report identified five patients who presented with AA who had GATA2 regulatory region mutations22. Mild chronic neutropenia with monocytopenia can also be a presentation of GATA2 deficiency, ten such patients were found in French severe congenital neutropenia registry23.
Hematopoietic Malignancies
GATA2 deficiency predisposes to leukemia: an original description was familial AML8,24,25. Myeloid neoplasms of any type eventually develop in up to 75% of patients26. The average age of onset for myeloid neoplasms varies, depending on the cohort studied, but overall has been shown to be 12–35 years old with a median of 19.7 years27. However, myeloid neoplasms due to this germline predisposition can develop into late adulthood, as late as the eighth decade has been reported and should be recognized26. The development of myeloid neoplasms is remarkably inconsistent within families. The underlying biology of these differences is not understood28.
MDS is very common in GATA2 deficiency, often accompanied by cytogenetic changes, most commonly monosomy 7 (41% of cases), trisomy 8 (15%) and trisomy 1q7,19,29. Interestingly, del5q has not been reported in GATA2 deficiency, nor have ringed sideroblasts. One study found 7% of primary pediatric MDS and 15% of children with advanced MDS to have GATA2 deficiency. Of those with pediatric MDS and monosomy 7, 37% had germline GATA2 mutations; in adolescents with MDS plus monosomy 7, 72% had underlying germline GATA2 mutations, suggesting that GATA2 deficiency may be the most common genetic defect predisposing to pediatric MDS16. Therefore, it is critical for all pediatric MDS and all AML patients with a somatic GATA2 mutation to be screened for germline mutations16,30.
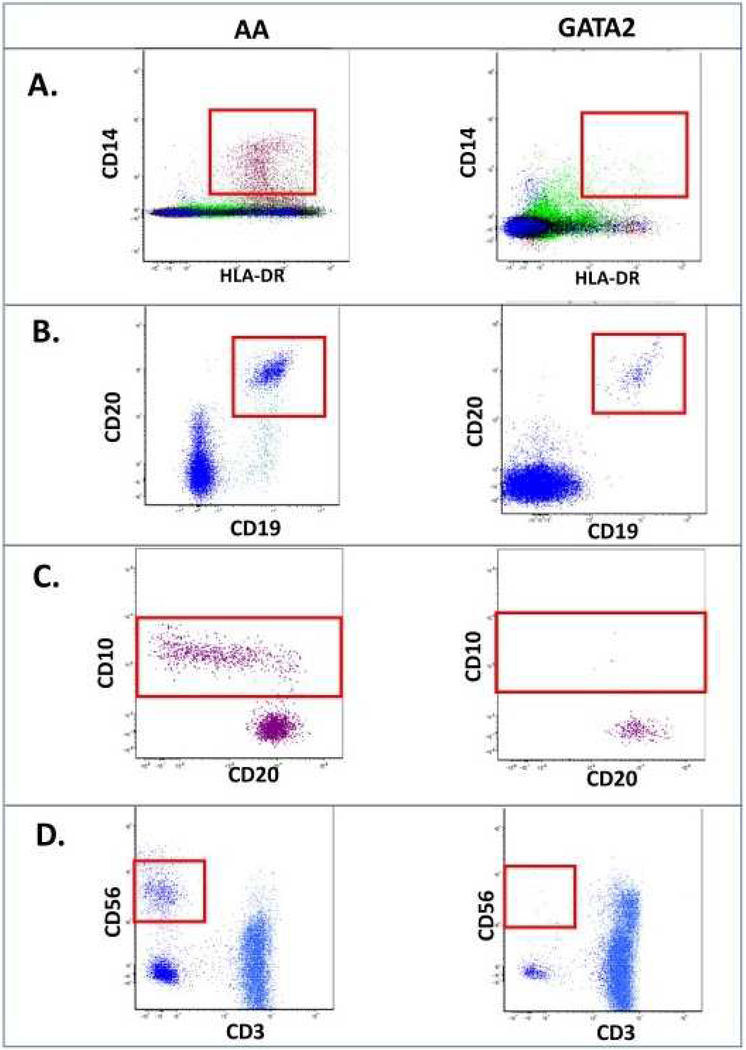
The mechanisms behind the development of MDS in GATA2 deficiency are only partially understood. It is hypothesized that the combination of HSC loss, bone marrow stress and clonal evolution drive the development of dysplasia and transformation to leukemia (Fig 3)27,31,32. Bone marrow stress occurs due the progressive cytopenias and recurrent infections33. Clonal evolution can occur through development of cytogenetic clones and progression to MDS/AML (Fig 1K-N). The most common gene with somatic mutation in hematopoietic clones is ASXL1, found in 29% in one series34. Wang et al. identified ASXL1 mutations in 2/6 patients screened within three families35. Bodor et al. identified a family with a pair of siblings with GATA2 mutation, one with a concurrent ASXL1 mutation and MDS and the other without either24. Acquisition of ASXL1 mutations may cooperate with germline GATA2 mutations to drive the hypoplastic bone marrow to a pro-leukemic hyperplastic state36.
Figure 3. Bone marrow failure and other manifestations of GATA2 deficiency.
GATA2 deficiency syndrome is caused by gene mutations in both the exonic and intronic regulatory regions of the gene leading to haploinsufficiency. Loss of GATA2 protein leads to hematopoietic stem and progenitor (HSPC) cell loss and dysfunction. This depletion leads to a combination of cytopenias- B, DC, NK and monocytes. The resultant immunodeficiency drives infections such as HPV and NTM, and pulmonary alveolar proteinosis. Overall, these factors lead to bone marrow stress, hypocellularity and the ultimate development of bone marrow failure, MDS and AML.
Other somatic mutations have also been reported. In the families described by Wang et al., two members had NRAS mutations, and others had mutations in RUNX1, TP53, STAG2 and SETBP135. Ding et al. described a father-son pair, both of whom had STAG2 mutations, but in different genomic locations37. Fujiwara et al. described a patient who progressed to MDS/AML with somatic mutations in EZH2, HECW1 and GATA138. Loyola and colleagues screened 60 GATA2 deficiency patients with MDS and found recurrent mutations in SETBP1, ASXL1, STAG2, RUNX1, CBL, EZH2, NRAS, JAK3 and PTPN1139. Fisher et al. described two pediatric GATA2 deficiency MDS patients- one with RUNX1, SETBP1 and IKZF1 somatic mutations, and the other with a CRLF2 mutation40.
MDS and AML are the best described myeloid neoplasms in GATA2 deficiency. However, chronic myelo-monocytic leukemia (CMML) in GATA2 deficiency has been associated with monocytosis and often have ASXL1 somatic mutations27,29,34.
A single case of pre-B cell acute lymphoblastic leukemia occurred in an adolescent girl who developed disseminated M kansasii after chemotherapy41. She also had other features of GATA2 deficiency including warts as a young child, megakaryocyte dysplasia, monosomy 7, disseminated fungal disease, PAP, and long standing monocytopenia.
GATA2 plays an important role in germline predisposition to AML, but also in de novo AML when GATA2 is somatically altered. A unique mechanism is involved in the pathogenesis of inv(3) AML, which repositions a GATA2 binding element upstream of EVI1, an oncogene, increasing EVI1 expression and decreasing GATA2 expression, thus creating somatic haploinsufficiency within the leukemic blasts42,43. Epigenetic alterations also lead to decreased GATA2 expression in normal karyotype AML44. GATA2 levels are tightly regulated throughout hematopoiesis, and dysregulation of the level of GATA2 promotes stem cell loss, dysfunction and leukemogenesis.
Immunologic
Much of the immune dysregulation in GATA2 deficiency is attributed to cytopenias and the underlying loss of stem cells45. However, quantitative cytopenia does not fully explain the breadth of immune dysregulation seen. Low numbers and dysfunction of NK cells is a key feature of GATA2 deficiency. GATA2 is required for normal and complete maturation of NK cells, maintenance of the CD56bright population, and full cytotoxicity46. Schlums and Jung found that GATA2 deficiency leads to loss of NK cell progenitors, thus decreasing canonical NK cell differentiation47. Adaptive NK cells may persist in GATA2 deficient patients, even after these patients have lost their canonical NK cells. The remaining NK cells have decreased CXCL12/CXCR4-dependent chemotaxis, while the B cells have increased CXCL12-induced chemotaxis48. Ruiz-Garcia studied four patients and showed that those with worse clinical phenotype had increased T cell senescence49.
GATA2 deficient patients also get panniculitis, erythema nodosum and arthritis. Autoimmune hepatitis in GATA2 deficiency has been reported50. It is important to keep the infection predisposition in mind, as erythema nodosum is more common in patients with active mycobacterial disease. There may also be a T regulatory cell, DC and increased CD38-CD21- B cell deficiency2. These autoimmune complaints may present initially to rheumatologists51. Hypogammaglobulinemia clinically consistent with common variable immunodeficiency also occurs52.
Infectious
NTM infections were found in up to in 53% of patients in one study29, often disseminated and difficult to control, due to slow (Mycobacterium avium complex (MAC), M kansasii, and M szulgai) and rapid growing (M fortuitum, M abscessus, and M chelonae) organisms29,53,54. M kansasii can cause a characteristic necrotizing mediastinal lymphadenitis55. Disseminated NTM disease correlates with cytopenias in adolescents or young adults; it is uncommon in childhood when the cell numbers are intact. Smoking may increase the risk for dissemination of NTM in the setting of already compromised alveolar macrophage function55.
Other severe bacterial infections include Clostridium difficile and group C streptococcal infections29. One case of P jiroveci pneumonia was reported56. Approximately 16% of patients had fungal infections including aspergillosis, disseminated histoplasmosis, and candidiasis29.
Human papilloma virus infections occur in two thirds as recalcitrant warts, condylomata, and/or cervical dysplasia. Persistent warts in a patient with cytopenias or bone marrow failure suggests GATA2 deficiency.
Epstein Barr virus (EBV) viremia was found in 11% of patients in one study29, including a variety of EBV-associated pathologies such as mononucleosis, chronic active EBV, hydroa vacciniforme-like lymphoma with hemophagocytic lymphohistiocytosis and smooth muscle cell tumors57. EBV associated spindle cell tumor and demyelinating sensorimotor polyradiculoneuropathy have been reported58,59. Severe disseminated herpes simplex infections are also important to watch for as they can lead to dire complications60,61. Overall, approximately 80% of patients will have infectious complications of their disease leading to significant morbidity and mortality.
Dermatologic
Warts are seen on extremities and genitalia (common or flat type) in about 50% of patients and are difficult to treat.62,63. Neutrophilic pustulosis (Sweet syndrome) can be seen in patients who go on to develop MDS or AML. Panniculitis, erythema nodosum and cellulitis have also been described64.
Vascular
Kazenwadel et al. showed that GATA2 is expressed in lymphatic vessels and is a critical regulator of development of lymphatic vessel valves65. Patients with gene deletions and stop codons appear to be at especially increased risk of lymphedema. Lymphedema, particularly in an adolescent or young adult with cytopenias, suggests GATA2 deficiency. GATA2 is also expressed in the vascular epithelium and platelets, and thrombosis occurs in a significant number of patients. The risk for thrombosis is likely multifactorial, since GATA2 deficient patients often have other risk factors, including infection and malignancy. Maternal-related preterm labor has been observed, as well as frequent miscarriage.
Pulmonary
Pulmonary alveolar proteinosis (PAP) is a common and life-threatening complication of GATA2 deficiency caused by over-accumulation of surfactant in alveoli impairing gas exchange. It is typically suspected on CT scan and confirmed on PAS staining or electron microscopy of bronchoalveolar lavage specimens66. PAP in GATA2 deficiency is likely due to alveolar macrophage dysfunction, in part due to abnormal GM-CSF-related signaling. Griese et al. identified two GATA2 deficiency patients in a cohort of GM-SCF autoantibody negative PAP patients67. Svobodova et al. identified a GATA2 deficient patient who presented with persistent diffuse parenchymal lung disease in childhood68. Pulmonary hypertension has been seen in GATA2 deficiency, both preceding and following the development of severe PAP69,70. Subpleural and paraseptal emphysematous changes in the lung may be seen long before overt clinical manifestations.
Eye and Ear
Congenital deafness has been observed in GATA2 deficiency, probably more often in those with gene deletions or stop codon mutations. Using conditional mouse models, Haugas et al. showed that GATA2 is required for proliferation of the epithelium in the semicircular duct and for clearance of mesenchymal cells to generate the vestibular perilymphatic space71. Berry and colleagues reported a case of central retinal vein occlusion in GATA2 deficiency, which may have represented underlying hypercoagulability due to cellular or vascular defects72.
Oncologic
Solid Tumors
A variety of solid malignancies have been reported, most related to HPV infection, such as genital dysplasias and invasive squamous cell carcinomas (SCC). HPV associated head and neck SCC have also been seen20,29. EBV-related tumors, such as leiomyosarcomas occur. Crall et al. reported a case of Merkel cell carcinoma in an adult with both GATA2 deficiency and neurofibromatosis type 173. Lastly, in the NIH cohort there is significant frequency of breast cancer in (approximately 20% in women above 35 years old), for unclear reasons29.
Hematopoietic stem/progenitor Cell Transplant
Hematopoietic stem/progenitor cell transplant (HSCT) is highly effective in the treatment of GATA2 deficiency. HSCT restores normal hematopoiesis, resolves MDS, clears long standing underlying infections, and resolves PAP and pulmonary hypertension in patients with GATA2 deficiency74. The optimal timing of transplant is unclear. Our institutional strategy has been to proceed with transplant when cytopenias are present, if life threatening infections have occurred, or if PAP or other significant organ dysfunction develops. However, the disease course is often difficult to predict and patients may be diagnosed only in the later stages of the disease.
The best choices for preparative regimen, donor source and graft versus host disease (GVHD) prophylaxis are areas of current investigation. Cuellar-Rodriguez and colleagues reported successful transplantation for GATA2 deficiency in six patients74. Since that time multiple other groups have reported good outcomes with HSCT for GATA2 deficiency. Both myeloablative and reduced intensity preparative regimens have been successful. Matched related and unrelated peripheral blood stem cells (PBMCs), matched and haploidential bone marrows, and umbilical cord grafts have all been reported.
Ciucilli et al. reported a young patient who received a transplant from his matched brother using an ablative regimen75. Ramazan et al. reported a very young patient with Emberger syndrome and MDS who underwent successful myeloablative HSCT with resolution of MDS76. Lubking et al. described an adult with MDS and ASXL1 somatic mutation who underwent successful HSCT from an unrelated PBMC donor with resolution of MDS and condylomata77. Maeurer et al. described a young adult with severe disseminated HPV skin disease treated with matched related HSCT78. Post HSCT her condylomata disappeared and her cervical cancer in situ resolved. These authors suggested pre-harvest donor HPV vaccination if not previously vaccinated. Malhi and colleagues reported two pediatric cases of HSCT using cord blood: the first patient had AML with MDS-related changes and received a myeloablative regimen; the other had MDS and received reduced intensity conditioning79. Saida et al. reported a pediatric patient with successful reduced intensity HSCT80.
Following up Cuellar-Rodriguez et al, Grossman et al. reported eight more patients (total 14), from an open HSCT trial specifically for GATA2 deficiency81. This group included both matched and haploidentical donors. The authors currently use a myeloablative regimen to improve engraftment and reduce the risk of relapse, beyond what was seen in the prior cohort.
GVHD has been seen in GATA2 HSCT at typical or increased rates, but different prophylaxis regimens make it difficult to generalize rates. Shah et al. reported a pair of monozygotic twins who both underwent HSCT for GATA2 deficiency from the same donor with the same conditioning regimen one twin developed severe acute GVHD of the skin and gastrointestinal tract requiring immunosuppression for 1.5 years, the other twin only had grade 1 GVHD of skin not requiring systemic corticosteroids82. This unique report highlights the nonheritable factors affecting GVHD outcomes. It is hypothesized that prior and peri-transplant exposures, especially infectious, may contribute to such differences.
Patient Monitoring and Screening
Currently, there are no guidelines for patient care and monitoring. However, expert opinion in the literature and our practice are given in Box 1. Once a GATA2 mutation has been identified and cytopenias of any degree are noted we start daily azithromycin prophylaxis for NTM. HPV vaccination should be given on the full schedule, as well as other childhood vaccines. We have not seen problems with live virus vaccines in children with normal counts or marrows. BCG vaccination has also not been a problem in GATA2 deficiency as long as counts are normal. Baseline bone marrow aspiration, biopsy and cytogenetics should be performed. We follow at least bi-annual CBC with differential, and yearly lymphocyte subset evaluation, bone marrow biopsy and aspiration, pulmonary function testing, comprehensive skin examinations and for females, gynecologic examinations. Good oral hygiene and regular dental visits are recommended, especially surveillance for HPV-related oral disease. If MDS develops, prompt referral to an HSCT team with disease specific knowledge is advisable. Likewise, if a GATA2 deficiency patient develops aplastic anemia, prompt HSCT is advisable as immunosuppressive therapy appears to increase risk and delays definitive treatment.
Box 1: Screening Recommendations.
Twice yearly CBC with differential
Yearly Lymphocyte Subset Evaluation- monitor monocyte loss or outgrowth
Yearly bone marrow biopsy and aspiration with mycobacterial culture,flow cytometry and cytogenetics; consider somatic mutation panel testing
Yearly Pulmonary Function Testing
Yearly Comprehensive Skin Examination
Yearly Gynecological Exam
Recommendations for testing and frequency listed here are only based on expert opinion and not evidence based at this time.
Overall, HSCT is the only curative option for GATA2 deficiency patients; gene therapy is unlikely to be available any time soon. Screening of potential donor family members for GATA2 mutation, in addition to HLA typing, is absolutely essential, given the wide variation in GATA2 deficiency phenotype and age at presentation. While the timing of transplantation is hard to precisely prescribe, the consequences of waiting until after major infections, organ dysfunction or malignancy develop are dire and baleful.
KEY POINTS.
- GATA2 deficiency has variable clinical presentations including warts, mycobacterial infections, fungal infections, lymphedema, pulmonary alveolar proteinosis, aplastic anemia, MDS or AML.
- Bone marrow histology in symptomatic GATA2 deficiency is typically hypocellular and has characteristic abnormal megakaryocytes with separated nuclear lobes; micromegakaryocytes with hypolobated nuclei; loss of monocytes and hematogones; and an inverted CD4:CD8 ratio. Overt morphologic evidence of dysplasia is variable.
- Historical features of bone marrow failure or MDS that suggest underlying GATA2 deficiency include persistent warts, lymphedema, pulmonary alveolar proteinosis or disseminated and/or unusual infections. However, in childhood MDS and leukemia can be initial presentations.
- Bone marrow transplantation can reverse the infectious, hematopoietic and pulmonary complications seen in GATA2 deficiency. Without bone marrow transplantation, there is a significant risk of transformation to MDS/AML or CMML.
Footnotes
DISCLOSURE STATEMENT
None of the authors have any disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Crispino JD, Horwitz MS. GATA factor mutations in hematologic disease. Blood. 2017;129(15):2103–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collin M, Dickinson R, Bigley V. Haematopoietic and immune defects associated with GATA2 mutation. British journal of haematology. 2015;169(2):173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai FY, Keller G, Kuo FC, et al. An early haematopoietic defect in mice lacking the transcription factor GATA-2. Nature. 1994;371(6494):221–226. [DOI] [PubMed] [Google Scholar]
- 4.Rodrigues NP, Janzen V, Forkert R, et al. Haploinsufficiency of GATA-2 perturbs adult hematopoietic stem-cell homeostasis. Blood. 2005;106(2):477–484. [DOI] [PubMed] [Google Scholar]
- 5.Lim KC, Hosoya T, Brandt W, et al. Conditional Gata2 inactivation results in HSC loss and lymphatic mispatterning. The Journal of clinical investigation. 2012;122(10):3705–3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu AP, Sampaio EP, Khan J, et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood. 2011;118(10):2653–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinh DC, Patel SY, Uzel G, et al. Autosomal dominant and sporadic monocytopenia with susceptibility to mycobacteria, fungi, papillomaviruses, and myelodysplasia. Blood. 2010;115(8):1519–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn CN, Chong CE, Carmichael CL, et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nature genetics. 2011;43(10):1012–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigley V, Haniffa M, Doulatov S, et al. The human syndrome of dendritic cell, monocyte, B and NK lymphoid deficiency. The Journal of experimental medicine. 2011;208(2):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostergaard P, Simpson MA, Connell FC, et al. Mutations in GATA2 cause primary lymphedema associated with a predisposition to acute myeloid leukemia (Emberger syndrome). Nature genetics. 2011;43(10):929–931. [DOI] [PubMed] [Google Scholar]
- 11.Hsu AP, Johnson KD, Falcone EL, et al. GATA2 haploinsufficiency caused by mutations in a conserved intronic element leads to MonoMAC syndrome. Blood. 2013;121(19):3830–3837, s3831–3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong CE, Venugopal P, Stokes PH, et al. Differential effects on gene transcription and hematopoietic differentiation correlate with GATA2 mutant disease phenotypes. Leukemia. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cortes-Lavaud X, Landecho MF, Maicas M, et al. GATA2 germline mutations impair GATA2 transcription, causing haploinsufficiency: functional analysis of the p.Arg396Gln mutation. Journal of immunology (Baltimore, Md : 1950). 2015;194(5):2190–2198. [DOI] [PubMed] [Google Scholar]
- 14.Dorn JM, Patnaik MS, Van Hee M, et al. WILD syndrome is GATA2 deficiency: A novel deletion in the GATA2 gene. The journal of allergy and clinical immunology In practice. 2017;5(4):1149–1152.e1141. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KD, Hsu AP, Ryu MJ, et al. Cis-element mutated in GATA2-dependent immunodeficiency governs hematopoiesis and vascular integrity. The Journal of clinical investigation. 2012;122(10):3692–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wlodarski MW, Hirabayashi S, Pastor V, et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood. 2016;127(11):1387–1397; quiz 1518. [DOI] [PubMed] [Google Scholar]
- 17.Novakova M, Zaliova M, Sukova M, et al. Loss of B cells and their precursors is the most constant feature of GATA-2 deficiency in childhood myelodysplastic syndrome. Haematologica. 2016;101(6):707–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calvo KR, Vinh DC, Maric I, et al. Myelodysplasia in autosomal dominant and sporadic monocytopenia immunodeficiency syndrome: diagnostic features and clinical implications. Haematologica. 2011;96(8):1221–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganapathi KA, Townsley DM, Hsu AP, et al. GATA2 deficiency-associated bone marrow disorder differs from idiopathic aplastic anemia. Blood. 2015;125(1):56–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dickinson RE, Milne P, Jardine L, et al. The evolution of cellular deficiency in GATA2 mutation. Blood. 2014;123(6):863–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Calvo KR, Hickstein DD, Holland SM. MonoMAC and GATA2 deficiency: overlapping clinical and pathological features with aplastic anemia and idiopathic CD4+ lymphocytopenia. Haematologica. 2012;97(4):e12–e13. [Google Scholar]
- 22.Townsley DMHA, Dumitriu B, Holland SM, Young NS. Regulatory Mutations in GATA2 Associated with Aplastic Anemia. Blood. 2014;120(21):3488. [Google Scholar]
- 23.Pasquet M, Bellanne-Chantelot C, Tavitian S, et al. High frequency of GATA2 mutations in patients with mild chronic neutropenia evolving to MonoMac syndrome, myelodysplasia, and acute myeloid leukemia. Blood. 2013;121(5):822–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bodor C, Renneville A, Smith M, et al. Germ-line GATA2 p.THR354MET mutation in familial myelodysplastic syndrome with acquired monosomy 7 and ASXL1 mutation demonstrating rapid onset and poor survival. Haematologica. 2012;97(6):890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hyde RK, Liu PP. GATA2 mutations lead to MDS and AML. Nature genetics. 2011;43(10):926–927. [DOI] [PubMed] [Google Scholar]
- 26.Wlodarski MW, Collin M, Horwitz MS. GATA2 deficiency and related myeloid neoplasms. Semin Hematol. 2017;54(2):81–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirabayashi S, Wlodarski MW, Kozyra E, Niemeyer CM. Heterogeneity of GATA2-related myeloid neoplasms. Int J Hematol. 2017;106(2):175–182. [DOI] [PubMed] [Google Scholar]
- 28.Brambila-Tapia AJL, Garcia-Ortiz JE, Brouillard P, et al. GATA2 null mutation associated with incomplete penetrance in a family with Emberger syndrome. Hematology (Amsterdam, Netherlands). 2017;22(8):467–471. [DOI] [PubMed] [Google Scholar]
- 29.Spinner MA, Sanchez LA, Hsu AP, et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood. 2014;123(6):809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stieglitz E, Loh ML. Pediatric MDS: GATA screen the germline. Blood. 2016;127(11):1377–1378. [DOI] [PubMed] [Google Scholar]
- 31.Hsu AP, McReynolds LJ, Holland SM. GATA2 deficiency. Current opinion in allergy and clinical immunology. 2015;15(1):104–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigues NP, Tipping AJ, Wang Z, Enver T. GATA-2 mediated regulation of normal hematopoietic stem/progenitor cell function, myelodysplasia and myeloid leukemia. The international journal of biochemistry & cell biology. 2012;44(3):457–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matatall KA, Jeong M, Chen S, et al. Chronic Infection Depletes Hematopoietic Stem Cells through Stress-Induced Terminal Differentiation. Cell reports. 2016;17(10):2584–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West RR, Hsu AP, Holland SM, Cuellar-Rodriguez J, Hickstein DD. Acquired ASXL1 mutations are common in patients with inherited GATA2 mutations and correlate with myeloid transformation. Haematologica. 2014;99(2):276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X, Muramatsu H, Okuno Y, et al. GATA2 and secondary mutations in familial myelodysplastic syndromes and pediatric myeloid malignancies. Haematologica. 2015;100(10):e398–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Micol JB, Abdel-Wahab O. Collaborating constitutive and somatic genetic events in myeloid malignancies: ASXL1 mutations in patients with germline GATA2 mutations. Haematologica. 2014;99(2):201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ding LW, Ikezoe T, Tan KT, et al. Mutational profiling of a MonoMAC syndrome family with GATA2 deficiency. Leukemia. 2017;31(1):244–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujiwara T, Fukuhara N, Funayama R, et al. Identification of acquired mutations by whole-genome sequencing in GATA-2 deficiency evolving into myelodysplasia and acute leukemia. Annals of hematology. 2014;93(9):1515–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loyola VBP HS, Pohl S, Kozyra EJ, Catala A, De Moerloose B, Dworzak M, Hasle H, Masetti R, Schmugge M, Smith O, Stary J, Ussowicz M, van den Heuvel-Eibrink HM, Mejstrikova E, Salzer U, Lübbert M, Heudobler D, Betts D, Cervera J, Gohring G, Haas OA, Haus O, Michalova K, Pasquali F, Tchinda J, van Roy N, Schlegelberger B, Beverloo HB, Noellke P, Yoshimi A, Locatelli F, Strahm B, Maciejewski JP, Rehli M, Niemeyer CM, Wlodarski MW. Somatic Genetic and Epigenetic Architecture of Myelodysplastic Syndromes Arising from GATA2 Deficiency. American Society of Hematology Meeting Abstracts. 2015;126(23):299. [Google Scholar]
- 40.Fisher KEHA, Williams CL, Sayeed H, Merritt BY, Elghetany MT, Holland SM, Bertuch AA, Gramatges MM. Somatic mutations in children with GATA2-associated myelodysplastic syndrome who lack other features of GATA2 deficiency. Blood Advance. 2017;1(7):443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koegel AK, Hofmann I, Moffitt K, Degar B, Duncan C, Tubman VN. Acute lymphoblastic leukemia in a patient with MonoMAC syndrome/GATA2 haploinsufficiency. Pediatric blood & cancer. 2016;63(10):1844–1847. [DOI] [PubMed] [Google Scholar]
- 42.Groschel S, Sanders MA, Hoogenboezem R, et al. A single oncogenic enhancer rearrangement causes concomitant EVI1 and GATA2 deregulation in leukemia. Cell. 2014;157(2):369–381. [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki H, Suzuki M, Otsuki A, et al. A remote GATA2 hematopoietic enhancer drives leukemogenesis in inv(3)(q21;q26) by activating EVI1 expression. Cancer cell. 2014;25(4):415–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Celton M, Forest A, Gosse G, et al. Epigenetic regulation of GATA2 and its impact on normal karyotype acute myeloid leukemia. Leukemia. 2014;28(8):1617–1626. [DOI] [PubMed] [Google Scholar]
- 45.Dotta L, Badolato R. Primary immunodeficiencies appearing as combined lymphopenia, neutropenia, and monocytopenia. Immunology letters. 2014;161(2):222–225. [DOI] [PubMed] [Google Scholar]
- 46.Mace EM, Hsu AP, Monaco-Shawver L, et al. Mutations in GATA2 cause human NK cell deficiency with specific loss of the CD56(bright) subset. Blood. 2013;121(14):2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schlums H, Jung M, Han H, et al. Adaptive NK cells can persist in patients with GATA2 mutation depleted of stem and progenitor cells. Blood. 2017;129(14):1927–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maciejewski-Duval A, Meuris F, Bignon A, et al. Altered chemotactic response to CXCL12 in patients carrying GATA2 mutations. Journal of leukocyte biology. 2016;99(6):1065–1076. [DOI] [PubMed] [Google Scholar]
- 49.Ruiz-Garcia R, Rodriguez-Vigil C, Marco FM, et al. Acquired Senescent T-Cell Phenotype Correlates with Clinical Severity in GATA Binding Protein 2-Deficient Patients. Frontiers in immunology. 2017;8:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Webb G, Chen YY, Li KK, et al. Single-gene association between GATA-2 and autoimmune hepatitis: A novel genetic insight highlighting immunologic pathways to disease. Journal of hepatology. 2016;64(5):1190–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson JA, Yu SS, Elist M, Arkfeld D, Panush RS. Rheumatologic manifestations of the “MonoMAC” syndrome. a systematic review. Clinical rheumatology. 2015;34(9):1643–1645. [DOI] [PubMed] [Google Scholar]
- 52.Chou J, Lutskiy M, Tsitsikov E, Notarangelo LD, Geha RS, Dioun A. Presence of hypogammaglobulinemia and abnormal antibody responses in GATA2 deficiency. The Journal of allergy and clinical immunology. 2014;134(1):223–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vila A, Dapas JI, Rivero CV, et al. Multiple Opportunistic Infections in a Woman with GATA2 Mutation. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2017;54:89–91. [DOI] [PubMed] [Google Scholar]
- 54.Camargo JF, Lobo SA, Hsu AP, Zerbe CS, Wormser GP, Holland SM. MonoMAC syndrome in a patient with a GATA2 mutation: case report and review of the literature. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013;57(5):697–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lovell JP, Zerbe CS, Olivier KN, et al. Mediastinal and Disseminated Mycobacterium kansasii Disease in GATA2 Deficiency. Annals of the American Thoracic Society. 2016;13(12):2169–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonzalez-Lara MF, Wisniowski-Yanez A, Perez-Patrigeon S, Hsu AP, Holland SM, Cuellar-Rodriguez JM. Pneumocystis jiroveci pneumonia and GATA2 deficiency: Expanding the spectrum of the disease. The Journal of infection. 2017;74(4):425–427. [DOI] [PubMed] [Google Scholar]
- 57.Cohen JI, Dropulic L, Hsu AP, et al. Association of GATA2 Deficiency With Severe Primary Epstein-Barr Virus (EBV) Infection and EBV-associated Cancers. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016;63(1):41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Parta M, Cuellar-Rodriguez J, Freeman AF, Gea-Banacloche J, Holland SM, Hickstein DD. Resolution of Multifocal Epstein-Barr Virus-Related Smooth Muscle Tumor in a Patient with GATA2 Deficiency Following Hematopoietic Stem Cell Transplantation. Journal of clinical immunology. 2017;37(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kazamel M, Klein CJ, Benarroch EE, Patnaik MM, Tracy JA. Subacute demyelinating polyradiculoneuropathy complicating Epstein-Barr virus infection in GATA2 haploinsufficiency. Muscle & nerve. 2017. [DOI] [PubMed] [Google Scholar]
- 60.Spinner MA, Ker JP, Stoudenmire CJ, et al. GATA2 deficiency underlying severe blastomycosis and fatal herpes simplex virus-associated hemophagocytic lymphohistiocytosis. The Journal of allergy and clinical immunology. 2016;137(2):638–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delgado-Marquez AM, Zarco C, Ruiz R, Simarro A, Vanaclocha F. Severe disseminated primary herpes simplex infection as skin manifestation of GATA2 deficiency. Journal of the European Academy of Dermatology and Venereology : JEADV. 2016;30(7):1248–1250. [DOI] [PubMed] [Google Scholar]
- 62.West ES, Kingsbery MY, Mintz EM, et al. Generalized verrucosis in a patient with GATA2 deficiency. The British journal of dermatology. 2014;170(5):1182–1186. [DOI] [PubMed] [Google Scholar]
- 63.Muszynski MA, Zerbe CS, Holland SM, Kong HH. A woman with warts, leg swelling, and deafness. Journal of the American Academy of Dermatology. 2014;71(3):577–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polat A, Dinulescu M, Fraitag S, et al. Skin manifestations among GATA2-deficient patients. The British journal of dermatology. 2017. [DOI] [PubMed] [Google Scholar]
- 65.Kazenwadel J, Secker GA, Liu YJ, et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2012;119(5):1283–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ballerie A, Nimubona S, Meunier C, et al. Association of pulmonary alveolar proteinosis and fibrosis: patient with GATA2 deficiency. Eur Respir J. 2016;48(5):1510–1514. [DOI] [PubMed] [Google Scholar]
- 67.Griese M, Zarbock R, Costabel U, et al. GATA2 deficiency in children and adults with severe pulmonary alveolar proteinosis and hematologic disorders. BMC pulmonary medicine. 2015;15:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svobodova T, Mejstrikova E, Salzer U, et al. Diffuse parenchymal lung disease as first clinical manifestation of GATA-2 deficiency in childhood. BMC pulmonary medicine. 2015;15:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanges S, Prevotat A, Fertin M, et al. Haemodynamically proven pulmonary hypertension in a patient with GATA2 deficiency-associated pulmonary alveolar proteinosis and fibrosis. Eur Respir J. 2017;49(5). [DOI] [PubMed] [Google Scholar]
- 70.Jouneau S, Ballerie A, Kerjouan M, Demant X, Blanchard E, Lederlin M. Haemodynamically proven pulmonary hypertension in a patient with GATA2 deficiencyassociated pulmonary alveolar proteinosis and fibrosis. Eur Respir J. 2017;49(5). [DOI] [PubMed] [Google Scholar]
- 71.Haugas M, Lillevali K, Hakanen J, Salminen M. Gata2 is required for the development of inner ear semicircular ducts and the surrounding perilymphatic space. Developmental dynamics : an official publication of the American Association of Anatomists. 2010;239(9):2452–2469. [DOI] [PubMed] [Google Scholar]
- 72.Berry D, Fekrat S. CENTRAL RETINAL VEIN OCCLUSION IN GATA2 DEFICIENCY. Retinal cases & brief reports. 2017. [DOI] [PubMed] [Google Scholar]
- 73.Crall C, Morley KW, Rabinowits G, Schmidt B, Dioun Broyles A, Huang JT. Merkel cell carcinoma in a patient with GATA2 deficiency: a novel association with primary immunodeficiency. The British journal of dermatology. 2016;174(1):169–171. [DOI] [PubMed] [Google Scholar]
- 74.Cuellar-Rodriguez J, Gea-Banacloche J, Freeman AF, et al. Successful allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Blood. 2011;118(13):3715–3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciullini Mannurita S, Vignoli M, Colarusso G, et al. Timely follow-up of a GATA2 deficiency patient allows successful treatment. The Journal of allergy and clinical immunology. 2016;138(5):1480–1483.e1484. [DOI] [PubMed] [Google Scholar]
- 76.Ramzan M, Lowry J, Courtney S, Krueger J, Schechter Finkelstein T, Ali M. Successful Myeloablative Matched Unrelated Donor Hematopoietic Stem Cell Transplantation in a Young Girl With GATA2 Deficiency and Emberger Syndrome. Journal of pediatric hematology/oncology. 2017;39(3):230–232. [DOI] [PubMed] [Google Scholar]
- 77.Lubking A, Vosberg S, Konstandin NP, et al. Young woman with mild bone marrow dysplasia, GATA2 and ASXL1 mutation treated with allogeneic hematopoietic stem cell transplantation. Leukemia research reports. 2015;4(2):72–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maeurer M, Magalhaes I, Andersson J, et al. Allogeneic Hematopoietic Cell Transplantation for GATA2 Deficiency in a Patient With Disseminated Human Papillomavirus Disease. Transplantation. 2014;98(12):e95–96. [DOI] [PubMed] [Google Scholar]
- 79.Mallhi K, Dix DB, Niederhoffer KY, Armstrong L, Rozmus J. Successful umbilical cord blood hematopoietic stem cell transplantation in pediatric patients with MDS/AML associated with underlying GATA2 mutations: two case reports and review of literature. Pediatric transplantation. 2016;20(7):1004–1007. [DOI] [PubMed] [Google Scholar]
- 80.Saida S, Umeda K, Yasumi T, et al. Successful reduced-intensity stem cell transplantation for GATA2 deficiency before progression of advanced MDS. Pediatric transplantation. 2016;20(2):333–336. [DOI] [PubMed] [Google Scholar]
- 81.Grossman J, Cuellar-Rodriguez J, Gea-Banacloche J, et al. Nonmyeloablative allogeneic hematopoietic stem cell transplantation for GATA2 deficiency. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2014;20(12):1940–1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shah NN, Parta M, Baird K, et al. Monozygotic twins with GATA2 deficiency: same haploidentical-related donor, different severity of GvHD. Bone marrow transplantation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]