Abstract
An in vitro evaluation of the antimicrobial activity of essential oil (EO) and methanol extract (ME) from Algerian Nigella sativa L. seeds against microbial strains isolated from the oral cavities of periodontal patients was performed. Twelve Gram-positive bacteria, eleven Gram-negative bacteria and three microscopic fungi strains were isolated and identified. The antimicrobial activities of EO and ME were tested against Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Enterococcus faecalis, Klebsiella pneumoniae, Proteus sp., Acinetobacter baumannii/calcoaceticus, Porphyromonas sp., Veillonella sp., Candida sp. and Saccharomyces sp.. The total polyphenol and flavonoids contents of ME were higher than those of EO. Thin layer chromatography showed that catechin, gallic acid and quercetin were most likely present in the extracts. Fourier transform infrared spectrometry analysis (FT-IR) indicated the presence of bands from the CO groups of acids, alcohols, phenols, and ethers and the C O band of aldehydes. Analysis of the antimicrobial activity of N. sativa extracts obtained by the microdilution method showed excellent bactericidal activity of the essential oil and moderate efficiency of the ME against all the microbes tested. Staphylococcus epidermidis and Porphyromonas sp. were the most sensitive to EO (minimum bactericidal concentration (MBC): 16,500 μg/ml) at 48 h of incubation and, 125,000 μg/ml of ME was the most active against all the microbes tested. However, after18 or 24 h, this efficiency was decreased in some strains. In addition, Saccharomyces sp. and Candida albicans were more sensitive to EO than ME during the incubation, while this efficiency was clearly not visible with the agar well method, and most microbes tested presented remarkable resistance to these extracts.
Keywords: Periodontal disease, Nigella sativa L. essential oil, Methanol extract, Bactericidal/fungicidal kinetics, Polyphenols, Flavonoids
1. Introduction
The oral flora consists of a complex ecosystem, and more than 700 bacterial species have been identified (Aas et al., 2005). It comprises Gram-positive and Gram-negative bacteria that can be facultatively anaerobic or strictly anaerobic (INSERM, 1999) living normally in an ecological balance state. However, an imbalance may encourage (stimulate) the appearance of pathology (gingivitis and periodontitis) (Mayrand and Grenier, 1998). Gingivitis is defined as the result of anon-specific inflammatory reaction caused by an increase in the amount of bacteria (Gram-positive or Gram-negative) at or below the marginal gingival level (Gendron et al., 2000). Periodontitis is an inflammatory lesion caused by bacterial infection that destructs the supporting tissues of the tooth (Silva et al., 2015, Holt and Bramanti, 1991). These pathologies are influenced by several factors, including smoking, sex hormones, drug intake, systemic diseases, allergic or infectious skin diseases and human immunodeficiency virus HIV (Mariotti and Hefti, 2015, Kinane, 2001, INSERM, 1999) and can be the result of some dominant bacterial pathogens acting on other bacteria species (Mayrand and Grenier, 1998). The infectious nature of most periodontal diseases and the limited results from conventional mechanical therapy justify the occasional use of antibiotics, which causes some risks, such as the development of resistance in various bacterial species (Bidault et al., 2007). Consequently, the search for alternative products isolated from plants used in traditional medicine is considered a good alternative to synthetic chemical treatments. Nigella sativa L. is a promising medicinal plant. It is a dicotyledon of the Ranunculaceae family that has angular and small sized seeds that are dark grey or black colour that is grown in Mediterranean Sea countries, such as Pakistan, India and Iran (Padhye et al., 2008). These plants are commonly known as black seed (English), Al-Habba Al-Sawdaa (Al-Gaby, 1998), and ‘Senouj’ in Algeria (Cheikh-Rouhou et al., 2007). It is frequently used as a spice and preservative in bread, pickles and other dishes (Aljabre et al., 2005) and has been traditionally used in Middle Eastern folk medicine as a natural remedy for various diseases for over 2000 years (Phillips, 1992). It is widely used in Arab and Islamic countries in traditional medicine for the treatment of diabetes, hypertension, bronchial pulmonary disorders, gastrointestinal disorders, various infections, inflammation and allergies. These therapeutic efficacies were recently proven by pharmacological and biological studies (Orsi-Llinares, 2005). Nigella sativa L. was also one of the valuable remedies provided by the personal physician of the pharaohs for digestive action after large meals and to treat headaches and toothaches (Toparslan, 2012). The black seed occupies an important place in Islamic civilization, as the Prophet Muhammad (peace be upon him) said “El Habbahsauda is a drug for all diseases except death”, and IbnSina (980-1037) cited the therapeutic effects of black seeds in his book “Kitab al-Shifa” (Toparslan, 2012). For these traditional uses of Nigella seeds, to valorise this plant in the pharmaceutical field, an in vitro study was carried out to determine the effect of the essential oil and methanol extract of Algerian N. sativa on the growth of micro-organisms isolated from the buccal cavity of periodontal patients.
2. Materials and methods
2.1. Plant material
Nigella sativa seeds were collected from Qsar Admor-Konta, Adrar-Algeria and identified by a plant botanist from Mustapha Stambouli University in Mascara, Algeria. The seeds were sorted and stored in clean bags for protection from light and moisture. The tested dry extract of N. sativa seeds was 2% ±0.35 (Fig. 1).
Fig. 1.
Saharian Nigella sativa (Adrar, Algeria).
2.2. Preparation of the extracts
2.2.1. Isolation of the essential oil
Thirty grams of N. sativa seeds was crushed into powder using an electric crusher (IKA WERKE M20) and submitted to ex situ steam distillation (Blanchaud-desce et al., 1987) for 90 min. After salting the distilled water, the essential oil (EO) was collected in n-hexane using liquid–liquid extraction. The traces of water in the organic phase were dried with a small amount of anhydrous sodium sulphate, concentrated under reduced pressure using a rotary evaporator at 40 °C and stored in sterile small glass tubes at −4 °C. The EO yield was estimated according to the total mass of the dry powder seeds.
2.2.2. Preparation of methanol extract
The methanol extract (ME) was extracted by the method provided by Houcher et al. (2007) modified in our laboratory. Fifty grams of seeds was ground into powder and soaked in 500 ml of 80% aqueous-methanol (1/10, w/v) for 24 h at room temperature. After filtration, the methanol extract (ME1) was concentrated under reduced pressure using a rotary evaporator at 40 °C until all the solvent was removed. A second extraction with residue and 50% aqueous-methanol (1/10 = w/v) was carried out for 24 h.
After filtration and concentration of the second extract (ME2), the two extracts were combined and stored in sterile small bottles (10 ml) at −40 °C until further use. The total crude methanol extract yield (ME) was estimated according to the total mass of the dry powder seeds.
2.3. Extracts analysis by thin layer chromatography
To ensure chromatographic separation of various chemical constituents of the extracts, we filed a drop of each prepared extract and standard substances on silica gel plates (stationary phase). We used different mobile phases for each extract (Table 1). The uncoloured spots were detected with short wave UV (366 nm), and the relative migration rates (Rfs) of different molecules were calculated (Touchstone and Dobbins, 1983).
Table 1.
Mobile phases of thin layer chromatography.
| Extract | Mobile phase | |
|---|---|---|
| 1 | EO | Toluene-methanol (95:5 v/v) |
| 2 | EO | Diethyl ether – n-hexane (1:1 v/v) |
| 3 | EO | Ethyl acetate – acétone – formic acid – distillated water (5:3:1:1 v/v/v/v) |
| 4 | EO, ME | Hexane – Diethyl ether – acetone (40:10:1.5 v/v/v) |
| 5 | EO, ME | Acetone-hexane (1:2 v/v) |
| 6 | EO, ME | Hexane – Diethyl ether – methanol (40:10:1.5 v/v/v) |
| 7 | EO, ME | Chloroform – Ethyl acétate – formic acid (50:40:10 v/v/v) |
| 8 | EO, ME | Hexane-acetone – Diethyl ether – methanol – distillated water (1:1:1:1 v/v/v/v/v) |
| 9 | ME | Toluene – Ethyl acetate – formic acid (5:4:1 v/v/v) |
| 10 | ME | Ethyl acetate – methanol – distillated water (77:13:10 v/v/v) |
| 11 | ME | Hexane – methanol-distillated water-acetone (1:1:1:1 v/v/v/v/v) |
EO: essential oil; ME: Methanol extract, v: volume.
2.4. Determination of total polyphenol content
Total poly phenol contents in the EO and ME of N. sativa seeds was determined with the Folin–Ciocalteu reagent according to the method described by Bourgou et al. (2008). In a test tube, 0.125 ml of Folin-Ciocalteu’s reagent (diluted 10%) was added to 0.5 ml of distilled water and 0.125 ml of diluted extract (or a standard solution of gallic acid (166 µg/ml) and it binary dilutions). The mixture was incubated for 3 min at room temperature, and 1.52 ml of Na2CO3 solution (7%) was then added. Finally, the volume was increased to 3 ml using distilled water. The solution was mixed by vortexing and incubated in the dark for 90 min at ambient temperature. Absorbance of the solution was measured at 760 nm against a blank solution using a spectrophotometer. The concentrations of the total phenolic compounds in each extract were calculated from the calibration curve of the gallic acid standard and expressed as milligrams of gallic acid equivalents (GAE) per gram of extract. All determinations were performed in duplicate.
2.5. Determination of total flavonoid content
In a 10 ml flask, 4 ml of distilled water was mixed with 1 ml of diluted extract (or a standard catechin solution (266 mg/L) and it binary dilutions), and 0.3 ml of NaNO2 (5%) was subsequently added. After five minutes, 0.3 ml of AlCl3 (10%) was added, and the mixture was incubated for six minutes at room temperature. Then, 2 ml of NaOH (4%) was added, and the total volume (10 ml) was immediately comprised with distilled water. After 15 min of incubation, the mixture was thoroughly stirred, and the absorbance was read against a blank at 510 nm (Salem et al., 2013a). The flavonoid contents were expressed as milligrams of catechin equivalent (CE) per gram of extract.
2.6. Fourier transform infrared spectrometry (FT-IR) analyses
Infrared spectrometry is used to identify various secondary metabolite groups in plants. It is based on the excitation of molecules by infrared radiation and the energy of molecular vibration changes, both of which represent the states of molecule rotation and vibration (Bertrand and Dufour, 1987). In the present study, the powder of N. sativa seeds, extracts and residues of extraction were characterized by the Fourier transform infrared spectrometry method (FT-IR-Agilent Type, Technologies Laboratory LMAE). The transmittance spectra were recorded at room temperature in the 4000–400 cm−1 range.
2.7. In vitro antimicrobial activity of essential oil and methanol extract
2.7.1. Clinical examination
The microbial strains used in this study were isolated from the oral cavities of 12 patients (4 men, 8 women) between 16 and 75 years of age that were suffering from periodontal diseases. These patients were selected after a questionnaire and a clinical examination (Periodontal Index, Gingival Index, Oral Hygiene Index, Plaque Index) (INSERM, 1999, Wei and Lang, 1982)by dentists from the health centre of Mehor Meheidine in Mascara, Algeria.
2.7.1.1. Periodontal index (PI) (Russel, 1956)
The periodontal index is an assessment system applied to each tooth with the following values:
0 = healthy tooth to periodontal
1 = gingival inflammation around a portion of the tooth
2 = gingival inflammation surrounding the tooth
6 = formation of a pocket
8 = loss-of-function by excessive mobility
2.7.1.2. Gingival index (GI) (Loe and Silness, 1963)
Four degrees of gingival inflammation were evaluated:
0 = no inflammation
1 = inflammation without bleeding
2 = inflammation causing bleeding
3 = ulceration and spontaneous bleeding
2.7.1.3. Oral Hygiene Index (OHI) (Greene and Vermillon, 1960)
This index comprises two distinct components: the debris index and the calculus index.
The debris index (DI) measures the coronary extension of soft deposits to the first, second or last third of the buccal or lingual surfaces of the teeth:
0 = no debris
1 = 1/3 of the surface is covered with debris
2 = 2/3 of the surface is covered with debris
3 = more than two-thirds of the exposed tooth surface is covered with debris
The calculus index (CI) measures the corresponding coronal extent of subgingival calculus in the form of isolated or deposits in a continuous strip:
0 = no calculus present
1 = 1/3 of the face is covered with calculus
2 = 2/3 of the face is covered with calculus
3 = entire face is covered with calculus
OHI is the total of the DI and CI.
2.7.1.4. Plaque Index (PlI) (Silness and Loe, 1964)
This index measures the thickness of the plaque in each of the four dental faces. The maximum number of patients is 28 × 4 = 112 dental faces.
0 = no plaque in the gingival area
1 = a film of plaque adhering to the free gingival margin and adjacent area of the tooth
2 = moderate accumulation of soft deposits within the gingival pocket, on the gingival margin and/or adjacent tooth surface, which can be seen by the naked eye
3 = abundance of soft matter within the gingival pocket and/or on the gingival margin and adjacent tooth surface
2.7.2. Microbiological sampling
After rinsing the mouth with sterile distilled water, supragingival plaques were removed by sterile swabs moistened with physiological saline solution, while subgingival plaques were collected on a Gracey curette inserted into the periodontal pocket. The samples were placed in sterile tubes and immediately transported to the laboratory (Yacoubi et al., 2010, Colombier et al., 2006).
2.7.3. Microbial strains
After an enrichment of levies in Brain Heart Infusion Broth (BHIB) (Denis et al., 2007), Gram-positive bacteria were isolated by the streak plate method on Agar Chapman, Bile Esculin Agar (BEA), De Man, Rogosa and Sharpe Agar (MRS), and Comombia Agar with 5% sheep blood media, while Gram-negative bacteria were isolated on Hecktoen Agar, Eosin Methylene blue Agar (EMB), Brain Heart Infusion (BHI), Mac Conkey Agar and Colombia Agar with 5% sheep blood. Facultative anaerobic bacteria were incubated under normal atmospheric conditions at an optimum temperature of 37 °C for 24–72 h, and strictly anaerobic bacteria were incubated in an anaerobic jar at 37 °C for 3–7 days. Microbial culture conditions, macroscopic characteristics (colony appearance, size, colour) and microscopic observations (bacterial form, Gram coloration) were supplemented with conventional biochemical tests (catalase, oxidase, mannitol, mobility, o-nitrophenyl-β-D-galactopyranoside (ONPG), coagulase, citrate, Kligler-Hajna, endospore stain) (Denis et al., 2007, Brossard et al., 2006, Djelouat, 1990, Leyral, 1973). Finally, bacteria identification diagrams of the main groups (Gram-positive bacilli, Gram-negative Bacilli & cocci, Gram-positive cocci, Gram-negative anaerobic bacilli) were used to guide the bacterial identification (Leyral and Joffin, 1998), and different miniaturized multi-test systems of API Staph (Staphylococcus, Micrococcus), API 20Strep (Streptococcus, Enterococcus), API 20E (Enterobacteriaceae and other Gram-negative bacilli bacteria), API 20NE (Gram-negative bacilli, no Enterobacteriaceae), and API 20A (strictly anaerobic bacteria) were applied according to the BioMerieux manual (Leyral and Joffin, 1998) to complete the identification of the microbes. Microscopic fungi were isolated on Sabouraud chloramphenicol gentamicin at 25 °C for 3–5 days and identified by API 20Candida. For preservation of the bacterial and microscopic fungi strains at −20 °C, nutrient and sabouraud broths with 12% glycerol were used successively.
2.7.4. Inoculum preparation
Microbial strains were thawed and inoculated in nutrient agar at 37 °C for 18 h, and one colony from each culture was subsequently diluted in nutrient broth to adjust the suspension to a final density of 108 UFC/ml at 620 nm (JENWAY, 6400 spectrophotometer).
2.7.5. Preparation of N. sativa extracts solutions
N. sativa extracts were dissolved in dimethyl sulfoxide (DMSO, 99% purity) to prepare 33,000 µg/ml and 250,000 µg/ml EO and ME solutions, respectively, and the solutions were sterilized by filtration (0.22-µm filters).
2.7.6. Effect of essential oil and methanol extract on microbial growth kinetics
This test was performed by the microdilution method in sterile 96-well microplates according to the method provided by Salem et al. (2013a). Briefly, 50 µl of Mueller Hinton broth (for bacteria test) or Sabouraud broth (for yeast test) was placed into wells of the microplates. Subsequently, 50 µl of EO (33,000 µg/ml) or ME (250,000 µg/ml) was added to the first column of the microplates. Then, the serial dilution of each extract was prepared and added to each well. Fifty microlitres of microbial suspension was adjusted to 108 UFC/ml. The microplates were covered with sterile aluminium foil and incubated under normal atmospheric conditions (facultatively anaerobic microbes) and in anaerobic jars (anaerobic microbes) at 37 °C. Microbial growth was determined by reading the respective absorbance (Abs) at 620 nm on a microplate reader (TECAN SPECTRA) five times (at 0, 2, 18, 24, 48 h). One well with specific medium (50 µl) and the microbial suspension (50 µl) was used as the growth control. Another well was inoculated with specific medium to assess the sterility and aseptic work conditions, and a negative control was prepared using DMSO. The microbial tests were prepared in triplicate.
2.7.7. Agar well diffusion assay
The antimicrobial effect of N. sativa extracts was tested according to the Abu-Al-Basal (2009) method modified in the laboratory. After the solidification of Mueller Hinton agar in Petri dishes (9 cm diameter), three wells 4 mm in diameter were cut in each dish. One hundred microlitres of diluted microbial suspension (108 UFC/ml) was inoculated on agar. The inocula were allowed to dry for 15 min. Then, 50 μl of different concentrations of EO (16,500 µg/ml and 8250 µg/ml) or ME (125,000 µg/ml and 62,500 µg/ml) was added separately to each well of the agar. DMSO was also used as a negative control. The Petri dishes were incubated under normal atmospheric conditions (facultatively anaerobic microbes) and in anaerobic jars (anaerobic microbes) at 37 °C for 18 h, and the diameters of the zones of inhibition were measured in millimetres (mm). All tests were carried out in triplicate.
3. Statistical analysis
All growth kinetics and agar well tests were performed in triplicate. The results are expressed as the mean ± SD. For microbial growth, a difference of 1 log UFC/ml (a microbiological standard) between the two times was considered significant (*P ≤ 0.05) (Molly et al., 1993).
4. Results
The relative yields (% w/w) of the EO and ME in N. sativa seeds were 0.45% ± 0.028 and 10.9 ± 1%, respectively. The EO was yellow to orange colour with aromatic characteristics. The ME had a patty appearance, characterized by a dark brown to black colour and a strong and specific odour.
4.1. Thin layer chromatography results
The results obtained show that the mobile phases hexane – diethyl ether – methanol (40:10:1.5 v/v/v) and chloroform – ethyl acetate – formic acid (50:40:10 v/v/v) are the most suitable for the separation of EO and ME molecules. Following observation under UV light (336 nm), compared with the relative migration rate (Rf) of separated spots and control components, we deduced that catechin, gallic acid and quercetin are probably present in the EO and ME, whereas ellagic acid and vanillin are present in EO and ME, respectively (Table 2).
Table 2.
The Rfs and Rt of extracts and witnesses.
| Eluent 6 | Eluent 7 | |
|---|---|---|
| Quercétrin | 0.09 | 0.45 |
| Gallic acid | 0.38 | 0.45 |
| élagic acid | 0.25 | 0.6 |
| Catéchine | 0.23 | 0.2 |
| Vanillin | – | 0.62 |
| Methanolic extract (ME) | 0.07; 0.10; 0.17; 0.23; 0.375; 0.826 | – |
| Essential oil (EO) | 0.1 0.22; 0.26; 0.34; 0.36; 0.76; 0.9 | 0.13; 0.19; 0.34; 0.48; 0.63 |
4.2. Total phenolics and flavonoids contents
Preliminary phytochemical screening indicated the presence of phenolic compounds and flavonoids in the ME (El-Agbar et al., 2008), and this present study shows that ME has higher total phenol and flavonoid contents (28.1 ± 3.5 mg GAE/g extract and 5.64 ± 0.4 mg CE/g extract) than EO (15.75 ± 2 mg GAE/g extract and 2.150 ± 0.5 mg CE/g extract).
4.3. Fourier transform infrared spectrometry (FT-IR) results
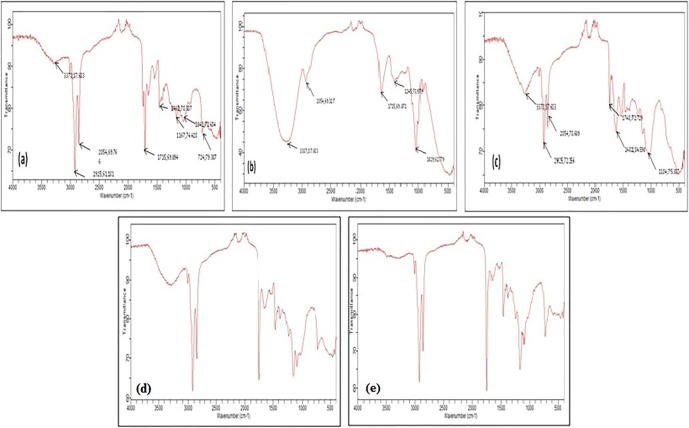
The FT-IR spectra of EO, ME and the powder seeds before and after extraction are given in Fig. 2(a–e). In the overall reading of all spectra, the average band elongations at 2925 cm−1 and 2854 cm−1 resulted in aliphatic CH (in an aromatic methoxyl group, in methyl and methylene side chains) and aromatic CH, respectively. The bands observed in all spectra at 1041 cm−1 and 1462 cm−1 may be attributed to the stretching band in the CO group of the acid, alcohol, phenol, ether or ester groups. In the region of 1300–1500 cm−1, several absorption bands were observed corresponding to a stretching band of CN vibration bands, and a peak was observed at 1600 cm−1, which corresponds to a deformation in the NH group plan amides. The band at 1715 cm−1 indicates the presence of C O stretching band aldehydes (Zawadzki, 1989). In the ME spectra, a large absorption band corresponding to OH observed at 3000–3500 cm−1, maximizing at approximately 3300 cm−1, which is characteristic of the vibration of elongation that can be attributed to alcohols or phenols, was intense because of the presence of water in the ME. The spectrum of powder seeds before and after extraction with methanol showed the presence of different absorption band intensities at 1715 cm−1, indicating the presence of a stretching band corresponding to the C O of aldehydes (Zawadzki, 1989)and a peak observed at 1700 cm−1 corresponding to the C O group of esters and lactones. For the EO, in the region of 500–1000 cm−1, low bands were observed corresponding to a band stretching vibration of C C—H. Another weak band was recorded at 2150 cm−1, which corresponds to the C C group of alkynes.
Fig. 2.
FT-IR results of N. Sativa: (a) fine powder of the seeds prior extraction: (b) ME, (c) residue extraction by maceration, (d) EO, (e) residue extraction by steam distillation.
4.4. Micro-organisms
Twelve Gram-positive bacteria, eleven Gram-negative bacteria and three microscopic fungi strains were isolated and identified from the oral cavities of periodontal patients (see Table 3). The microbial plaques of gingivitis consisted of Gram-positive facultative or strictly anaerobic bacteria, including the dominant species Actinomyces and Streptococcus in the supra and sub-gingival plaques. We can also isolated a low proportion of the strictly anaerobic Gram-negative bacilli bacteria (INSERM, 1999), while strictly anaerobic Gram-negative bacteria and spirochetes that adhere to Gram-positive bacteria were isolated from periodontitis pathologies (INSERM, 1999). Porphyromonas gingivalis and Prevotella intermedia are considered the major pathogens of periodontitis and Veillonella species that are frequently associated with it, but no pathogenic activity has been attributed to this genus (AFSSAPS, 2001, INSERM, 1999). Streptococcus species are the commensal flora of the human oral cavity and may play a protective role in periodontal diseases (Hillman et al., 1985). Some strains, such as group D streptococci and Enterococcus faecalis, are not commensal oral microflora (INSERM, 1999), and Streptococcus pneumoniae is not usually isolated from oral samples (Tanner et al., 1994). Staphylococcus species are associated with oral infections, such as gingivitis and local juvenile periodontitis (Rams et al., 1990). On the other hand, Tanner et al. (1994) showed that Micrococcus are not major pathogens, but they have occasionally been associated with infections in immunocompromised patients, and some Bacillus species are associated with human infections. Souto et al. (2014) showed that Pseudomonas aeruginosa and Acinetobacter spp. are frequently detected in subgingival biofilms and the saliva of chronic periodontitis patients, whereas Enterobacteria and yeast are strains more isolated from the oral cavities of refractory periodontitis patients (AFSSAPS, 2001).
Table 3.
Microbial strains isolated from the buccal cavity of periodontal patients.
| Sexe | Age (yaers) | PI | GI | DI | CI | OHI | PlI | Isolated microbial strains | |
|---|---|---|---|---|---|---|---|---|---|
| P1 | M | 56 | 8 | 2 | 2 | 2 | 4 | 2 | Streptococcus sp., Streptococcus sp., Streptococcus pyogenes, Enterococcus faecalis, |
| P2 | W | 28 | 8 | 3 | 1 | 1 | 2 | 1 | Streptococcus sp., Streptococcus pyogenes, Streptococcus D group, Staphylococcus aureus |
| P3 | M | 75 | 2 | 1 | 0 | 1 | 1 | 1 | S. aureus, Staphylococcus epidermidis, Enterococcus durans, Klebsiellapneumonia, Streptococcus pneumoniae Streptococcus sp., Veillonella sp. |
| P4 | W | 32 | 6 | 3 | 1 | 1 | 2 | 1 | S. epidermidis, S. aureus, E. durans Streptococcus sp., Candida sp., Saccharomyces sp., Veillonella sp., Klebsiellapneumonia, Lactobacillus sp |
| P5 | W | 50 | 8 | 3 | 2 | 1 | 3 | 2 | Streptococcus sp.,Streptocoque D group, Streptococcus sp., S. epidermidis, Proteussp, Escherichia coli., Veillonella sp., Lactobacillus sp., Candida sp. |
| P6 | W | 48 | 1 | 1 | 0 | 0 | 0 | 0 | Escherichia sp., S. epidermidis, Streptococcus D group, Candida sp. |
| P7 | M | 56 | 1 | 1 | 0 | 0 | 0 | 0 | S. epidermidis, Klebsiella pneumonia Streptococcus sp.,Candida sp. |
| P8 | W | 49 | 8 | 2 | 1 | 1 | 2 | 1 | S. aureus, Escherichia sp., Streptococcus D group, Lactobacillus sp., Klebsiella pneumonia |
| P9 | W | 41 | 9 | 3 | 2 | 2 | 4 | 3 | Klebsiella pneumonia ssp. Pneumonia, Porphyromonas sp., Enterococcus faecalis, Acinetobacter baumannii/calcoacetecus Candida sp. |
| P10 | W | 24 | 6 | 2 | 2 | 2 | 4 | 3 | Micrococcus sp., Bacillus sp., S. epidermidis, Pseudomonas sp., Enterobacter aerogenes, Saccharomyces sp. |
| P11 | W | 16 | 6 | 2 | 3 | 2 | 5 | 2 | Streptocoque D group Enterobacter aerogenes, Acinetobacter baumannii/calcoacetecus, Klebsiella pneumonia, Klebsiella sp., Candida sp., |
| P12 | M | 60 | 8 | 3 | 2 | 2 | 4 | 3 | Acinetobacter, baumannii/calcoacetecus, Pseudomonas maltophilia, Porphyromonas sp., Klebsiella pneumonia ssp., Pneumonia, Bacillus sp., Streptococcus D group, S. aureus, Candida albicans |
P: Periodontal patients; M: Man; W:Woman; PI: Periodontal index; GI: gingivitis index; DI: Debri index; CI: Calcus index; OHI: Oral hygien index; PlI:plaque index.
4.5. Antimicrobial activity results
The biological activities of N. sativa EO and ME were tested against four Gram-positive bacteria (S. pneumoniae, E. faecalis, S. aureus, S. epidermidis), five Gram-negative bacteria (Porphyromonas sp., Veillonella sp., Klebsiella pneumoniae, Acinetobacter baumannii/calcoaceticus, Proteus sp.) and two microscopic fungi strains (Candida sp. and Saccharomyces sp.).
4.5.1. Results of essential oil and methanol extract effects on microbial growth kinetics
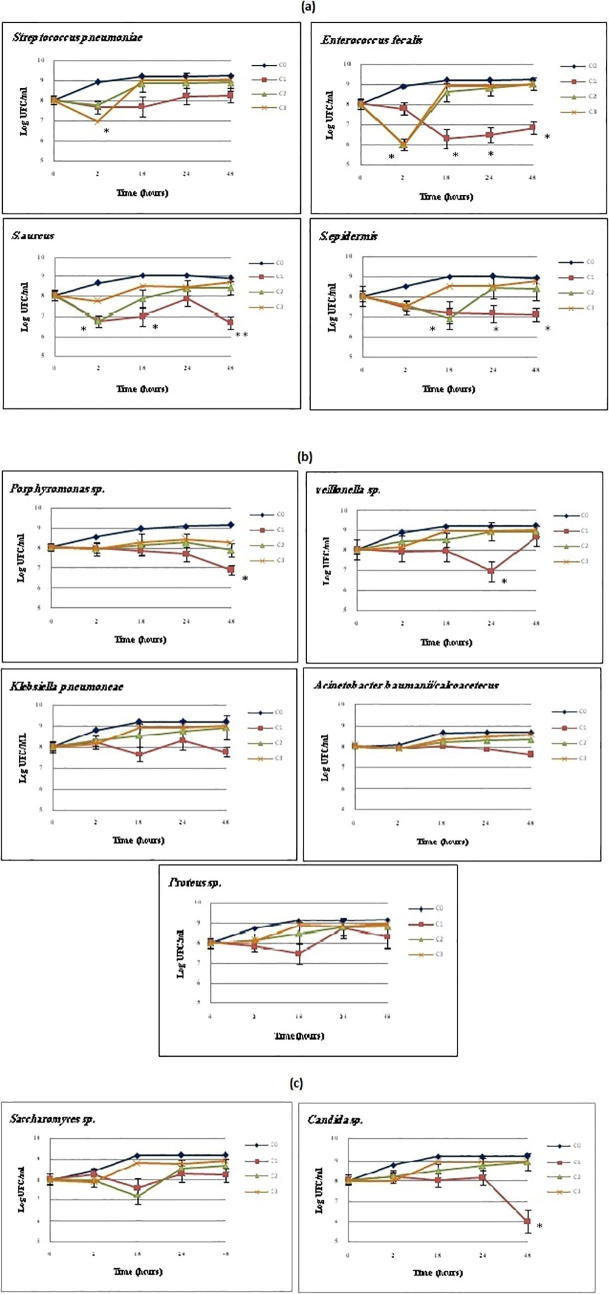
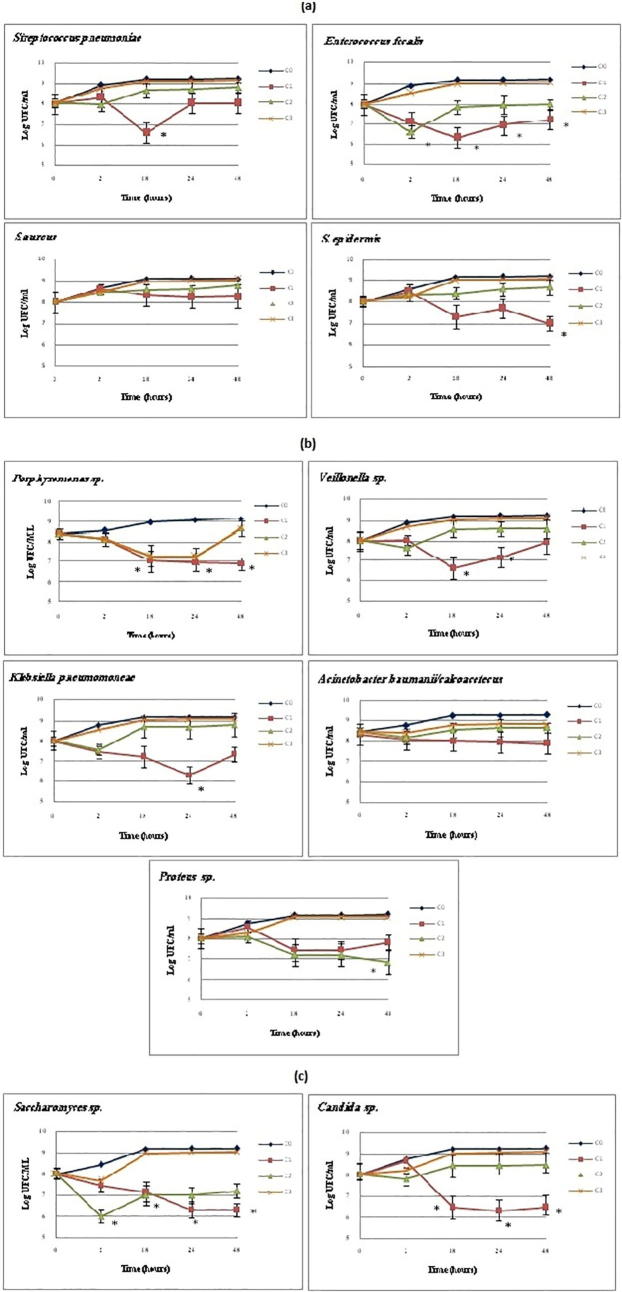
We tested the bactericide, fungicide kinetic, minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and minimum fungicidal concentration (MFC) values of N. sativa EO and ME against the microbes tested. The EO (MBC: 16,500 μg/ml) has specific bactericidal activity against Gram-positive and Gram-negative bacteria (Fig. 3a, b), but this efficiency did not reach statistical significance (P ≥ 0.05) in some stains. On the other hand, the bactericidal activity decreased, and some strains resumed their growth after 18 h. The obtained results showed no activity at the lowest concentrations against all the micro-organisms tested, and S. epidermis was the most sensitive to EO (MBC: 8250 μg/ml for18 h). EO has a specific effect on anaerobic strains, whereas Saccharomyces sp. was more sensitive (MFC: 8250 μg/ml) than Candida sp. (MCI: 16,500 μg/ml) to this extract in the first 18 h (Fig. 3c). The results of the ME antimicrobial activity showed a moderate antibacterial activity (MBC: 125,000 μg/ml) against all the microbes tested (Fig. 4a–c). Staphylococcus aureus was the most resistant, and Porphyromonas sp. (MBC: 31,250 μg/ml for 24 h) and Proteus sp. (MBC: 62,500 μg/ml for 24 h) were the most sensitive bacteria to ME during the incubation (*P ≤ 0.05). Furthermore, the bactericidal activity of some strains decreased after 18 h of incubation, and growth resumed.
Fig. 3.
Effect if N. sativa essential oil on growth of: (a) Gram postive bacteria; (b) Gram negative bacteria; (c) Saccharomyces sp and Candida sp. (*P = 0.05).
Fig. 4.
Effect of N. sativa methanol extract on growth of: (a) Gram positive bacteria; (b) Gram negative bacteria; (c) Saccharomyces sp and Candida sp. (*P = 0.05).
4.5.2. Results of the agar well diffusion method
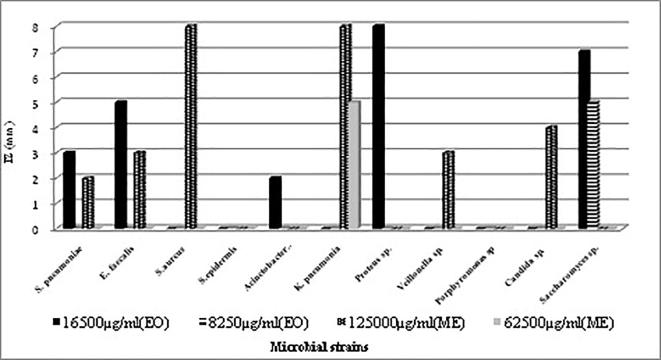
The agar well diffusion method confirmed the antimicrobial activities of EO and ME against some microbes, but this efficiency was not clearly visible against other micro-organisms that have exhibited a high sensitivity to these extracts according to the microdilution method. EO was more active against Proteus sp. and Saccharomyces sp., while Staphylococcus aureus and Klebsiella pneumoniae were the most sensitive to ME (Fig. 5).
Fig. 5.
Biological activity of essential il (EO) and methanol extract (ME) of N. sativa seeds by agar well diffusion method.
5. Discussion
These results showed the excellent antimicrobial activity of EO (MBC: 16,500 μg/ml) and moderate efficiency of ME (MBC: 125,000 μg/ml) against all the microbes tested. The results depended on the incubation time, the concentration of extract and the microbial strain tested. Our results confirmed that the efficiency of N. sativa EO against cariogenic bacteria and other Gram-positive bacterial strains (Jrah Harzallah et al., 2011, Ara et al., 2005). On the other hand, the bactericidal activity of some microbe strains tested was decreased, and the strains resumed their growth. This phenomenon could be the result of the reduced concentration of active substances in the culture medium or the altered physicochemical conditions that impair the efficiency of these active substances against micro-organisms; however, many studies have shown the resistance of Gram-negative bacteria to EO (Ali and Blunden, 2003, Toama et al., 1974). The current results confirmed the activity of the volatile Algerian N. sativa seed oil against numerous strains of this bacterial group (Ara et al., 2005). Biochemical and FT-IR results confirmed the richness of N. sativa extracts in polyphenols, flavonoids, ellagic acid, alcohols, phenols, and ketones. Generally, these molecules possess antimicrobial and therapeutic properties. Moreover, many active compounds (thymoquinone, thymohydroquinones, p-cymène, thymol, carvacrol, α and β pinene, alcohols, ketones, etc.) were characterized in the EO of N. sativa seeds collected from Adrar (Algeria) (Benkaci et al., 2007). They have bactericidal or bacteriostatic activity against different microbes (Salman et al., 2016, Halawani, 2009, Zhiri, 2006). Scandorieiro et al. (2016) suggested that hydrophobic bioactive compounds damage the cell membrane, increase cell permeability and affect biomolecule synthesis. According to Zhiri (2006), these activities depend on the chemical composition, functional groups (alcohols, phenols, ketones and terpene compounds) and synergistic effects of the major compounds. Methanol extract had a specific antimicrobial activity against all the microbes tested. This efficiency has been confirmed in many studies (Salman et al., 2016, Tanis et al., 2009, Aljabre et al., 2005, Kökdil et al., 2005), and Toppozada et al. (1965) reported the antimicrobial activity of N. sativa seed phenolic compounds by inhibiting bacterial proteins synthesis (Mason and Wasserman, 1987), whereas thymoquinone and tannins can be extracted from ME (Salman et al., 2016, Eloff, 1998) and also have antimicrobial activity (Hashem and El-Kiey, 1982). Our study showed that the polyphenol and flavonoid contents in ME were higher than those in EO, but the latter had a stronger antimicrobial effect (MBC of 16,000 µg/ml), which could be explained by the difference in the EO and ME compounds and confirm the antimicrobial activity of the non-polar fraction of N. sativa extracts. Moreover, among all the microbial growth kinetic results indicated the bactericidal actions of EO and ME. On the other hand, the results of the agar well diffusion method did not reflect the effectiveness of the N. sativa extracts against the microbes tested. According to Mahmoudi et al., 2016, Salem et al., 2013b, the inhibition zone (IZ) values are potentially affected by the solubility of the oil, the diffusion range in the agar, and the evaporation. Additionally, the strains that were the most sensitive (lower MIC values) did not always have the biggest inhibition zones (diffusion method) because the diameter of the inhibition zones does not reflect the antibacterial activity of a compound.
6. Conclusion
Remarkable efficiencies of the essential oil and methanol extract of N. sativa seeds collected from South Algeria against the growth of microbes isolated from the oral cavity of periodontitis patients were elucidated by the microdilution method, while the agar well diffusion method did not reflect these results. This proves that several factors can influence the antimicrobial activity of these extracts, and these results must be reproduced by other studies (in vitro and in vivo) to support the hypothesis regarding the efficacy of N. sativa against oral pathologies.
Conflicts of interest statement
We have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aas J.A., Paster B.J., Stoke L.N., Olsen I., Dewhirst F.E. Defining the normal bacterial flora of the oral cavity. J. Clin Microbiol. 2005;11:5721–5732. doi: 10.1128/JCM.43.11.5721-5732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Al-Basal M.A. In vitro and In vivo anti-microbial effects of Nigella sativa Linn. seed extracts against clinical isolates from skin wound infections. Am. J. Appl. Sci. 2009;6:1440–1447. [Google Scholar]
- AFSSAPS, 2001. Agence française de sécurité sanitaire des produits de santé, Prescription des antibiotiques en odontologie et stomatologie-Argumentaire.
- Al-Gaby A.M.A. Amino acid composition and biological effects of supplementing broad bean and corn proteins with Nigella sativa (black cumin) cake protein. Nahrung. 1998;42:290–294. doi: 10.1002/(SICI)1521-3803(199810)42:05<290::AID-FOOD290>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Ali B.H., Blunden G. Pharmacological and toxicological properties of Nigella sativa. Phytother. Res. 2003;17:299–305. doi: 10.1002/ptr.1309. [DOI] [PubMed] [Google Scholar]
- Aljabre S.H.M., Randhawa M.A., Akhtar N., Alakloby O.M., Alqurashi A.M., Aldossary A. Antidermatophyte activity of ether extract of Nigella sativa and its active principle, thymoquinone. J. Ethnopharmacol. 2005;101:116–119. doi: 10.1016/j.jep.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ara N., Choudhury S.A.R., Ruhul A. In vitro antimicrobial activity of the volatile oil of Nigella sativa Linn Seeds. TAJ. 2005;18:109–112. [Google Scholar]
- Benkaci A.F., Baaliouamer A., Meklati B.Y., Chemat F. Chemical composition of seed essential oils from Algerian Nigella sativa extracted by microwave and hydrodistillation. Flavour Fragr. J. 2007;22:148–153. doi: 10.1002/ffj.1773. [DOI] [Google Scholar]
- Bertrand D., Dufour E. 1ière ed. Lavoisier Tec et doc; Paris: 1987. La spectroscopie infrarouge et ses applications analytiques. [Google Scholar]
- Bidault P., Chandad F., Grenier D. Risques de résistance bactérienne liée à l’antibiothérapie systémique en parodontie-Pratique clinique. J. Can. Dent. Assoc. 2007;73:721–725. [PubMed] [Google Scholar]
- Blanchaud-desce, M., Fosset, B., Guyot, F., Jullien, L., Palacin, S., 1987. Chimie organique experimentale. Ed Hermann, 1987, pp. 27–37.
- Bourgou S., Ksouri R., Bellila A., Skandrani I., Falleh H., Marzouk B. Phenolic composition and biological activities of Tunisian Nigella sativa L. C. R. Biol. 2008;331:48–55. doi: 10.1016/j.crvi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Brossard, H.,Leyral, G.,Terry, O., 2006. Activités technologiques en microbiologie, Tome 2 Bacteriologie systématique, ed. CRDP Aquitaine, France.
- Cheikh-Rouhou S., Besbes S., Hentati B., Blecker C., Deroanne C., Attia H. Nigella sativa L., chemical composition and physicochemical characteristics of lipid fraction. Food Chem. 2007;101:673–681. doi: 10.1016/j.foodchem.2006.02.022. [DOI] [Google Scholar]
- Colombier M.L., Leroy P., Yasukawa K. Bacterial tests on periodontitis. Bull. Acad. Natle Chir. Dent. 2006;49:97–102. [Google Scholar]
- Denis F., Ploy M.C., Martin C., Bingen E., Quentin R. Elsevier Masson; 2007. Bactériologie médicale, techniques usuelles. [Google Scholar]
- Djelouat S. sciences et techniques; Constantine, Algerie: 1990. Le diagnostic biochimique bacterien. Collection guides pratiques, microbiologie medicale; p. 118. [Google Scholar]
- El-Agbar Z.A., Shakya A.K., Khalaf N.A., AL-Haroon M. Comparative antioxidant activity of some edible plants. Turk. J. Biol. 2008;32:193–196. [Google Scholar]
- Eloff J.N. Which extractant should be used for the screening and isolation of antimicrobial components from plants. J. Ethnopharmacol. 1998;60:1–8. doi: 10.1016/s0378-8741(97)00123-2. [DOI] [PubMed] [Google Scholar]
- Gendron R., Grenier D., Maheu-Robert L.F. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000;2:897–906. doi: 10.1016/s1286-4579(00)00391-9. [DOI] [PubMed] [Google Scholar]
- Halawani E. Antibacterial activity of thymoquinone and thymohydroquinone of Nigella sativa L. and their interaction with some antibiotics. Advan. Biol. Res. 2009;3:148–152. [Google Scholar]
- Hashem F.M., El-Kiey M.A. Nigella sativa seeds of Egypt. J. Pharm. sci. 1982;3:121–133. [Google Scholar]
- Hillman, J.D., Socransky, S.S., Shivers, M., 1985. The relationships between streptococcal species and periodontopathic bacteria in human dental plaque. Arch Oral Biol 30, 791–795. In: Tanner, A., Maiden, M.F.J, Paster, B.J., Dewhirst, F.E., 1994. The impact of 16S ribosomal RNA-based phylogeny on the taxonomy of oral bacteria. Periodontol. 2000 5, 26–51.
- Holt S.C., Bramanti T.E. Factors in virulence expression and their role in periodontal disease pathogenesis. Crit. Rev. Oral Biol. Med. 1991;2:177–281. doi: 10.1177/10454411910020020301. [DOI] [PubMed] [Google Scholar]
- Houcher Z., Boudiaf K., benboubetra M., Houcher B. Effects of methanolic extract and commercial oil of Nigella sativa L. on blood glucose and antioxidant capacity in alloxan-induced diabetic rats. Pteridines. 2007;18:8–18. [Google Scholar]
- INSERM, Institut national de la santé et de la recherche médicale, 1999. Maladies parodontales «Thérapeutiques et prévention» Expertise Collective. Rapport établi à la demande de la Mutuelle Générale de l'Education Nationale-ed. INSERM, Paris.
- Jrah Harzallah H., Kouidhi B., Flamini G., Bakhrouf A., Mahjoub T. Chemical composition, antimicrobial potential against cariogenic bacteria and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chem. 2011;129:1469–1474. doi: 10.1016/j.foodchem.2011.05.117. [DOI] [Google Scholar]
- Kinane D.F. Causation and pathogenesis of periodontal disease. Periodontology. 2001;25:8–20. doi: 10.1034/j.1600-0757.2001.22250102.x. [DOI] [PubMed] [Google Scholar]
- Kökdil G., Delialioğlu N., Özbilgin B., Emekdaş G. Antilisterial activity of ballotaspecies growing in turkey- antibacterial activity screening of Nigella L. species growing in Turkey. J. Fac. Pharm. 2005;34:183–190. [Google Scholar]
- Leyral, G., 1973. Bacteriologie-BA73, sequences 1et 2, ed CNED-Toulouse.
- Leyral G., Joffin J.N. 2ième ed. CRDP d’Aquitaine; Bordeaux: 1998. Microbiologie technique: tome 2, Documentation technique (Biologie technique) [Google Scholar]
- Mahmoudi H., Aouadhi C., Kaddour R., Gruber M., Zargouni H., Zaouali W., Ben Hamida N., Ben Nasri M., Ouerghi Z., Hosni K. Comparison of antioxidant and antimicrobial activities of two cultivated Cistus species from Tunisia. Biosci. J. 2016;32:226–237. [Google Scholar]
- Mariotti A., Hefti A.F. Defining periodontal health. BMC Oral Health. 2015;15(Suppl 1):S6. doi: 10.1186/1472-6831-15-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason T.L., Wasserman B.P. Inactivation of red beet beta-glucan synthase by native and oxidized phenolic compounds. Phytochemistry. 1987;26:2197–2202. [Google Scholar]
- Mayrand D., Grenier D. Bacterial interactions in periodontal diseases. Bull. Inst. Pasteur. 1998;96:125–133. [Google Scholar]
- Molly, K., Vande Woestyne, M., Verstaete, W., 1993. Development of a 5 step multi-camber reactor as a simulation of human intestinal microbial ecosystem. Appl. Microbiol. Biotechnol. 39, 254–258. In: Chelli-Chentouf, N., TirTouilMeddah, A., Mullie, C., Aoues, A., Meddah, B., 2012. In vitro and in vivo antimicrobial activity of Algerian Hoggar Salvadora persica L. extracts against microbial strains from children’ soral cavity. J. Ethnopharmacol. 144, 57–66. [DOI] [PubMed]
- Orsi Llinares, F., 2005. La nigelle, une épice d’intérêt médicinal, thèse doctorat en pharmacie, faculté de pharmacie de Grenoble, Universite Joseph Fourier.
- Padhye S., Banerjee S., Ahmad A., Mohammad R., Sarkar F.H. From here to eternity, the secret of Pharaohs: therapeutic potential of black cumin seeds and beyond. Cancer Ther. 2008;6:495–510. [PMC free article] [PubMed] [Google Scholar]
- Phillips J.D. Medicinal plants. Biologist. 1992;39:187–191. [Google Scholar]
- Rams, T.E., Feik, D., Slots, J., 1990.Staphylococci in human periodontal diseases. Oral Microbiol. Immunol. 5, 29–32. In: Tanner, A., Maiden, M.F.J, Paster, B.J., Dewhirst, F.E., 1994. The impact of 16S ribosomal RNA-based phylogeny on the taxonomy of oral bacteria. Periodontol. 2000 5, 26–51. [DOI] [PubMed]
- Salem M.Z.M., Ali H.M., El-Shanhorey N.A., Abdel-Megeed A. Evaluation of extracts and essential oil from Callistemon viminalis leaves: Antibacterial and antioxidant activities, total phenolic and flavonoid contents. Asian Pac. J. Trop. Biomed. 2013:785–791. doi: 10.1016/S1995-7645(13)60139-X. [DOI] [PubMed] [Google Scholar]
- Salem M.Z.M., Aly H., Gohar Y., El-Sayed A.W. Biological activity of extracts from Morus alba L., Albizzia lebbeck (L.) Benth. and Casuarina glauca Sieber against the growth of some pathogenic bacteria. IJAFR. 2013;2:9–22. [Google Scholar]
- Salman M.T., Khan R.A., Shukla I. Antibacterial activity of Nigella Sativa Linn. seeds against multiple antibiotics resistant clinical strains of Staphylococcus aureus. Int. Arch. BioMed Clin. Res. 2016;2:96–99. doi: 10.21276/iabcr.2016.2.3.24. [DOI] [Google Scholar]
- Scandorieiro S., Camargo L.C., Lancheros C.A.C., Yamada-Ogatta S.F., Nakamura C.V., Oliveira A.G., Andrade C.G.T.J., Duran N., Nakazato G., Kobayashi R.K.T. Synergistic and additive effect of Oregano essential oil and biological Silver nanoparticles against Multidrug-Resistant bacterial strains. Front. Microbiol. 2016;7:760. doi: 10.3389/fmicb.2016.00760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva N., Abusleme L., bravo D., Dutzan N., Garcia-Sesnich J., Vernal R., Hernández M., Gamonal J. Host response mechanisms in periodontal diseases. J. Appl. Oral Sci. 2015;23:329–355. doi: 10.1590/1678-775720140259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souto R., Silva-Boghossian C.M., Colombo A.P. Prevalence of Pseudomonas aeruginosa and Acinetobacter spp. in subgingival biofilm and saliva of subjects with chronic periodontal infection. Braz. J. Microbiol. 2014;45:495–501. doi: 10.1590/s1517-83822014000200017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanis H., Aygan A., Digrak M. Antimicrobial activity of four Nigella species Grown in Southern Turkey. Int. J. Agric. Biol. 2009;11:771–774. [Google Scholar]
- Tanner A., Maiden M.F.J., Paster B.J., Dewhirst F.E. The impact of 16S ribosomal RNA-based phylogeny on the taxonomy of oral bacteria. Periodontology. 1994;2000(5):26–51. doi: 10.1111/j.1600-0757.1994.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Toama M.A., EL-Alfy T.S., EL-Fatatry H.M. Antimicrobial activity of the volatile oil of Nigella sativaLinneaus Seeds. Antimicrobial agents and chemotherapy. Antimicrob. Ag. Chemother. 1974;6:225–226. doi: 10.1128/aac.6.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toparslan, C., 2012. À propos de Nigella sativa L., These de doctorat en pharmacie, faculte de pharmacie, universite de Lorraine.
- Toppozada, H.H., Masloum, H., El-Dakhakhany, M., 1965. The anti-bacterial properties of Nigella sativa seeds: active principle with some clinical application. J. Egypt. Med. Asso., 48(suppl): 187-202. In: Shohayeb, M., Halawani, E., 2012. Comparative antimicrobial activity of some active constituents of N. sativa L. World Appl. Sci. J. 20, 182–189. 10.5829/idosi.wasj.2012.20.02.7156. [DOI] [PubMed]
- Touchstone J., Dobbins M.F. Practice of thin layer chromatography, wiley Interscience. Berlin. 1983:1–13. [Google Scholar]
- Wei S.H.Y., Lang N.P. Periodontal epidemiological indices for children and adolescents: II. Evaluation of oral hygiene; III. Clinical applications. Pediatr. Dent. 1982;4:64–73. [PubMed] [Google Scholar]
- Yacoubi A., Djamila B., Makhrelouf L., Bensoltane A. Microbiological Study of Periodontitis in the West of Algeria. World J. Med. Sci. 2010;5:7–12. [Google Scholar]
- Zawadzki, J., 1989. Infrared spectroscopy in surface chemistry of ethylene. In: Yakout, S.M., Sharaf El-Deen, G., 2016.Characterization of activated carbon prepared by phosphoric acid activation of olive stones. Arab. J. Chem. 9, 1155–1162. 10.1016/j.arabjc.2011.12.002. [DOI]
- Zhiri, A., 2006. Aromathérapie. Nutra news, science, nutrition, prévention et santé, ed Fondation pour le libre choix, pp. 2–16.