Abstract
Periodontium regeneration is a highly challenging process as it requires the regeneration of three different tissues simultaneously. The aim of this study was to develop a composite material that can be easily applied and can sufficiently deliver essential growth factors and progenitor cells for periodontal tissue regeneration.
Freeze-dried platelet concentrate (FDPC) was prepared and incorporated in a thermo-sensitive chitosan/β-glycerol phosphate (β-GP) hydrogel at concentrations of 5, 10, or 15 mg/ml. The viscosity of the hydrogels was investigated as the temperature rises from 25 °C to 37 °C and the release kinetics of transforming growth factor (TGF-β1), platelet-derived growth factor (PDGF-BB) and insulin-like growth factor (IGF-1) were investigated at four time points (1 h, 1 day, 1 week, 2 weeks). Periodontal ligament stem cells (PDLSCs) were isolated from human third molars and encapsulated in the different hydrogel groups. Their viability was investigated after 7 days in culture in comparison to standard culture conditions and non FDPC-loaded hydrogel.
Results showed that loading FDPC in the hydrogel lowered the initial viscosity in comparison to the unloaded control group and did not affect the sol-gel transition in any group. All FDPC-loaded hydrogel groups exhibited sustained release of TGF-β1 and PDGF-BB for two weeks with significant difference between the different concentrations. The loading of 10 and 15 mg/ml of FDPC in the hydrogel increased the PDLSCs viability significantly compared to the unloaded hydrogel and was comparable to the standard culture conditions.
Accordingly, it may be concluded that loading FDPC in a chitosan/β-GP hydrogel can offer enhanced injectability, a sustained release of growth factors and increased viability of encapsulated stem cells which can be beneficial in periodontium tissue regeneration.
Keywords: Periodontal regeneration, Thermo-sensitive hydrogel, Chitosan, Platelet concentrate, Growth factors
1. Introduction
One of the most prevalent destructive conditions to the teeth supportive structures is the inflammatory condition of the periodontium, namely periodontitis (Albandar, 2005). Periodontitis destroys the alveolar bone, cementum and periodontal ligament (PDL), the three constituent tissues of the periodontium. This destruction may ends up by losing the tooth if not treated properly. This in consequence results in esthetic, speech and masticatory deficiencies, in addition to claims of its link to heart diseases, which all have impact on the patient's emotional and general health (Pihlstrom et al., 2005, Kjellstrom et al., 2016).
Due to the great development in the regenerative science and understanding in the wound healing process, interest has been drawn toward using the body’s own mechanisms to stimulate and in some cases control the regeneration (Chen et al., 2010). This was accomplished by implementing the tissue engineering and regenerative medicine science which utilizes mainly three elements to build/regenerate new functional tissues. These three elements are a scaffold to support the new tissues being built, chemical cues to guide and enhance the tissue formation and cells to aid in the building process. According to the intended tissue to be regenerated, scaffold properties, type of the chemical cues and cells phenotype are tailored (Vacanti and Vacanti, 2000, Salgado et al., 2013).
Thermo-sensitive hydrogels have drawn a lot of attention in the biomedical and tissue engineering fields as they can deliver therapeutic molecules and cells to a body defect through simple injection with minimum surgical procedure. They are injected in their fluid state and this offers the feasibility to easily incorporate therapeutic agents or cells before the injection. Once injected, their flowing nature allows them to perfectly fill and adapt to the implantation site before gelation in comparison to a prefabricated scaffold (Liu et al., 2016). They contain low amount of dry mass (between 1 and 20% of total mass), which makes them less irritant to tissues and hence less inflammation is expected to happen in the peri-implantation area (Pakulska et al., 2012). Hydrogels loaded with drugs, growth factors, and bioactive compounds allow sustained release of their load. In addition, hydrogels can encapsulate cells as their permeable structure permits nutrients and metabolites diffusion (Fedorovich et al., 2007).
Different materials, such as chitosan, can be formulated to give thermo-sensitive hydrogels. Chitosan offers several biological advantages being antibacterial, bioadhesive and hemostatic which makes it good candidate for wound healing (Dash et al., 2011). Chitosan in the form of hydrogel is a 3D polymer network that can absorb and retain a large amount of water inside it due to the presence of high number of hydrophilic groups (Giri et al., 2012). However, chitosan lacks the required ligands for cell adhesion and several studies have reported delayed cells attachment and spreading on its surface (Wang and Stegemann, 2010, Srivastava et al., 2014, Zhang et al., 2014, Debnath et al., 2015). Cell adhesion to chitosan mainly depends on the proteins adsorption on its surface that allows the cells to recognize it. Incorporating bioactive molecules within the chitosan matrix that can present the required proteins on the chitosan surface for cell adhesion would enhance its performance as a scaffold in supporting the cells viability and proliferation (Rajendran et al., 2017). In addition, these bioactive molecules can be released from the chitosan to aid in the regeneration process through its interaction with the building cells (Ruel-gariepy et al., 2000).
A lot of researches have been conducted on using one or couple of natural or recombinant growth factors in relatively high concentrations to enhance the properties of different scaffold materials and this approach has been proven successful (Maskarinec and Tirrell, 2005, Morra, 2006). However, the isolation of natural growth factors or the development of the recombinant ones is very costly. Also, in the natural healing events, many growth factors are released in specific ratios and sequence which is hard to be replicated (Gurtner et al., 2008, Ganapathy et al., 2012). A way to deliver high concentrations of many natural growth factors is through the use of high concentration of platelets that can release more than twenty types of growth factors and plasma components in their biologically determined ratios (Creeper, 2009). The preparation of platelets in high concentration has been given many names according to its final form. These names are platelets concentrate (PC), platelet rich concentrate (PRC), platelet enriched plasma, platelet rich plasma (PRP), platelet releasate (PR), autologous platelet gel and preparation rich in growth factors (PRGF). These preparations have been introduced as a cheaper, safer and more effective way to locally deliver high concentration of many growth factors for enhanced healing and regeneration of tissues (Foster et al., 2009).
Platelet concentrate has short shelf-life span (4–5 days) to be used effectively. Therefore, different methods for platelets preservation have been investigated to extend their shelf-life like cryopreservation through the use of dimethyl sulfoxide (DMSO) (Valeri et al., 1974) or their fixation by paraformaldheyde (Read et al., 1995). However, freeze-drying has been proven to be the most effective approach for platelets long-time preservation (Pan et al., 2016). In addition, freeze-dried platelet concentrate is easy in transportation due to its low volume and weight, stable at room temperature for long times and easily manipulated and applied (Fan et al., 2009, Nakatani et al., 2017). Another shortcoming of the platelet concentrate is the rapid release of the platelet contents when delivered as it is and short benefit of its therapeutic effect (Yung et al., 2017). The incorporation of platelet concentrate in a carrier/scaffold like a hydrogel can sustain the release of the growth factors over the longer period of the regeneration process and present the required bioactive molecules for cells adhesion and proliferation on the scaffold surface.
Therefore, the current study aims at investigating the effect of loading a thermo-sensitive chitosan-based hydrogel with different concentrations of freeze-dried platelet concentrate (FDPC) on the sol-gel transition and the viscosity of the forming hydrogel, the release kinetics of growth factors and the viability of encapsulated periodontal ligament-derived stem cells.
2. Materials and methods
2.1. Materials
Chitosan (medium molecular weight and degree of deacetylation equal to 83% as determined by H NMR) was obtained from Sigma Aldrich, USA. Beta-glycerol phosphate (β-GP) was purchased from Biotech, India. Phosphate buffer saline (PBS) was obtained from Lonza, USA. Alpha–Minimum Essential Medium (α-MEM) and Trypsin/EDTA were obtained from Lonza, Belgium. Fetal Bovine Serum (FBS) was obtained from Seralab, UK. MTT Dye was purchased from Bio Basic Inc., USA. All other reagents were of analytical grade.
2.2. Thermo-sensitive chitosan hydrogel preparation and gelation
The thermo-sensitive chitosan hydrogel was prepared according to Khodaverdi et al. (2012). All steps were done in an aseptic environment and ultra-pure water was used to prepare the required solutions. Briefly, chitosan solution was prepared by dissolving 200 mg of chitosan powder in 9 ml of 0.1 M acetic acid solution of pH = 4. The pH of the acetic acid was monitored using a pH meter and adjusted by the addition of potassium hydroxide (1M). Chitosan powder was added to the solvent incrementally over 15 min to prevent clumping of the powder and then left under continuous magnetic stirring for two hours at room temperature for complete dissolution. β-GP aqueous solution of 0.45 M concentration was prepared and filtered through 0.22 µm filter. Both solutions were then stored at 4 °C until mixing.
Preparation of the thermo-sensitive hydrogel was accomplished through the addition of 1 ml of the β-GP solution dropwise to 9 ml of the chitosan solution while stirring in an ice bath. Stirring was done for 5 min and a 2% w/v chitosan/β-GP solution was obtained. This solution was then incubated at 37 °C for 10 min to allow gelation to occur.
2.3. Preparation of freeze-dried platelets concentrate (FDPC)
Six units platelet bag was purchased from Shabrawishi hospital blood bank, El-Dokki, Giza, Egypt. The platelet units were divided into polypropylene tubes, each containing 10 ml of plasma. The tubes were then centrifuged at 4450 rpm for 10 min with zero acceleration to concentrate the platelets in each tube into a pellet. After the centrifugation, 95% of the supernatant (platelet poor plasma) was aspirated carefully without disturbing the formed platelet pellets and discarded. Each platelet pellet was then re-suspended into 2 ml of Tyrode’s solution buffer (136 mM NaCl, 11.9 mM NaHCO3, 5.6 mM glucose, 5 mM HEPES, 2.7 mM KCl, 2 mM MgCl2, 0.42 mM NaH2PO4, pH 7.4) (Pietramaggiori, 2006) and platelets concentration was adjusted to 1 × 106/µl in each tube. The solution was then incubated in an orbital shaker rotating at 60 rpm at 37 °C for two hours. The platelet concentrate solutions were then gradually frozen down by freezing in a −20 °C freezer for one hour then in a −80 °C ultra-freezer for additional two hours before freeze-drying for 24 h (Alpha 2–4 LDplus, Christ, Germany). After the freeze-drying process was completed, the tubes were immediately tightly closed and saved at −80 °C until use.
2.4. FDPC loading of the hydrogel
The prepared FDPC powder was mixed with the chitosan solution before the addition of the β-GP solution in different concentrations to create four groups as listed in Table 1.
Table 1.
Groups description.
| Groups | Description |
|---|---|
| Group I | Chitosan hydrogel only |
| Group II | Chitosan hydrogel loaded with 5 mg/ml FDPC |
| Group III | Chitosan hydrogel loaded with 10 mg/ml FDPC |
| Group IV | Chitosan hydrogel loaded with 15 mg/ml FDPC |
2.5. Viscosity measurements
The viscosity was measured as a sample of the chitosan/β-GP solution was heated from 25 °C to 37.5 °C to record the changes in the viscosity during the sol-gel transition. A rheometer (DV3T, Brookfield, USA) with cone/plate attachment and a temperature controller was used. Chitosan solution mixed with FDPC powder according to each group specified weight (Table 1) was mixed with the β-GP solution just before each run. After the plate attachment of the rheometer was cooled to 23 °C, a 0.5 ml of the freshly mixed cold solution was injected in the middle of the plate. The cone was then inserted in place with a separation distance of 0.013 mm between the cone tip and the plate. The resistance of the hydrogel solution to flow with the rotating cone was measured as the temperature ramped up and readings were recorded every 2.5 °C rise starting from 25 °C to 37.5 °C.
2.6. Growth factor release assessment
Groups II, III and IV of the chitosan hydrogel loaded with FDPC were tested for the release of three growth factors: transforming growth factor-β1 (TGF-β1), platelets derived growth factor (PDGF-BB) and insulin growth factor (IGF-1). A total of fifteen disc-shaped hydrogel samples (n = 5), 10 mm in diameter and 4 mm in thickness, were prepared. After gel formation, samples were removed from the molds and each sample was immersed in 2 ml PBS in polypropylene tubes. The samples were then incubated at 37 °C in an orbital shaker rotating at a speed of 100 rpm. At time points 1 h, 1 day, 1 week and 2 weeks, a volume of 0.5 ml of the PBS solution from each sample was withdrawn and saved at −80 °C in an ultra-freezer in a separate Eppendorf tube. The original solution around each sample was refilled with an equal volume (0.5 ml) of new PBS to maintain the volume around the samples during testing.
After collecting the sample solutions at all-time points, sandwich enzyme-linked immunosorbent assay (ELISA) steps were done according to the manufacturer instructions for TGF-β1 (Kit #: EIA-1864, DRG International, Inc., USA), PDGF-BB (kit #: E-EL-H1577, Elabscience, China) and IGF-1 (kit #: EIA-4140, DRG International, Inc., USA). Optical density of the developed color was measured using a microplate reader (Stat Fax 2100, Awareness Technology Inc., USA) and cumulative release values were calculated. Empty wells (Control samples) were included in the test and their results were subtracted from the values of the tested groups.
2.7. Stem cells separation and propagation
Cell separation protocol of the periodontal ligament stem cells (PDLSCs) that was established by Seo et al. (2004), was followed with modification. After informed consent from the patients, three human third molar teeth with complete healthy roots were collected from patients (age 25–35) who have undergone impaction surgery at the Department of Oral Surgery, Faculty of Oral and Dental Medicine, Future University, Egypt. Immediately after extraction, the teeth were put in α-MEM with 15% FBS, 100 U/ml Penicillin and 100 µg/ml streptomycin.
The teeth were washed three times by a sterile PBS and the PDL from the middle third of the roots was scraped by no. 15 scalpel. The collected periodontal tissues were minced and cultured in a 10 cm diameter tissue culture plate with α-MEM supplemented with 15% FBS, 100 µM ascorbic acid 2-phosphate, 2 mM L-glutamine, 100 U/ml Penicillin and 100 µg/ml streptomycin. The cultured explants were maintained at 37 °C in a humidified atmosphere and 5% CO2. The culture medium was changed every 3–4 days and the cells were evaluated daily during culture period using phase contrast microscope (model: CKX41, Olympus Global, Japan). After 8 days of culture, the cells that migrated out of the tissue explants were detached from the plate floor (trypsinized) and passaged into new culture flasks. Passaging was repeated as culture flasks reached 80% confluence.
2.8. PDLSCs encapsulation and viability assessment
PDLSCs at the fourth passage were gently added and mixed in the chitosan/β-GP hydrosol FDPC-unloaded (negative control) and FDPC-loaded groups to be homogenously incorporated inside the gel. The added volume of the cell suspension allowed the cells concentration to be 10,000 cells per each 150 μl of the hydrogel. Before gelation of the hydrosol, 150 µl of the sol were injected in the middle of each well of a 24 well plate (n = 5) and the plate was incubated for 10 min at 37 °C to allow for gelation to occur. Then, a volume of 0.5 ml of the full medium was added to each well. In addition, cells only (with same concentration) were cultured as positive control group in different wells (n = 5).
After 7 days of incubation, a volume of 50 µl of MTT solution of concentration of 5 mg/ml was added to the medium of each well and incubated for 4 h to allow for the formation of formazan crystals. The media were then discarded from all wells and 200 µl of dimethyl sulfoxide (DMSO) were added to each well. The plate was then put on shaker with rotation speed of 80 rpm for 30 min to dissolve the formed formazan crystals. The solution in each well was then transferred to an Eppendorf tube and diluted with additional 1 ml of DMSO. The solutions color absorption values were measured at a wavelength of 560 nm using UV/Vis Spectrophotometer (Libra S50, Biochrom, UK). Mean absorbance of the samples of each group was measured and the viability percentage was calculated taking the positive control (cells only) as 100% survival. The control and test groups are described in Table 2. An unloaded-hydrogel without cells group was also tested to subtract its values from the values of the tested groups to exclude any effect of the hydrogel on the color absorption values.
Table 2.
MTT assay groups.
| Groups | Description |
|---|---|
| Positive control | Cells only |
| Group I (Negative control) | Hydrogel + cells |
| Group II | Hydrogel loaded with 5 mg/ml FDPC + cells |
| Group III | Hydrogel loaded with 10 mg/ml FDPC + cells |
| Group IV | Hydrogel loaded with 15 mg/ml FDPC + cells |
2.9. Statistical analysis
Data was analyzed and tabulated in the form of mean ± standard deviation using statistical package for the social sciences (SPSS) version 20 for windows. Data was tested for normality using Kolmogorov Smirnov’s test. Comparisons between the groups were based on ANOVA for parametric data with Tukey’s test as post hoc test and Kruskal-Wallis test for non-parametric data with automated Dunn-Benferroni post hoc method. The intra group comparison was performed using Friedman’s Analysis of Variance by rank with automated Dunn-Benferroni post hoc method. All comparisons were done at statistical significance level of P < 0.05. Graphical depictions of mean and standard deviation data were constructed with Microsoft Excel 2016.
3. Results
3.1. Thermo-sensitive chitosan hydrogel preparation and gelation
Gelation of the thermo-sensitive chitosan hydrogel was confirmed. The flow of the solution stopped after 10 min at 37 °C which confirmed the transformation to the gel state (Fig. 1). The gelation was irreversible.
Fig. 1.
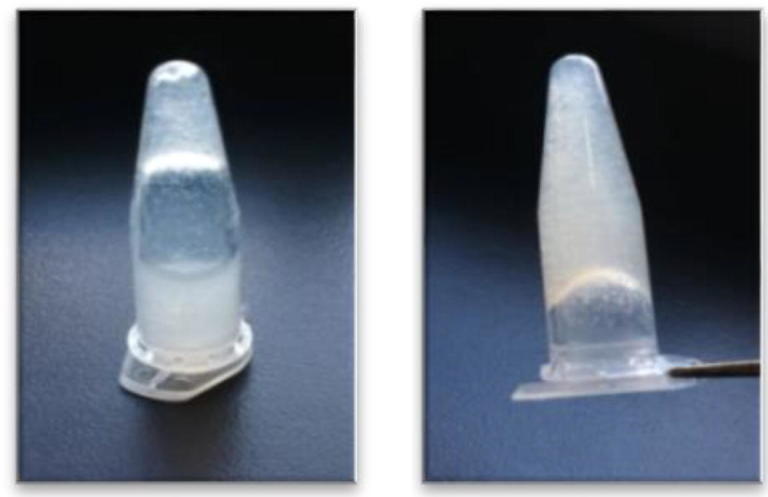
The hydrogel before setting (left) and after setting at 37 °C (right).
3.2. Viscosity measurements
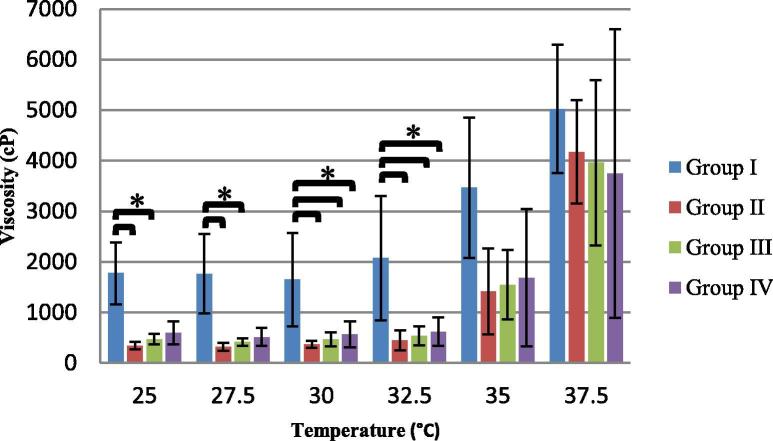
The values of the viscosity as the temperature rises of the chitosan/β-GP solution (Group I) showed that the viscosity plateaued at temperatures below 30 °C and started to increase gradually from temperature 30 °C to 37.5 °C to reach almost three fold increase (Fig. 2). The three groups containing FDPC showed viscosity increase following the same trend that was observed with the control group (Group I). Their viscosity values almost plateaued from the starting temperature of the test (25 °C) until about temperature 32.5 °C with small values of standard of deviation. Starting from temperature 32.5 °C, the viscosity started to increase gradually until temperature 37.5 °C where the standard of deviation increased markedly.
Fig. 2.
Mean viscosity (cP) values (±SD) of the four groups at each temperature (°C) point (*indicates significant difference).
Intra-group comparison between all temperature points revealed that significant difference (p < 0.05) was present between temperature 37.5 °C and the first three temperatures of the test (25, 27.5 and 30 °C) in all groups. On the other hand, statistical comparison of the viscosity values between the groups (Fig. 2) showed that at temperatures 25 °C and 27.5 °C, group I exhibited significantly higher viscosity values than groups II and III and at temperatures 30 °C and 32.5 °C, it had significantly higher viscosity values than all the other three groups. At temperatures 35 °C and 37.5 °C, no significant differences were found between any of the groups.
3.3. Growth factor release assessment
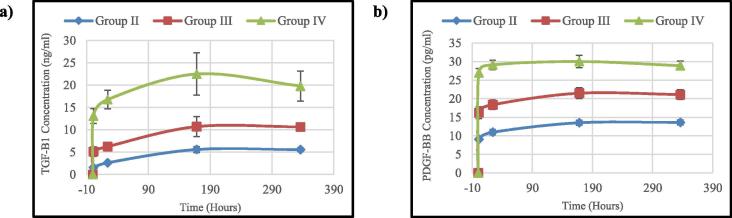
The cumulative release assessment results (Table 3, Table 4) for the TGF-β1 and PDGF-BB showed that the tested samples were able to sustain the release of the growth factors throughout the test duration. While, the IGF-1 results from all the tested groups were negative. The release profile of both TGF-β1 and PDGF-BB (Fig. 3) showed that there was an initial burst release during the first hour of the test from all groups followed by gradual increase to the next one day time point for all groups. This initial burst release increased from group II to group IV as the platelet concentration increased and it was very pronounced in group IV which had the highest platelet concentration.
Table 3.
Mean (ng/ml) cumulative release values of TGF-β1of all groups at the four time points.
| Group | 1 Hour | 1 Day | 1 Week | 2 Weeks |
|---|---|---|---|---|
| Group II | 1.53 ± 0.29Aa | 2.63 ± 0.20Ba | 5.60 ± 0.66Ca | 5.55 ± 0.41Ca |
| Group III | 5.11 ± 1.07Ab | 6.25 ± 0.52Bb | 10.73 ± 2.26Cb | 10.66 ± 0.68Cb |
| Group IV | 13.10 ± 1.67Ac | 16.77 ± 2.08Bc | 22.50 ± 4.72Cc | 19.79 ± 3.35Dc |
–Different capital superscript letters in each raw indicate significant difference between different time points within the same group.
–Different small superscript letters in each column indicate significant difference between the different groups at each time point.
Table 4.
Mean (pg/ml) cumulative release values of PDGF-BB of all groups at the four time points.
| Group | 1 Hour | 1 Day | 1 Week | 2 Weeks |
|---|---|---|---|---|
| Group II | 9.08 ± 0.46Aa | 10.97 ± 0.72Ba | 13.54 ± 0.63Ca | 13.62 ± 0.66Ca |
| Group III | 16.24 ± 1.57Ab | 18.37 ± 1.28Bb | 21.50 ± 1.48Cb | 21.12 ± 1.32Cb |
| Group IV | 27.00 ± 1.12Ac | 29.13 ± 1.29Bc | 30.04 ± 1.65Bc | 28.91 ± 1.21Bc |
–Different capital superscript letters in each raw indicate significant difference between different time points within the same group.
–Different small superscript letters in each column indicate significant difference between the different groups at each time point.
Fig. 3.
Real time cumulative release profile of: (a) TGF-β1 and (b) PDGF-BB.
At the one week time point, TGF- β1 release profile showed more increase for all the groups, while PDGF-BB release profile showed that only group II and group III had increased release but there was no significant difference in group IV in the release between the one day and one week time points. At the two weeks time point, the concentration of the released TGF-β1 showed almost similar value as one week time point in groups II and III. In group IV, the released TGF-β1 showed decrease in its concentration than the one week time point, while for PDGF-BB, the release concentrations were almost the same as one week time point in all groups.
3.4. Stem cells separation and propagation
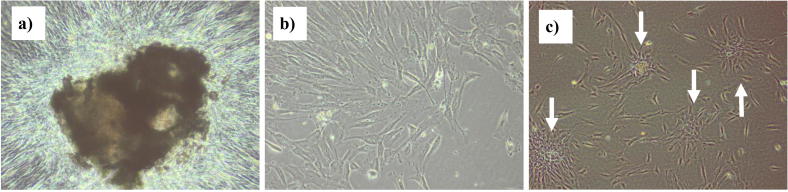
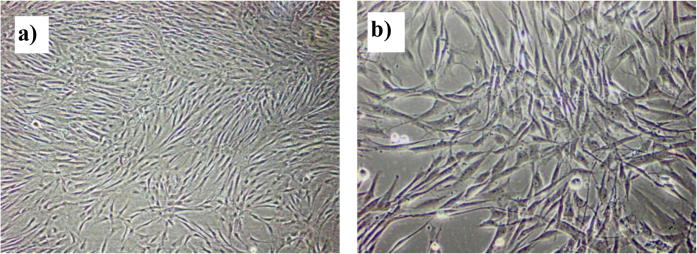
After four days in culture, spindle shaped cells started to migrate out of the tissue pieces from all their circumferences and moved toward less density spaces in the culture plate. By the eighth day of culture, more cells migrated out and filled more spaces of the culture plate (Fig. 4a, b). Cells formed colonies (Fig. 4c) as they were passaged for the first time. Cells were successively passaged as they reached 80% confluence of the culture flask floor (Fig. 5).
Fig. 4.
Phase contrast images showing: (a) tissue explants, (b) cells migrated out of the explant at day 8 of culture and (c) cells forming colonies (white arrows) after passaging (100× magnification).
Fig. 5.
Phase contrast images showing PDLSCs filling the culture flask floor (80% confluence): (a) 100× magnification, (b) 200× magnification.
3.5. Encapsulated PDLSCs viability assessment
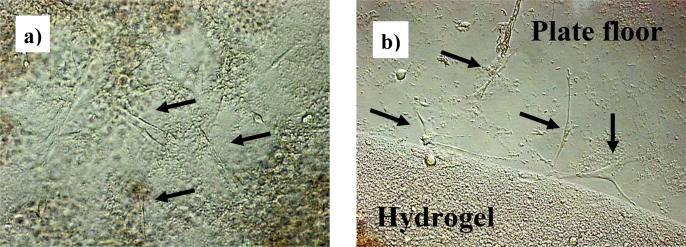
Phase contrast microscope examination of the PDLSCs encapsulated in the hydrogel revealed viability of cells as they were seen through and at the edges of the hydrogel spreading and adherent to the floor of the plate (Fig. 6).
Fig. 6.
Phase contrast images showing viable PDLSCs (Black arrows) adhering to the plate floor: (a) Through the hydrogel, (b) at the edge of the hydrogel (200× magnification).
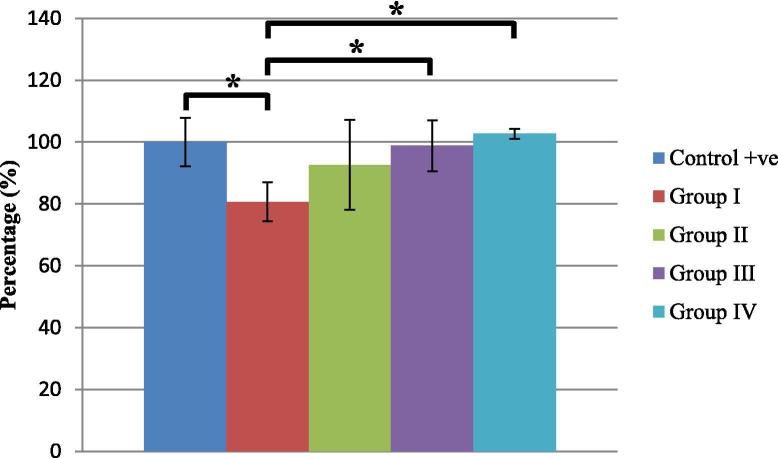
MTT assay results revealed that PDLSCs were able to stay viable during the test period (7 days) encapsulated inside the hydrogel. Cells viability percentages compared to the positive control group (taken as 100% viability) are presented in Fig. 7. Group I (FDPC-unloaded group) was significantly lower in cells viability than the positive control group (cells only), while group III and group IV were significantly higher in cells viability than the unloaded group (negative control group). In addition, group III and group IV were comparable to the positive control. The cells viability in group II did not significantly differ from any of the other four groups.
Fig. 7.
Cell viability percentages (±SD) in the different groups after 7 days of culture (*indicates significant difference).
4. Discussion
The composite hydrogel prepared in the current study is aimed to be injected in the bone defects around teeth while it is in the sol state and to change to the gel state inside the defect at the body temperature. Viscosity measurements were done in this study to investigate the state of the chitosan/β-GP solution as temperature rises from room temperature (25 °C) to the temperature of the human body (37 °C). It was important to confirm that the sol-gel transition starts at temperatures near the body temperature and that the chitosan/β-GP solution has a relatively low viscosity at room temperature to facilitate its injectability. Moreover, it was necessary to investigate the effect of adding FDPC on the sol viscosity and the sol-gel transition temperature.
The results showed that the chitosan/β-GP system prepared in this study maintained its viscosity values from room temperature until 30 °C and then started to increase gradually until temperature 37.5 °C. The rise in the viscosity reached about three fold increase as it reached temperature 37.5 °C. This change in the viscosity values is a result of the sol-gel transition in the chitosan/β-GP system due to the increase in the hydrophobic interaction between the chitosan chains as the temperature rises (Chenite et al., 2001).
The addition of FDPC at different concentrations to the hydrogel did not affect the sol-gel transition profile as the temperature was raised. However, it lowered the starting viscosity values rendering the hydrogel less viscous. This could be attributed to the positively charged growth factors that are released from the platelets (King and Krebsbach, 2012, Zhao et al., 2015). These positively charged molecules may have increased the repulsion forces between the chitosan chains leading to increased inter-chain distances and easier chains sliding. This effect could ease the injectability of the material rendering it more practical in its application.
The periodontal regeneration process and healing period can be enhanced by incorporating signaling molecules that modulate cells functions. Therefore, the FDPC was incorporated in the chitosan hydrogel to sustain the release of the natural bioactive agents contained inside the platelets. The growth factors investigated in the current study were selected for their important role in regulating and enhancing the regeneration of tissues (Barrientos et al., 2008). Both TGF-β and IGF-1 induce matrix formation. In addition, IGF-1 regulates cells proliferation and maturation and inhibits cell death. On the other hand, PDGF-BB has been found to be a potent mitogenic factor and to have a chemotactic effect on mesenchymal cells including periodontal progenitor cells in comparison to its other isoforms (PDGF-AA, PDGF-AB). PDGF-BB also enhances the stabilization of the IGF receptors, preventing their down-regulation (Raja et al., 2009, Javed et al., 2011).
In the current investigation, the release assay of TGF-β1 and PDGF-BB from the FDPC-loaded hydrogels showed cumulative sustained release of these two growth factors for two weeks. The release profiles of both growth factors showed almost the same trend but different in the amounts released. They were characterized by initial burst release during the first hour that increased with the increase in FDPC loading concentration. This was followed by a gradual increase in their release at one day and one week time points and leveled off for most of the groups at the two weeks time points.
The initial burst release phase could be attributed to several factors. First, chitosan is known to induce platelet aggregation and activation which may lead to fast release of the platelets load (Shen et al., 2006). Second, the hydrogel is a very porous structure and when immersed in the release medium (PBS), it swells and its pores widen (Berger et al., 2005). This leads to fast initial diffusion of the growth factors near the surface and inside the pores (Khodaverdi et al., 2012). Third, both TGF-β1 and PDGF-BB are positively charged molecules (Yamamoto et al., 2000, Chen et al., 2013a, Chen et al., 2013b). This may leads to some repulsive forces between them and any remaining positive charges on the chitosan chains that may have not been neutralized by the β-GP during the hydrogel preparation. These repulsive forces may also contribute to their rapid initial release. This initial fast release could be advantageous considering the chemotactic effect of PDGF-BB and the benefit of recruiting high number of progenitor cells at the early stages of the defect regeneration. In addition, the effect of TGF-β1 and PDGF-BB in increasing the cells proliferation and matrix formation is also needed in the early stages of defect regeneration as to fasten the building of new tissues (Park et al., 2000).
The second phase of slower gradual release can be attributed to the diffusion of the growth factors through the smaller pores and the deeper sections of the hydrogel which takes more time than the diffusion of the surface-adsorbed growth factors. Also, the decrease in the availability of these growth factors by time leads to decrease in the released amount. Finally, at the two weeks time point, results showed that the growth factors concentration leveled off giving a plateau pattern in groups II and III, and decreased in group IV for TGF-β1 while plateaued in all groups of PDGF-BB. This level off or decrease in the release rate may indicate the depletion of the mobile growth factors moieties. Moreover, one should consider the stability of the growth factors in the release medium at 37 °C. Most of the growth factors denature at such temperature and are only stable for a relatively short period of time (Bradley et al., 2009). Taking in mind this fact, a lot of the released growth factors may have been deactivated during the immersion and they were not measured. Therefore, as the release rate decreases at the end of the test period, it may approach the rate of denaturation of the growth factors, thus the measured release profile plateaus.
Regarding the IGF-1, the results were all negative for all the groups. This could be attributed to the fact that this growth factor is present mainly in the plasma not inside the platelets (Eppley et al., 2004). In the preparation steps of platelet concentrate in the current study, only a very small amount of the plasma was left with the platelets so as to enhance the effect of the preservation buffer. It seems that this amount of plasma contained only a very low level of IGF-1 that could not be detected. In addition, IGF-1 has a very short half-life and its serum levels differ greatly among individuals according to many variables like nutritional status, exercise and genetics (Foster et al., 2009). Therefore, it should be noted that following the FDPC preparation protocol used in the current study, the levels of the IGF-1 could be undetectable.
To test the ability of cells to be encapsulated in the chitosan hydrogel and to retain their viability, PDLSCs were isolated, propagated and encapsulated in the different groups of the prepared chitosan hydrogel/FDPC. The PDLSCs were selected to be used in the current study as the intention of using this hydrogel was mainly for periodontium regeneration. The PDLSCs have the potential to regenerate the different tissues of the periodontium. PDLSCs had been shown to be the most favorable candidate in periodontal regeneration in different studies compared to other stem cells (Wada et al., 2009, Tsumanuma et al., 2011, Lymperi et al., 2013, Han et al., 2014, Zhu and Liang, 2015).
The results of testing the viability of the PDLSCs encapsulated in the chitosan/β-GP hydrogel showed that the different hydrogel groups were able to maintain the viability of the cells during the 7 days test period. The viability of the PDLSCs encapsulated in the hydrogel unloaded with FDPC (negative control) was lower than that of the positive control group which represents the standard culture environment in vitro. This could be attributed to the fact that cells like to adhere to a surface to effectively proliferate, a phenomena called anchorage dependence (Dike and Farmer, 1988, Chen et al., 2013a, Chen et al., 2013b) and cells take some time to attach to the chitosan surface (Wang and Stegemann, 2010) in comparison to the polystyrene-treated tissue culture plate surface. Therefore, it could be that the chitosan hydrogel unloaded with FDPC in the current study only offered a biocompatible environment for the cells but without the required attachment platform to maintain its viability and to induce cells proliferation.
However, the FDPC-loaded groups were comparable to the positive control group and groups III and IV were significantly higher in cells viability than the unloaded group (negative control). This could be attributed to that the released platelets contents had modulated the chitosan surface through the presentation of adhesive proteins like fibronectin and fibrinogen for cells to adhere and proliferate. This is in addition to the role of many growth factors released from the platelets that have indisputable role in enhancing cells function and proliferation (Kakudo et al., 2008, Mishra et al., 2008, Crespo-Diaz et al., 2011, Creeper and Ivanovski, 2012, Jo et al., 2012, Shani et al., 2014). Finally, the comparable results between the FDPC loaded groups could be due to the small difference in the FDPC concentrations loaded in the hydrogel.
5. Conclusions
Within the limits of the present study, freeze-dried platelet concentrate (FDPC) can be incorporated in a chitosan thermo-sensitive hydrogel without adversely affecting its sol-gel transition and viscosity. In addition, the FDPC improves the hydrogel’s ability to maintain the viability of cells encapsulated within its matrix to levels comparable to standard culture environment and to sustain the release of growth factors essential for tissue regeneration for up to two weeks. Finally, pre-clinical and clinical studies need to be performed before these results could be extrapolated to clinical application.
Ethical statement
There are no animal experiments carried out for this article.
Acknowledgments
Acknowledgments
The authors would like to thank the Faculty of Pharmacy, Future University, Egypt for providing all the help and facilities for doing the materials preparation and characterization. Also, the authors would like to thank the Biochemistry Department, Faculty of Medicine, Cairo University, Egypt for helping in conducting the ELISA testing.
Conflict of interest
The authors declared that there is no conflict of interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Peer review under responsibility of King Saud University.
References
- Albandar J.M. Epidemiology and risk factors of periodontal diseases. Dent. Clin. North Am. 2005;49:517–532. doi: 10.1016/j.cden.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Barrientos S., Stojadinovic O., Golinko M.S., Brem H., Tomic-Canic M. Perspective article: growth factors and cytokines in wound healing. Wound Repair Regen. 2008;16:585–601. doi: 10.1111/j.1524-475X.2008.00410.x. [DOI] [PubMed] [Google Scholar]
- Berger J., Reist M., Chenite A., Felt-Baeyens O., Mayer J.M., Gurny R. Pseudo-thermosetting chitosan hydrogels for biomedical application. Int. J. Pharm. 2005;288:17–25. doi: 10.1016/j.ijpharm.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Bradley J.C., Simoni J., Bradley R.H., McCartney D.L., Brown S.M. Time- and temperature-dependent stability of growth factor peptides in human autologous serum eye drops. Cornea. 2009;28:200–205. doi: 10.1097/ICO.0b013e318186321e. [DOI] [PubMed] [Google Scholar]
- Chen F., Zhang J., Zhang M., An Y., Chen F., Wu Z. A review on endogenous regenerative technology in periodontal regenerative medicine. Biomaterials. 2010;31:7892–7927. doi: 10.1016/j.biomaterials.2010.07.019. [DOI] [PubMed] [Google Scholar]
- Chen P.H., Chen X., He X. Platelet-derived growth factors and their receptors: structural and functional perspectives. Biochim. Biophys. Acta. 2013;1834:2176–2186. doi: 10.1016/j.bbapap.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S., Lewallen M., Xie T. Adhesion in the stem cell niche: biological roles and regulation. Development. 2013;140:255–265. doi: 10.1242/dev.083139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenite A., Buschmann M., Wang D., Chaput C., Kandani N. Rheological characterisation of thermogelling chitosan/glycerol-phosphate solutions. Carbohydr. Polym. 2001;46:39–47. [Google Scholar]
- Creeper F., Lichanska A.M., Marshall R.I., Seymour G.J., Ivanovski S. The effect of platelet-rich plasma on osteoblast and periodontal ligament cell migration, proliferation and differentiation. J. Periodontal. Res. 2009;44:258–265. doi: 10.1111/j.1600-0765.2008.01125.x. [DOI] [PubMed] [Google Scholar]
- Creeper F., Ivanovski S. Effect of autologous and allogenic platelet-rich plasma on human gingival fibroblast function. Oral Dis. 2012;18:494–500. doi: 10.1111/j.1601-0825.2011.01897.x. [DOI] [PubMed] [Google Scholar]
- Crespo-Diaz R., Behfar A., Butler G.W., Padley D.J., Sarr M.G., Bartunek J., Dietz A.B., Terzic A. Platelet lysate consisting of a natural repair proteome supports human mesenchymal stem cell proliferation and chromosomal stability. Cell Transplant. 2011;20:797–811. doi: 10.3727/096368910X543376. [DOI] [PubMed] [Google Scholar]
- Dash M., Chiellini F., Ottenbrite R.M., Chiellini E. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011;36:981–1014. [Google Scholar]
- Debnath T., Ghosh S., Potlapuvu U.S., Kona L., Kamaraju S.R., Sarkar S., Gaddam S., Chelluri L.K. Proliferation and differentiation potential of human adipose-derived stem cells grown on chitosan hydrogel. PLoS One. 2015;10 doi: 10.1371/journal.pone.0120803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dike L.E., Farmer S.R. Cell adhesion induces expression of growth-associated genes in suspension-arrested fibroblasts. Proc. Natl. Acad. Sci. USA. 1988;85:6792–6796. doi: 10.1073/pnas.85.18.6792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eppley B.L., Woodell J.E., Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast. Reconstr. Surg. 2004;114:1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- Fan J., Xu X., Zhang S., Zhu F., Chen G., Yan L. Experimental study on rehydration conditions of freeze-dried platelets. J. Zhejiang Univ. Sci. A. 2009;10:697–703. [Google Scholar]
- Fedorovich N.E., Alblas J., de Wijn J.R., Hennink W.E., Verbout A.J., Dhert W.J. Hydrogels as extracellular matrices for skeletal tissue engineering: state-of-the-art and novel application in organ printing. Tissue Eng. 2007;13:1905–1925. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- Foster T.E., Puskas B.L., Mandelbaum B.R., Gerhardt M.B., Rodeo S.A. Platelet-rich plasma: from basic science to clinical applications. Am. J. Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- Ganapathy N., Venkataraman S.S., Daniel R., Aravind R.J., Kumarakrishnan V.B. Molecular biology of wound healing. J. Pharm. Bioallied Sci. 2012;4:S334–S337. doi: 10.4103/0975-7406.100294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giri T.K., Thakur A., Alexander A., Badwaik H., Ajazuddin, Tripathi D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: present status and applications. Acta Pharm. Sin. B. 2012;2:439–449. [Google Scholar]
- Gurtner G.C., Werner S., Barrandon Y., Longaker M.T. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Han J., Menicanin D., Gronthos S., Bartold P. Stem cells, tissue engineering and periodontal regeneration. Aust. Dent. J. 2014;59:117–130. doi: 10.1111/adj.12100. [DOI] [PubMed] [Google Scholar]
- Javed F., Al-Askar M., Al-Rasheed A., Al-Hezaimi K. Significance of the platelet-derived growth factor in periodontal tissue regeneration. Arch. Oral Biol. 2011;56:1476–1484. doi: 10.1016/j.archoralbio.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Jo C.H., Kim J.E., Yoon K.S., Shin S. Platelet-rich plasma stimulates cell proliferation and enhances matrix gene expression and synthesis in tenocytes from human rotator cuff tendons with degenerative tears. Am. J. Sports Med. 2012;40:1035–1045. doi: 10.1177/0363546512437525. [DOI] [PubMed] [Google Scholar]
- Kakudo N., Minakata T., Mitsui T., Kushida S., Notodihardjo F.Z., Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast. Reconstr. Surg. 2008;122:1352–1360. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- Khodaverdi E., Tafaghodi M., Ganji F., Abnoos K., Naghizadeh H. In vitro insulin release from thermosensitive chitosan hydrogel. AAPS PharmSciTech. 2012;13:460–466. doi: 10.1208/s12249-012-9764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King W.J., Krebsbach P.H. Growth factor delivery: how surface interactions modulate release in vitro and in vivo. Adv. Drug Deliv. Rev. 2012;64:1239–1256. doi: 10.1016/j.addr.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellstrom B., Ryden L., Klinge B., Norhammar A. Periodontal disease-important to consider in cardiovascular disease prevention. Exp. Rev. Cardiovasc. Ther. 2016;14:987–989. doi: 10.1080/14779072.2016.1202112. [DOI] [PubMed] [Google Scholar]
- Liu L., Gao Q., Lu X., Zhou H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016;11:673–683. [Google Scholar]
- Lymperi S., Ligoudistianou C., Taraslia V., Kontakiotis E., Anastasiadou E. Dental stem cells and their applications in dental tissue engineering. Open Dent. J. 2013;7:76–81. doi: 10.2174/1874210601307010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskarinec S.A., Tirrell D.A. Protein engineering approaches to biomaterials design. Curr. Opin. Biotechnol. 2005;16:422–426. doi: 10.1016/j.copbio.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Mishra A., Tummala P., King A., Lee B., Kraus M., Tse V., Jacobs C.R. Buffered platelet-rich plasma enhances mesenchymal stem cell proliferation and chondrogenic differentiation. Tissue Eng Part C, Methods. 2008;15:431–435. doi: 10.1089/ten.tec.2008.0534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morra M. Biochemical modification of titanium surfaces: peptides and ECM proteins. Eur. Cell. Mater. 2006;12:1–15. doi: 10.22203/ecm.v012a01. [DOI] [PubMed] [Google Scholar]
- Nakatani Y., Agata H., Sumita Y., Koga T., Asahina I. Efficacy of freeze-dried platelet-rich plasma in bone engineering. Arch. Oral Biol. 2017;73:172–178. doi: 10.1016/j.archoralbio.2016.10.006. [DOI] [PubMed] [Google Scholar]
- Pakulska M.M., Ballios B.G., Shoichet M.S. Injectable hydrogels for central nervous system therapy. Biomed. Mater. 2012;7 doi: 10.1088/1748-6041/7/2/024101. [DOI] [PubMed] [Google Scholar]
- Pan L., Yong Z., Yuk K.S., Hoon K.Y., Yuedong S., Xu J. Growth factor release from lyophilized porcine platelet-rich plasma: quantitative analysis and implications for clinical applications. Aesthetic Plast. Surg. 2016;40:157–163. doi: 10.1007/s00266-015-0580-y. [DOI] [PubMed] [Google Scholar]
- Park Y.J., Lee Y.M., Park S.N., Sheen S.Y., Chung C.P., Lee S.J. Platelet derived growth factor releasing chitosan sponge for periodontal bone regeneration. Biomaterials. 2000;21:153–159. doi: 10.1016/s0142-9612(99)00143-x. [DOI] [PubMed] [Google Scholar]
- Pietramaggiori G., Kaipainen A., Czeczuga J.M., Wagner C.T., Orgill D.P. Freeze-dried platelet-rich plasma shows beneficial healing properties in chronic wounds. Wound Repair Regen. 2006;14:573–580. doi: 10.1111/j.1743-6109.2006.00164.x. [DOI] [PubMed] [Google Scholar]
- Pihlstrom B.L., Michalowicz B.S., Johnson N.W. Periodontal diseases. Lancet. 2005;366:1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- Raja S., Byakod G., Pudakalkatti P. Growth factors in periodontal regeneration. Int. J. Dent. Hyg. 2009;7:82–89. doi: 10.1111/j.1601-5037.2009.00380.x. [DOI] [PubMed] [Google Scholar]
- Rajendran D., Hussain A., Yip D., Parekh A., Shrirao A., Cho C.H. Long-term liver-specific functions of hepatocytes in electrospun chitosan nanofiber scaffolds coated with fibronectin. J. Biomed. Mater. Res. A. 2017;105:2119–2128. doi: 10.1002/jbm.a.36072. [DOI] [PubMed] [Google Scholar]
- Read M.S., Reddick R.L., Bode A.P., Bellinger D.A., Nichols T.C., Taylor K., Smith S.V., McMahon D.K., Griggs T.R., Brinkhous K.M. Preservation of hemostatic and structural properties of rehydrated lyophilized platelets: potential for long-term storage of dried platelets for transfusion. Proc. Natl. Acad. Sci. USA. 1995;92:397–401. doi: 10.1073/pnas.92.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel-Gariepy E., Chenite A., Chaput C., Guirguis S., Leroux J. Characterization of thermosensitive chitosan gels for the sustained delivery of drugs. Int. J. Pharm. 2000;203:89–98. doi: 10.1016/s0378-5173(00)00428-2. [DOI] [PubMed] [Google Scholar]
- Salgado A.J., Oliveira J.M., Martins A., Teixeira F.G., Silva N.A., Neves N.M., Sousa N., Reis R.L. Tissue engineering and regenerative medicine: past, present, and future. Int. Rev. Neurobiol. 2013;108:1–33. doi: 10.1016/B978-0-12-410499-0.00001-0. [DOI] [PubMed] [Google Scholar]
- Seo B.M., Miura M., Gronthos S., Bartold P.M., Batouli S., Brahim J., Young M., Robey P.G., Wang C.Y., Shi S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004;364:149–155. doi: 10.1016/S0140-6736(04)16627-0. [DOI] [PubMed] [Google Scholar]
- Shani S., Ahmad R.E., Naveen S.V., Murali M.R., Puvanan K., Abbas A.A., Kamarul T. Platelet rich concentrate promotes early cellular proliferation and multiple lineage differentiation of human mesenchymal stromal cells in vitro. Scient. World J. 2014;2014 doi: 10.1155/2014/845293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen E.C., Chou T.C., Gau C.H., Tu H.P., Chen Y.T., Fu E. Releasing growth factors from activated human platelets after chitosan stimulation: a possible bio-material for platelet-rich plasma preparation. Clin. Oral Implants Res. 2006;17:572–578. doi: 10.1111/j.1600-0501.2004.01241.x. [DOI] [PubMed] [Google Scholar]
- Srivastava G.K., Rodriguez-Crespo D., Singh A.K., Casado-Coterillo C., Fernandez-Bueno I., Garcia-Gutierrez M.T., Coronas J., Pastor J.C. Chitosan feasibility to retain retinal stem cell phenotype and slow proliferation for retinal transplantation. Biomed. Res. Int. 2014;2014 doi: 10.1155/2014/287896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumanuma Y., Iwata T., Washio K., Yoshida T., Yamada A., Takagi R., Ohno T., Lin K., Yamato M., Ishikawa I., Okano T., Izumi Y. Comparison of different tissue-derived stem cell sheets for periodontal regeneration in a canine 1-wall defect model. Biomaterials. 2011;32:5819–5825. doi: 10.1016/j.biomaterials.2011.04.071. [DOI] [PubMed] [Google Scholar]
- Vacanti C.A., Vacanti J.P. The science of tissue engineering. Orthop. Clin. North Am. 2000;31:351–355. doi: 10.1016/s0030-5898(05)70155-3. [DOI] [PubMed] [Google Scholar]
- Valeri C.R., Feingold H., Marchionni L.D. A simple method for freezing human platelets using 6% dimethylsulfoxide and storage at -80 °C. Blood. 1974;43:131. [PubMed] [Google Scholar]
- Wada N., Menicanin D., Shi S., Bartold P.M., Gronthos S. Immunomodulatory properties of human periodontal ligament stem cells. J. Cell. Physiol. 2009;219:667–676. doi: 10.1002/jcp.21710. [DOI] [PubMed] [Google Scholar]
- Wang L., Stegemann J.P. Thermogelling chitosan and collagen composite hydrogels initiated with β-glycerophosphate for bone tissue engineering. Biomaterials. 2010;31:3976–3985. doi: 10.1016/j.biomaterials.2010.01.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Tabata Y., Hong L., Miyamoto S., Hashimoto N., Ikada Y. Bone regeneration by transforming growth factor β1 released from a biodegradable hydrogel. J. Control. Release. 2000;64:133–142. doi: 10.1016/s0168-3659(99)00129-7. [DOI] [PubMed] [Google Scholar]
- Yung Y., Fu S., Cheuk Y., Qin L., Ong M.T., Chan K., Yung P.S. Optimization of platelet concentrates therapy: Composition, localisation, and duration of action. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2017;7:27–36. doi: 10.1016/j.asmart.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Wu Y., Thote T., Lee E.H., Ge Z., Yang Z. The influence of scaffold microstructure on chondrogenic differentiation of mesenchymal stem cells. Biomed. Mater. 2014;9 doi: 10.1088/1748-6041/9/3/035011. [DOI] [PubMed] [Google Scholar]
- Zhao Q.L., Zhou Y., Wang M. Controlled release of growth factors from tissue engineering scaffolds made by positive and negative voltage electrospinning. Mater. Sci. Forum. 2015;815:385–389. [Google Scholar]
- Zhu W., Liang M. Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cells Int. 2015;2015 doi: 10.1155/2015/972313. [DOI] [PMC free article] [PubMed] [Google Scholar]