Absract
Aim
Chemokines released by different host cells when exposed to the components of periodontopathic bacteria induce and maintain an inflammatory response in the periodontium. The aim of the study was to estimate the salivary levels of two chemokines, macrophage inflammatory protein-1 alpha (MIP-1α) and monocyte chemo attractant protein-1 (MCP-1) in health, gingivitis and periodontitis and to evaluate their role as reliable salivary biomarkers in discriminating gingivitis and periodontitis from health.
Methods
A cross sectional study was designed to estimate the levels of MIP-1α and MCP-1 in whole unstimulated saliva from 75 patients who were divided into healthy (Group 1, n = 25), gingivitis (Group 2, n = 25) and chronic generalized periodontitis (Group 3, n = 25). MIP-1α and MCP-1 levels were estimated by using ELISA and were correlated with clinical parameters. ROC curve analysis was done to determine the sensitivity and specificity of these biomarkers in distinguishing periodontal disease from health.
Results
Both the biomarkers were detected in all the saliva samples. There was a statistically significant difference in the concentration of both the analytes in Group 3 and Group 2 compared with Group 1 (p < 0.001). ROC curve analysis showed 100% sensitivity and specificity for MIP-1α and MCP-1 in discriminating periodontitis from health. For discriminating gingivitis from health, MIP-1α had a higher sensitivity and specificity (100% & 88% respectively) compared to MCP-1(84.1% & 80% respectively).
Conclusion
There is a substantial increase in the concentration of both MIP-1α and MCP-1 with increasing severity of periodontal disease. Both the analytes showed promising results as biomarkers for discriminating periodontal disease from health.
Keywords: Chemokines, Periodontal diseases, Saliva, Macrophage inflammatory protein-1, Monocyte chemoattractant protein-1
1. Introduction
The biochemical signalling involved in the periodontal disease inflammatory disease process consists of three biological phases i.e. inflammation, connective tissue degradation, and alveolar bone turnover. An intermediate mechanism that lies between bacterial stimulation and tissue destruction is the production of cytokines, which stimulates inflammatory events that play an important role in leukocyte recruitment and may directly or indirectly modulate osteoclast formation (Graves, 2008). A constellation of circulating molecules in these biological phases have been detected at elevated levels in the gingival crevicular fluid and whole saliva of patients who have periodontal disease (Craig and Loberg, 2006). Among the mediators potentially involved in leukocyte migration to periodontal environment, chemokines have been investigated with special interest (Silva et al., 2007).
Chemokines are class of chemotactic cytokines that are capable of activating and promoting the migration of a variety of leukocytes and bone cells. They induce leukocyte migration and activation by binding to specific G protein coupled seven transmembrane spanning cell surface receptors (Graves and Jiang, 1995). Chemokines are found in gingival tissue and crevicular fluid and they are produced by number of cell types in the periodontium such as fibroblasts, endothelial cells, macrophages, osteoclasts, epithelial cells, polymorphonuclear leukocytes, monocytes, lymphocytes and mast cells. Some chemokines have important pro-inflammatory effects and are related to periodontal tissue destruction that involves the stimulation of bone resorption and induction of tissue damage (Hanazawa et al., 1993).
MIP-1α and MCP-1are chemotactic chemokines secreted by a variety of cell types, including macrophages, fibroblasts, epithelial cells, and endothelial cells (Furutani et al., 1989, Gong et al., 1997, Pype et al., 1999). These play an important role in the pathogenesis of various inflammatory diseases and conditions such as periodontitis, multiple myeloma, Sjögren syndrome, and rheumatoid arthritis (Maurer and von Stebut, 2004). They perform various biological functions, such as recruiting inflammatory cells, wound healing, inhibition of stem cells, and maintaining effector immune response (Gemmell, 2001).
Early detection of disease plays a crucial role for treatment planning and prognosis. Saliva has great potential as a diagnostic fluid and offers advantage over serum and other biological fluids by its non-invasive method of collection, smaller sample aliquots, cost effectiveness, early storage and transportation (Giannobile et al., 2011, Lamster et al., 1990). Salivary biomarkers are potentially important for determining the presence, risk and progress of periodontal disease. However, clinical translation of biomarker technology from lab to chair side requires studies that identify bio-markers associated with health, gingivitis and periodontitis (Syndergaard et al., 2014). Unlike periodontal disease, gingivitis has lacked evidence of salivary biomarkers with strong discriminatory capacity, a feature critical for the translational utility of salivary biomarkers to chair side diagnostics (Ebersole et al., 2015). Because of the relationship of gingivitis with periodontitis and the unidentified biologic processes that lead to the transition between these two inflammatory conditions, diagnostic tools for early detection are desirable (Syndergaard et al., 2014). Thus, the present study aimed to estimate the levels of salivary MIP-1α and MCP-1 in healthy individuals, patients with gingivitis and chronic periodontitis and to correlate their levels with the clinical parameters of periodontal disease. Also, the study aimed to evaluate the discriminatory capacity of MIP-1α and MCP-1 to distinguish periodontal disease from health.
2. Material and methods
2.1. Study population
This cross-sectional study was approved by Institutional Ethical Committee, Vydehi Institute of Dental Sciences and Research Centre, Bangalore, India. Patients aged between 18 and 50 years, who reported to the outpatient unit of Department of Periodontics, between September 2015 and June 2016 and were willing to participate in study and give informed consent were considered for the study. Patients using tobacco and tobacco related products or alcohol, patients having any systemic diseases, pregnant and lactating women, those who underwent periodontal treatment in the last 6 months, patients who have taken antibiotics or anti-inflammatory drugs within one month prior to the study, patients who had any oral mucosal inflammatory conditions, xerostomia or salivary gland disorders and those who were having <20 teeth were excluded from the study. 75 adults (40 males and 35 females) conforming to the inclusion criteria were included in the study. Plaque Index (PI) (Loe, 1967), Gingival Index (GI) (Loe, 1967), presence or absence of bleeding on probing (BOP) (Lang et al., 1986), probing pocket depth (PPD) and clinical attachment loss (CAL) (Ramfjord, 1974) were recorded using a mouth mirror and UNC 15 probe.1
Based on the clinical findings, patients were categorized into three groups. Group 1 consisted of 25 individuals with clinically healthy periodontium, with Mean GI ≤ 1, BOP < 20% of sites, no sites with PPD ≥ 3 mm and no CAL, Group 2 consisted of 25 individuals who showed clinical signs of gingival inflammation, Mean GI > 1, BOP > 20% of sites, no sites with PPD ≥ 3 mm and CAL ≥ 2 mm. Group 3 included patients who were diagnosed with chronic periodontitis. Diagnosis of chronic periodontitis was made according to the criteria given by 1999 International Workshop for classification of Periodontal Diseases and Conditions (Armitage, 1999). 25 patients who showed signs of clinical inflammation, mean GI > 1, CAL > 3 mm and PPD ≥ 5 mm in at least 30% of sites were included in the study.
2.2. Saliva sample collection
Unstimulated whole saliva was collected according to a previously described method (Miller et al., 2006). Individuals were recalled the following day for saliva sample collection to avoid contamination by blood during periodontal examination. 5 ml of unstimulated whole expectorated saliva was collected from each patient between 9 and 11 am while seated in upright position. Patients were asked to avoid all oral hygiene measures, eating, drinking, or gum chewing for 1 h before collection. Patients rinsed their mouth with water, and then expectorated whole saliva into sterile tubes. Collected samples were stored at −80 °C prior to analysis.
2.3. MIP-1α and MCP-1 assay
The levels of MIP-1α and MCP-1 in saliva were estimated using an enzyme-linked immunoassay kit.2 All analyses were performed in duplicate within six months of sample procurement. Standards were included on all runs and all results were reported within the linearity of the assays. Readings were made at 450 nm with a micro plate spectrophotometer.3
2.4. Statistical analyses
All data were analysed using SPSS (version 18.0, Chicago, IL, USA). Descriptive and inferential statistical analysis has been carried out in the present study. Results on continuous measurements are presented as Mean ± SD and results on categorical measurements are presented in Number (%). P < 0.05 was considered statistically significant. Analysis of Variance (ANOVA) was carried out to find the significance of study parameters on categorical scale between two or more groups. Scheffe’s multiple comparison test was applied to know the difference between the groups. Since the three groups had a statistically significant difference with respect to age, to rule out the possibilities of age acting as a confounding factor on the levels of the chemokines, ANCOVA test was done. Also, Bonferroni multiple comparison test was applied to compare the adjusted mean values. Pearson correlation co-efficient test was used to find the degree of correlation between the clinical parameters and salivary MIP-1α and MCP-1 concentration in all the three groups. Partial correlation coefficient has been calculated after the controlling the effect of the age of the subjects on the levels of the biomarkers studied. ROC curve analysis was done to determine the sensitivity and specificity of these biomarkers in distinguishing periodontal disease from health.
3. Results
Descriptive statistics of the study population is shown in Table 1. The significant P value for age between the groups revealed that all the three groups have been statistically different. In order to get more details of the variation between the three groups, repeated contrast test was applied. The result indicated the chronic periodontitis patients belonged to a higher age range when compared to individuals in other two groups. All three groups have been similar with respect to the gender of the individuals.
Table 1.
Descriptive statistics of demographics and clinical parameters of the study population.
| Category | Group 1 (n = 25) | Group 2 (n = 25) | Group 3 (n = 25) | P value |
|---|---|---|---|---|
| Age in years* | 24.8 ± 3.1 | 30 ± 7.6 | 46.2 ± 9.9 | 0.000† |
| Gender (M/F) | 11/14 | 12/13 | 17/8 | 0.190‡ |
| PI* | 0.44 ± 0.48 | 0.67 ± 0.44 | 1.87 ± 0.38 | 0.000† |
| GI* | 0.26 ± 0.23 | 1.37 ± 0.27 | 1.72 ± 0.35 | 0.000† |
| % BOP* | 12.04 ± 3.76 | 25.48 ± 3.42 | 31.64 ± 3.8 | 0.000† |
| PPD* | 1.58 ± 0.58 | 2.37 ± 0.25 | 5.5 ± 0.66 | 0.000† |
| CAL* | – | 1.43 ± 0.30 | 3.46 ± 0.34 | 0.000† |
Mean ± Standard Deviation.
Significant P value using One-way ANOVA test.
Non-significant P value using Chi-square test.
The clinical parameters used to differentiate the three groups (gingival index, bleeding on probing, probing pocket depth and clinical attachment level) were found to be significantly higher in group 3 relative to group 1 and 2 therefore conforming to the inclusion criteria (Table 1).
3.1. Salivary analyte levels
Scheffe’s multiple comparison test showed that the mean values of MIP-1α and MCP-1 levels in saliva was higher in Group 3 when compared to Group 1 and 2 (Table 2). The difference in levels of the analytes was found to be statistically significant. Since age can act as a confounding factor, ANCOVA test was applied to see whether the difference in the mean level of the chemokines has occurred due to periodontal inflammation or due to age of the subjects. The non-significant P value for age of the subjects revealed that age has no specific role in the difference of mean levels of MIP-1α and MCP-1 and the difference in the mean values of the groups are due to the clinical conditions of the subjects only (Table 2). Further, Bonferroni multiple comparison test has been applied to compare the adjusted mean values. The result indicated that all the three comparisons were statistically different (Table 3).
Table 2.
Scheffe’s multiple comparison test for MIP-1α and MCP-1 and the ANCOVA test result after controlling age of the subjects.
| Comparison |
Mean difference | P-value | ANCOVA test result |
|||
|---|---|---|---|---|---|---|
| Mean | Mean | Source | F-value | P-value | ||
| MIP-1α | ||||||
| Group 1 | Group 2 | −11.3320* | .000 | Age | 0.315 | 0.576 |
| Group 3 | −28.1440* | .000 | Group | 70.207 | 0.000* | |
| Group 2 | Group 3 | −16.8120* | .000 | |||
| MCP-1 | ||||||
| Group 1 | Group 2 | −176.1320* | .000 | Age | 1.623 | 0.207 |
| Group 3 | −550.1240* | .000 | Group | 75.321 | 0.000* | |
| Group 2 | Group 3 | −373.9920* | .000 | |||
P value statistically significant.
Table 3.
Estimated marginal Mean for MIP-1α and MCP-1 and its Bonferroni Multiple Comparison test result.
| Estimated marginal value |
95% Confidence value |
Bonferroni multiple comparison test result |
||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SE* | Lower | Upper | Comparison | Mean difference | P value | ||
| MIP-1α | ||||||||
| Group 1 | 7.89 | 1.27 | 5.35 | 10.44 | ||||
| Group 2 | 18.99 | 1.08 | 16.82 | 21.15 | Group 1 | Group 2 | −11.091 | .000 |
| Group 3 | 35.05 | 1.47 | 32.11 | 37.98 | Group 3 | −27.151 | .000 | |
| Group 2 | Group 3 | −16.061 | .000 | |||||
| MCP-1 | ||||||||
| Group 1 | 141.57 | 27.01 | 87.69 | 195.45 | ||||
| Group 2 | 329.30 | 23.03 | 283.38 | 375.22 | Group 1 | Group 2 | −187.728* | .000 |
| Group 3 | 739.42 | 31.15 | 677.30 | 801.53 | Group 3 | −597.848* | .000 | |
| Group 2 | Group 3 | −410.119* | .000 | |||||
Standard error.
3.2. Relationship between salivary analyte levels and clinical parameters of periodontal disease
Pearson correlation coefficient value between the clinical parameters and MIP-1α level and MCP-1 level demonstrated a significant positive correlation (Table 4). Also, Partial correlation coefficient has been calculated after controlling the effect of the age of the individuals. All the partial correlation coefficient values were lesser than their corresponding Pearson correlation coefficient values, however, the significant P value shows that these variables correlate with MIP-1α and MCP-1 values even after controlling the effect of age of the individuals (Table 4).
Table 4.
Correlation coefficient value between the clinical parameters and MIP-1α level and MCP-1 level.
| Clinical parameters | MIP-1α |
MCP-1 |
||||
|---|---|---|---|---|---|---|
| r-Value | r-Value* | p-Value | r-Value | r-Value* | p-Value | |
| PI | 0.769 | 0.555 | <0.001 | 0.706 | 0.470 | <0.001 |
| GI | 0.819 | 0.694 | <0.001 | 0.703 | 0.507 | <0.001 |
| BOP | 0.792 | 0.624 | <0.001 | 0.750 | 0.569 | <0.001 |
| PPD | 0.880 | 0.739 | <0.001 | 0.859 | 0.730 | <0.001 |
| CAL | 0.879 | 0.730 | <0.001 | 0.862 | 0.737 | <0.001 |
Partial correlation coefficient value after adjusting for age.
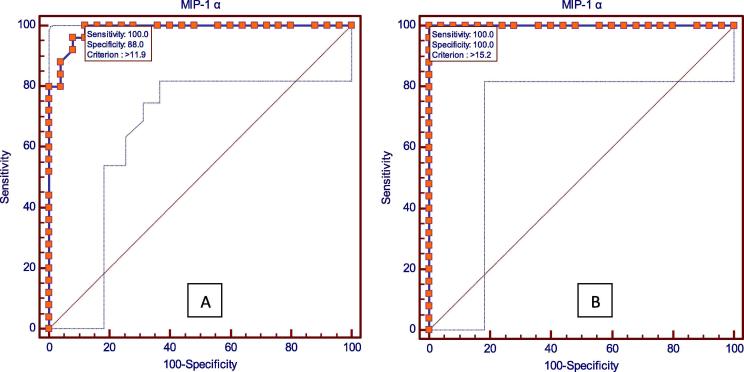
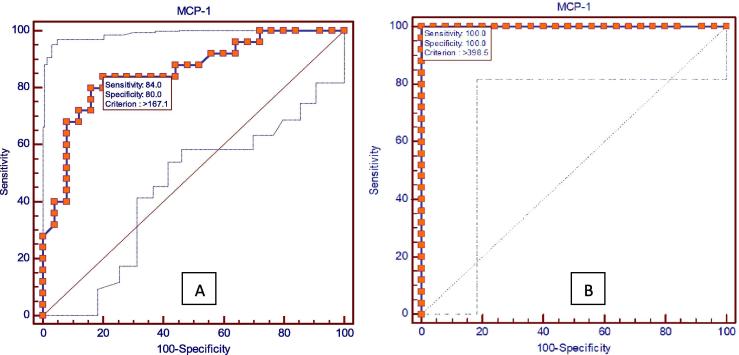
Fig. 1, Fig. 2 shows the results of receiver operating characteristic (ROC) analysis which was done to find out whether these two chemokines could be used as biomarkers for gingivitis and periodontitis and which among the two is a better predictor for disease progression. The P value was found to be highly significant for both MIP-1α and MCP-1 in gingivitis and periodontitis, which shows the utility of these two chemokines as biomarkers for gingivitis and periodontitis.
Fig. 1.
Receiver operating characteristic (ROC) analysis showing MIP-1α in (A) gingivitis and (B) periodontitis.
Fig. 2.
Receiver operating characteristic (ROC) analysis showing MCP-1 in (A) gingivitis and (B) periodontitis.
4. Discussion
This cross-sectional study evaluated the levels of two chemokines, MIP-1α and MCP-1 in whole unstimulated saliva of 75 adults who were categorized into three groups- healthy controls, patients having gingivitis and chronic generalized periodontitis. At the time of designing this study, literature search did not reveal any studies comparing healthy, gingivitis and periodontitis patients for the two biomarkers used, and hence the third group of gingivitis was included. Ebersole et al. in 2015, conducted a similar study for evaluating IL-1β, IL-16, MIP-1α, and MMP-8 in whole saliva in periodontal health, gingivitis and periodontitis. The results of this study had a close resemblance to the present study in that the levels of MIP-1α was significantly increased in the periodontitis group compared to both gingivitis and healthy groups. Few other studies have also reported similar results for MIP-1α in gingivitis (Syndergaard et al., 2014) and periodontitis (Al Sabbagh et al., 2012). In contrary to all the previously reported studies, Emingil et al. in 2005 reported that generalized aggressive periodontitis, chronic periodontitis, gingivitis and healthy groups had comparable GCF MIP-1α levels and concluded that the MIP-1α levels in GCF do not seem to play a discriminatory role in periodontitis. They explained that the low MIP-1α levels in periodontitis group could be because of the lack of macrophages as well as subsets lymphocytes with specific receptors for MIP-1α.
The salivary concentrations of MCP-1was found to be higher in group 3 when compared to group 1 and 2. Of the available literature, the only report on the levels of MCP-1 in saliva was the study done by Gupta et al. (2013). The results of the present study are in accordance with this study, which compared the MCP-1 levels in saliva, GCF and serum in individuals with chronic periodontitis. Same authors published a study in 2015 in which the MCP-1 levels were correlated with soluble CD-40 ligand, a cardiac marker in both GCF and serum. Correlation studies revealed that both these markers demonstrated a strong correlation among each other both in GCF and serum before and after periodontal therapy. Various other recent studies also reported higher GCF levels of MCP-1 in periodontitis patients when compared to healthy individuals (Hanazawa et al., 1993, Kurtis et al., 2005 Nov, Pradeep et al., 2009 Sep, Pradeep et al., 2009). Comparable results were also obtained with MCP-1 levels in serum and gingival tissues also (Gemmell et al., 2001, Tonetti et al., 1994). Tonetti et al. demonstrated mRNA for MCP-1 in tissue biopsies taken from diseased periodontal sites. The presence of MCP-1 was detected in the basal layers of oral epithelium as well as in the inflammatory infiltrate in the diseased periodontal tissues.
The present study showed a positive statistical correlation between the levels of MIP-1α and MCP-1 with all the clinical parameters evaluated. Other studies which evaluated these two biomarkers in saliva, GCF or serum have shown similar correlation with clinical parameters of periodontal disease (Emingil et al., 2005, Pradeep et al., 2009, Sexton et al., 2011 May). The elevated levels of MIP-1α and MCP-1 and its statistically significant positive correlation with clinical parameters suggests their role in the pathogenesis of inflammatory periodontal disease. MIP-1α is the most abundantly expressed chemokine in periodontitis tissues, with its expression localized in the connective tissue subjacent to the pocket epithelium of inflamed gingival tissues (De Lima et al., 2016). Both MIP-1α and MCP-1 are involved in the migration of macrophages to periodontal tissues (Gemmell et al., 2001). Products derived from macrophages such as IL-1 and TNF- α are pro-inflammatory cytokines which are known to induce bone resorption. Thus, these chemoattractants play a significant role in maintenance of chronic inflammatory reaction and the bone loss characteristic of periodontal disease.
Numerous studies have been conducted across the globe to identify biomarkers for distinguishing the individuals with periodontal disease from periodontally healthy individuals. But clinical relevance of these studies can be considered only if the sensitivity and specificity of the biomarker to discriminate the appropriate disease are evaluated (Fine et al., 2009). The results of our present study revealed that MIP-1α was the better biomarker in terms of sensitivity and specificity in differentiating gingivitis from health. Whereas in terms of differentiating periodontitis, both MIP-1α and MCP-1 had 100% sensitivity and specificity. Fine et al. in 2009 reported a sensitivity of 90.3% and specificity of 85.7% for salivary MIP-1α for the diagnosis of aggressive periodontitis. Later, the same authors reported the sensitivity and specificity of MIP-1α in GCF to be 93.33% and 98.73% respectively (Fine et al., 2014). The systematic review and meta-analysis published by De Lima et al. in 2016, which reviewed the clinical utility of various salivary biomarkers (MMP 8, MMP 9, IL-1β, IL-6, IL-10, PGE2, OPG, TNF α, MIP-1α, calprotectin, albumin, ICTP), identified that only MIP-1α had a high accuracy of identifying or excluding the occurrence of periodontal disease in adults.
5. Conclusion
The results of the present study suggest that salivary levels of both MIP-1α and MCP-1 could have clinical utility as a screening tool for discriminating the various stages of periodontal disease. Future longitudinal studies are recommended to determine whether these two biomarkers could be of help in predicting periodontal breakdown in susceptible individuals.
6. Conflict of interest and source of funding statement
The authors declare that they do not have a conflict of interest. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
7. Ethical statement
This cross-sectional study was approved by Institutional Ethical Committee, Vydehi Institute of Dental Sciences and Research Center, Bangalore, India. Informed consent was obtained from all participants prior to start of the study.
Footnotes
Peer review under responsibility of King Saud University.
Hu-Friedy PCP UNC 15 Qulix, Chicago, IL.
EMD Millipore, Billerica, MA.
SpectraMAX 340, Molecular Devices, Sunnyvale, CA, USA.
References
- Al-Sabbagh M., Alladah A., Lin Y. Bone remodeling-associated salivary biomarker MIP-1α distinguishes periodontal disease from health. J. Periodontal Res. 2012;47:389–395. doi: 10.1111/j.1600-0765.2011.01445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- Craig M.J., Loberg R.D. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev. 2006;25:611–619. doi: 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- De Lima C.L., Acevedo A.C., Grisi D.C., Taba M., Jr, Guerra E., De Luca Canto G. Host-derived salivary biomarkers in diagnosing periodontal disease: systematic review and meta-analysis. J. Clin. Periodontol. 2016;43:492–502. doi: 10.1111/jcpe.12538. [DOI] [PubMed] [Google Scholar]
- Ebersole J.L., Nagarajan R., Akers D., Miller C.S. Targeted salivary biomarkers for discrimination of periodontal health and disease (s) Front. Cell. Infect. Microbiol. 2015;5:62. doi: 10.3389/fcimb.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emingil G., Atilla G., Başkesen A., Berdeli A. Gingival crevicular fluid EMAP-II, MIP-1α and MIP-1β levels of patients with periodontal disease. J. Clin. Periodontol. 2005;32:880–885. doi: 10.1111/j.1600-051X.2005.00780.x. [DOI] [PubMed] [Google Scholar]
- Fine D.H., Markowitz K., Fairlie K. Macrophage inflammatory protein-1α shows predictive value as a risk marker for subjects and sites vulnerable to bone loss in a longitudinal model of aggressive periodontitis. PLoS One. 2014;9(6):e98541. doi: 10.1371/journal.pone.0098541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine D.H., Markowitz K., Furgang D., Fairlie K., Ferrandiz J., Nasri C., McKiernan M., Donnelly R., Gunsolley J. Macrophage inflammatory protein-1α: a salivary biomarker of bone loss in a longitudinal cohort study of children at risk for aggressive periodontal disease? J. Periodontol. 2009;80:106–113. doi: 10.1902/jop.2009.080296. [DOI] [PubMed] [Google Scholar]
- Furutani Y., Nomura H., Notake M. Cloning and sequencing of the cDNA for human monocyte chemotactic and activating factor (MCAF) Am. J. Pathol. 1989;159:249–255. doi: 10.1016/0006-291x(89)92430-3. [DOI] [PubMed] [Google Scholar]
- Gemmell E., Carter C.L., Seymour G.J. Chemokines in human periodontal disease tissues. Clin. Exp. Immunol. 2001;125:134–141. doi: 10.1046/j.1365-2249.2001.01511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannobile W.V., McDevitt J.T., Niedbala R.S., Malamud D. Translational and clinical applications of salivary diagnostics. Adv. Dent. Res. 2011;23:375–380. doi: 10.1177/0022034511420434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong X., Gong W., Kuhns D.B., Ben-Baruch A., Howard O.Z., Wang J.M. Monocyte chemotactic protein-2 (MCP-2) uses CCR1 and CCR2B as its functional receptors. J. Biol. Chem. 1997;272:11682–11685. doi: 10.1074/jbc.272.18.11682. [DOI] [PubMed] [Google Scholar]
- Graves D. Cytokines that promote periodontal tissue destruction. J. Periodontol. 2008;79:1585–1591. doi: 10.1902/jop.2008.080183. [DOI] [PubMed] [Google Scholar]
- Graves D.T., Jiang Y. Chemokines, a family of chemotactic cytokines. Crit. Rev. Oral Biol. Med. 1995;6:109–118. doi: 10.1177/10454411950060020101. [DOI] [PubMed] [Google Scholar]
- Gupta M., Chaturvedi R., Jain A. Role of monocyte chemoattractant protein-1 (MCP-1) as an immune-diagnostic biomarker in the pathogenesis of chronic periodontal disease. Cytokine. 2013;61:892–897. doi: 10.1016/j.cyto.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Hanazawa S., Kawata Y., Takeshita A., Kumada H., Okithu M., Tanaka S., Yamamoto Y., Masuda T., Umemoto T., Kitano S. Expression of monocyte chemoattractant protein 1 (MCP-1) in adult periodontal disease: increased monocyte chemotactic activity in crevicular fluids and induction of MCP-1 expression in gingival tissues. Infect. Immun. 1993;61:5219–5224. doi: 10.1128/iai.61.12.5219-5224.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtis B., Tüter G., Serdar M., Akdemir P., Uygur C., Firatli E., Bal B. Gingival crevicular fluid levels of monocyte chemoattractant protein-1 and tumor necrosis factor-alpha in patients with chronic and aggressive periodontitis. J. Periodontol. 2005;76(11):1849–1855. doi: 10.1902/jop.2005.76.11.1849. [DOI] [PubMed] [Google Scholar]
- Lamster I.B., Celenti R., Ebersole J.L. The relationship of serum IgG antibody titres to periodontal pathogens to indicators of the host response in crevicular fluid. J. Clin. Periodontol. 1990;17:419–425. doi: 10.1111/j.1600-051x.1990.tb02340.x. [DOI] [PubMed] [Google Scholar]
- Lang N.P., Joss A., Orsanic T., Gusberti F.A., Siegrist B.E. Bleeding on probing. A predictor for the progression of periodontal disease? J. Clin. Periodontol. 1986;13:590–596. doi: 10.1111/j.1600-051x.1986.tb00852.x. [DOI] [PubMed] [Google Scholar]
- Loe H. The gingival index, the plaque index and the retention index system. J. Periodontol. 1967;38:610–616. doi: 10.1902/jop.1967.38.6.610. [DOI] [PubMed] [Google Scholar]
- Maurer M., von Stebut E. Macrophage inflammatory protein-1. Int. J. Biochem. Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Miller C.S., King C.P., Jr, Langub M.C., Kryscio R.J., Thomas M.V. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J. Am. Dent. Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Daisy H., Hadge P., Garg G., Thorat M. Correlation of gingival crevicular fluid interleukin-18 and monocyte chemoattractant protein protein-1 levels in periodontal health and disease. J. Periodontol. 2009;80:1454–1461. doi: 10.1902/jop.2009.090117. [DOI] [PubMed] [Google Scholar]
- Pradeep A.R., Daisy H., Hadge P. Serum levels of monocyte chemoattractant protein-1 in periodontal health and disease. Cytokine. 2009;47:77–81. doi: 10.1016/j.cyto.2009.05.012. [DOI] [PubMed] [Google Scholar]
- Pype J.L., Dupont L.J., Menten P. Expression of monocyte chemotactic protein (MCP)-1, MCP-2, and MCP-3 by human airway smooth-muscle cells: modulation by corticosteroids and T-helper 2 cytokines. Am. J. Respir. Cell Mol. Biol. 1999;21:528–536. doi: 10.1165/ajrcmb.21.4.3660. [DOI] [PubMed] [Google Scholar]
- Ramfjord S.P. Design of studies or clinical trials to evaluate the effectiveness of agents or procedures for the prevention, or treatment, of loss of the periodontium. J. Periodontal Res. 1974;9(suppl 14):78–93. doi: 10.1111/j.1600-0765.1974.tb01767.x. [DOI] [PubMed] [Google Scholar]
- Sexton W.M., Lin Y., Kryscio R.J., Dawson D.R., Ebersole J.L., Miller C.S. Salivary biomarkers of periodontal disease in response to treatment. J. Clin. Periodontol. 2011;1(38):434–441. doi: 10.1111/j.1600-051X.2011.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva T.A., Garlet G.P., Fukada S.Y., Silva J.S., Cunha F.Q. Chemokines in oral inflammatory diseases: apical periodontitis and periodontal disease. J. Dent. Res. 2007;86(4):306–319. doi: 10.1177/154405910708600403. [DOI] [PubMed] [Google Scholar]
- Syndergaard B., Al-Sabbagh M., Kryscio R.J. Salivary biomarkers associated with gingivitis and response to therapy. J. Periodontol. 2014;85:e295–e303. doi: 10.1902/jop.2014.130696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti M.S., Imboden M.A., Gerber L., Lang N.P., Laissue J., Mueller C. Localized expression of mRNA for phagocyte-specific chemotactic cytokines in human periodontal infections. Infect. Immun. 1994;62:4005–4014. doi: 10.1128/iai.62.9.4005-4014.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]