Abstract
Introduction
The EXIST-2 (NCT00790400) study demonstrated the superiority of everolimus over placebo for the treatment of renal angiomyolipomas associated with tuberous sclerosis complex (TSC) or sporadic lymphangioleiomyomatosis (LAM). This post hoc analysis of EXIST-2 study aimed to assess angiomyolipoma tumor behavior among patients who submitted to continued radiographic examination following discontinuation of everolimus in the noninterventional follow-up phase.
Methods
For patients who discontinued everolimus at the completion of extension phase for reasons other than angiomyolipoma progression, a single CT/MRI scan of the kidney was collected after 1 year of treatment discontinuation. Changes from baseline and from the time of everolimus discontinuation in the sum of volumes of target angiomyolipoma lesions were assessed in the non-interventional follow-up phase (data cutoff date, November 6, 2015).
Results
Of the 112 patients who received ≥1 dose of everolimus and discontinued treatment by the end of extension phase, 34 (30.4%) were eligible for participation in the non-interventional follow-up phase. Sixteen of 34 patients were evaluable for angiomyolipoma tumor behavior as they had at least one valid efficacy assessment (i.e. kidney CT/MRI scan) after everolimus discontinuation. During the non-interventional follow-up phase, compared with baseline, two patients (12.5%) experienced angiomyolipoma progression (angiomyolipoma-related bleeding [n = 1], increased kidney volume [n = 1]). Five patients out of 16 (31.3%) experienced angiomyolipoma progression when compared with the angiomyolipoma tumor assessment at everolimus discontinuation. The median (range) percentage change in angiomyolipoma tumor volume (cm3) from baseline was −70.56 (−88.30; −49.64) at time of everolimus discontinuation (n = 11), and −50.55 (−79.40; −23.16) at week 48 (n = 7) after discontinuation of everolimus. One patient death was reported due to angiomyolipoma hemorrhage.
Conclusions
Angiomyolipoma lesions displayed an increase in volume following discontinuation of everolimus in patients with renal angiomyolipoma or sporadic LAM associated with TSC, but there was no evidence of rapid regrowth.
Trial registration
ClinicalTrials.gov NCT00790400
Introduction
Angiomyolipomata are the most common renal manifestations in patients with tuberous sclerosis complex (TSC) developing during later childhood and adolescence.[1, 2] Renal angiomyolipomata are benign tumors composed of blood vessels, smooth muscle, and adipose tissue.[3] They belong to a family of neoplasms called perivascular epithelioid cell tumors (PEComas) which include pulmonary lymphangioleiomyomatosis (LAM).[3] LAM is a progressive lung disease characterized by infiltration of smooth muscle cells and formation of parenchymal cysts. The cells comprising the LAM lesions and angiomyolipomata appear to arise from a common source.[3, 4] Approximately, 80% of patients with TSC develop renal angiomyolipomata and mostly because of a definitive mutation of the TSC1 or TSC2 gene.[4] Renal angiomyolipomata may occur unilaterally or bilaterally and historically were the most common cause of premature mortality in adults with TSC.[5] Angiomyolipomata are mostly asymptomatic and slow growing. Studies that have explored the growth rates of angiomyolipomata showed mean growth rates within a range of ≥0.25 cm/year, 0.37 cm/year, and 0.5 cm/year.[6–8] Angiomyolipomata display linear growth and tend to be significantly more symptomatic as they grow.[6, 9] Large angiomyolipomata measuring >3 to 4 cm in diameter may develop a vascular aneurysm and life-threatening hemorrhage or compress normal renal tissue, potentially leading to renal failure.[5, 10] Treatment with mammalian target of rapamycin (mTOR) inhibitors is recommended for asymptomatic, growing angiomyolipomata measuring larger than 3 cm in diameter.[11] In the phase 3 EXIST-2 trial, the mTOR inhibitor everolimus reduced renal angiomyolipoma volume by ≥50% from baseline to the end of the core phase in 42% of patients after a median treatment duration of 38 weeks. Long-term results from this study demonstrated sustained tumor regression with everolimus. The proportion of patients with a ≥50% reduction in renal angiomyolipoma increased over time to 54% at ~2.5 years, and 58% at ~4 years.[12, 13] Although angiomyolipoma lesions shrink and stabilize with mTOR inhibitor treatment, there is a tendency to increase volume after the therapy is discontinued.[4, 14] Limited evidence is currently available to understand the rebound growth following discontinuation.[14] The aim of this post hoc analysis of the EXIST-2 study was to better characterize tumor response status for up to 1-year after discontinuation of everolimus treatment in patients with renal angiomyolipoma associated with TSC or sporadic LAM who submitted to continued radiographic assessment in the non-interventional follow-up phase.
Methods
EXIST-2 (NCT00790400) was a prospective, phase 3, multicenter, randomized, double-blind trial with a core phase followed by an extension phase and a non-interventional follow-up phase. A complete description of the study participants, design, and outcomes has been published.[13, 15] In brief, eligible patients were ≥18 years of age with at least one renal angiomyolipoma measuring ≥3 cm in its longest diameter, as measured by computed tomography (CT) or magnetic resonance imaging (MRI), and had a definitive diagnosis of TSC according to the TSC consensus criteria or sporadic LAM as confirmed by biopsy or chest CT scan. Patients were excluded if they had angiomyolipoma-related bleeding or embolization during the 6 months prior to randomization or if their angiomyolipoma required surgery at the time of randomization or had impaired lung function. The primary analysis of the data from core phase (data cutoff June 30, 2011) favored everolimus over placebo in reducing angiomyolipoma volume. The study was then unblinded on September 9, 2011 and all patients (including those who had received placebo during the core phase) received everolimus in the open-label extension phase, until 4 years after the last patient was randomized (last patient, last treatment, January 28, 2015). Everolimus was initiated at oral dose of 10 mg/day and dose adjusted based on tolerability. Patients were treated with everolimus until angiomyolipoma progression, intolerable toxicity or any other reason for discontinuation. The definition of angiomyolipoma progression is described in detail previously.[13, 15]
All patients who had either completed the extension phase or who discontinued everolimus for reasons other than angiomyolipoma progression or due to adverse events (AEs) were allowed to participate in the non-interventional follow-up phase. Patients were excluded to participate in the non-interventional follow-up phase if they started treatment with systemic mTOR inhibitors, or had embolization or angiomyolipoma requiring partial/complete nephrectomy immediately after everolimus discontinuation. A single kidney CT/MRI scan was to be performed approximately one year after the discontinuation of treatment with everolimus in the non-interventional follow-up phase. If a patient needed any intervention following treatment discontinuation, a kidney CT/MRI was to be performed prior to the start of intervention, and then the non-interventional follow-up phase ended. Interventions included commercial everolimus, off label rapamycin, embolization, or nephrectomy. Data collected during the non-interventional follow-up phase included angiomyolipoma-related bleeding events, AEs (only spontaneously reported by the patient were captured), use of concomitant medications and non-drug therapies for the treatment of angiomyolipoma or angiomyolipoma-related disease progression. End-of study assessment was planned after all patients have either completed the one-year non-interventional follow-up phase or have exited the study. In addition to the initial definition based on baseline volume, a sensitivity analysis was performed using a different definition for angiomyolipoma progression during the non-interventional follow-up phase: angiomyolipoma volume increased by ≥25% from end of treatment or ≥20% increase in the volume of either kidney from end of treatment, appearance of new angiomyolipoma ≥1 cm, an increase in the longest diameter of angiomyolipoma lesion that was <1 cm at the end of treatment to ≥1 cm during the non-interventional follow-up phase, or grade ≥2 angiomyolipoma-related bleeding according to the NCI-CTCAE, version 3.0.[16] Angiomyolipoma progression was presented as a percentage rate along with an exact 95% confidence interval (CI) obtained using the Clopper-Pearson method. Baseline data was summarized by descriptive statistics. The assessment of efficacy and safety was restricted to patients without angiomyolipoma progression or anti-TSC therapy (non-study systemic anti-angiomyolipoma therapies or angiomyolipoma-related surgeries) at the time of everolimus discontinuation. Patients with at least one valid efficacy assessment during the non-interventional follow-up period were evaluable for efficacy analysis. Statistical analysis was performed using SAS software (SAS Institute Inc., Cary, NC, USA). Here we present the angiomyolipoma tumor response status among patients who submitted to continued radiographic assessment following discontinuation of everolimus in the non-interventional follow-up phase at the data cut-off date of November 6, 2015.
All patients and or legal representatives provided written informed consent according to local guidelines before enrolment and before participating in the non-interventional follow-up phase. Independent ethics committees and/or local review boards approved the protocol (S1 and S2 Appendices), which was executed according to the International Conference on Harmonization Good Clinical Practice guidelines.
Results
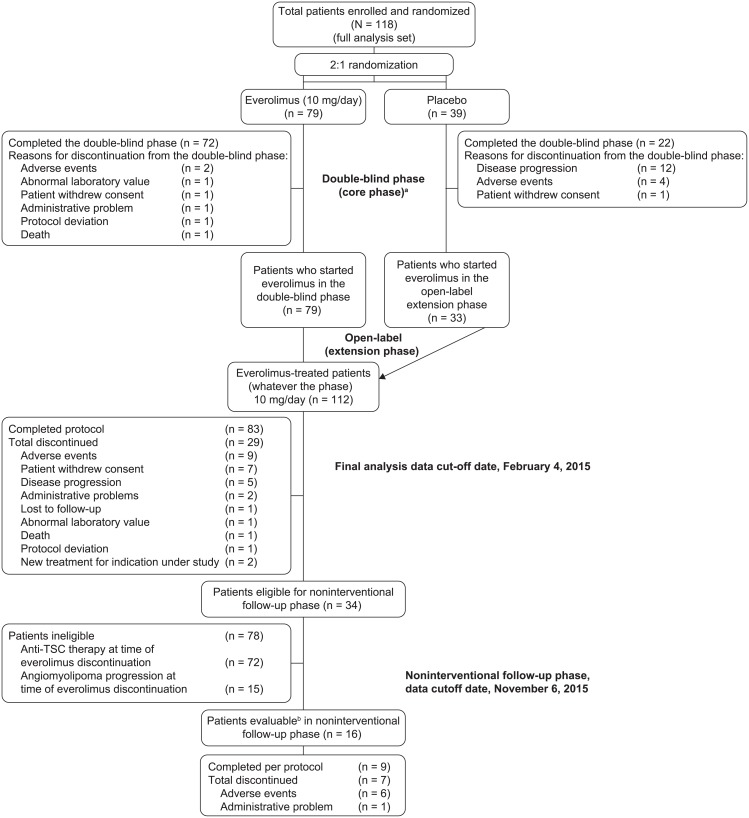
A total of 118 patients with renal angiomyolipoma associated with TSC or sporadic LAM were enrolled from 24 centers in 11 countries between April 28, 2009 and December 30, 2010. Overall, 112 patients received at least one dose of everolimus during the core phase and/or the extension phase, and had discontinued treatment with everolimus by the end of extension phase. Only 34 patients out of 112 (30.4%) were eligible for assessments in the noninterventional follow-up phase (Fig 1).
Fig 1. CONSORT flow diagram of patient disposition in the double-blind core phase followed by open-label extension phase and noninterventional follow-up phase.
aPatients with angiomyolipoma progression were unblinded at the end of the double-blind phase, and patients who were on placebo were allowed to cross over to open-label everolimus. bPatients were evaluable for noninterventional follow-up phase as they had at least one adequate efficacy assessment (CT/MRI scan).
The remaining 78 patients (69.6%) were not eligible either because they received commercial everolimus (or another anti-TSC therapy) after everolimus discontinuation (64.3%) or had an angiomyolipoma progression at the time of everolimus discontinuation (13.4%). The most common reason for everolimus discontinuation among the 34 patients was AEs, reported in nine patients (26.5%). Thirteen patients completed the extension phase as per the study protocol, six withdrew consent, one withdrew due to protocol deviation, two withdrew due to administrative problems, one withdrew due to loss to follow-up, and one withdrew due to abnormal laboratory value. One patient (originally randomized to everolimus) with a history of intractable seizures died; this fatal case was reported with the initial study report.[13] Only 16 patients of the 34 were evaluable for efficacy as they had at least one valid efficacy assessment (i.e. kidney CT/MRI scan) after everolimus discontinuation. Eleven patients were randomized to everolimus starting in the core phase while the remaining five patients were randomized to placebo and switched to everolimus starting in the extension phase. The reasons for treatment discontinuation for the 16 patients were treatment completion as per protocol (56.3%), AEs (37.5%), and administrative problems (6.3%).
The median age of the 16 patients evaluable for efficacy in the non-interventional follow-up phase was 34.9 years (range, 20.5–49.4) at baseline and 38.2 years (range, 25.1–52.1) at end of treatment, and 50% were female (Table 1). The median sum of volumes of target lesions at baseline was 154.67 cm3 (range, 24.1–861.4). The median duration of study follow-up since discontinuation of everolimus was 11.1 months (range, 3.2–39.1). The median duration of everolimus exposure was 43.7 months (range, 0.46–60.5), and the median dose intensity was 9.89 mg/day (range, 3.9–10).
Table 1. Patient demographics and disease characteristics.
| Characteristic | Evaluable patientsa (N = 16) |
|---|---|
| Median age at baseline, years (range) | 34.9 (20.5–49.4) |
| Median age at time of treatment discontinuation, years (range) | 38.2 (25.1–52.1) |
| Sex, n (%) | |
| Male | 8 (50.0) |
| Female | 8 (50.0) |
| Race, n (%) | |
| Caucasian | 14 (87.5) |
| Asian | 2 (12.5) |
| WHO performance status at baseline, n (%) | |
| 0 | 11 (68.8) |
| 1 | 4 (25.0) |
| 2 | 1 (6.3) |
| Median body mass index at baseline, kg/m2 (range) | 24.25 (19.8–29.0) |
| Median body surface area at baseline, m2 (range) | 1.83 (1.62–2.14) |
| Sum of volumes of target renal angiomyolipoma lesions, cm3 | |
| Mean (standard deviation) | 248.72 (254.9) |
| Median (range) | 154.67 (24.1–861.4) |
aPatients with at least one valid efficacy assessment during the non-interventional follow-up phase.
Efficacy outcomes
Angiomyolipoma tumor progression at the time of primary analysis was observed in 3 patients treated with everolimus (3/79, 3.8%) and 8 patients treated with placebo (8/39, 20.5%).[13] At the end of the extension phase, 16 patients reported angiomyolipoma progression (16/112, 14.3%).[17] At the end of the non-interventional follow-up phase, angiomyolipoma progression was observed in 2/16 patients (12.5%) when compared to the baseline. Among the 2 patients, one patient was already considered to have angiomyolipoma progression at the end of the extension phase. Primary reasons for progression in 2 patients were angiomyolipoma-related bleeding of grade ≥2 in one patient (6.3%) and increase in kidney volume only in another patient (6.3%). Further detailed evaluation of the CT scans by the investigator for the patient with increased kidney volume revealed an increase in cyst volume which may have contributed to the overall kidney volume increase as the kidney volume measurements also included cyst volume. However, cyst volume was not centrally collected and assessed separately. Five patients (31.3%) reported angiomyolipoma progression at the end of the non-interventional follow-up phase when compared with the end of treatment, as per the sensitivity analysis. The most common reasons for progression in these 5 patients included increase in both tumor and kidney volume in three patients (18.8%), angiomyolipoma-related bleeding of grade ≥2 in one patient (6.3%), and increase in tumor volume only in one patient (6.3%).
The median percentage change in the sum of volumes of target angiomyolipoma lesions (cm3) from baseline (119.31; range, 24.06–861.36) to end of treatment (27.49; range, 4.03–253.61) was −70.56% (range, −88.30 to −49.64; n = 11 evaluable patients), and from baseline (146.79; range, 24.06–861.36) to week 48 of non-interventional follow-up phase (87.32; range, 4.96–312.68) was −50.55% (range, −79.40 to −23.16; n = 7 evaluable patients; Table 2). The median percentage change in the sum of volumes of target angiomyolipoma lesions (cm3) from end of treatment (46.63; range, 4.03–253.61) to week 48 of the non-interventional follow-up phase (87.32; range, 4.96–312.68) was 52.53% (range, −23.16 to 87.25; n = 7 evaluable patients; Table 2).
Table 2. Median sum of volumes of target angiomyolipoma lesions at baseline, EOT, and week 48 of non-interventional follow-up phase, and percentage change from baseline to EOT/week 48 of non-interventional follow-up phase and from EOT to week 48 of non-interventional follow-up phase.
| Median (range) | Evaluable patients at EOT (N = 11) | Evaluable patients at week 48 of non-interventional follow-up phase (N = 7) |
|---|---|---|
| Sum of volumes at baseline, cm3 | 119.31 (24.06–861.36) | 146.79 (24.06–861.36) |
| Sum of volumes at EOT, cm3 | 27.49 (4.03–253.61) | 46.63 (4.03–253.61) |
| Sum of volumes at week 48 of non-interventional follow-up phase, cm3 | NA | 87.32 (4.96–312.68) |
| Percentage change from baseline, % | −70.56 (−88.30 to −49.64) | −50.55 (−79.40–23.16) |
| Percentage change from EOT, % | NA | 52.53 (−23.16–87.25) |
Abbreviations: EOT = end of treatment; NA = not applicable.
Safety outcomes
Safety results during treatment with everolimus were reported in detail in prior publications.[12, 13, 15] No renal hemorrhages were observed during the cumulative 390.8 patient-years of everolimus treatment.[17] During the non-interventional follow-up phase, one patient died after 5.8 months of treatment discontinuation due to angiomyolipoma hemorrhage. The patient was exposed to everolimus treatment for approximately 4.1 years. Five patients reported AEs after 28-days of treatment discontinuation which included post-procedural complications, procedural pain and arthralgia for the same patient, hypersensitivity, decrease in weight, anemia and hemorrhage reported in one patient each.
Discussion
This post hoc analysis of EXIST-2 demonstrated tumor growth after discontinuation of everolimus in patients with renal angiomyolipoma associated with TSC or sporadic LAM. However, there was no evidence of rapid regrowth as the increase in angiomyolipoma lesion volume following treatment discontinuation did not exceed the lesion volume at baseline, even among patients who displayed progression. The angiomyolipoma progression rates were comparable between patients of the non-interventional follow-up phase (31.3%) and the patients treated with placebo during the double-blind core phase (20.5%).[13] To the best of our knowledge, this is the first ever study of everolimus reporting the tumor behavior after treatment discontinuation. Previously, an increase in angiomyolipoma volume was reported with discontinuation from sirolimus therapy, but did not return completely to the baseline values. The reason for angiomyolipoma progression could be due to impaired apoptosis or increase in angiomyolipoma size or volume.[4] In EXIST-2, 12 of the 16 evaluable patients experienced an increase in angiomyolipoma volume compared to their most recent tumor volume assessed before treatment discontinuation (EOT). A median angiomyolipoma growth of 52.53% was observed at 48 weeks after everolimus discontinuation.
Long-term safety analysis of EXIST-2 had shown that everolimus was well-tolerated and the AEs remained consistent with the previous reports.[13, 15] Angiomyolipoma-related bleeding event was reported only in one patient during the non-interventional follow-up phase, and during an overall 390.8 patient-years of treatment with everolimus, no bleeding events were reported.[17] Six patients reported angiomyolipoma-related bleeding events in the year before randomization. These observations suggest a considerably lower annual rate of angiomyolipoma-related bleeding for patients receiving everolimus when compared with patients not receiving everolimus either before or after the treatment portion of the study. Previous studies suggested a correlation between angiomyolipoma size and risk of hemorrhage.[18] The aneurysm and tumor sizes are considered as the main predictors of renal hemorrhage.[18] Angiomyolipomata tend to become symptomatic as they grow. Previous studies showed tumor growth in 27% to 53% of lesions measuring <4 cm and in 46% to 75% of lesions measuring >4 cm over a follow-up period of up to 4 years.[7, 8] This suggests that treatment that maintains or reduces angiomyolipoma lesions may reduce the risk of bleeding.
Fewer evaluable patients to assess tumor response after treatment discontinuation is a limitation of this analysis as more robust predictions of tumor behavior after cessation of everolimus treatment are difficult. In summary, angiomyolipoma lesion volume increased for up to one year after discontinuation of everolimus in patients with renal angiomyolipoma associated with TSC or sporadic LAM, compared to their most recent tumor volume assessment performed prior to treatment discontinuation; though the angiomyolipoma volume did not exceed that measured at baseline. The results of our study show that persistence of clinically significant angiomyolipoma volume reduction may require ongoing treatment with everolimus in patients with TSC.
Supporting information
(DOCX)
(PDF)
(DOC)
Acknowledgments
We thank the patients and their families who participated in the EXIST-2 trial; the investigators, study nurses, and clinical research associates from the individual trial centers who provided ongoing support; and Rama Mylapuram (Novartis Healthcare Pvt. Ltd.) for providing medical editorial assistance with this manuscript.
Data Availability
Novartis supports the publication of scientifically rigorous analysis that is relevant to patient care, regardless of a positive or negative outcome. Qualified external researchers can request access to anonymized patient-level data, respecting patient informed consent, through www.clinicalstudydatarequest.com, according to requirements noted on the web portal.
Funding Statement
The study sponsor Novartis Pharmaceuticals Corporation contributed to the study design, data analysis, and the decision to publish. Novartis authors reviewed the draft for submission.
References
- 1.Brakemeier S, Bachmann F, Budde K. Treatment of renal angiomyolipoma in tuberous sclerosis complex (TSC) patients. Pediatr Nephrol. 2016. 10.1007/s00467-016-3474-6 . [DOI] [PubMed] [Google Scholar]
- 2.Northrup H, Krueger DA, International Tuberous Sclerosis Complex Consensus G. Tuberous sclerosis complex diagnostic criteria update: recommendations of the 2012 Iinternational Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):243–54. 10.1016/j.pediatrneurol.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabenou RA, Charles HW. Differentiation of Sporadic Versus Tuberous Sclerosis Complex-Associated Angiomyolipoma. AJR Am J Roentgenol. 2015;205(2):292–301. 10.2214/AJR.14.14255 . [DOI] [PubMed] [Google Scholar]
- 4.Bissler JJ, McCormack FX, Young LR, Elwing JM, Chuck G, Leonard JM, et al. Sirolimus for angiomyolipoma in tuberous sclerosis complex or lymphangioleiomyomatosis. N Engl J Med. 2008;358(2):140–51. 10.1056/NEJMoa063564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Callaghan FJ, Noakes MJ, Martyn CN, Osborne JP. An epidemiological study of renal pathology in tuberous sclerosis complex. BJU Int. 2004;94(6):853–7. 10.1111/j.1464-410X.2004.05046.x . [DOI] [PubMed] [Google Scholar]
- 6.Bhatt JR, Richard PO, Kim NS, Finelli A, Manickavachagam K, Legere L, et al. Natural History of Renal Angiomyolipoma (AML): Most Patients with Large AMLs >4cm Can Be Offered Active Surveillance as an Initial Management Strategy. Eur Urol. 2016;70(1):85–90. 10.1016/j.eururo.2016.01.048 . [DOI] [PubMed] [Google Scholar]
- 7.Lemaitre L, Robert Y, Dubrulle F, Claudon M, Duhamel A, Danjou P, et al. Renal angiomyolipoma: growth followed up with CT and/or US. Radiology. 1995;197(3):598–602. 10.1148/radiology.197.3.7480725 . [DOI] [PubMed] [Google Scholar]
- 8.Steiner MS, Goldman SM, Fishman EK, Marshall FF. The natural history of renal angiomyolipoma. J Urol. 1993;150(6):1782–6. . [DOI] [PubMed] [Google Scholar]
- 9.Patel U, Simpson E, Kingswood JC, Saggar-Malik AK. Tuberose sclerosis complex: analysis of growth rates aids differentiation of renal cell carcinoma from atypical or minimal-fat-containing angiomyolipoma. Clin Radiol. 2005;60(6):665–73; discussion 3–4. 10.1016/j.crad.2005.01.009 . [DOI] [PubMed] [Google Scholar]
- 10.Budde K, Gaedeke J. Tuberous sclerosis complex-associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis. 2012;59(2):276–83. 10.1053/j.ajkd.2011.10.013 . [DOI] [PubMed] [Google Scholar]
- 11.Krueger DA, Northrup H, International Tuberous Sclerosis Complex Consensus G. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 International Tuberous Sclerosis Complex Consensus Conference. Pediatr Neurol. 2013;49(4):255–65. 10.1016/j.pediatrneurol.2013.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Belousova E, Frost MD, et al. Everolimus long-term use in patients with tuberous sclerosis complex: Four-year update of the EXIST-2 study. PLoS One. 2017;12(8):e0180939 10.1371/journal.pone.0180939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for angiomyolipoma associated with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis (EXIST-2): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2013;381(9869):817–24. 10.1016/S0140-6736(12)61767-X . [DOI] [PubMed] [Google Scholar]
- 14.Sheth RA, Feldman AS, Paul E, Thiele EA, Walker TG. Angiographic and volumetric effects of mammalian target of rapamycin inhibitors on angiomyolipomas in tuberous sclerosis. World J Radiol. 2016;8(3):308–15. 10.4329/wjr.v8.i3.308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bissler JJ, Kingswood JC, Radzikowska E, Zonnenberg BA, Frost M, Belousova E, et al. Everolimus for renal angiomyolipoma in patients with tuberous sclerosis complex or sporadic lymphangioleiomyomatosis: extension of a randomized controlled trial. Nephrol Dial Transplant. 2016;31(1):111–9. 10.1093/ndt/gfv249 . [DOI] [PubMed] [Google Scholar]
- 16.Krueger DA, Care MM, Holland K, Agricola K, Tudor C, Mangeshkar P, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363(19):1801–11. 10.1056/NEJMoa1001671 . [DOI] [PubMed] [Google Scholar]
- 17.Bissler JJ R E, Zonnenberg B, Belousova E, Frost MD, Sauter M, Kingswood JC, Brakemeier S, De Vries PJ, Berkowitz N, Voi M, Peyrard S, Budde K, Franz DN. Everolimus for Renal Angiomyolipoma Associated With Tuberous Sclerosis Complex or Sporadic Lymphangioleiomyomatosis: Final Long-term Results From EXIST-2. Eur Urol Supplements. 2016;15(3):e757–e7571. [Google Scholar]
- 18.Yamakado K, Tanaka N, Nakagawa T, Kobayashi S, Yanagawa M, Takeda K. Renal angiomyolipoma: relationships between tumor size, aneurysm formation, and rupture. Radiology. 2002;225(1):78–82. 10.1148/radiol.2251011477 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(PDF)
(DOC)
Data Availability Statement
Novartis supports the publication of scientifically rigorous analysis that is relevant to patient care, regardless of a positive or negative outcome. Qualified external researchers can request access to anonymized patient-level data, respecting patient informed consent, through www.clinicalstudydatarequest.com, according to requirements noted on the web portal.