Abstract
Background and aims
The predictive value of traditional risk factors for vascular events in patients with manifest vascular disease is limited, underscoring the need for novel biomarkers to improve risk stratification. Since hematological parameters are routinely assessed in clinical practice, they are readily available candidates.
Methods
We used data from 3,922 vascular patients, who participated in the Second Manifestations of ARTerial Disease (SMART) study. We first investigated associations between recurrent vascular events and 22 hematological parameters, obtained from the Utrecht Patient Oriented Database (UPOD), and then assessed whether parameters associated with outcome improved risk prediction.
Results
After adjustment for all SMART risk score (SRS) variables, lymphocyte %, neutrophil count, neutrophil % and red cell distribution width (RDW) were significantly associated with vascular events. When individually added to the SRS, lymphocyte % improved prediction of recurrent vascular events with a continuous net reclassification improvement (cNRI) of 17.4% [95% CI: 2.1, 32.1%] and an increase in c-statistic of 0.011 [0.000, 0.022]. The combination of lymphocyte % and neutrophil count resulted in a cNRI of 22.2% [3.2, 33.4%] and improved c-statistic by 0.011 [95% CI: 0.000, 0.022]. Lymphocyte % and RDW yielded a cNRI of 18.7% [3.3, 31.9%] and improved c-statistic by 0.016 [0.004, 0.028]. However, the addition of hematological parameters only modestly increased risk estimates for patients with an event during follow-up.
Conclusions
Several hematological parameters were independently associated with recurrent vascular events. Lymphocyte % alone and in combination with other parameters enhanced discrimination and reclassification. However, the incremental value for patients with a recurrent event was limited.
Introduction
The most common underlying cause of cardiovascular disease is atherosclerosis, leading to over 13 million deaths per year worldwide [1]. The implementation of preventive therapies critically depends on the reliable identification of individuals at risk. In clinical practice, vascular risk assessment is primarily based on risk factors, such as smoking, hypertension, diabetes, obesity and hyperlipidemia [2]. While a large body of evidence has underpinned the significance of such traditional risk factors in primary prevention [3–5], their predictive value for vascular risk in patients with established vascular disease is less clear [6–8]. Thus, novel risk factors are needed to improve risk stratification in secondary prevention and to establish the pathophysiological processes underlying recurrent vascular risk.
The SMART risk score (SRS) has been specifically developed to predict recurrent vascular events in patients with established atherosclerotic vascular disease [9]. This score not only includes traditional risk factors, but also vascular disease history, renal function and high-sensitive C-reactive protein (hs-CRP), an inflammatory marker associated with vascular risk [10]. Besides hs-CRP, several other biomarkers have been linked to prognosis of vascular disease, including N‐terminal pro‐type brain natriuretic peptide, troponins, ST2 and growth-differentiation factor-15 [6,11]. A recent study identified different routinely-measured hematological parameters that predict outcomes in patients with coronary artery disease [12]. Because these parameters are measured by most hematology analyzers, they are readily available for use in clinical practice without the need to rely on expensive equipment. Despite their potential clinical utility, no study has yet assessed whether hematological parameters improve prediction of recurrent events beyond established secondary risk factors used in the SRS. Combining data from the Second Manifestations of ARTerial Disease (SMART) study and the Utrecht Patient Oriented Database (UPOD), we investigated the incremental value of routinely measured hematological parameters for the prediction of recurrent vascular events. We first investigated associations between 22 hematological parameters and recurrent vascular events. Then, we assessed whether parameters independently associated with recurrent events improved risk prediction compared to the SRS.
Methods
Study population
We conducted this study in patients with a clinical manifestation of atherosclerotic vascular disease (cerebrovascular disease, coronary artery disease, peripheral artery disease or abdominal aortic aneurysm) who participated in the SMART study. Details on disease definitions and recruitment procedures have been published previously [9,13]. Briefly, the SMART study, an ongoing, single-center, prospective cohort study, enrolled patients aged 18–80 who were referred to the University Medical Center Utrecht for clinical manifestations of atherosclerotic vascular disease or the treatment of vascular risk factors. Because complete hematological parameters were not available before 2005, we restricted our analysis to a subset of patients enrolled from January 2005 onwards. For this study, follow-up data were available until March, 2014. At baseline, patients were requested to fill in a questionnaire on medical history, symptoms of vascular disease and vascular risk factors. During follow-up, questionnaires were sent to patients or their general practitioner twice a year to obtain information on their health status. Moreover, hospital discharge letters were collected to verify vascular events. All events were adjudicated by three members of the Endpoint Committee. The outcome of interest was a composite endpoint of vascular death, ischemic or hemorrhagic stroke or myocardial infarction, as previously described in more detail [9]. All patients provided written informed consent. The SMART study was approved by the Ethics Committee of the University Medical Center Utrecht.
Hematological parameters
We enriched the SMART cohort with 22 routinely measured hematological parameters, obtained from UPOD, which comprises clinically relevant data from all patients admitted to the University Medical Center Utrecht, including laboratory measurements. Hematology measurements were performed as part of clinical routine in EDTA blood on the Sapphire hematology analyzers (Abbott, Santa Clara, CA). It uses the multi-angle polarized scatter separation technique. Further details on the quantification of hematological parameters in UPOD have recently been published elsewhere [12].
Clinical chemistry
Clinical chemistry measurements, i.e. creatinine, total cholesterol, triglycerides, HDL-cholesterol and hs-CRP, were performed in Li-heparin plasma on clinical routine IVD analyzers (AU5800, Beckman Coulter, Brea, CA) at the central diagnostic laboratory of the UMC Utrecht according to international standards (ISO9001, ISO15189). LDL-cholesterol was calculated using the Friedewald equation; eGFR was calculated from creatinine levels according to the MDRD formula.
Statistical analysis
As for the derivation of the SRS, we truncated all continuous variables, including all hematological parameters, at the 1st and the 99th percentile to reduce the impact of outliers [9]. Using single imputation by additive regression, we imputed missing values for all variables included in the SRS (total n = 126; 0.2%). The variable with the highest percentage of missing values was hs-CRP (n = 75; 1.9%). To facilitate comparison between different hematological parameters, all values were scaled to SD units prior to analysis.
We first evaluated associations between each of the 22 hematological parameters and recurrent vascular events, using Cox proportional hazards modeling adjusted for all SRS variables [age, sex, diabetes mellitus, current smoking, systolic blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, hs-CRP, estimated glomerular filtration rate (eGFR), years since first vascular event, history of cerebrovascular disease, history of coronary artery disease, history of abdominal aortic aneurysm, history of peripheral artery disease]. Analogous to the SRS, hs-CRP was loge-transformed and quadratic terms were added for age and eGFR [9]. Since none of hematological parameters showed a skewness >2, loge-transformation was not applied. Hematological parameters were entered as quadratic polynomials if the addition of a quadratic term improved model fit, as indicated by the likelihood ratio test (p<0.05). Accordingly, we added a quadratic term for hematocrit. The proportional hazards assumption was tested for each model using scaled Schoenfeld residuals. Associations between hematological parameters and outcome were adjusted for multiple testing. Since several of the 22 parameters were highly correlated (Figure A in S1 File), we estimated the effective number of independent tests for multiple testing correction using principal component analysis. The first 11 principal components explained over 95% of the variance in the hematology data, yielding a significance threshold of 0.05/11 = 0.0045.
We next evaluated the added predictive value of hematological parameters, significantly associated with outcome, by comparing different biomarker models to a reference model in terms of discrimination and reclassification. The reference model was constructed by fitting the SRS variables to our dataset. The single biomarker models included the SRS variables and one of the hematological parameters significantly associated with recurrent event risk. We additionally assessed the performance of multi-biomarker models that included combinations of hematological parameters. To evaluate discrimination, we calculated Harrell’s c for each model and compared c-statistics between each biomarker model and the reference model, using the jackknife approach proposed by Antolini et al [14]. Extending the area under the receiver operating characteristic (ROC) curve to censored outcomes, Harrell’s c measures the ability of a risk prediction model to discriminate individuals with a target events from event-free individuals. Reclassification was assessed by continuous net reclassification improvement (cNRI), as implemented in the nricens R package (https://cran.r-project.org/web/packages/nricens/index.html), which computes NRI for censored survival data. Confidence intervals for NRI were computed by bootstrapping. To obtain robust reclassification indices, we assessed cNRI at 7 years, given a median follow-up of 4.6 years (IQR: 2.5–6.9 years). 7 years also corresponds to the follow-up period for which the SRS was initially calibrated before risk estimates were extrapolated to 10-year risk predictions [9]. Due to the absence of established categories for the 7-year risk of recurrent vascular events, we did not assess categorical NRI.
Results
3,922 patients with manifest vascular disease enrolled in the SMART cohort were included in this study. Baseline characteristics of the study population are summarized in Table 1. During a median follow-up of 4.6 years (IQR: 2.5–6.9 years), 310 recurrent vascular events occurred. In contrast to Dorresteijn et al. [9], we only included patients recruited from 2005 onwards. Compared to this study, we observed lower event rates (1.7% vs. 2.6%), most likely reflecting improved secondary prevention therapies. In line with this, the proportion of patients treated with statins was higher in our study. Table 2 shows baseline values of all 22 hematological parameters stratified by event status.
Table 1. Baseline characteristics.
| All (N = 3922) |
No vascular event (N = 3612) |
Vascular event (N = 310) |
|
|---|---|---|---|
| Age, years | 61 (54–68) | 61 (54–67) | 64 (56–71) |
| Male sex | 2850 (73) | 2610 (72) | 240 (77) |
| Type of vascular disease | |||
| Cerebrovascular disease | 1125 (29) | 1032 (29) | 93 (30) |
| Coronary artery disease | 2588 (66) | 2373 (66) | 215 (69) |
| Peripheral artery disease | 531 (14) | 481 (13) | 50 (16) |
| Abdominal aortic aneurysm | 236 (6) | 213 (6) | 23 (7) |
| Years since first vascular event | |||
| less than 1 year | 2283 (60) | 2140 (61) | 143 (48) |
| 1–2 years | 389 (10) | 363 (10) | 26 (9) |
| over 2 years | 1110 (29) | 980 (28) | 130 (44) |
| Current smoking | 1060 (27) | 954 (27) | 106 (34) |
| Diabetes mellitus | 704 (18) | 628 (17) | 76 (25) |
| Systolic blood pressure, mm Hg | 136 (124–149) | 135 (124–149) | 140 (129–155) |
| Diastolic blood pressure, mm Hg | 80 (73–88) | 80 (74–88) | 81 (73–90) |
| eGFR, ml/min/1.73 m2 | 77 (66–88) | 77 (67–88) | 70 (60–84) |
| Total cholesterol, mmol/l | 4.3 (3.7–5.1) | 4.3 (3.7–5.1) | 4.3 (3.7–5.1) |
| LDL cholesterol, mmol/l | 2.4 (1.9–3.0) | 2.4 (1.9–3.0) | 2.4 (1.9–3.1) |
| HDL cholesterol, mmol/l | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.1 (1.0–1.4) |
| Triglycerides, mmol/l | 1.2 (0.9–1.8) | 1.2 (0.9–1.8) | 1.3 (0.9–1.9) |
| hs-CRP, nmol/l | 16 (8–36) | 15 (8–34) | 26 (12–62) |
| Medication | |||
| Lipid-lowering drugs | 3140 (80) | 2888 (80) | 252 (81) |
| Blood pressure-lowering drugs | 3086 (79) | 2829 (78) | 257 (83) |
| Glucose-lowering drugs | 560 (14) | 497 (14) | 63 (20) |
| Antithrombotic drugs | 3493 (89) | 3206 (89) | 287 (93) |
Discrete variables are expressed as count (%), continuous variables as median (IQR). Type of vascular disease is not mutually exclusive as patients may have experienced several manifestations of vascular disease. eGFR: estimated glomerular filtration rate (see [9]); HDL: high-density lipoprotein; hs-CRP: high-sensitivity C-reactive protein; IQR: inter-quartile range; LDL: low-density lipoprotein.
Table 2. Hematological parameters.
| Unit | No vascular event | Vascular event | |
|---|---|---|---|
| White blood cells | 109/l | 6.6 (5.5–7.9) | 7.2 (5.9–8.7) |
| Neutrophils | 109/l | 3.8 (3.0–4.7) | 4.2 (3.5–5.4) |
| Lymphocytes | 109/l | 1.9 (1.5–2.4) | 1.9 (1.5–2.3) |
| Monocytes | 109/l | 0.54 (0.44–0.67) | 0.58 (0.49–0.70) |
| Eosinophils | 109/l | 0.19 (0.12–0.28) | 0.21 (0.15–0.28) |
| Basophils | 109/l | 0.04 (0.02–0.06) | 0.04 (0.03–0.06) |
| Neutrophil % | % | 57.9 (52.1–63.7) | 60.3 (55.1–66.2) |
| Lymphocyte % | % | 29.4 (24.4–34.7) | 26.2 (21.8–32.0) |
| Monocyte % | % | 8.2 (6.9–9.7) | 8.2 (6.8–9.8) |
| Eosinophil % | % | 2.9 (1.9–4.2) | 3.0 (2.1–4.1) |
| Basophil % | % | 0.61 (0.39–0.88) | 0.58 (0.36–0.78) |
| Red blood cells | 1012/l | 4.7 (4.4–5.0) | 4.6 (4.2–4.9) |
| Hemoglobin | mmol/l | 8.8 (8.3–9.3) | 8.8 (8.2–9.3) |
| MCV | fl | 89.8 (87.1–92.5) | 89.9 (86.9–92.8) |
| RDW | % | 12.1 (11.7–12.7) | 12.3 (11.8–13.3) |
| MCH | fmol | 1.9 (1.8–2.0) | 1.9 (1.8–2.0) |
| MCHC | mmol/l | 21.1 (20.7–21.5) | 21.1 (20.5–21.5) |
| Hematocrit | % | 41.7 (39.3–44.1) | 41.7 (38.6–44.4) |
| Platelets | 109/l | 237 (202–280) | 235 (203–276) |
| MPV | fl | 7.7 (7.2–8.4) | 7.9 (7.3–8.6) |
| Plateletcrit | % | 0.19 (0.17–0.22) | 0.20 (0.17–0.23) |
| PDW | 10xGSD | 16.1 (15.8–16.6) | 16.2 (15.8–16.6) |
Values are expressed as median (IQR) and stratified by event status. GSD: geometric standard deviation; IQR: inter-quartile range; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; MPV: mean platelet volume; PDW: platelet distribution width; RDW: red cell distribution width.
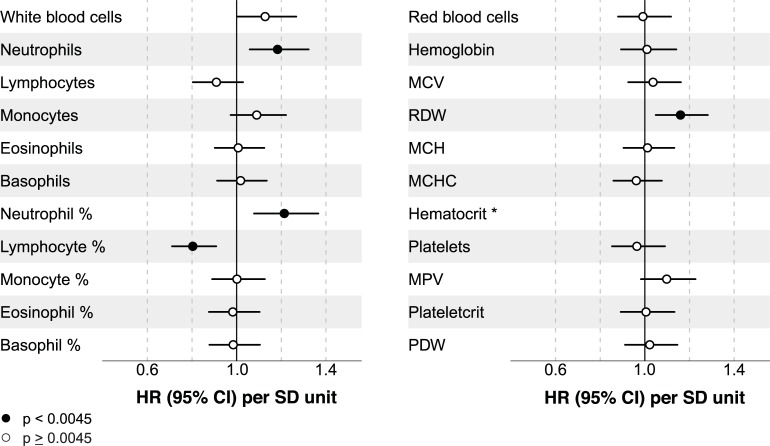
First, we studied associations between hematological parameters and secondary vascular outcomes. Table A in S1 File displays unadjusted and adjusted hazard ratios (HRs) for all hematological parameters. HRs for all SRS variables (reference model) are shown in Table B in S1 File. Since most hematological parameters are directly or indirectly related to immunological processes, we assessed whether these associations were independent of hs-CRP. The addition of hs-CRP particularly attenuated effect estimates for white blood cell count, neutrophil count, monocyte count and neutrophil % (Fig B in S1 File). Four parameters remained significantly associated with vascular events after adjustment for the SRS variables (Fig 1). Lymphocyte % showed a negative association with the recurrent vascular events (HR in SD units: 0.80 [95% CI: 0.71, 0.91]), whereas neutrophil count (HR in SD units: 1.19 [1.06, 1.33]), neutrophil % (HR in SD units: 1.22 [1.08, 1.37]), and RDW (HR in SD units: 1.16 [1.05, 1.28]) were positively associated with recurrent vascular events.
Fig 1. Each of the 22 hematological parameters was analyzed separately.
HRs are given per SD-unit increase adjusted for all SRS variables. CI: confidence interval; HR: hazard ratio; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; MCV: mean corpuscular volume; MPV: mean platelet volume; PDW: platelet distribution width; RDW: red cell distribution width; SD: standard deviation; SRS: SMART risk score. *A quadratic term was added for hematocrit. Significance test for quadratic polynomial after adjustment for all SRS variables: χ2(df = 2) = 6.2; p = 0.045.
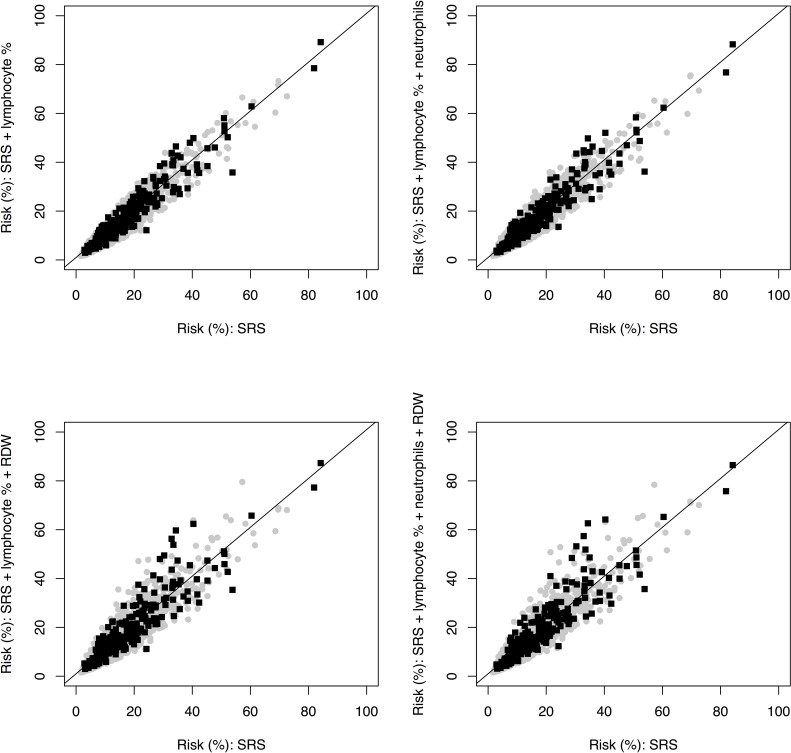
To assess discrimination and continuous reclassification, we next added each of the four hematological parameters that were independently associated with recurrent event risk to a reference model composed of the SRS variables (Table 3). We observed the largest cNRI for lymphocyte %. For events, this parameter improved continuous reclassification by 13.6%, for non-events by 3.8%, yielding a cNRI of 17.4% [95% CI: 2.1, 32.1%]. Additionally, lymphocyte % improved discrimination (c-statistic) by 0.0110 [95% CI: 0.0004, 0.0216]. We also tested whether lymphocyte % combined with other parameters further improved the predictive performance of the SRS. Neutrophil % was not included into a multi-biomarker model because this parameter was highly correlated with lymphocyte % (r = -0.92). Lymphocyte % and neutrophil count improved cNRI by 22.2% [3.2, 33.4%]. The increase in c-statistic was 0.0112 [0.0004, 0.220], which was comparable to that achieved by lymphocyte % alone. For lymphocyte % and RDW combined, the cNRI was 18.7% [3.3, 31.9%], the improvement in c-statistic was 0.016 [0.004, 0.028]. With a cNRI of 17.2% [4.1, 32.8%], all three parameters yielded a lower reclassification improvement than the combination of lymphocyte % and neutrophil count. Lymphocyte % in combination with RDW improved discrimination with an increase in c-statistic of 0.016 [0.004, 0.028]. Fig 2 illustrates the change in predicted risk for different biomarker models, stratified by event status. While lymphocyte % alone and the combination of lymphocyte % and neutrophil count showed the largest continuous reclassification improvement (Table 3) for events, risk estimates increased only modestly in patients who experienced an event. Lymphocyte % and RDW combined predominantly increased risk estimates for events in the higher risk range.
Table 3. Predictive performance of hematological parameters.
| Reclassification improvement % | ||||
|---|---|---|---|---|
| Change in c-statistic [95% CI] | with event |
without event |
Net [95% CI] |
|
|
Neutrophils |
0.006 [-0.002, 0.014] |
-9.1 |
15.6 |
6.5 [-6.0, 22.7] |
| Neutrophil % | 0.008 [-0.002, 0.018] | 7.2 | 6.7 | 13.9 [-0.3, 27.7] |
| Lymphocyte % | 0.011 [0.000, 0.022] | 13.6 | 3.8 | 17.4 [2.1, 32.1] |
| RDW | 0.007 [-0.001, 0.015] | -11.3 | 25.0 | 13.6 [-1.9, 26.4] |
|
Lymphocyte % + neutrophils |
0.011 [0.000, 0.022] |
14.8 |
7.4 |
22.2 [3.2, 33.4] |
| Lymphocyte % + RDW |
0.016 [0.004, 0.028] | 9.0 | 9.7 | 18.7 [3.3, 31.9] |
| Lymphocyte % + neutrophils + RDW |
0.016 [0.004, 0.028] | 5.1 | 12.0 | 17.2 [4.1, 32.8] |
First, hematological parameters significantly associated with outcome were individually added to a reference model composed of the SRS variables. For each single biomarker model (SRS + hematological parameter), we evaluated improvement in discrimination (c-statistic) and reclassification (NRI) compared to the reference model (SRS). We then assessed the predictive performance of multi-biomarker models comprising combinations of lymphocyte % and other hematological parameters. NRI: net reclassification improvement; RDW: red cell distribution width; SRS: SMART risk score.
Fig 2. Predicted 7-year risks for reference model (SRS) vs. selected biomarker models (SRS + hematological parameters) stratified by event status.
Patients who did not experience a recurrent vascular event during 7-years of follow up (gray circles) were correctly reclassified if there predicted risk was lower after the addition of hematological parameters to the SRS (below the black line). Patients who experienced an event (black squares) were correctly reclassified if there predicted risk was higher after the addition of hematological parameters to the SRS (above the black line). RDW: red cell distribution width; SRS: SMART risk score.
Discussion
In this study, we evaluated the incremental predictive value of routinely measured hematological parameters for the prediction of recurrent vascular events in patients with established vascular disease. We first investigated associations between 22 parameters and recurrent event risk and then assessed whether parameters associated with outcome improved risk prediction. Out of the four parameters significantly associated with outcome, lymphocyte % showed the largest cNRI when individually added to the SRS. Overall, the combination of lymphocyte % and neutrophil count yielded the largest cNRI compared to the SRS, but only modestly improved discrimination (c-statistic) and risk estimates for patients who experienced an event during follow-up.
Lymphocytes have been implicated in the modulation of inflammatory processes at distinct stages of atherogenesis [15]. Numerous observational studies in patients with coronary artery disease have reported associations of low absolute and relative lymphocyte levels with poor cardiovascular outcomes [12,16–21]. However, some studies found no link between absolute lymphocyte count and all-cause mortality in pre-existing coronary artery disease [22–24]. Consistent with a role of low lymphocyte levels in vascular disease progression, lymphocyte apoptosis is enhanced in myocardial infarction, but not in stable angina, indicating that low lymphocyte levels may specifically reflect inflammatory processes in advanced atherosclerosis (e.g. plaque rupture) [25]. In our study, however, lymphocyte % rather than absolute lymphocyte count was associated with recurrent vascular events. Accordingly, lymphocyte levels were comparable between patients with and without a recurrent event during follow-up–unlike concentrations of other white blood cell types, such as neutrophils and monocytes (Table 2). Low lymphocyte % may thus reflect increased levels of other white blood cell types in patients at risk.
Besides lymphocyte %, both absolute and relative neutrophil count were independently associated with recurrent vascular risk without improving risk prediction when individually added to the SRS. The combination of lymphocyte % and absolute neutrophil count showed the largest cNRI of all models assessed, but only moderately increased risk estimates for events. The discrimination improvement with lymphocyte % and absolute neutrophil count was likewise limited with an increase in c-statistic equal to that achieved by lymphocyte % alone. The neutrophil to lymphocyte ratio has been widely studied as a marker of cardiovascular risk, suggesting that neutrophil levels are associated with poor prognosis of coronary and peripheral artery disease [26]. There is mounting evidence that neutrophils play an important role in early and advanced atherosclerosis by exacerbating endothelial dysfunction, recruiting monocytes to atherosclerotic lesions, promoting foam cell formation and by destabilizing atherosclerotic plaques [27].
RDW was also independently associated with clinical outcome. Several studies have linked increased RDW to poor outcomes in patients with coronary artery disease, stroke or peripheral artery disease [12,28–31]. RDW is a measure of the variation in erythrocyte volume. The mechanisms by which RDW relates to cardiovascular risk are unknown. Severe inflammation is associated with inhibition of erythrocyte maturation, which results in anisocytosis, suggesting that RDW reflects enhanced inflammation in atherosclerosis, potentially relevant to disease progression [32]. However, RDW did not improve risk prediction and, when combined with lymphocyte %, yielded a cNRI comparable to that achieved by lymphocyte % alone. Moreover, RDW and lymphocyte % predominantly increased risk estimates for events in the higher risk range. Since patients with a high SRS would already be eligible for increased surveillance and more extensive treatment, the added value of RDW for clinical risk prediction is limited.
In the unadjusted analysis, total white blood cell count and monocyte count were strongly associated with recurrent events. However, adjustment for all SRS variables attenuated effect estimates for both parameters, especially due to the inflammatory marker hs-CRP (Fig B in S1 File). In vitro findings suggest that CRP interacts with monocytes to enhance inflammation in acute coronary syndrome [33]. Thus, hs-CRP and monocytes may share a common pathophysiological pathway, whereas other hematological parameters may reflect inflammatory processes that do not, or to a lesser extent, involve CRP. Overall, our findings lend further support to the inflammatory hypothesis of atherothrombosis and add to recent clinical trial data suggesting that anti-inflammatory therapy reduces cardiovascular risk in secondary prevention [34].
Hematological parameters are routinely measured in many hospitals and do not require expensive equipment for analysis, underscoring their clinical potential. In our study, lymphocyte % alone and combined with other hematological parameters yielded the largest cNRI. However, these models only marginally improved discrimination and absolute risk estimates for events. Thus, it remains to be determined whether the incorporation of hematological parameters into risk prediction algorithms would influence clinical decision making in secondary prevention. Since many clinical and demographic characteristics are not assessed systematically in clinical routine, it is often not possible to calculate clinical scores, such as the SRS. Routine hematology testing may be combined with other emerging biomarker technologies suitable for clinical laboratory use to construct biomarker risk scores that do not depend on the availability of clinical information. Such biomarker-based scores could routinely be computed by clinical chemistry laboratories, facilitating the implementation of risk assessment tools for secondary prevention in clinical practice. Besides adding hematological parameters to established clinical scores, future studies also evaluate their predictive value in combination with other biomarkers.
Moreover, the ability of hematological parameters to predict recurrent vascular risk may vary between different manifestations of vascular disease, such as myocardial infarction and ischemic stroke. Since hematological parameters were not available from all SMART patients, the sample size of our study population was limited. As a result, we could not perform stratified analyses for different vascular disease groups. Therefore, further research is required to corroborate our findings in larger cohorts and establish the predictive value of hematological parameters for different manifestations of vascular disease.
In conclusion, we identified several hematological parameters that were independently associated recurrent vascular event in patients with vascular disease. When added to a model comprising the SRS variables, lymphocyte % alone and in combination with other hematological parameters, especially with neutrophil count, improved risk prediction, but only modestly increased risk estimates for patients who experienced a recurrent vascular event.
Supporting information
(PDF)
Acknowledgments
We gratefully acknowledge the contribution of the SMART research nurses; R.van Petersen (data-manager); B.G.F. Dinther (vascular manager) and the participants of the SMART Study Group: A. Algra MD, PhD; Y. van der Graaf, MD, PhD; D.E. Grobbee, MD, PhD; G.E.H.M. Rutten, MD, PhD, Julius Center for Health Sciences and Primary care; F.L.J.Visseren, MD, PhD, Department of Vascular Medicine; G.J. de Borst, MD, PhD, Department of Vascular Surgery; L.J. Kappelle, MD, PhD, Department of Neurology; T. Leiner, MD, PhD, Department of Radiology; P.A. Doevendans, MD,PhD, Department of Cardiology.
Data Availability
Data may not be made publicly available due to legal restrictions. Data are available upon request from jwester3@umcutrecht.nl (SMART) and upod@umcutrecht.nl (UPOD). The authors confirm they accessed the data used in their study in the same manner they expect future researchers to do so, and did not receive special privileges.
Funding Statement
Folkert W. Asselbergs is supported by a Dekker scholarship-Junior Staff Member 2014T001 - Netherlands Heart Foundation and UCL Hospitals NIHR Biomedical Research Centre.
References
- 1.Mendis S, Puska P, Norrving B. Global atlas on vascular disease prevention and control Geneva: World Health Organization, 2011. [Google Scholar]
- 2.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European guidelines on vascular disease prevention in clinical practice: the sixth joint task force of the European Society of Cardiology and other societies on vascular disease prevention in clinical practice. Eur Heart J. 2016;37:2315–2381. 10.1093/eurheartj/ehw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Agostino RB, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General vascular risk profile for use in primary care. Circulation. 2008;117:743–753. 10.1161/CIRCULATIONAHA.107.699579 [DOI] [PubMed] [Google Scholar]
- 4.Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. 10.1001/jama.2009.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364: 937–952. 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 6.Beatty AL, Ku IA, Bibbins‐Domingo K, Christenson RH, DeFilippi CR, Ganz P, et al. Traditional risk factors versus biomarkers for prediction of secondary events in patients with stable coronary heart disease: from the heart and soul study. J. Am Heart Assoc. 2015;4:e001646 10.1161/JAHA.114.001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Agostino RB, Belanger AJ, Kannel WB, Cruickshank JM. Relation of low diastolic blood pressure to coronary heart disease death in presence of myocardial infarction: the Framingham Study. BMJ 1991;303:385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero-Corral A, Montori VM, Somers VK, Korinek J, Thomas RJ, Allison TG, et al. Association of bodyweight with total mortality and with vascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006;368:666–678. 10.1016/S0140-6736(06)69251-9 [DOI] [PubMed] [Google Scholar]
- 9.Dorresteijn JAN, Visseren FLJ, Wassink AMJ, Gondrie MJA, Steyerberg EW, Ridker PM, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99:866–872. 10.1136/heartjnl-2013-303640 [DOI] [PubMed] [Google Scholar]
- 10.Buckley DI, Fu R, Freeman M, Rogers K, Helfand M. C-reactive protein as a risk factor for coronary heart disease: a systematic review and meta-analyses for the US Preventive Services Task Force. Ann Intern Med. 2009;151;483–495. [DOI] [PubMed] [Google Scholar]
- 11.Eggers KM, Lindahl B. Prognostic biomarkers in acute coronary syndromes: risk stratification beyond cardiac troponins. Curr Cardiol Rep. 2017;19:29 10.1007/s11886-017-0840-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gijsberts CM, den Ruijter HM, de Kleijn DP, Huisman A, ten Berg MJ, van Wijk RH, et al. Hematological parameters improve prediction of mortality and secondary adverse events in coronary angiography patients: a longitudinal cohort study. Medicine. 2015;94:e1992 10.1097/MD.0000000000001992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simons PC, Algra A, van de Laak MF, Grobbee DE, van der Graaf Y. Second Manifestations of ARTerial disease (SMART) study: rationale and design. Eur J Epidemiol. 1999;15:773–81. [DOI] [PubMed] [Google Scholar]
- 14.Antolini L, Nam BH, D'Agostico RB. Inference on correlated discrimination measures in survival analysis: a nonparametric approach. Commun Statist Theory Meth. 2004;33 2117–2135. [Google Scholar]
- 15.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Ann Rev Immunol. 2009;27:165–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dragu R, Huri S, Zuckerman R, Suleiman M, Mutlak D, Agmon Y, et al. Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis. 2008;196: 405–412. 10.1016/j.atherosclerosis.2006.11.022 [DOI] [PubMed] [Google Scholar]
- 17.Horne BD, Anderson J, John JM, Weaver A, Bair TL, Jensen KR, et al. Which white blood cell subtypes predict increased cardiovascular risk?. J Am Coll Cardiol. 2005;45: 1638–1643. 10.1016/j.jacc.2005.02.054 [DOI] [PubMed] [Google Scholar]
- 18.Núñez J, Sanchis J, Bodí V, Núñez E, Mainar L, Heatta AM, et al. Relationship between low lymphocyte count and major cardiac events in patients with acute chest pain, a non-diagnostic electrocardiogram and normal troponin levels. Atherosclerosis. 2009;206:251–257. 10.1016/j.atherosclerosis.2009.01.029 [DOI] [PubMed] [Google Scholar]
- 19.ó Hartaigh B, Bosch JA, Thomas GN, Lord JM, Pilz S, Loerbroks A, et al. Which leukocyte subsets predict cardiovascular mortality? From the LUdwigshafen RIsk and Cardiovascular Health (LURIC) Study. Atherosclerosis. 2012:224;161–169. 10.1016/j.atherosclerosis.2012.04.012 [DOI] [PubMed] [Google Scholar]
- 20.Ommen SR, Gibbons RJ, Hodge DO, Thomson SP. Usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am J Cardiol. 1997:79;812–814. [DOI] [PubMed] [Google Scholar]
- 21.Zouridakis EG, Garcia-Moll X, Kaski JC. Usefulness of the blood lymphocyte count in predicting recurrent instability and death in patients with unstable angina pectoris. Am J Cardiol. 2000;86:449–51. [DOI] [PubMed] [Google Scholar]
- 22.Azab B, Shah N, Akerman M, McGinn JT. Value of platelet/lymphocyte ratio as a predictor of all-cause mortality after non-ST-elevation myocardial infarction. J Thromb Thrombolysis. 2012:34(3); 326–334. 10.1007/s11239-012-0718-6 [DOI] [PubMed] [Google Scholar]
- 23.Azab B, Zaher M, Weiserbs KF, Torbey E, Lacossiere K, Gaddam S, et al. Usefulness of neutrophil to lymphocyte ratio in predicting short-and long-term mortality after non–ST-elevation myocardial infarction. Am J Cardiol. 2010;106:470–476. 10.1016/j.amjcard.2010.03.062 [DOI] [PubMed] [Google Scholar]
- 24.Gijsberts CM, Ellenbroek GH, Ten Berg MJ, Huisman A, van Solinge WW, Asselbergs FW, et al. Routinely analyzed leukocyte characteristics improve prediction of mortality after coronary angiography. Eur J Prev Cardiol. 2016;23:1211–1220. 10.1177/2047487315621832 [DOI] [PubMed] [Google Scholar]
- 25.Pasqui AL, Di Renzo M, Bova G, Bruni F, Puccetti L, Pompella G, et al. T cell activation and enhanced apoptosis in non-ST elevation myocardial infarction. Clin Exp Med. 2003; 3:37–44. 10.1007/s102380300014 [DOI] [PubMed] [Google Scholar]
- 26.Balta S, Celik T, Mikhailidis DP, Ozturk C, Demirkol S, Aparci M, et al. The relation between atherosclerosis and the neutrophil–lymphocyte ratio. Clin Appl Thromb Hemost. 2016;22:405–411. 10.1177/1076029615569568 [DOI] [PubMed] [Google Scholar]
- 27.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ Res. 2012;110;875–888. 10.1161/CIRCRESAHA.111.257535 [DOI] [PubMed] [Google Scholar]
- 28.Dabbah S, Hammerman H, Markiewicz W, Aronson D. Relation between red cell distribution width and clinical outcomes after acute myocardial infarction. Am J Cardiol. 2010;105: 312–317. 10.1016/j.amjcard.2009.09.027 [DOI] [PubMed] [Google Scholar]
- 29.Tonelli M, Sacks F, Arnold M, Moye L, Davis B, Pfeffer M. Relation between red blood cell distribution width and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. 10.1161/CIRCULATIONAHA.107.727545 [DOI] [PubMed] [Google Scholar]
- 30.Ani C, Ovbiagele B. Elevated red blood cell distribution width predicts mortality in persons with known stroke. J Neurol Sci. 2009;277:103–108. 10.1016/j.jns.2008.10.024 [DOI] [PubMed] [Google Scholar]
- 31.Ye Z, Smith C, Kullo IJ. Usefulness of red cell distribution width to predict mortality in patients with peripheral artery disease. Am J Cardiol. 2001;107:1241–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Montagnana M, Cervellin G, Meschi T, Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. Clin Chem Lab Med. 2012;50: 635–641. [DOI] [PubMed] [Google Scholar]
- 33.Liuzzo G, Santamaria M, Biasucci LM, Narducci M, Colafrancesco V, Porto A, et al. Persistent activation of nuclear factor kappa-B signaling pathway in patients with unstable angina and elevated levels of C-reactive protein: evidence for a direct proinflammatory effect of azide and lipopolysaccharide-free C-reactive protein on human monocytes via nuclear factor kappa-B activation. J Am Coll Cardiol. 2007;49:185–194. 10.1016/j.jacc.2006.07.071 [DOI] [PubMed] [Google Scholar]
- 34.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
Data may not be made publicly available due to legal restrictions. Data are available upon request from jwester3@umcutrecht.nl (SMART) and upod@umcutrecht.nl (UPOD). The authors confirm they accessed the data used in their study in the same manner they expect future researchers to do so, and did not receive special privileges.